Computational Study of Cresyl Violet Covalently Attached to the Silane Coupling Agents: Application to TiO2-Based Photocatalysts and Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Characteristics of CV
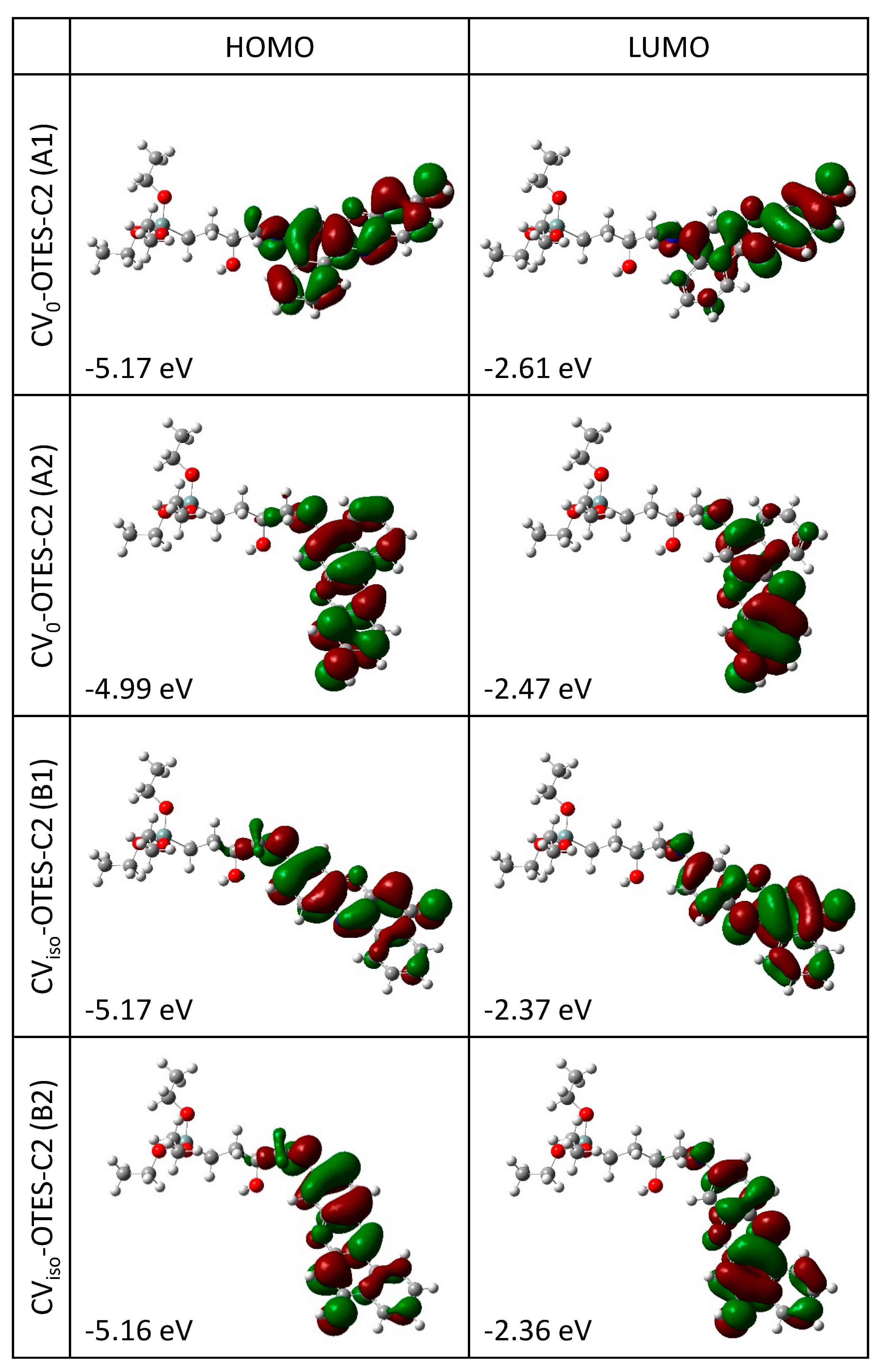
3.2. Geometry Structures and Characteristics of CV–OTES–C2
3.3. Effect of the Alkyl Chain Length of OTES–Cn
3.4. Configuration of CV–OTES–C2 Adsorbed on a TiO2 Cluster
3.5. FMO, DOS Spectra and MEP of CV–OTES–C2 Adsorbed on the TiO2 Cluster
3.6. Simulated Short-Circuit Current Density
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, G.; Liu, X.; Zhao, L.; Zhang, P.; Gao, Y. Synergistic photocatalytic performance of chemically modified amino phthalocyanine-GPTMS/TiO2 for the degradation of Acid Black 1. Inorg. Chem. Commun. 2020, 113, 107795. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.T.; Duspara, P.A.; Seo, S.; Abu Bakar, N.S.B.; Greaney, M.F. Visible light promoted thiol-ene reactions using titanium dioxide. Chem. Commun. 2015, 51, 4383–4385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Gonzalez, V.; Obregon, S.; Patron-Soberano, O.A.; Terashima, C.; Fujishima, A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl. Catal. B Environ. 2020, 270, 118853. [Google Scholar] [CrossRef]
