Nano Porous Carbon Derived from Citrus Pomace for the Separation and Purification of PMFs in Citrus Processing Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis and Characterization of the Carbon Adsorbents
2.3. Preparation of Crude Extracts from Citrus Processing Wastes
2.4. UPLC Analysis of PMFs
2.5. Optimization of the Enrichment Process
2.5.1. Adsorption and Desorption Properties of Activated Carbons
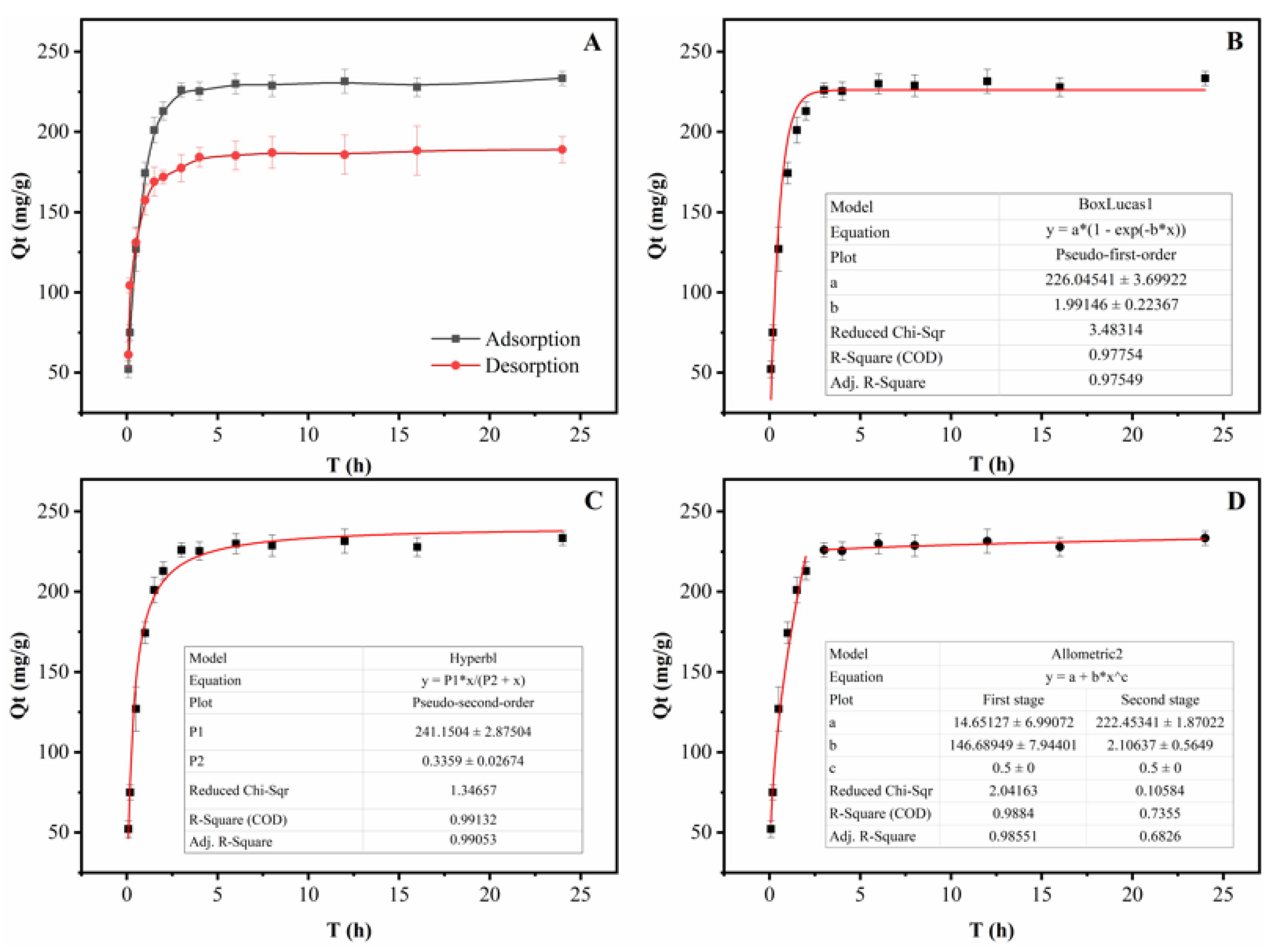
2.5.2. Adsorption and Desorption Kinetics of the Selected Porous Carbon
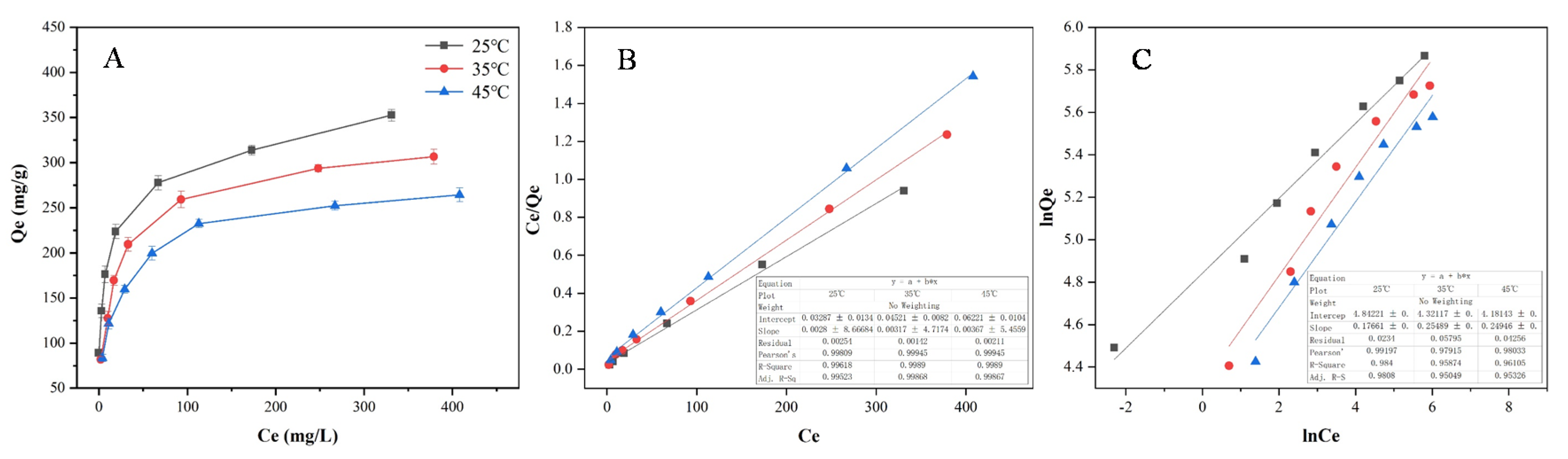
2.5.3. Adsorption Isotherms on the Selected Activated Carbons
2.5.4. Experimental Design for Optimization of PMFs Enrichment Process
2.6. Separation of Polymethoxyflavones by Prep-HPLC
2.7. Crystallization
3. Results and Discussion
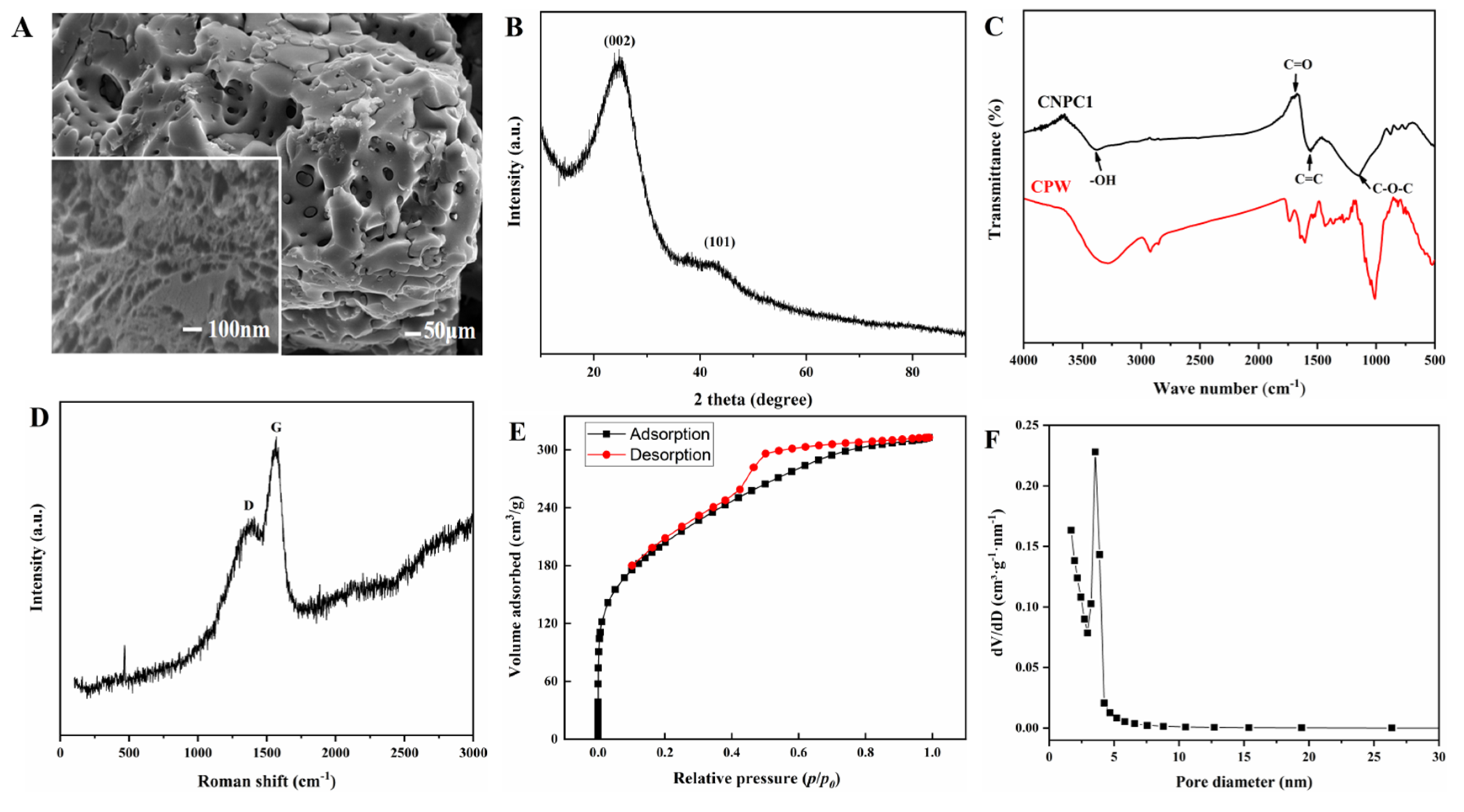
3.1. Characterization of Citrus Nanoporous Carbon
3.2. Static Adsorption and Desorption Capacities
3.3. Adsorption Kinetics and Isotherms
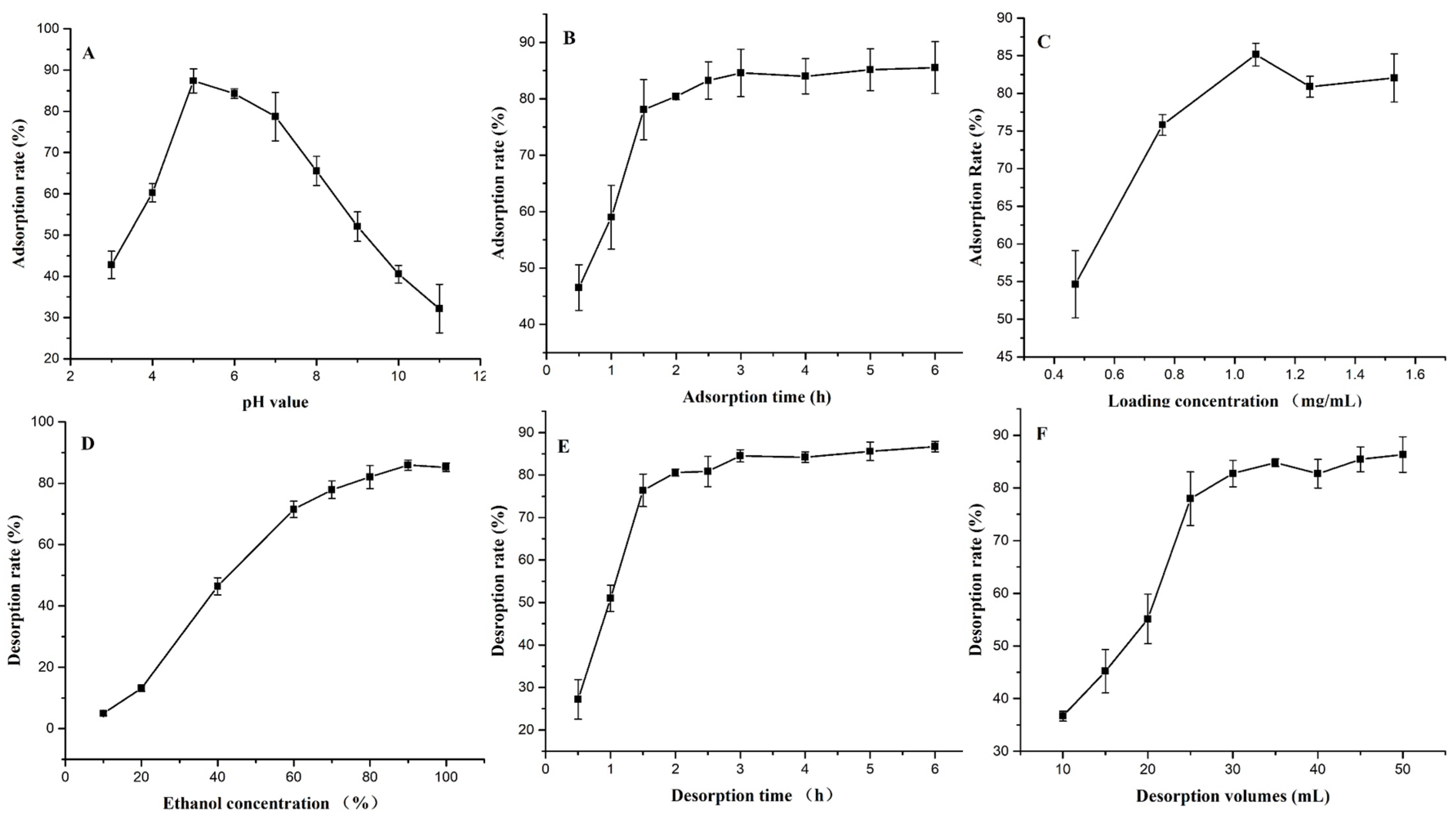
3.4. Single-Factor Test Results of Static Adsorption and Desorption
3.5. Effect of Enrichment
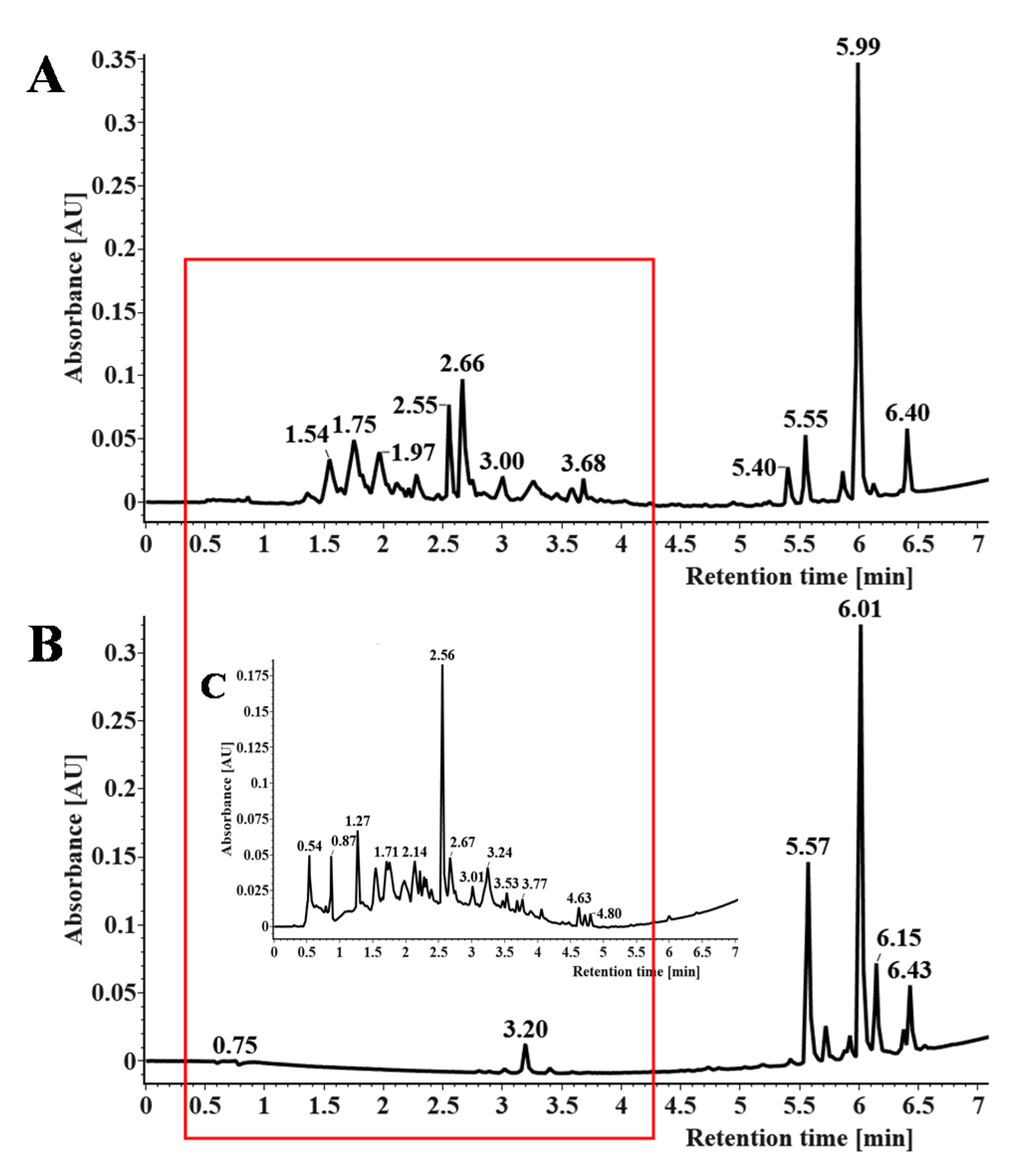
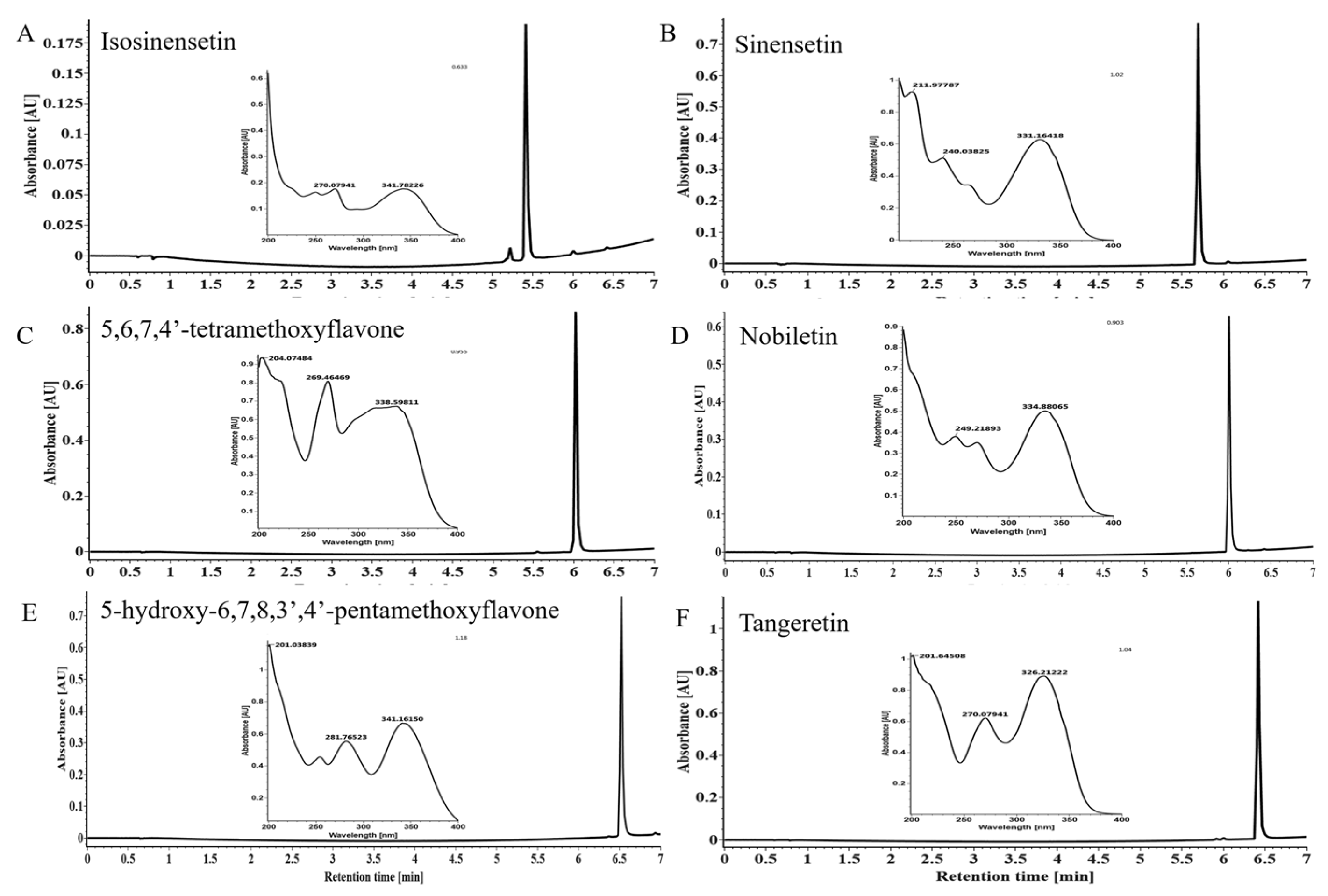
3.6. Isolation and Identification of PMFs
3.7. Comparison with Other Methods
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Satari, B.; Karimi, K. Resources, Conservation & Recycling Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Resources, Conservation and Recycling Environmental challenges impeding the composting of biodegradable municipal solid waste: A critical review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Ángel, J.; López, S.