Investigation of Plasma-Assisted Functionalization of Graphitic Materials for Epoxy Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Manufacture
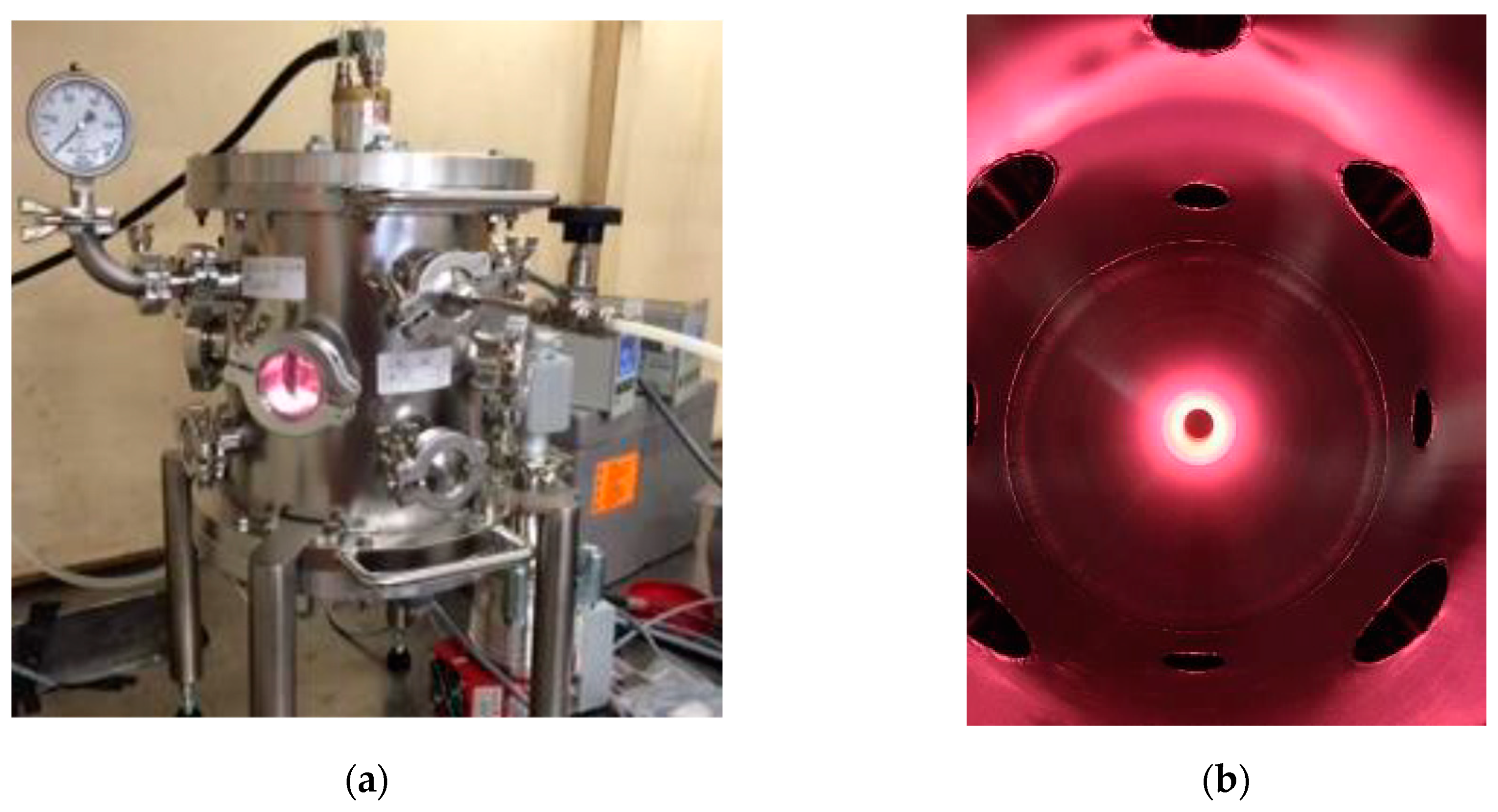
2.3. Plasma Functionalization
2.4. UV-vis Spectroscopy
2.5. Contact Angle Measurements
2.6. Mechanical Testing
2.7. Electrical Resistivity Measurements
2.8. Dynamic-Mechanical Analysis (DMA)
2.9. Environmental Scanning Electron Microscope (ESEM)
3. Results
3.1. Plasma Functionalization
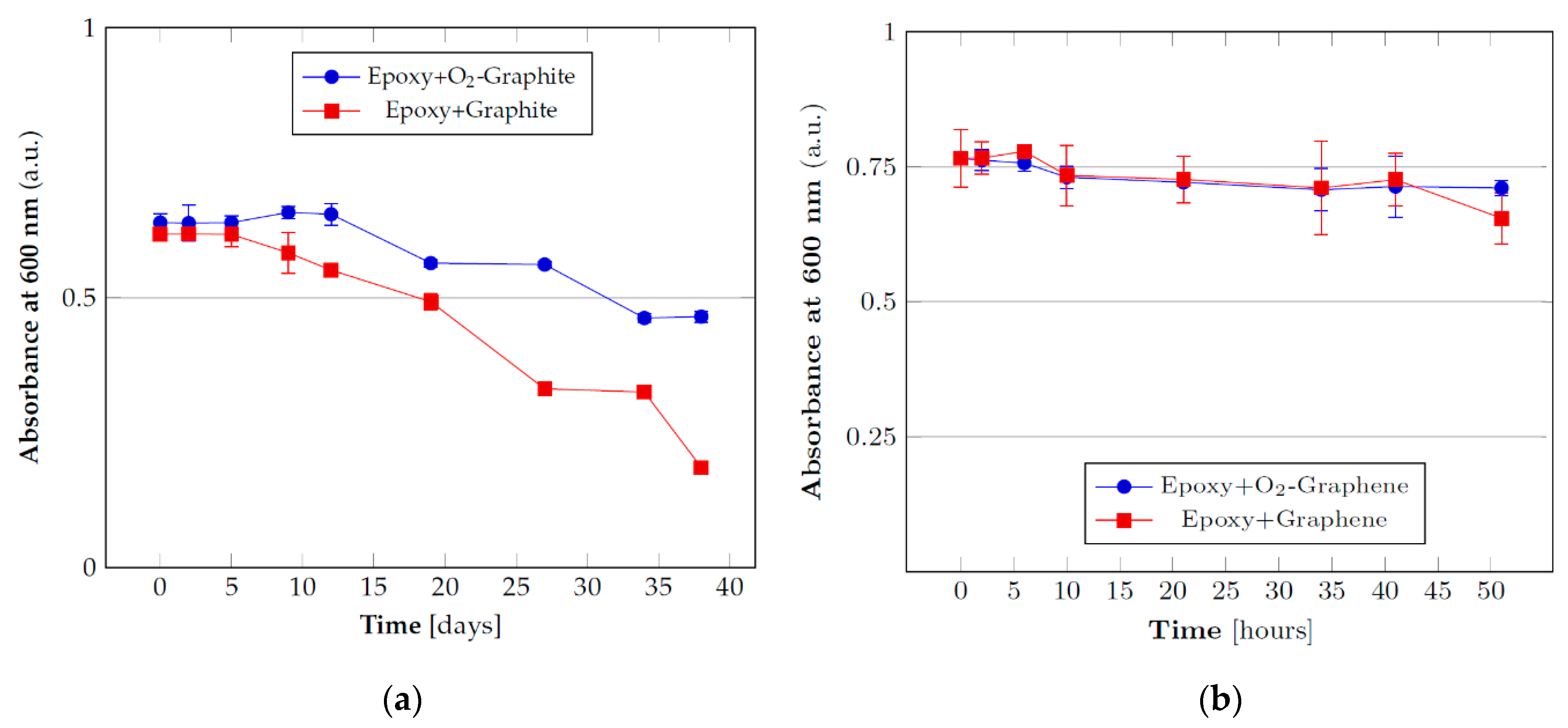
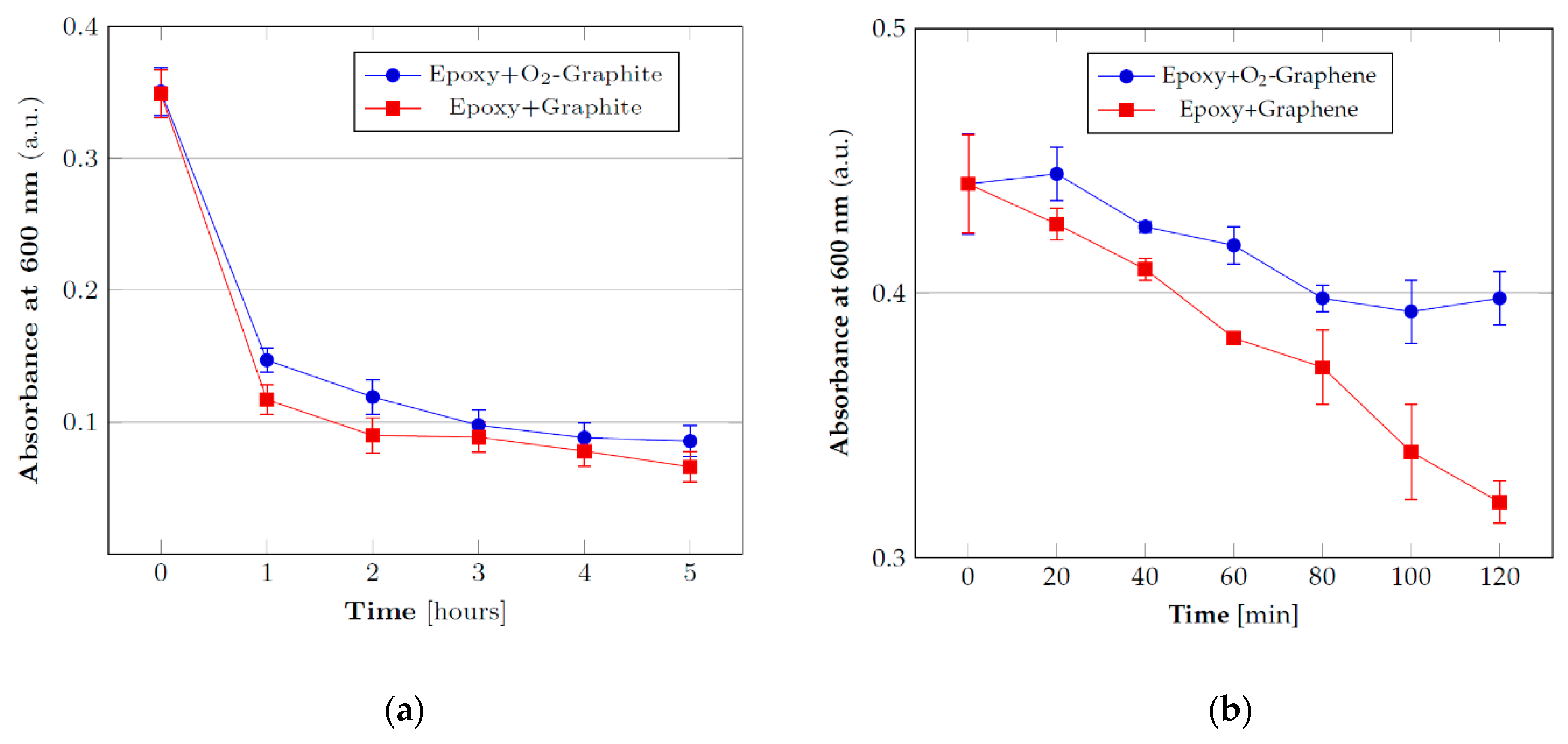
3.2. Stability Tests
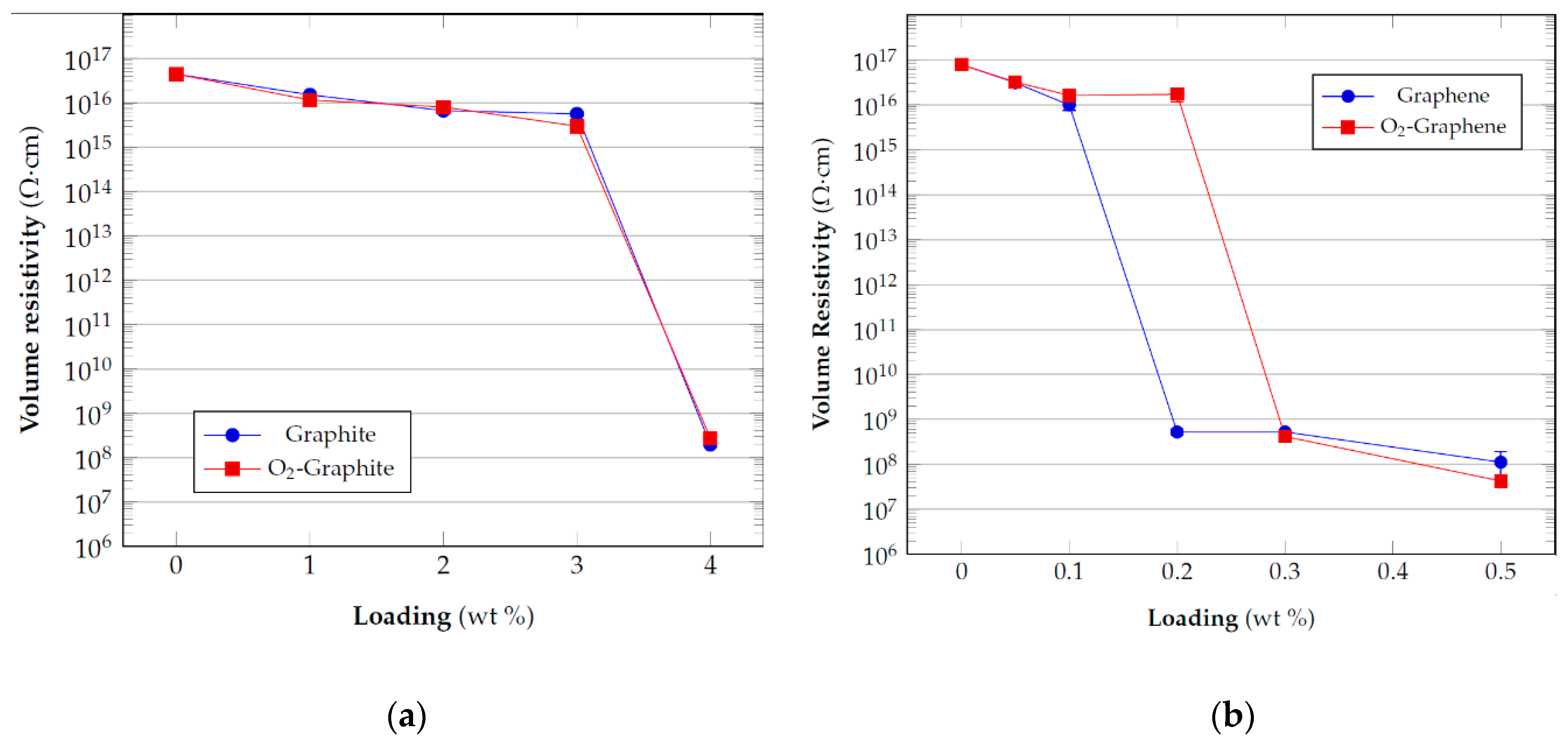
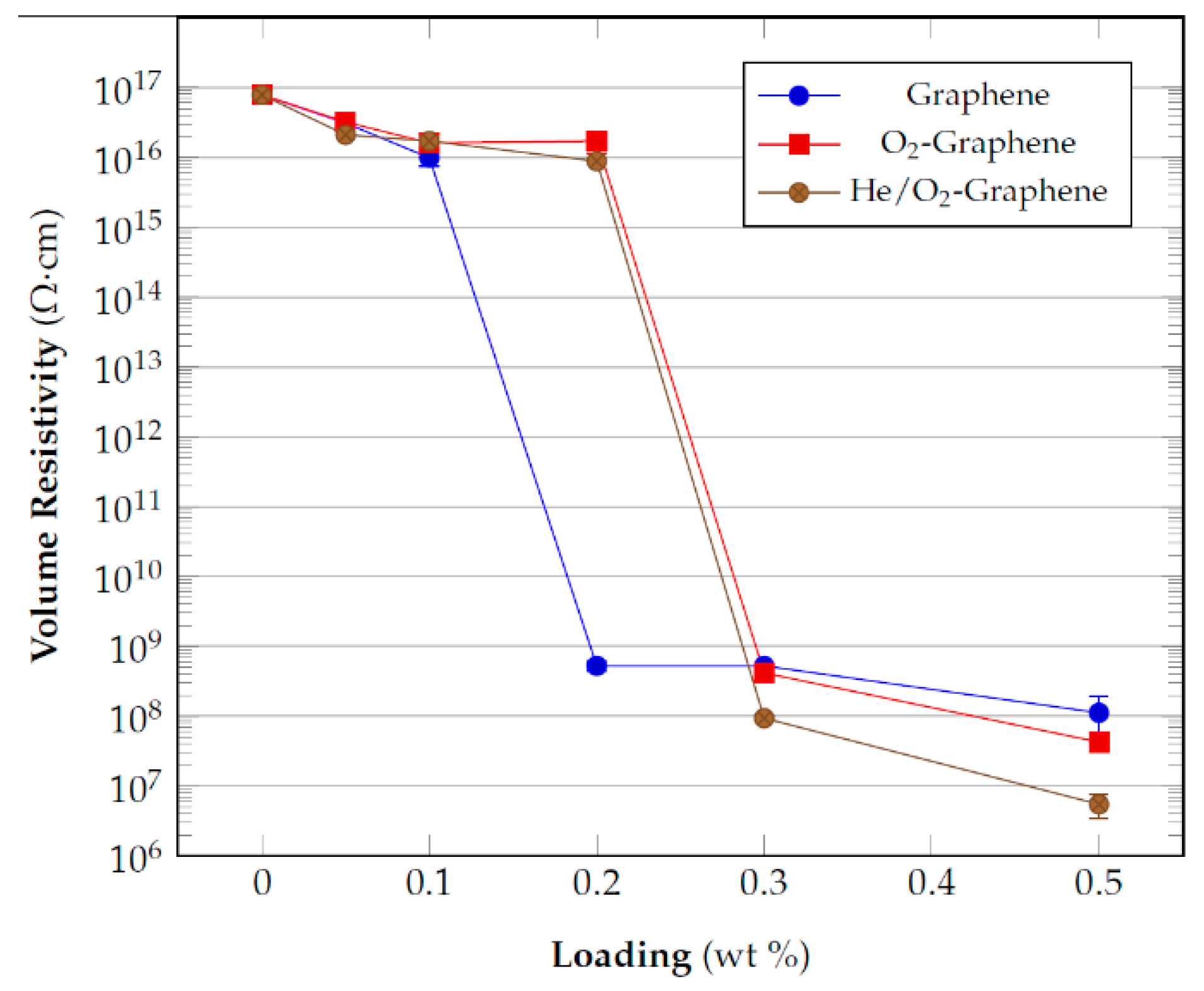
3.3. Electrical Resistivity Measurements
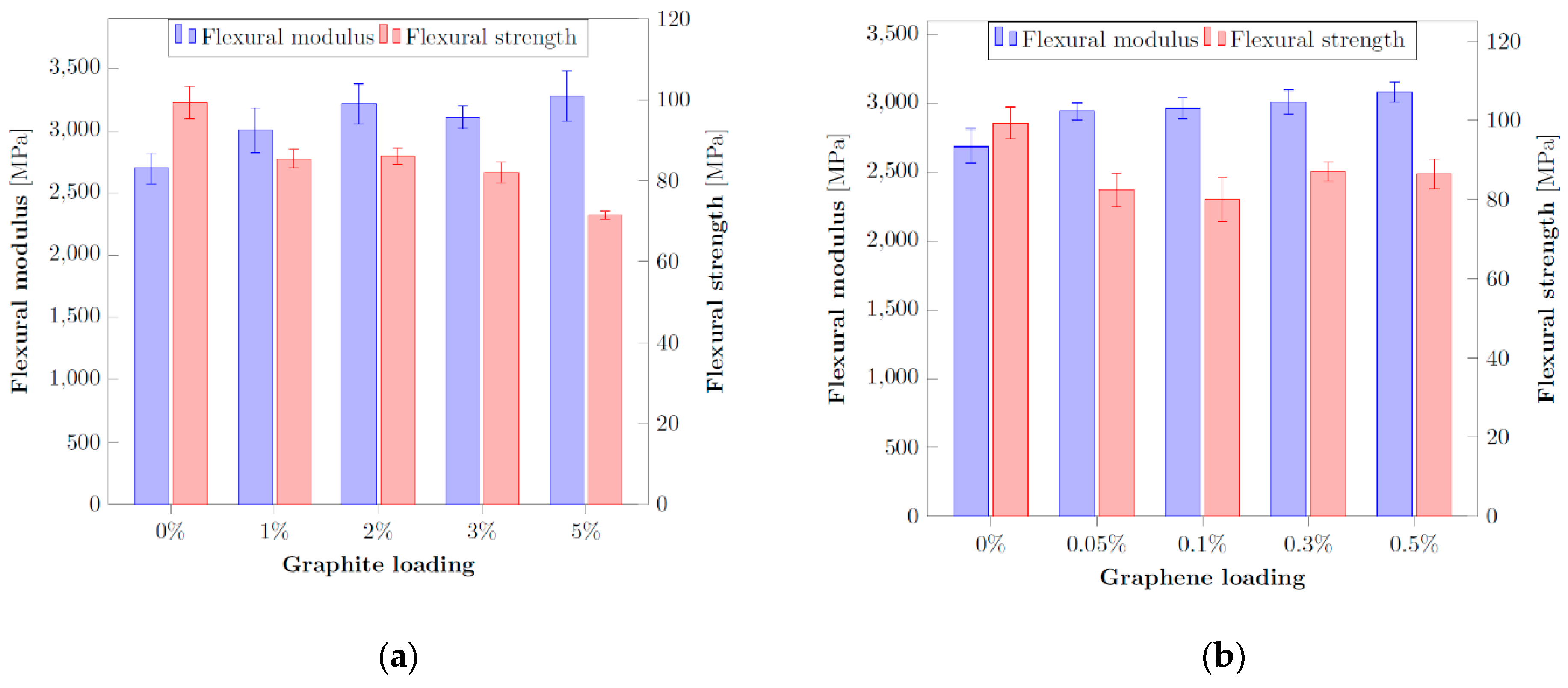
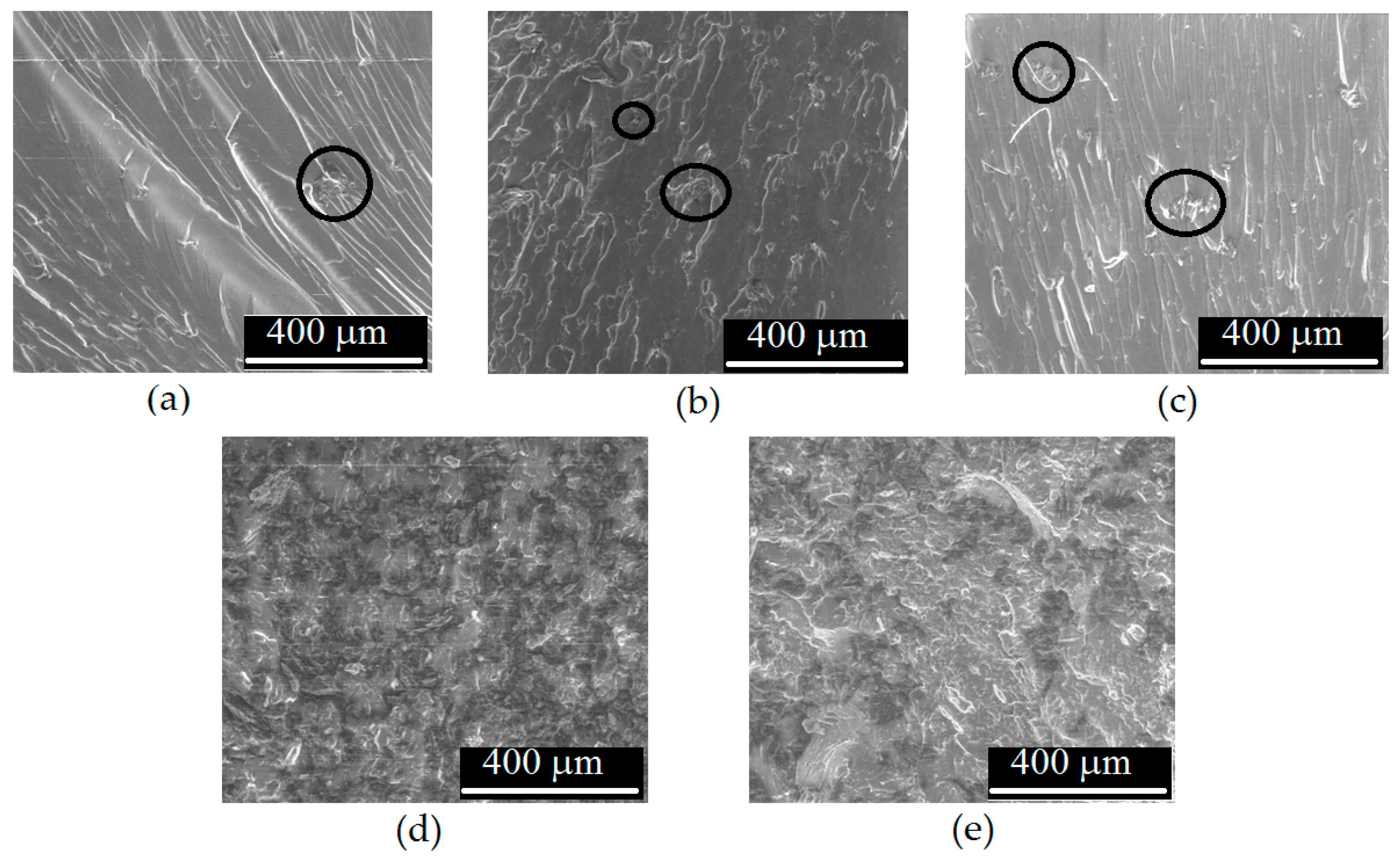
3.4. First Mechanical Results
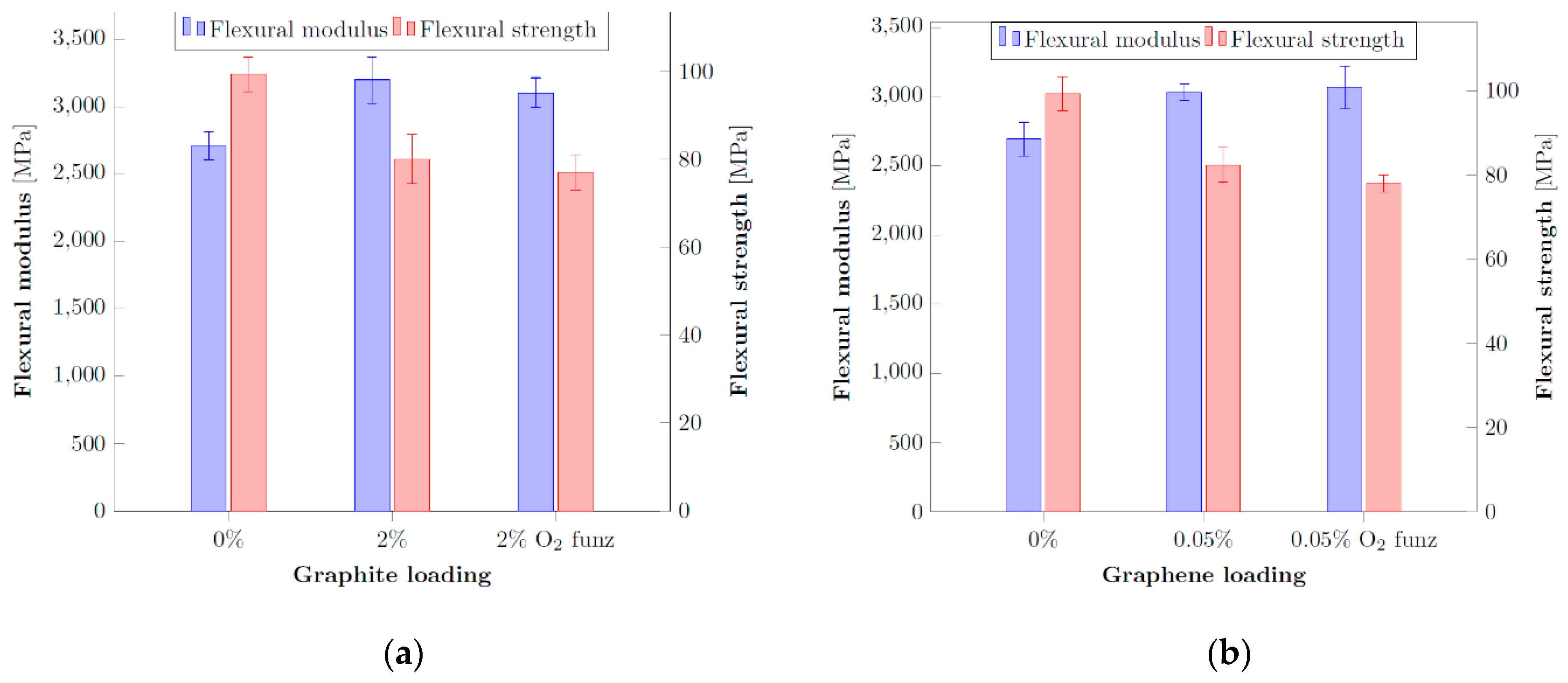
3.5. Effect of Thermal Treatment on Graphene Plasma Functionalization
- The dispersion (resin + filler) is subjected to heating up to 80 °C for a reduced amount of time (about 1 h) both during the preparation of the samples before the dispersion by homogenization and before the curing starts, during the vacuum treatment to remove the presence of bubbles in the dispersion;
- The curing process is carried out at high temperatures (120 °C) for 12 h but the main concern is related to the first hours of the process where the high temperature lowers the viscosity of the dispersion and agglomeration/sedimentation can take place. After this time the extent of the curing reaction is such to significantly increase the viscosity and “freeze” the particles of the fillers inside the matrix, preventing sedimentation and aggregation.
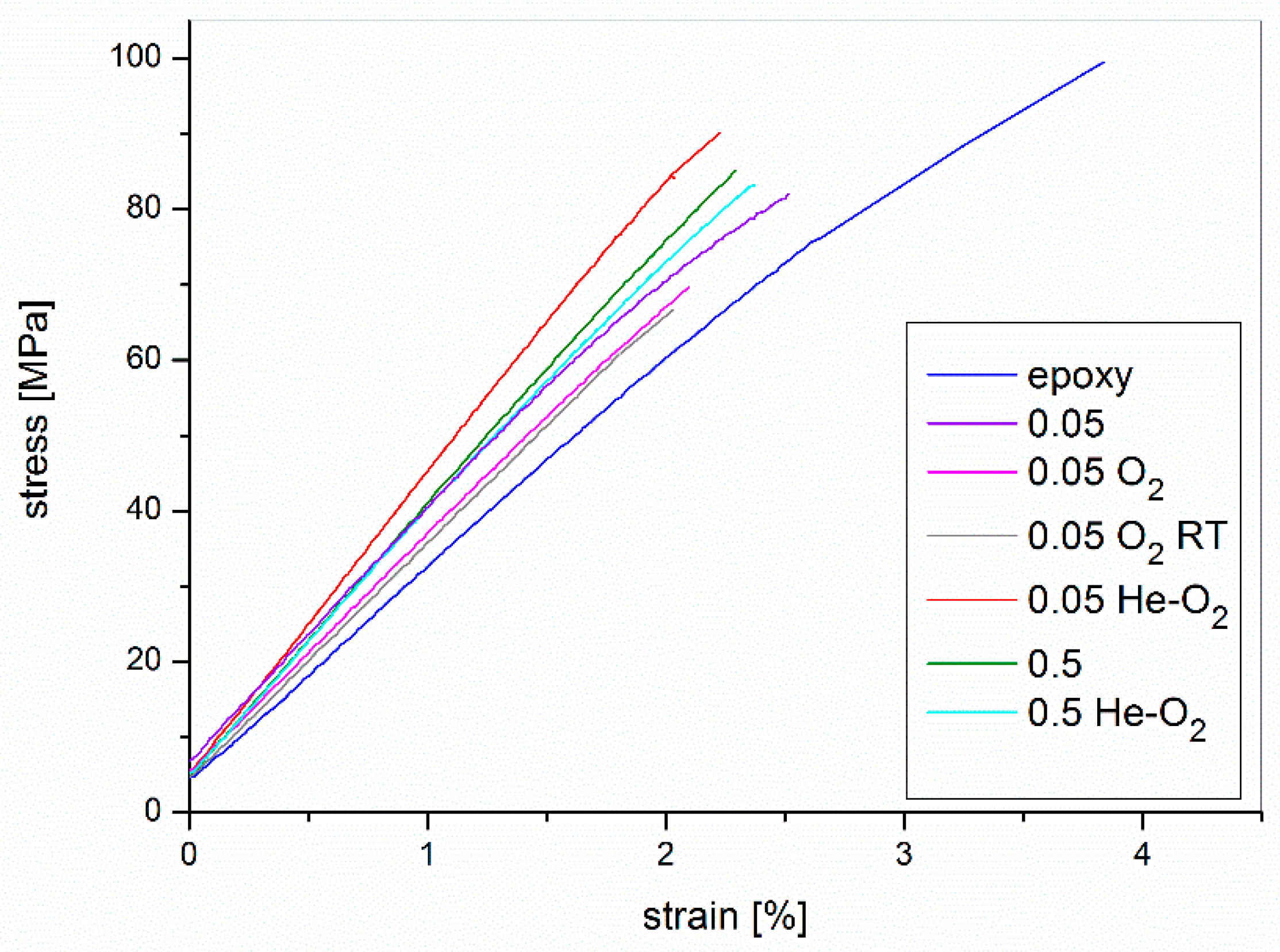
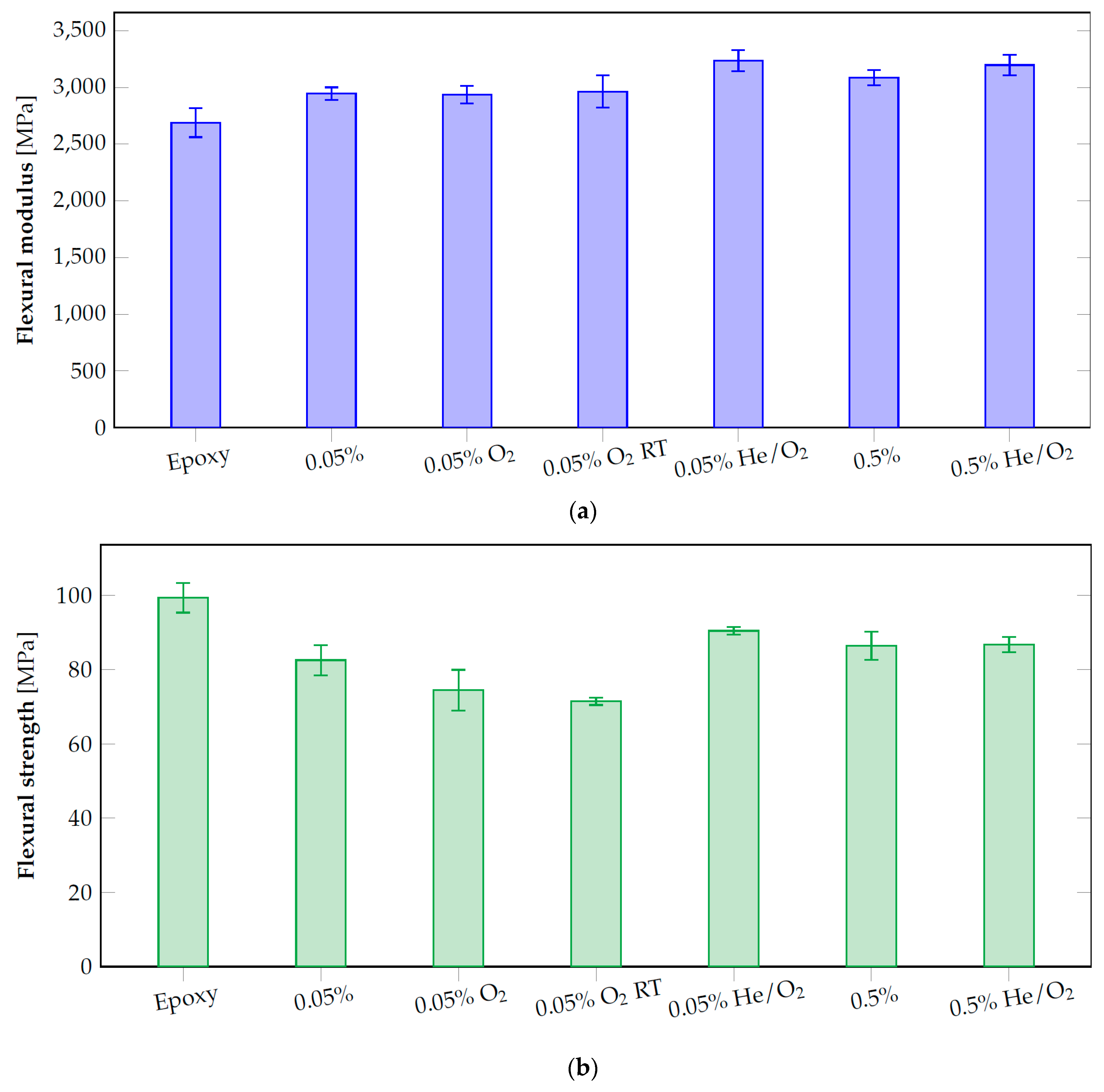
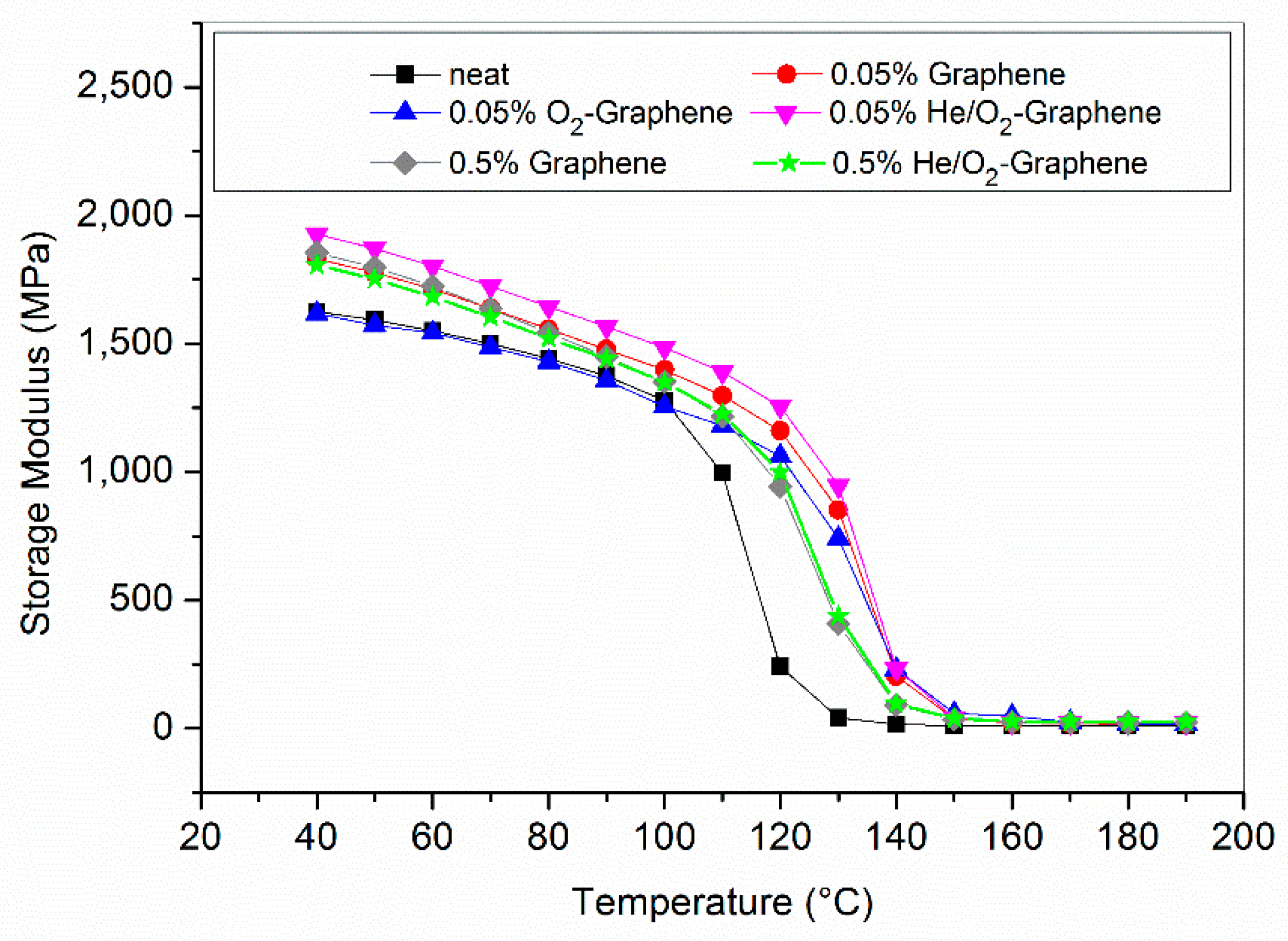
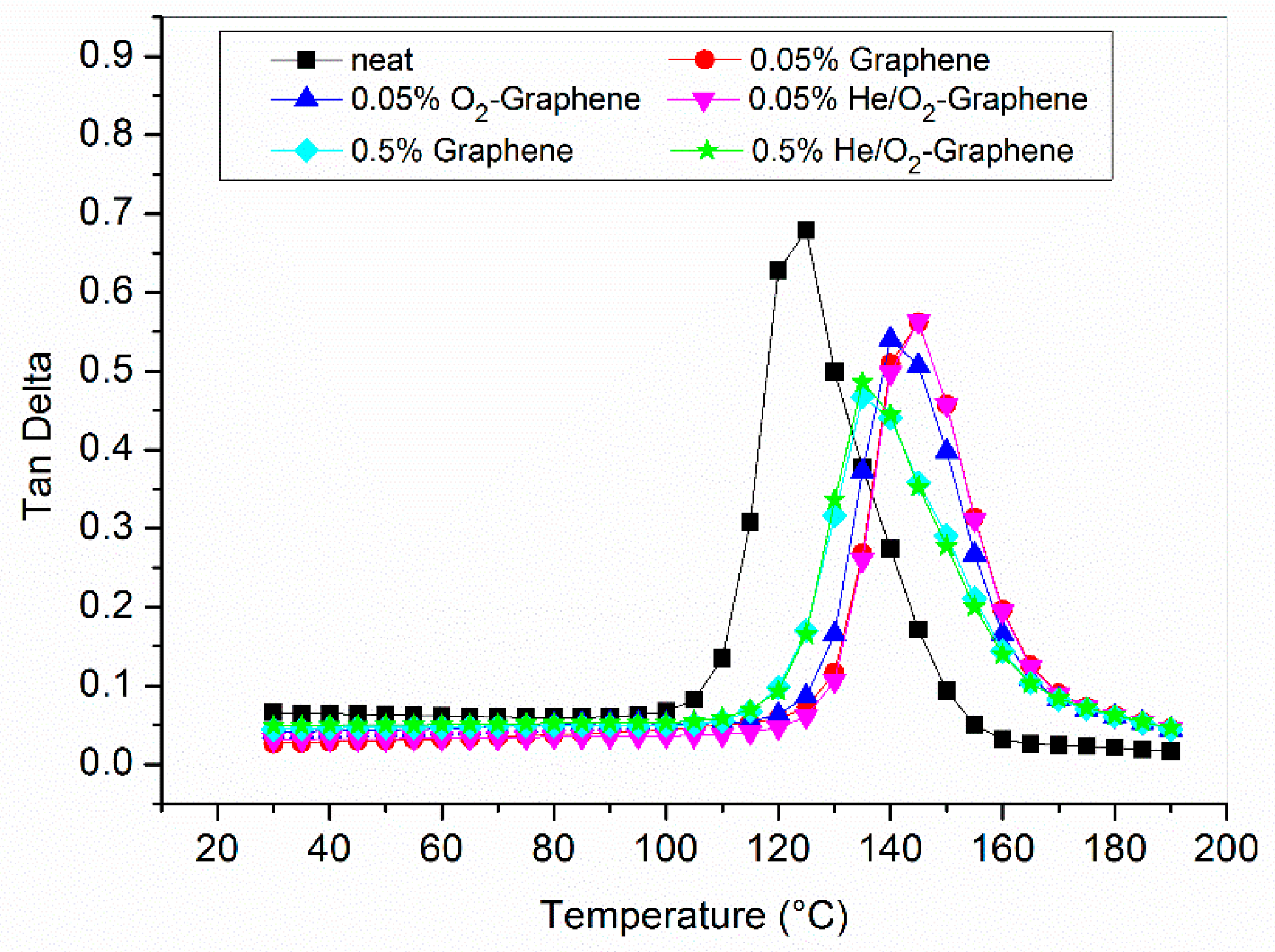
3.6. Optimized Composites Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pappas, D. Status and potential of atmospheric plasma processing of materials. J. Vac. Sci. Technol. A 2011, 29, 20801. [Google Scholar] [CrossRef]
- Tsai, P.S.; Blinder, P.; Migliori, B.J.; Neev, J.; Jin, Y.; Squier, J.A.; Kleinfeld, D. Plasma-mediated ablation: An optical tool for submicrometer surgery on neuronal and vascular systems. Curr. Opin. Biotechnol. 2009, 20, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, V.M.; Kornblit, A. Plasma etching: Yesterday, today, and tomorrow. J. Vac. Sci. Technol. A 2013, 31, 50825. [Google Scholar] [CrossRef]