- Paszkiewicz-Gawron, M.; Makurat, S.; Rak, J.; Zdrowowicz, M.; Lisowski, W.; Zaleska-Medynska, A.; Kowalska, E.; Mazierski, P.; Luczak, J. Theoretical and experimental studies on the visible light activity of TiO2 modified with halide-based ionic liquids. Catalysts 2020, 10, 371. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Moghaddas, M.M.; Zargari, S. Investigation of the anchoring silane coupling reagent effect in porphyrin sensitized mesoporous V-TiO2 on the photodegradation efficiency of methyl orange under visible light irradiation. J. Sol-Gel Sci. Technol. 2013, 65, 420–429. [Google Scholar] [CrossRef]
- Sil, M.C.; Kavungathodi, M.F.M.; Nithyanandhan, J. Effect and position of spiro-bipropylenedioxythiophene π-spacer in donor-π-spacer-acceptor dyes for dye-sensitized solar cell. Dyes Pigment. 2019, 161, 313–323. [Google Scholar] [CrossRef]
- Zhu, H.-C.; Zhang, J.; Wang, Y.-L. Adsorption orientation effects of porphyrin dyes on the performance of DSSC: Comparison of benzoic acid and tropolone anchoring groups binding onto the TiO2 anatase (101) surface. Appl. Surf. Sci. 2018, 433, 1137–1147. [Google Scholar] [CrossRef]