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason. Sonochem. 2015, 24, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Pourbafrani, M.; Forgács, G.; Sárvári, I.; Niklasson, C. Bioresource Technology Production of biofuels, limonene and pectin from citrus wastes. Bioresour. Technol. 2010, 101, 4246–4250. [Google Scholar] [CrossRef]
- Balu, A.M.; Budarin, V.; Shuttleworth, P.S.; Pfaltzgraff, L.A.; Waldron, K.; Luque, R.; Clark, J.H. Valorisation of Orange Peel Residues: Waste to Biochemicals and Nanoporous Materials. ChemSusChem 2012, 5, 1694–1697. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Z. Citrus pectin-derived carbon microspheres with superior adsorption ability for methylene blue. Nanomaterials 2017, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Baseggio, A.M. Subcritical water extraction of fl avanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Owis, A.I. Citrus Polymethoxyflavones: Biofunctional Molecules of Therapeutic Interest, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, ISBN 9780444641793. [Google Scholar]
- Zhao, Z.; He, S.; Hu, Y.; Yang, Y.; Jiao, B.; Fang, Q.; Zhou, Z. Fruit flavonoid variation between and within four cultivated Citrus species evaluated by UPLC-PDA system. Sci. Hortic. 2017, 224, 93–101. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Kou, G.; Li, Z.; Wu, C.; Liu, Y.; Hu, Y.; Guo, L.; Xu, X.; Zhou, Z. Citrus Tangeretin Improves Skeletal Muscle Mitochondrial Biogenesis via Activating the AMPK-PGC1-α Pathway in Vitro and in Vivo: A Possible Mechanism for Its Beneficial Effect on Physical Performance. J. Agric. Food Chem. 2018, 66, 11917–11925. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Zhao, Z.; Zhao, Y.; Xu, X.; Li, Y.; Tan, S.; Huang, C. PMFs-rich Citrus extract prevents the development of non-alcoholic fatty liver disease in C57BL/6J mice induced by a high-fat diet. J. Funct. Foods 2018, 47, 28–39. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Liu, Z.; Feng, S.; Zhou, H.; Liu, C.; Liu, L.; Xie, Y. Role of tangeretin as a potential bioavailability enhancer for silybin: Pharmacokinetic and pharmacological studies. Pharmacol. Res. 2018, 128, 153–166. [Google Scholar] [CrossRef]
- Li, S.; Lambros, T.; Wang, Z.; Goodnow, R.; Ho, C. Efficient and scalable method in isolation of polymethoxyflavones from orange peel extract by supercritical fluid chromatography. J. Chromatogr. B 2007, 846, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lv, X.; Yang, G.; Zhan, J.; Li, M.; Long, T.; Ho, C. Simultaneous separation of six pure polymethoxy flavones from sweet orange peel extract by high performance counter current chromatography. Food Chem. 2019, 292, 160–165. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Z.; Zhou, Z. Simultaneous separation and purification of five polymethoxylated flavones from “dahongpao” tangerine (Citrus tangerina tanaka) using macroporous adsorptive resins combined with prep-HPLC. Molecules 2018, 23, 2660. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Z.; Guo, Y.; Chu, C.; Zheng, G.; Liu, E.; Li, F. Enrichment of polymethoxyflavones from Citrus reticulata ‘Chachi’ peels and their hypolipidemic effect. J. Chromatogr. B 2019, 1124, 226–232. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, I.; Bajpai, V.K.; Lee, H.; Kim, T.; Upadhyay, A.; Huh, Y.S.; Han, Y.K.; Tripathi, K.M. Sustainable Graphene Aerogel as an Ecofriendly Cell Growth Promoter and Highly Efficient Adsorbent for Histamine from Red Wine. ACS Appl. Mater. Interfaces 2019, 11, 18165–18177. [Google Scholar] [CrossRef]
- Myung, Y.; Jung, S.; Tung, T.T.; Tripathi, K.M.; Kim, T. Graphene-Based Aerogels Derived from Biomass for Energy Storage and Environmental Remediation. ACS Sustain. Chem. Eng. 2019, 7, 3772–3782. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Khan, I.; Kang, S.M.; Haldorai, Y.; Tripathi, K.M.; Jung, S.; Chen, L.; Kim, T.; Huh, Y.S.; et al. A Sustainable Graphene Aerogel Capable of the Adsorptive Elimination of Biogenic Amines and Bacteria from Soy Sauce and Highly Efficient Cell Proliferation. ACS Appl. Mater. Interfaces 2019, 11, 43949–43963. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Tang, J.; Lu, H.; Liu, Y. Ginger Straw Waste-Derived Porous Carbons. Molecules 2019, 24, 469. [Google Scholar] [CrossRef]