- Khelifa, F.; Ershov, S.; Habibi, Y.; Snyders, R.; Dubois, P. Free-Radical-Induced Grafting from Plasma Polymer Surfaces. Chem. Rev. 2016, 116, 3975–4005. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, R.J.; Adams, P.M.; Nokes, J.; Kim, H.I. Surface functionalization of graphenelike materials by carbon monoxide atmospheric plasma treatment for improved wetting without structural degradation. J. Vac. Sci. Technol. B, Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2012, 30, 03D107. [Google Scholar] [CrossRef]
- Novák, I.; Števiar, M.; Popelka, A.; Chodák, I.; Mosnáček, J.; Špírková, M.; Janigová, I.; Kleinová, A.; Sedliačik, J.; Šlouf, M. Surface modification of polyethylene by diffuse barrier discharge plasma. Polym. Eng. Sci. 2013, 53, 516–523. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Kulkarni, H.B.; Tambe, P.M.; Joshi, G. Influence of covalent and non-covalent modification of graphene on the mechanical, thermal and electrical properties of epoxy/graphene nanocomposites: A review. Compos. Interfaces 2018, 25, 381–414. [Google Scholar] [CrossRef]
- Kemp, M. Low temperature plasma process: Effectively modifies surface of graphene. Adv. Mater. Process. 2014, 172, 15–18. [Google Scholar]
- Fulcheri, L.; Schwob, Y.; Fabry, F.; Flamant, G.; Chibante, L.F.P.; Laplaze, D. Fullerene production in a 3-phase AC plasma process. Carbon N. Y. 2000, 38, 797–803. [Google Scholar] [CrossRef]
- Okuno, H.; Charlier, J.-C.; Fulcheri, L.; Gruenberger, T.; Gonzalez-Aguilar, J. Plasma processing of carbon nanomaterials. High Temp. Mater. Process. 2004, 8, 119–138. [Google Scholar]
- Keidar, M.; Shashurin, A.; Li, J.; Volotskova, O.; Kundrapu, M.; Zhuang, T. Sen Arc plasma synthesis of carbon nanostructures: Where is the frontier? J. Phys. D. Appl. Phys. 2011, 44, 174006. [Google Scholar] [CrossRef]
- Dey, A.; Chroneos, A.; Braithwaite, N.S.J.; Gandhiraman, R.P.; Krishnamurthy, S. Plasma engineering of graphene. Appl. Phys. Rev. 2016, 3, 21301. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Graphene for High-Performance Ultracapacitors and the Importance of Nitrogen-Doped Sites at Basal Planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Imran Jafri, R.; Rajalakshmi, N.; Ramaprabhu, S. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. 2010, 20, 7114–7117. [Google Scholar] [CrossRef]
- Sim, U.; Yang, T.-Y.; Moon, J.; An, J.; Hwang, J.; Seo, J.-H.; Lee, J.; Kim, K.Y.; Lee, J.; Han, S.; et al. N-doped monolayer graphene catalyst on silicon photocathode for hydrogen production. Energy Environ. Sci. 2013, 6, 3658–3664. [Google Scholar] [CrossRef]