- Ooyama, Y.; Shimada, Y.; Inoue, S.; Nagano, T.; Fujikawa, Y.; Komaguchi, K.; Imae, I.; Harima, Y. New molecular design of donor-π-acceptor dyes for dye-sensitized solar cells: Control of molecular orientation and arrangement on TiO2 surface. New J. Chem. 2011, 35, 111–118. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Shao, C.; Lin, C.; Liu, Y. First-Principles Screening and Design of Novel Triphenylamine-Based D-π-A Organic Dyes for Highly Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. C 2015, 119, 21852–21859. [Google Scholar] [CrossRef]
- Ud-Din Khan, S.; Mahmood, A.; Rana, U.A.; Haider, S. Utilization of electron-deficient thiadiazole derivatives as π-spacer for the red shifting of absorption maxima of diarylamine-fluorene based dyes. Theor. Chem. Acc. 2015, 134, 1–7. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Zhang, H. Rigidified and expanded N-annulated perylenes as efficient donors in organic sensitizers for application in solar cells. Phys. Chem. Chem. Phys. 2019, 21, 10488–10496. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Xiong, L.; Liu, F.; Li, C. Grafting hyperbranched polymers onto TiO2 nanoparticles via thiol-yne click chemistry and its effect on the mechanical, thermal and surface properties of polyurethane coating. Materials 2019, 12, 