- Niazi, L.; Lashanizadegan, A.; Shari, H. Chestnut oak shells activated carbon: Preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J. Clean. Prod. 2018, 185, 554–561. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, W.; Cao, S.; Wang, G.; Zhou, Z. Carbon-Based Fe3O4 Nanocomposites Derived from Waste Pomelo Peels for Magnetic Solid-Phase Extraction of 11 Triazole Fungicides in Fruit Samples. Nanomaterials 2018, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Datta, D.; Joshi, A.; Gupta, S.; Gote, Y. Adsorption of isonicotinic acid from aqueous solution using multi-walled carbon nanotubes/Fe3O4. J. Mol. Liq. 2019, 276, 163–169. [Google Scholar] [CrossRef]
- Qin, G.; Ma, J.; Wei, W.; Li, J.; Yue, F. The enrichment of chlorogenic acid from Eucommia ulmoides leaves extract by mesoporous carbons. J. Chromatogr. B 2018, 1087, 6–13. [Google Scholar] [CrossRef]
- Zhang, W.; Ying, L.; Juan, X.; Zhou, Z. Citrus pectin derived porous carbons as a superior adsorbent toward removal of methylene blue. J. Solid State Chem. 2016, 243, 101–105. [Google Scholar] [CrossRef]
- Wang, L. Purification of Ginkgo biloba Extract by Antisolvent Recrystallization. Chem. Eng. Technol. 2016, 39, 1301–1308. [Google Scholar] [CrossRef]
- Nieto-delgado, C.; Terrones, M.; Rangel-mendez, J.R. Development of highly microporous activated carbon from the alcoholic beverage industry organic by-products. Biomass Bioenergy 2010, 35, 103–112. [Google Scholar] [CrossRef]
- Jie, Z.; Tao, H.; Ying, X. Zinc citrate-based nanoporous carbon materials: Large capacitive enhancement using redox active electrolyte of p-phenylenediamine. J. Alloys Compd. 2015, 651, 414–422. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Fu, C.; Li, J.; Li, Z. Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, P.F.; Yang, Z.J.; Jiang, T.W.; Yao, K.L.; Han, R.; Huo, X.X.; Xiong, Y.Y. Bi-functional porous carbon spheres derived from pectin as electrode material for supercapacitors and support material for Pt nanowires towards electrocatalytic methanol and ethanol oxidation. Electrochim. Acta 2015, 163, 140–148. [Google Scholar] [CrossRef]
- Dai, J.; Sun, J.; Xie, A.; He, J.; Li, C.; Yan, Y. Designed preparation of 3D hierarchically porous carbon material via solvothermal route and in situ activation for ultrahigh-efficiency dye removal: Adsorption isotherm, kinetics and thermodynamics characteristics. RSC Adv. 2016, 6, 3446–3457. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, W.; Cao, S.; Xi, C.; Wang, G.; Zhou, Z. Determination of Organophosphorus Pesticide Residues in Fruits and Vegetables Using Porous Carbon Nanoparticles Based on Citrus Compose. Chin. J. Anal. Chem. 2013, 53, 1689–1699. (In Chinese) [Google Scholar] [CrossRef]
- Correia-Sá, L.; Fernandes, V.C.; Calhau, C.; Domingues, V.F.; Delerue-Matos, C. Optimization of QuEChERS Procedure Coupled to GC-ECD for Organochlorine Pesticide Determination in Carrot Samples. Food Anal. Methods 2013, 6, 587–597. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Jiao, B. Determination of ten pyrethroids in various fruit juices: Comparison of dispersive liquid-liquid microextraction sample preparation and QuEChERS method combined with dispersive liquid-liquid microextraction. Food Chem. 2014, 159, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.B.; Zhao, Q.; Mo, J.Z.; Huang, Y.Q.; Luo, Y.B.; Yu, Q.W.; Feng, Y.Q. Quick, easy, cheap, effective, rugged and safe method with magnetic graphitized carbon black and primary secondary amine as adsorbent and its application in pesticide residue analysis. J. Chromatogr. A 2013, 1300, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, L.; Zhou, L.; Zhang, F.; Kang, S.; Pan, C. Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J. Chromatogr. A 2012, 1225, 17–25. [Google Scholar] [CrossRef]
- Liu, S.; Yang, X. Separation and Purification of Polymethoxylated Flavones from Orange Peel Using Macroporous Resin. Nat. Prod. Res. Dev. 2014, 26, 1562–1567. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Yang, Q.; Huang, Y. Optimization of separation and enrichment process of four polymethoxyflavones from Bauhinia championii (Benth.) Benth by macroporous resin. J. Guangdong Pharm. Univ. 2017, 33, 317–321. (In Chinese) [Google Scholar] [CrossRef]
| Adsorbents Type | Adsorption Capacity (mg/g) | Desorption Capacity (mg/g) | Desorption Rate (%) |
|---|---|---|---|
| CNPC1 | 494.64 ± 5.87 | 435.62 ± 19.49 | 88.07 ± 6.36 |
| OPC | 479.98 ± 9.60 | 393.72 ± 4.01 | 82.03 ± 1.75 |
| ZPC | 410.02 ± 3.67 | 344.76 ± 1.51 | 84.09 ± 1.73 |
| CNPC2 | 490.84 ± 3.32 | 419.42 ± 0.40 | 85.45 ± 0.83 |
| MCNPC | 474.80 ± 2.71 | 383.06 ± 2.51 | 80.68 ± 0.03 |
| AC | 479.85 ± 1.82 | 405.85 ± 2.66 | 84.58 ± 1.78 |
| Adsorbents | PMFs Number | Adsorption Capacity (mg/g) | Desorption Capacity (mg/g) | Sample | Ref. |
