Information-Driven Design as a Potential Approach for 3D Printing of Skeletal Muscle Biomimetic Scaffolds
Abstract
:1. Introduction: the Current Scenario
2. Moving Towards Biomimetic Engineered Muscular Constructs
2.1. SM dECM-Based Hydrogel: A Material for Biomimetic Engineered Muscular Constructs?
2.2. Stereolithography: A Suitable Fabrication Technique for Engineered Muscular Constructs?
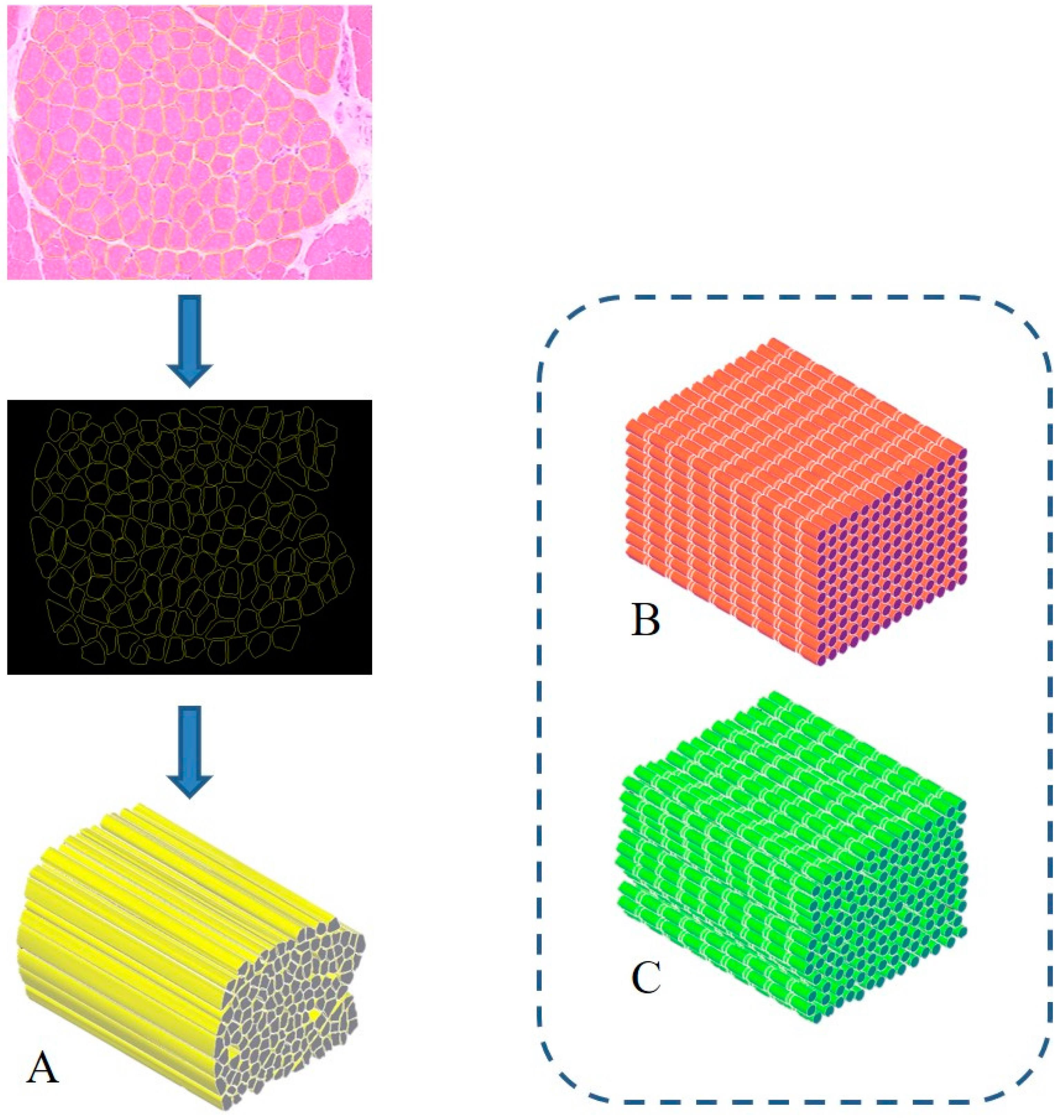
3. Proposal of a Biomimetic Scaffold for the Treatment of Severe Muscle Injuries
Author Contributions
Funding
Conflicts of Interest
References
- Mase, V.J.; Hsu, J.R.; Wolf, S.E.; Wenke, J.C.; Baer, D.G.; Owens, J.; Badylak, S.F.; Walters, J.T. Clinical Application of an Acellular Biologic Scaffold for Surgical Repair of a Large, Traumatic Quadriceps Femoris Muscle Defect. Orthopedics 2010, 33, 511. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Badylak, S.F. Regeneration of skeletal muscle. Cell Tissue Res. 2011, 347, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Kasif, S.; Murali, T.M. Systems Biology Characterization of Engineered Tissues. Ann. Rev. Biomed. Eng. 2013, 15, 55–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigodarzere, G.C.; Mantero, S. Skeletal muscle tissue engineering: Strategies for volumetric constructs. Front. Physiol. 2014, 5, 362. [Google Scholar] [CrossRef] [Green Version]
- Grasman, J.M.; Zayas, M.J.; Page, R.L.; Pins, G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015, 25, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Mulbauer, G.D.; Matthew, H. Biomimetic Scaffolds in Skeletal Muscle Regeneration. Discoveries 2019, 7, e90. [Google Scholar] [CrossRef]
- Orlando, G.; Soker, S.; Stratta, R.J. Organ Bioengineering and Regeneration as the New Holy Grail for Organ Transplantation. Ann. Surg. 2013, 258, 221–232. [Google Scholar] [CrossRef]
- Trevisan, C.; Maghin, E.; Dedja, A.; Caccin, P.; De Cesare, N.; Franzin, C.; Boso, D.; Pesce, P.; Caicci, F.; Boldrin, F.; et al. Allogenic tissue-specific decellularized scaffolds promote long-term muscle innervation and functional recovery in a surgical diaphragmatic hernia model. Acta Biomater. 2019, 89, 115–125. [Google Scholar] [CrossRef]
- Urciuolo, A.; De Coppi, P. DecellularizedTissue for Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2392. [Google Scholar] [CrossRef] [Green Version]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geißler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.; Lee, J.; Yoo, J.J.; Kim, G.H.; Lee, S.J. Effect of Hierarchical Scaffold Consisting of Aligned dECM Nanofibers and Poly(lactide-co-glycolide) Struts on the Orientation and Maturation of Human Muscle Progenitor Cells. ACS Appl. Mater. Interfaces 2019, 11, 39449–39458. [Google Scholar] [CrossRef] [PubMed]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D Bioprinting in Skeletal Muscle Tissue Engineering. Small 2019, 15, e1805530. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Farr, A.C.; Hogan, K.J.; Mikos, A.G. Nanomaterial Additives for Fabrication of Stimuli-Responsive Skeletal Muscle Tissue Engineering Constructs. Adv. Health Mater. 2020, 2000730. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sarrafian, T.L.; Bodine, S.C.; Murphy, B.; Grayson, J.K.; Stover, S.M. Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review. Vet. Surg. 2018, 47, 524–535. [Google Scholar] [CrossRef]