- Muhammad Hafiz, S.; Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M.; et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B Chem. 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Hwang, J.; Kyw Choi, H.; Moon, J.; Yong Kim, T.; Shin, J.-W.; Woong Joo, C.; Han, J.-H.; Cho, D.-H.; Woo Huh, J.; Choi, S.-Y.; et al. Multilayered graphene anode for blue phosphorescent organic light emitting diodes. Appl. Phys. Lett. 2012, 100, 133304. [Google Scholar] [CrossRef]
- Das, S.; Sudhagar, P.; Verma, V.; Song, D.; Ito, E.; Lee, S.Y.; Kang, Y.S.; Choi, W. Amplifying Charge-Transfer Characteristics of Graphene for Triiodide Reduction in Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2011, 21, 3729–3736. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Baraket, M.; Stine, R.; Lee, W.K.; Robinson, J.T.; Tamanaha, C.R.; Sheehan, P.E.; Walton, S.G. Aminated graphene for DNA attachment produced via plasma functionalization. Appl. Phys. Lett. 2012, 100, 233123. [Google Scholar] [CrossRef]
- Zaldivar, R.J.; Kim, H.I.; Steckel, G.L.; Nokes, J.P.; Morgan, B.A. Effect of Processing Parameter Changes on the Adhesion of Plasma-treated Carbon Fiber Reinforced Epoxy Composites. J. Compos. Mater. 2009, 44, 1435–1453. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D. Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Paredes, J.; Martínez-Alonso, A.; Tascón, J.M. Comparative study of the air and oxygen plasma oxidation of highly oriented pyrolytic graphite: A scanning tunneling and atomic force microscopy investigation. Carbon N. Y. 2000, 38, 1183–1197. [Google Scholar] [CrossRef]
- Cvelbar, U.; Markoli, B.; Poberaj, I.; Zalar, A.; Kosec, L.; Spaić, S. Formation of functional groups on graphite during oxygen plasma treatment. Appl. Surf. Sci. 2006, 253, 1861–1865. [Google Scholar] [CrossRef]
- Baraket, M.; Walton, S.G.; Lock, E.H.; Robinson, J.T.; Perkins, F.K. The functionalization of graphene using electron-beam generated plasmas. Appl. Phys. Lett. 2010, 96, 231501. [Google Scholar] [CrossRef]
- Zaldivar, R.J.; Adams, P.M.; Kim, H.I.; Nokes, J.P.; Patel, D.N. Strength improvements in toughened epoxy composites using surface treated GnPs. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Moosburger-Will, J.; Lachner, E.; Löffler, M.; Kunzmann, C.; Greisel, M.; Ruhland, K.; Horn, S. Adhesion of carbon fibers to amine hardened epoxy resin: Influence of ammonia plasma functionalization of carbon fibers. Appl. Surf. Sci. 2018, 453, 141–152. [Google Scholar] [CrossRef]
- Pötschke, P.; Zschoerper, N.P.; Moller, B.P.; Vohrer, U. Plasma Functionalization of Multiwalled Carbon Nanotube Bucky Papers and the Effect on Properties of Melt-Mixed Composites with Polycarbonate. Macromol. Rapid Commun. 2009, 30, 1828–1833. [Google Scholar] [CrossRef]