2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meroni, D.; Lo Presti, L.; Di Liberto, G.; Ceotto, M.; Acres, R.G.; Prince, K.C.; Bellani, R.; Soliveri, G.; Ardizzone, S. A Close Look at the Structure of the TiO2-APTES Interface in Hybrid Nanomaterials and Its Degradation Pathway: An Experimental and Theoretical Study. J. Phys. Chem. C 2017, 121, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, B.; Balakrishna, A.; Arranja, C.T.; Dias, C.M.F.; Sobral, A.J. Chemically modified amino porphyrin/TiO2 for the degradation of Acid Black 1 under day light illumination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 176, 134–141. [Google Scholar] [CrossRef]
- Luitel, T.; Zamborini, F.P. Covalent Modification of Photoanodes for Stable Dye-Sensitized Solar Cells. Langmuir 2013, 29, 13582–13594. [Google Scholar] [CrossRef]
- Pandit, B.; Luitel, T.; Cummins, D.R.; Thapa, A.K.; Druffel, T.; Zamborini, F.; Liu, J. Spectroscopic Investigation of Photoinduced Charge-Transfer Processes in FTO/TiO2/N719 Photoanodes with and without Covalent Attachment through Silane-Based Linkers. J. Phys. Chem. A 2013, 117, 13513–13523. [Google Scholar] [CrossRef]