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef]
- Popov, A.; Malferrari, S.; Kalaskar, D.M. 3D bioprinting for musculoskeletal applications. J. 3D Print. Med. 2017, 1, 191–211. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Motaung, K.S.; Adesida, A.B. Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628. [Google Scholar] [CrossRef] [Green Version]
- Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: Prospects for the future. Methods 2020, 171, 108–118. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Pati, F.; Cho, D.-W. Bioprinting of 3D Tissue Models Using Decellularized Extracellular Matrix Bioink. Breast Cancer 2017, 1612, 381–390. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, T.G.; Jeong, J.; Yi, H.-G.; Park, J.W.; Hwang, W.; Cho, D.-W. 3D Cell Printing of Functional Skeletal Muscle Constructs Using Skeletal Muscle-Derived Bioink. Adv. Health Mater. 2016, 5, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Jun, Y.-J.; Kim, D.Y.; Yi, H.-G.; Chae, S.-H.; Kang, J.; Lee, J.; Gao, G.; Kong, J.-S.; Jang, J.; et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Devarasetty, M.; Kang, H.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S.; et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Lee, J.; Atala, A.; Yoo, J.J.; Lee, S.J.; Kim, G. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials 2019, 230, 119632. [Google Scholar] [CrossRef]
- Teodori, L.; Costa, A.; Marzio, R.; Perniconi, B.; Coletti, D.; Adamo, S.; Gupta, B.; Tárnok, A. Native extracellular matrix: A new scaffolding platform for repair of damaged muscle. Front. Physiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef]
- Ciocci, M.; Mochi, F.; Carotenuto, F.; Di Giovanni, E.; Prosposito, P.; Francini, R.; De Matteis, F.; Reshetov, I.V.; Casalboni, M.; Melino, S.; et al. Scaffold-in-Scaffold Potential to Induce Growth and Differentiation of Cardiac Progenitor Cells. Stem Cells Dev. 2017, 26, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VijayaVenkataRaman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg, A.; Hobeika, N.; Stehlin, F.; Malval, J.; Wieder, F.; Prabhakaran, P.; Baldeck, P. Recent advances in two-photon stereolithography. In Updates in Advanced Lithography; Hosaka, S., Ed.; IntechOpen: London, UK, 2013; pp. 35–64. [Google Scholar] [CrossRef] [Green Version]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Electron. 2013, 25, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.-W.; Kwon, S.M.; Cho, D.