- Malik, R.; McConnell, C.; Alvarez, N.T.; Haase, M.; Gbordzoe, S.; Shanov, V. Rapid, in situ plasma functionalization of carbon nanotubes for improved CNT/epoxy composites. RSC Adv. 2016, 6, 108840–108850. [Google Scholar] [CrossRef]
- Steffen, T.T.; Fontana, L.C.; Nahorny, J.; Becker, D. Role of nitrogen–oxygen plasma functionalization of carbon nanotubes in epoxy nanocomposites. Polym. Compos. 2019, 40, E1162–E1171. [Google Scholar] [CrossRef]
- Alam, A.; Wan, C.; McNally, T. Surface amination of carbon nanoparticles for modification of epoxy resins: Plasma-treatment vs. wet-chemistry approach. Eur. Polym. J. 2017, 87, 422–448. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Liaghat, G.; Vahid, S.; Spacie, C.; Paton, K.R.; Sainsbury, T. Improving the fracture toughness properties of epoxy using graphene nanoplatelets at low filler content. Nanocomposites 2017, 3, 85–96. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. D790-17 Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. D638-14 Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. D257-14 Standard Test Methods for DC Resistance or Conductance of Insulating Materials; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Ashraf, A.; Wu, Y.; Wang, M.C.; Aluru, N.R.; Dastgheib, S.A.; Nam, S.W. Spectroscopic investigation of the wettability of multilayer graphene using highly ordered pyrolytic graphite as a model material. Langmuir 2014, 30, 12827–12836. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Karumuri, A.; Mukhopadhyay, S.M. Wettability tailoring of nanotube carpets: Morphology-chemistry synergy for hydrophobic–hydrophilic cycling. RSC Adv. 2017, 7, 25265–25275. [Google Scholar] [CrossRef]
- Lin, C.H.; Tsai, M.S.; Chen, W.T.; Hong, Y.Z.; Chien, P.Y.; Huang, C.H.; Woon, W.Y.; Lin, C.T. A low-damage plasma surface modification method of stacked graphene bilayers for configurable wettability and electrical properties. Nanotechnology 2019, 30, 245709. [Google Scholar] [CrossRef]
- Gürsoy, M.; Özcan, F.; Karaman, M. Improvement of carbon nanotube dispersion in electrospun polyacrylonitrile fiber through plasma surface modification. J. Appl. Polym. Sci. 2019, 136, 1–6. [Google Scholar] [CrossRef]
- Chen, C.; Liang, B.; Ogino, A.; Wang, X.; Nagatsu, M. Oxygen Functionalization of Multiwall Carbon Nanotubes by Microwave-Excited Surface-Wave Plasma Treatment. J. Phys. Chem. C 2009, 113, 7659–7665. [Google Scholar] [CrossRef]
- Mohan, J.; Ramamoorthy, A.; Ivanković, A.; Dowling, D.; Murphy, N. Effect of an Atmospheric Pressure Plasma Treatment on the Mode I Fracture Toughness of a Co-Cured Composite Joint. J. Adhes. 2014, 90, 733–754. [Google Scholar] [CrossRef]