- Abdulelah, H.; Ali, B.; Mahdi, M.A.; Abdullah, A.Q.; Hassan, J.J.; Al-Taay, H.F.; Jennings, P. Fabrication and characterization of porous CdS/dye sensitized solar cells. J. Sol. Energy 2016. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Pan, C.; Wang, G.; Wu, Y.; Zhang, Y.; Yu, G.; Zhang, M. A comparative study on properties of two phenoxazine-based dyes for dye-sensitized solar cells. Dye. Pigment. 2014, 101, 67–73. [Google Scholar] [CrossRef]
- Hong, Y.; Iqbal, Z.; Yin, X.; Cao, D. Synthesis of double D-A branched organic dyes employing indole and phenoxazine as donors for efficient DSSCs. Tetrahedron 2014, 70, 6296–6302. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for potassium to gold including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals radii of elements. Inorg. Mater. (Transl. Neorg. Mater.) 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinzaru, S.C.; Falamas, A.; Dehelean, C.; Morari, C.; Venter, M. Double amino functionalized Ag nanoparticles as SERS tags in Raman diagnostic. Croat. Chem. Acta 2013, 86, 233–244. [Google Scholar] [CrossRef]
- Gong, Q.; Shi, W.; Li, L.; Ma, H. Leucine aminopeptidase may contribute to the intrinsic resistance of cancer cells toward cisplatin as revealed by an ultrasensitive fluorescent probe. Chem. Sci. 2016, 7, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Al