-W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Stress-strain measurements of human and porcine corneas after riboflavin–ultraviolet-A-induced cross-linking. J. Cataract. Refract. Surg. 2003, 29, 1780–1785. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Stachon, T.; Wang, J.; Song, X.; Langenbucher, A.; Seitz, B.; Szentmáry, N. Impact of crosslinking/riboflavin-UVA-photodynamic inactivation onviability, apoptosis and activation of human keratocytes in vitro. J. Biomed. Res. 2015, 29, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Ahearne, M.; Coyle, A. Application of UVA-riboflavin crosslinking to enhance the mechanical properties of extracellular matrix derived hydrogels. J. Mech. Behav. Biomed. Mater. 2016, 54, 259–267. [Google Scholar] [CrossRef]
- Arrizabalaga, J.H.; Nollert, M.U. Riboflavin-UVA crosslinking of amniotic membranes and its influence on the culture of adipose-derived stem cells. J. Mech. Behav. Biomed. Mater. 2020, 106, 103729. [Google Scholar] [CrossRef]
- Bajaj, P.; Chan, V.; Jeong, J.H.; Zorlutuna, P.; Kong, H.; Bashir, R. 3-D biofabrication using stereolithography for biology and medicine. IEEE Eng. Med. Biol. Soc. 2012, 2012, 6805–6808. [Google Scholar] [CrossRef]
- Steier, A.; Muñiz, A.; Neale, D.; Lahann, J. Emerging Trends in Information-Driven Engineering of Complex Biological Systems. Adv. Mater. 2019, 31, e1806898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joung, D.; Lavoie, N.S.; Guo, S.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D Printed Neural Regeneration Devices. Adv. Funct. Mater. 2019, 30, 1906237. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Teodori, L.; Crupi, A.; Costa, A.; Diaspro, A.; Melzer, S.; Tárnok, A. Three-dimensional imaging technologies: A priority for the advancement of tissue engineering and a challenge for the imaging community. J. Biophotonics 2016, 10, 24–45. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiguera, S.; Del Gaudio, C.; Carotenuto, F.; Di Nardo, P.; Teodori, L. Information-Driven Design as a Potential Approach for 3D Printing of Skeletal Muscle Biomimetic Scaffolds. Nanomaterials 2020, 10, 1986. https://doi.org/10.3390/nano10101986
Baiguera S, Del Gaudio C, Carotenuto F, Di Nardo P, Teodori L. Information-Driven Design as a Potential Approach for 3D Printing of Skeletal Muscle Biomimetic Scaffolds. Nanomaterials. 2020; 10(10):1986. https://doi.org/10.3390/nano10101986
Chicago/Turabian StyleBaiguera, Silvia, Costantino Del Gaudio, Felicia Carotenuto, Paolo Di Nardo, and Laura Teodori. 2020. "Information-Driven Design as a Potential Approach for 3D Printing of Skeletal Muscle Biomimetic Scaffolds" Nanomaterials 10, no. 10: 1986. https://doi.org/10.3390/nano10101986
APA StyleBaiguera, S., Del Gaudio, C., Carotenuto, F., Di Nardo, P., & Teodori, L. (2020). Information-Driven Design as a Potential Approach for 3D Printing of Skeletal Muscle Biomimetic Scaffolds. Nanomaterials, 10(10), 1986. https://doi.org/10.3390/nano10101986