- Yousefi, N.; Lin, X.; Zheng, Q.; Shen, X.; Pothnis, J.R.; Jia, J.; Zussman, E.; Kim, J.-K. Simultaneous in situ reduction, self-alignment and covalent bonding in graphene oxide/epoxy composites. Carbon N. Y. 2013, 59, 406–417. [Google Scholar] [CrossRef]
- Meng, Q.; Jin, J.; Wang, R.; Kuan, H.-C.; Ma, J.; Kawashima, N.; Michelmore, A.; Zhu, S.; Wang, C.H. Processable 3-nm thick graphene platelets of high electrical conductivity and their epoxy composites. Nanotechnology 2014, 25, 125707. [Google Scholar] [CrossRef]
- Tschoppe, K.; Beckert, F.; Beckert, M.; Mülhaupt, R. Thermally Reduced Graphite Oxide and Mechanochemically Functionalized Graphene as Functional Fillers for Epoxy Nanocomposites. Macromol. Mater. Eng. 2015, 300, 140–152. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, D.; Williams, T.S.; Severino, J.; De Rosa, I.M.; Carlson, L.; Hicks, R.F. Rapid oxidative activation of carbon nanotube yarn and sheet by a radio frequency, atmospheric pressure, helium and oxygen plasma. Carbon N. Y. 2013, 57, 11–21. [Google Scholar] [CrossRef]
- Hernández, S.C.; Wheeler, V.D.; Osofsky, M.S.; Jernigan, G.G.; Nagareddy, V.K.; Nath, A.; Lock, E.H.; Nyakiti, L.O.; Myers-Ward, R.L.; Sridhara, K.; et al. Plasma-based chemical modification of epitaxial graphene with oxygen functionalities. Surf. Coat. Technol. 2014, 241, 8–12. [Google Scholar] [CrossRef]
- Wei, J.; Atif, R.; Vo, T.; Inam, F. Graphene Nanoplatelets in Epoxy System: Dispersion, Reaggregation, and Mechanical Properties of Nanocomposites. J. Nanomater. 2015, 2015, 12. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Ureña, A. Advantages and disadvantages of the addition of graphene nanoplatelets to epoxy resins. Eur. Polym. J. 2014, 61, 206–214. [Google Scholar] [CrossRef]
- Debelak, B.; Lafdi, K. Use of exfoliated graphite filler to enhance polymer physical properties. Carbon N. Y. 2007, 45, 1727–1734. [Google Scholar] [CrossRef]
- Zaman, I.; Kuan, H.-C.; Meng, Q.; Michelmore, A.; Kawashima, N.; Pitt, T.; Zhang, L.; Gouda, S.; Luong, L.; Ma, J. A Facile Approach to Chemically Modified Graphene and its Polymer Nanocomposites. Adv. Funct. Mater. 2012, 22, 2735–2743. [Google Scholar] [CrossRef]
- King, J.A.; Klimek, D.R.; Miskioglu, I.; Odegard, G.M. Mechanical properties of graphene nanoplatelet/epoxy composites. J. Appl. Polym. Sci. 2013, 128, 4217–4223. [Google Scholar] [CrossRef]
- Wang, X.; Jin, J.; Song, M. An investigation of the mechanism of graphene toughening epoxy. Carbon N. Y. 2013, 65, 324–333. [Google Scholar] [CrossRef]