Mamunur Rashid, M.; Hayati, D.; Kwak, K.; Hong, J. Theoretical investigation of azobenzene-based photochromic dyes for dye-sensitized solar cells. Nanomaterials 2020, 10, 914. [Google Scholar] [CrossRef]
- Jafari, A.; Ghanadzadeh, A.; Tajalli, H.; Yeganeh, M.; Moghadam, M. Electronic absorption spectra of cresyl violet acetate in anisotropic and isotropic solvents. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66A, 717–725. [Google Scholar] [CrossRef]
- Banik, S.; Hussain, S.A.; Bhattacharjee, D. Modified aggregation pattern of cresyl violet acetate adsorbed on nano clay mineral layers in Langmuir Blodgett film. J. Photochem. Photobiol. A Chem. 2018, 353, 570–580. [Google Scholar] [CrossRef]
- Ostrowski, P.P.; Fairn, G.D.; Grinstein, S.; Johnson, D.E. Cresyl violet: A superior fluorescent lysosomal marker. Traffic 2016, 17, 1313–1321. [Google Scholar] [CrossRef]
- Itoh, Y.; Nakashima, Y.; Tsukamoto, S.; Kurohara, T.; Suzuki, M.; Sakae, Y.; Oda, M.; Okamoto, Y.; Suzuki, T. N+-C-H···O Hydrogen bonds in protein-ligand complexes. Sci. Rep. 2019, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Oprea, C.I.; Girtu, M.A. Structure and electronic properties of TiO2 nanoclusters and dye-nanocluster systems appropriate to model hybrid photovoltaic or photocatalytic applications. Nanomaterials 2019, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