|---|---|---|---|---|---|
| D101 | 2 | 8.92 | 8.23 | Citrus reticulata Blanco | [41] |
| NKA | 4 | 10.68 | 8.75 | Bauhinia championii Benth | [42] |
| HPD 450 | - | 14.55 | 13.22 | Citrus reticulata Blanco | [20] |
| AB-8 | 5 | 27.1 | 26.3 | Citrus reticulata Blanco | [33] |
| HPD 100 | 5 | 271.52 | 248.17 | Citrus tangerina tanaka | [14] |
| HPD 300 | 5 | 301.75 | 274.01 | Citrus tangerina tanaka | [19] |
| CNPC1 | 6 | 494.64 | 435.62 | Citrus sinensis Osbeck | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, X.; Qiu, L.; Wang, Y.; Zhou, Z. Nano Porous Carbon Derived from Citrus Pomace for the Separation and Purification of PMFs in Citrus Processing Wastes. Nanomaterials 2020, 10, 1914. https://doi.org/10.3390/nano10101914
Li Z, Chen X, Qiu L, Wang Y, Zhou Z. Nano Porous Carbon Derived from Citrus Pomace for the Separation and Purification of PMFs in Citrus Processing Wastes. Nanomaterials. 2020; 10(10):1914. https://doi.org/10.3390/nano10101914
Chicago/Turabian StyleLi, Zhenqing, Xin Chen, Lulu Qiu, Yu Wang, and Zhiqin Zhou. 2020. "Nano Porous Carbon Derived from Citrus Pomace for the Separation and Purification of PMFs in Citrus Processing Wastes" Nanomaterials 10, no. 10: 1914. https://doi.org/10.3390/nano10101914
APA StyleLi, Z., Chen, X., Qiu, L., Wang, Y., & Zhou, Z. (2020). Nano Porous Carbon Derived from Citrus Pomace for the Separation and Purification of PMFs in Citrus Processing Wastes. Nanomaterials, 10(10), 1914. https://doi.org/10.3390/nano10101914