- Ribeiro, H.; Silva, W.M.; Rodrigues, M.-T.F.; Neves, J.C.; Paniago, R.; Fantini, C.; Calado, H.; Seara, L.M.; Silva, G.G. Glass transition improvement in epoxy/graphene composites. J. Mater. Sci. 2013, 48, 7883–7892. [Google Scholar] [CrossRef]
- Yu, J.W.; Jung, J.; Choi, Y.-M.; Choi, J.H.; Yu, J.; Lee, J.K.; You, N.-H.; Goh, M. Enhancement of the crosslink density, glass transition temperature, and strength of epoxy resin by using functionalized graphene oxide co-curing agents. Polym.Chem. 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Monti, M.; Rallini, M.; Puglia, D.; Peponi, L.; Torre, L.; Kenny, J.M. Morphology and electrical properties of graphene–epoxy nanocomposites obtained by different solvent assisted processing methods. Compos. Part A Appl. Sci. Manuf. 2013, 46, 166–172. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, Z.; Li, J.; Zhang, H.; Lu, H.; Yang, Y. Constructing hierarchically structured interphases for strong and tough epoxy nanocomposites by amine-rich graphene surfaces. J. Mater. Chem. 2010, 20, 9635–9643. [Google Scholar] [CrossRef]
- Liao, S.-H.; Liu, P.-L.; Hsiao, M.-C.; Teng, C.-C.; Wang, C.-A.; Ger, M.-D.; Chiang, C.-L. One-Step Reduction and Functionalization of Graphene Oxide with Phosphorus-Based Compound to Produce Flame-Retardant Epoxy Nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; Yan, C.; Liu, M.; Motta, N.; Waclawik, E.R. Fabrication and characterisation of graphene oxide-epoxy nanocomposite. In Proceedings of the 4th International Conference on Smart Materials and Nanotechnology in Engineering (SMN 2013); SPIE—Society of Photo-Optical Instrumentation Engineers, Gold Coast, Australia, 10–12 July 2013; pp. 1–7. [Google Scholar]
- Liu, F.; Guo, K. Reinforcing epoxy resin through covalent integration of functionalized graphene nanosheets. Polym. Adv. Technol. 2014, 25, 418–423. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Processing and characterization of high content multilayer graphene/epoxy composites with high electrical conductivity. Polym. Compos. 2016, 37, 2897–2906. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci. 2011, 36, 191–217. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
| Variable | Level/Condition of Treatment |
|---|---|
| Gas for plasma atmosphere, Gas | O2, Ar, Air, N2 |
| Plasma power, MW [W] | 80, 100, 200 |
| Residence time, RT [min] | 1, 2, 5, 10, 30 |
| Chamber pressure, P [mbar] | 0.01, 0.06 |
| Plasma source-sample distance, d [cm] | 3, 6 |
| Amount of treated material, m [g] | 0.25, 1, 2, 3, 4, 5 |
| m [g] | MW [W] | Gas | RT [min] | P [mbar] | d [cm] | Contact Angle [°] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 4 days | 7 days | 14 days | 30 days | 45 days | 60 days | 75 days | ||||||