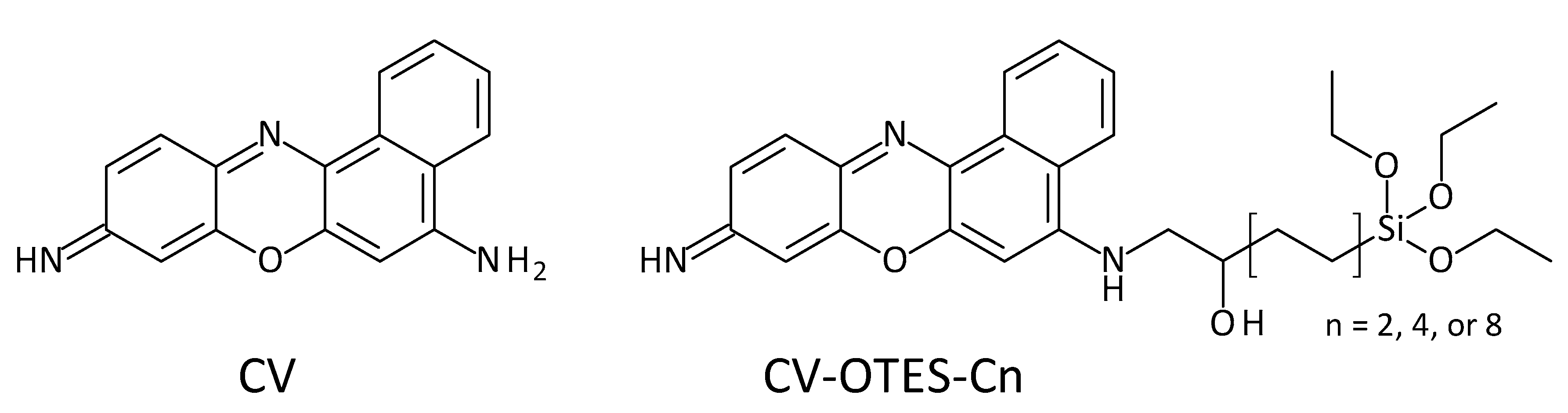
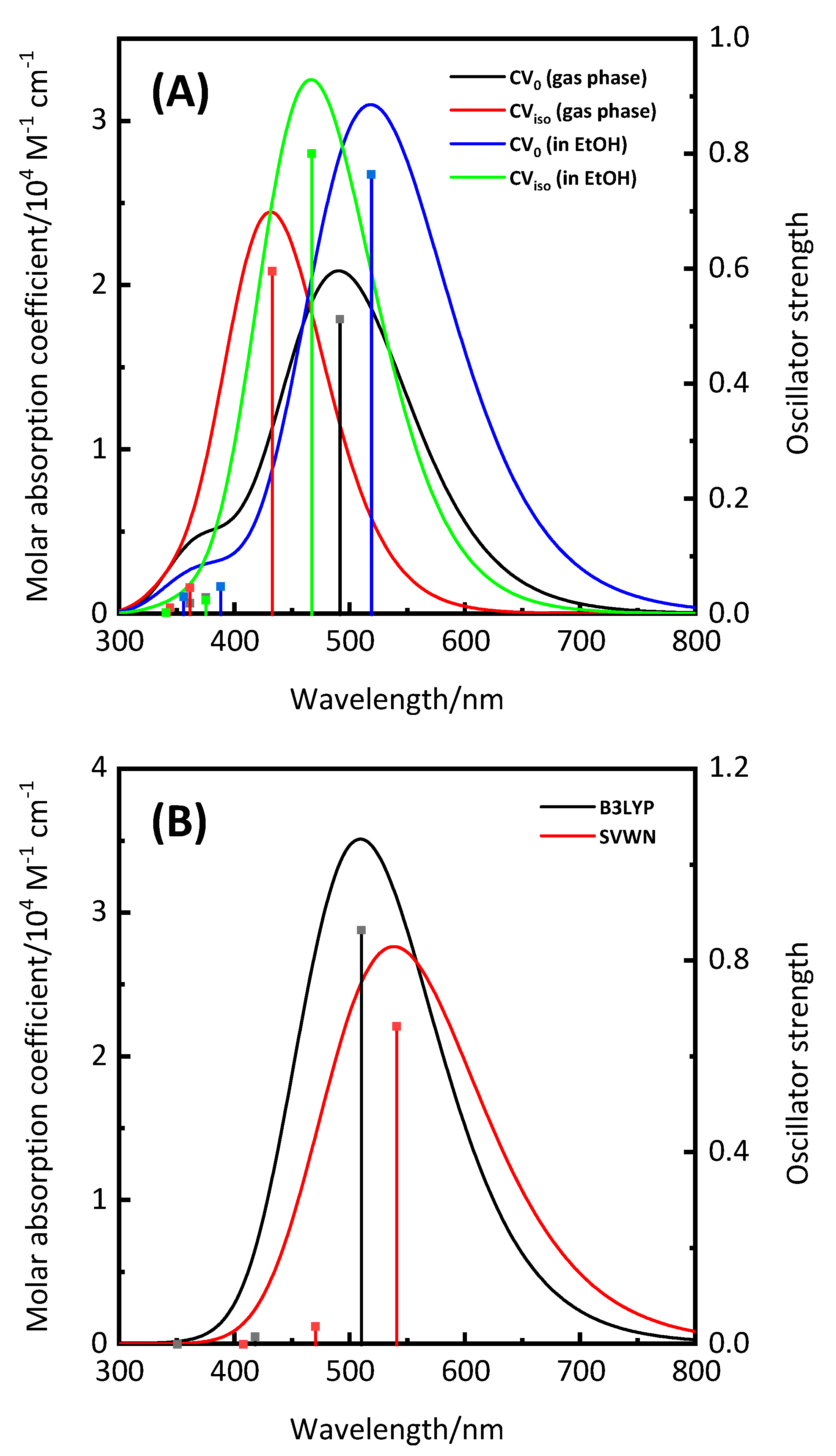

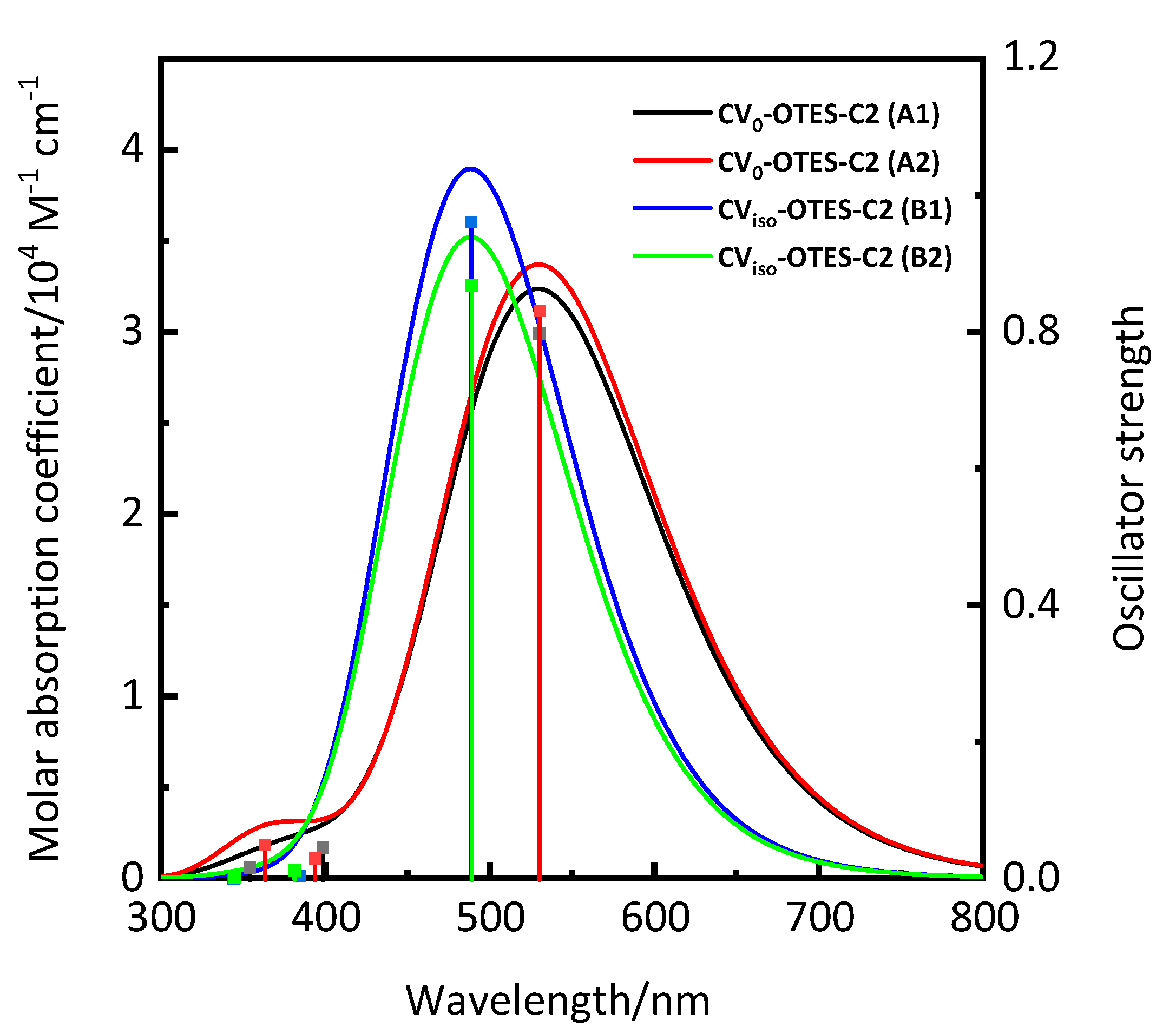
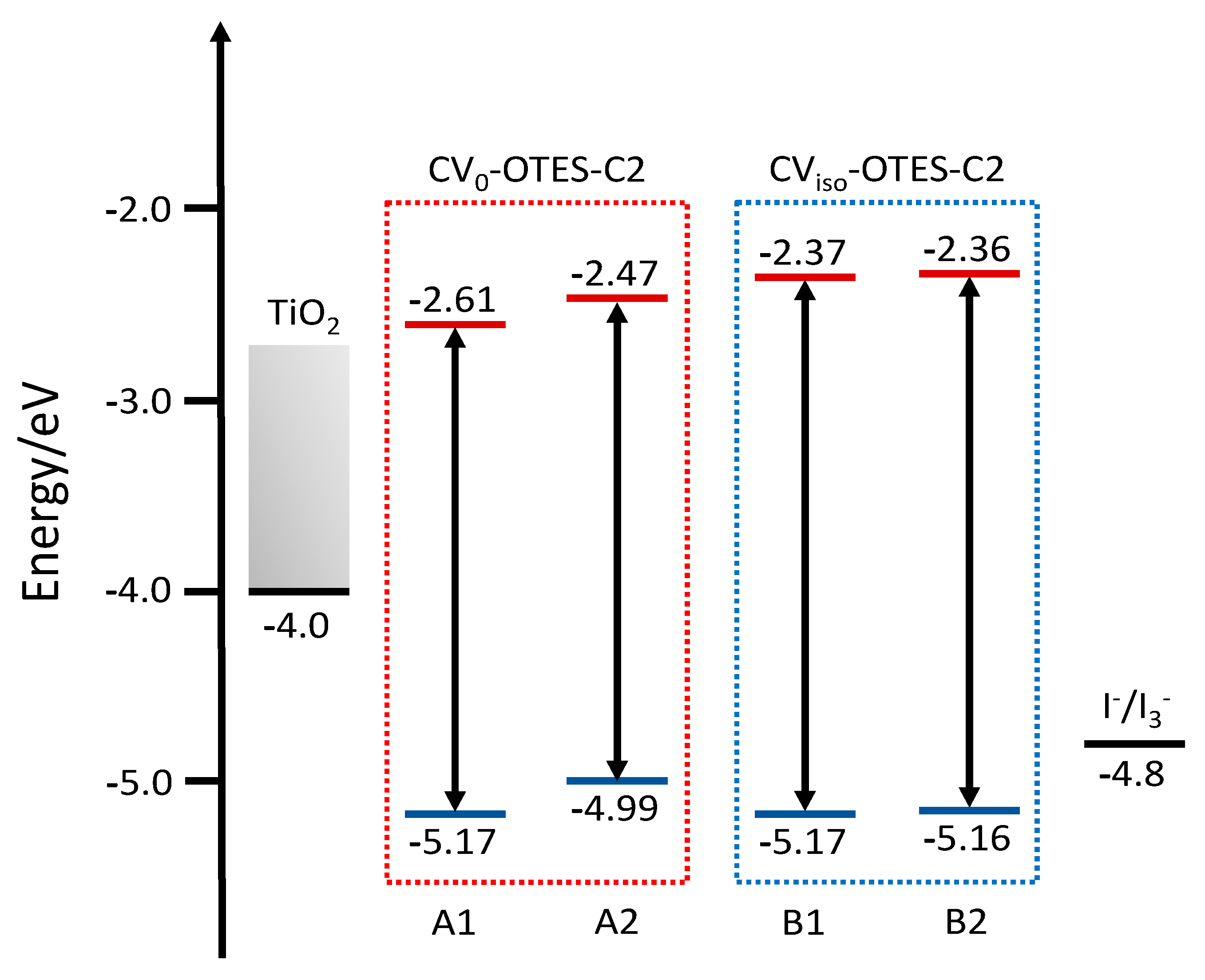

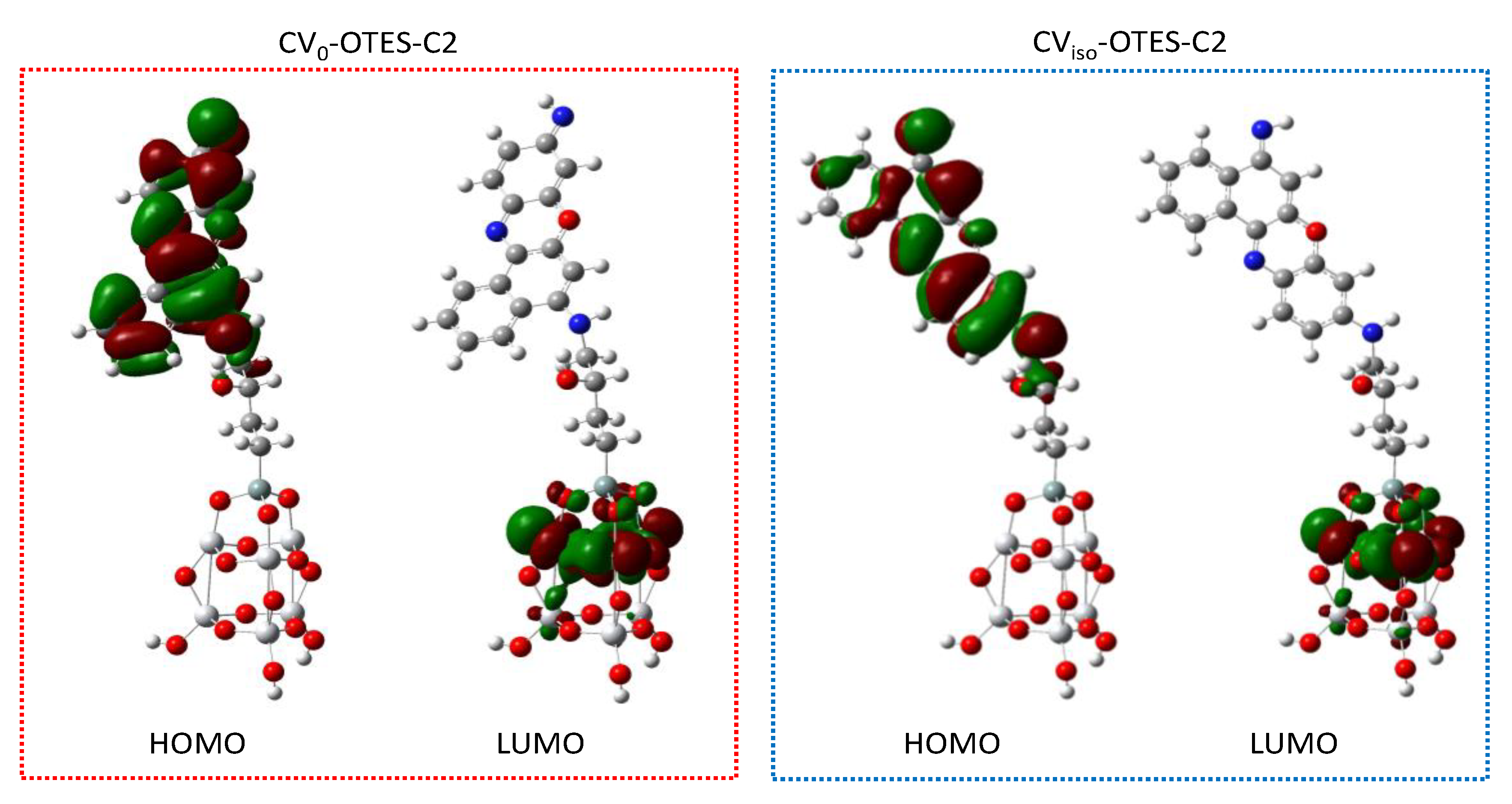
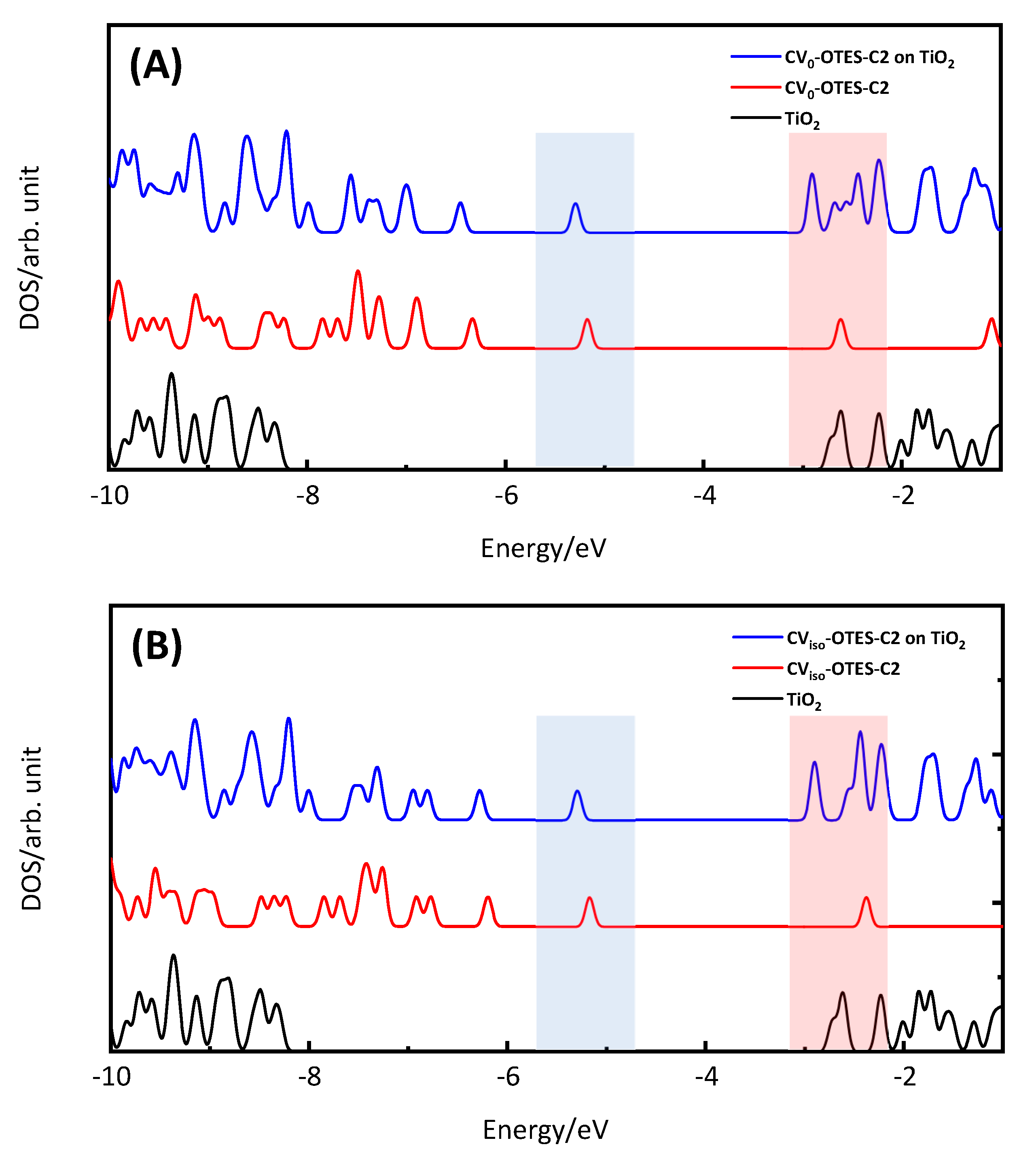
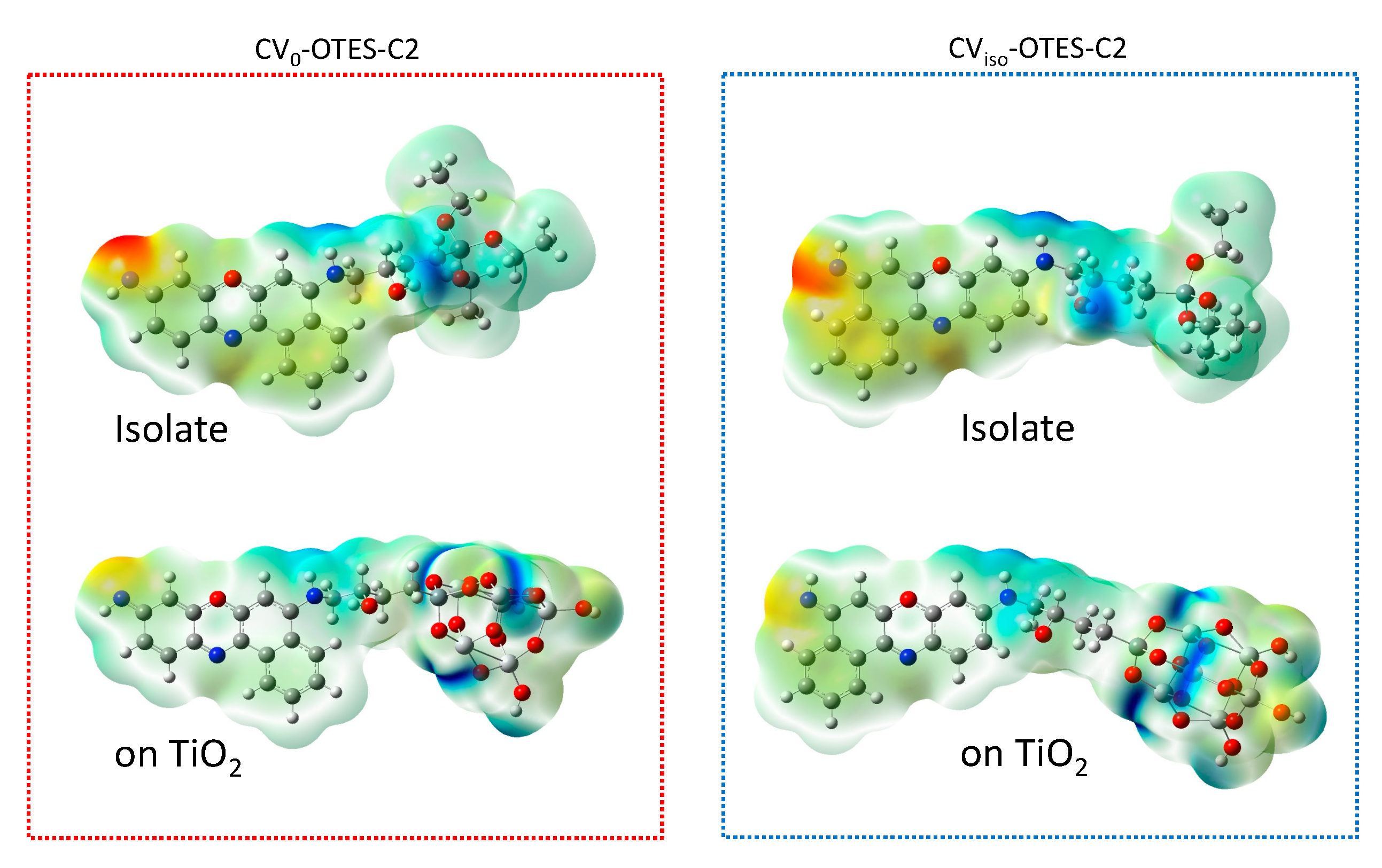
| Dyes | Conditions | λmax (nm) | ε (104 M−1 cm−1) | ES1 (eV) | f | LHE |
|---|---|---|---|---|---|---|
| CV0 | gas phase | 491 | 2.09 | 2.52 | 0.513 | 0.69 |
| in EtOH | 518 | 3.10 | 2.39 | 0.7643 | 0.83 | |
| CViso | gas phase | 432 | 2.44 | 2.87 | 0.5968 | 0.75 |
| in EtOH | 467 | 3.25 | 2.66 | 0.8008 | 0.84 | |
| CV+ | in EtOH | 510 | 3.51 | 2.43 | 0.8641 | 0.86 |
| CV+ a | in EtOH | 541 | 2.76 | 2.29 | 0.6637 | 0.78 |
| Dyes | λmax (nm) | ε (104 M−1 cm−1) | ES1 (eV) | f | LHE |
|---|---|---|---|---|---|
| CV0–OTES–C2 (A1) | 530 | 3.24 | 2.34 | 0.7979 | 0.84 |
| CV0–OTES–C2 (A2) | 530 | 3.37 | 2.34 | 0.8317 | 0.85 |
| CViso–OTES–C2 (B1) | 488 | 3.89 | 2.53 | 0.9614 | 0.89 |
| CViso–OTES–C2 (B2) | 489 | 3.52 | 2.54 | 0.8688 | 0.86 |
| Dyes | Edye (eV) | Edye* (eV) | ∆Ginject (eV) |
|---|---|---|---|