| 0.25 | 80 | Air | 10 | 0.06 | 6 | 60.4 ± 1.4 | - | - | - | - | - | - | - |
| O2 | 56.3 ± 1.6 | - | - | - | - | - | - | - | |||||
| 0.25 | 80 | O2 | 5 | 0.06 | 6 | 55.9 ± 2.1 | - | - | - | - | - | - | - |
| 3 | CW | CW | CW | CW | <30 | <30 | <30 | <30 | |||||
| 0.25 | 80 | O2 | 1 | 0.06 | 3 | CW | CW | CW | CW | <30 | <30 | <30 | 55.5 ± 2.3 |
| 2 | CW | CW | CW | CW | CW | <30 | <30 | <30 | |||||
| 5 | CW | CW | CW | CW | <30 | <30 | <30 | <30 | |||||
| 10 | CW | CW | CW | CW | <30 | <30 | <30 | <30 | |||||
| 0.25 | 80 | O2 | 2 | 0.01 | 3 | <30 | <30 | <30 | <30 | - | - | - | - |
| 0.06 | CW | CW | CW | CW | CW | <30 | <30 | <30 | |||||
| 0.25 | 80 | O2 | 2 | 0.06 | 3 | CW | CW | CW | CW | CW | <30 | <30 | <30 |
| N2 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | 51.3 ± 2.1 | |||||
| Air | <30 | <30 | <30 | <30 | <30 | <30 | <30 | 55.2 ± 1.4 | |||||
| Ar | <30 | <30 | <30 | <30 | <30 | <30 | <30 | 46.4 ± 0.8 | |||||
| 0.25 | 80 | O2 | 2 | 0.06 | 3 | CW | CW | CW | CW | CW | <30 | <30 | <30 |
| 100 | CW | CW | CW | CW | CW | <30 | <30 | <30 | |||||
| 200 | CW | CW | CW | CW | CW | <30 | <30 | <30 | |||||
| 0.25 | 80 | O2 | 2 | 0.06 | 3 | CW | CW | CW | CW | CW | <30 | <30 | <30 |
| 1 | <30 | <30 | <30 | <30 | - | <30 | 38.4 ± 1.9 | 40.3 ± 2.0 | |||||
| 2 | <30 | <30 | <30 | <30 | - | 51.8 ± 3.2 | 59.1 ± 1.5 | 61.4 ± 1.5 | |||||
| 3 | <30 | <30 | <30 | <30 | - | 43.2 ± 1.8 | 50.1 ± 1.8 | 53.4 ± 2.0 | |||||
| 4 | <30 | <30 | <30 | <30 | - | 57.4 ± 1.8 | 66.3 ± 2.0 | 67.1 ± 1.4 | |||||
| 5 | <30 | <30 | <30 | <30 | - | 61.5 ± 2.2 | 67.2 ± 0.5 | 71.4 ± 1.1 | |||||
| 0.25 | 80 | O2 | 2 | 0.01 | 3 | <30 | <30 | <30 | <30 | - | - | - | - |
| 100 | 56.0 ± 2.3 | - | - | - | - | - | - | - | |||||
| 200 | 50.0 ± 3.1 | - | - | - | - | - | - | - | |||||
| m [g] | MW [W] | Gas | RT [min] | P [mbar] | d [cm] | Contact Angle [°] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 4 days | 7 days | 14 days | 30 days | 45 days | 60 days | 75 days | ||||||
| 0.05 | 80 | O2 | 2 | 0.06 | 3 | CW | CW | CW | CW | CW | - | CW | CW |
| 200 | CW | CW | CW | CW | CW | - | 62.4 ± 3.4 | 64.6 ± 1.3 | |||||
| 80 | O2 | 2 | 0.06 | 3 | CW | CW | CW | CW | CW | - | CW | CW | |
| Air | CW | CW | CW | CW | CW | - | 45.4 ± 2.2 | 44.1 ± 1.5 | |||||
| 80 | O2 | 2 | 0.01 | 3 | CW | CW | CW | CW | CW | - | CW | CW | |
| 0.06 | CW | CW | CW | CW | CW | - | CW | CW | |||||
| 80 | O2 | 1 | 0.06 | 3 | CW | CW | CW | CW | CW | - | CW | 65.2 ± 1.2 | |
| 2 | CW | CW | CW | CW | CW | - | CW | CW | |||||
| m [g] | MW [W] | Gas | RT [min] | P [mbar] | d [cm] | Contact Angle [°] |
|---|---|---|---|---|---|---|
| 12 h | ||||||
| 0.25 | 80 | He | 2 | 0.06 | 3 | CW |
| Ar | ||||||
| He/O2 (10:1) | ||||||
| He/O2 (5:1) | ||||||
| He/O2 (2:1) | ||||||
| Ar/O2 (10:1) |
| Gas | Plasma Parameters | Treatment | Contact Angle [°] | |||
|---|---|---|---|---|---|---|
| Microwave Power [W] | Time [min] | Pressure [mbar] | Distance [cm] | |||
| None | 80 | 2 | 0.06 | 3 | 12 h @ 120 °C | 109 ± 2 |
| O2 | 80 | 2 | 0.06 | 3 | Not treated | CW |
| 1 h @ 80 °C | 58 ± 1 | |||||
| 12 h @ 80 °C | 98 ± 1 | |||||
| He | 80 | 2 | 0.06 | 3 | Not treated | CW |
| 1 h @ 80 °C | 56 ± 2 | |||||
| 12 h @ 80 °C | 51 ± 1 | |||||
| 30′ @ 160 °C | 47 ± 1 | |||||
| 2 h @ 160 °C | 49 ± 1 | |||||
| Ar | 80 | 2 | 0.06 | 3 | Not treated | CW |
| 1 h @ 80 °C | 49 ± 3 | |||||
| 12 h @ 80 °C | 56 ± 1 | |||||
| 30′ @ 160 °C | 44 ± 1 | |||||
| 2 h @ 160 °C | 61 ± 2 | |||||
| He/O2 (10:1) | 80 | 2 | 0.06 | 3 | Not treated | CW |
| 1 h @ 120 °C | CW | |||||
| 12 h @ 120 °C | 81 ± 3 | |||||
| He/O2 (5:1) | 80 | 2 | 0.06 | 3 | Not treated | CW |
| 1 h @ 120 °C | CW | |||||
| 12 h @ 120 °C | 67 ± 2 | |||||
| He/O2 (2:1) | 80 | 2 | 0.06 | 3 | Not treated | CW |
| 1 h @ 120 °C | CW | |||||
| 12 h @ 120 °C | 93 ± 2 | |||||
| Sample | Toughness [J/m3 *104] |
|---|---|
| neat | 213 |
| 0.05% | 114 |
| 0.05% O2 | 80 |
| 0.05% O2 RT | 73 |
| 0.05% He–O2 | 121 |
| 0.5% | 105 |
| 0.5% He–O2 | 109 |
| Sample | Storage Modulus [MPa] | Tg from Tan δ [°C] |
|---|---|---|
| Neat resin | 1624 | 123 |
| 0.05% Graphene | 1831 | 143 |
| 0.05% O2-Graphene | 1617 | 140 |
| 0.05% He/O2-Graphene | 1927 | 144 |
| 0.5% Graphene | 1854 | 136 |
| 0.5% He/O2-Graphene | 1807 | 135 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boaretti, C.; Roso, M.; Bonora, R.; Modesti, M.; Lorenzetti, A. Investigation of Plasma-Assisted Functionalization of Graphitic Materials for Epoxy Composites. Nanomaterials 2020, 10, 78. https://doi.org/10.3390/nano10010078
Boaretti C, Roso M, Bonora R, Modesti M, Lorenzetti A. Investigation of Plasma-Assisted Functionalization of Graphitic Materials for Epoxy Composites. Nanomaterials. 2020; 10(1):78. https://doi.org/10.3390/nano10010078
Chicago/Turabian StyleBoaretti, Carlo, Martina Roso, Renato Bonora, Michele Modesti, and Alessandra Lorenzetti. 2020. "Investigation of Plasma-Assisted Functionalization of Graphitic Materials for Epoxy Composites" Nanomaterials 10, no. 1: 78. https://doi.org/10.3390/nano10010078
APA StyleBoaretti, C., Roso, M., Bonora, R., Modesti, M., & Lorenzetti, A. (2020). Investigation of Plasma-Assisted Functionalization of Graphitic Materials for Epoxy Composites. Nanomaterials, 10(1), 78. https://doi.org/10.3390/nano10010078