| CV0–OTES–C2 (A1) | 5.17 | 2.83 | −1.17 |
| CV0–OTES–C2 (A2) | 4.99 | 2.65 | −1.35 |
| CViso–OTES–C2 (B1) | 5.17 | 2.64 | −1.36 |
| CViso–OTES–C2 (B2) | 5.16 | 2.62 | −1.38 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshita, T. Computational Study of Cresyl Violet Covalently Attached to the Silane Coupling Agents: Application to TiO2-Based Photocatalysts and Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 1958. https://doi.org/10.3390/nano10101958
Takeshita T. Computational Study of Cresyl Violet Covalently Attached to the Silane Coupling Agents: Application to TiO2-Based Photocatalysts and Dye-Sensitized Solar Cells. Nanomaterials. 2020; 10(10):1958. https://doi.org/10.3390/nano10101958
Chicago/Turabian StyleTakeshita, Tatsuya. 2020. "Computational Study of Cresyl Violet Covalently Attached to the Silane Coupling Agents: Application to TiO2-Based Photocatalysts and Dye-Sensitized Solar Cells" Nanomaterials 10, no. 10: 1958. https://doi.org/10.3390/nano10101958
APA StyleTakeshita, T. (2020). Computational Study of Cresyl Violet Covalently Attached to the Silane Coupling Agents: Application to TiO2-Based Photocatalysts and Dye-Sensitized Solar Cells. Nanomaterials, 10(10), 1958. https://doi.org/10.3390/nano10101958





