Gold Nanoparticles Supported on Urchin-Like CuO: Synthesis, Characterization, and Their Catalytic Performance for CO Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. CuO Support Preparation
2.2. Catalyst Preparation
2.3. Characterization
2.4. Catalytic Activity
3. Results
3.1. Effect of Time of Dropping of NaOH on CuO Support
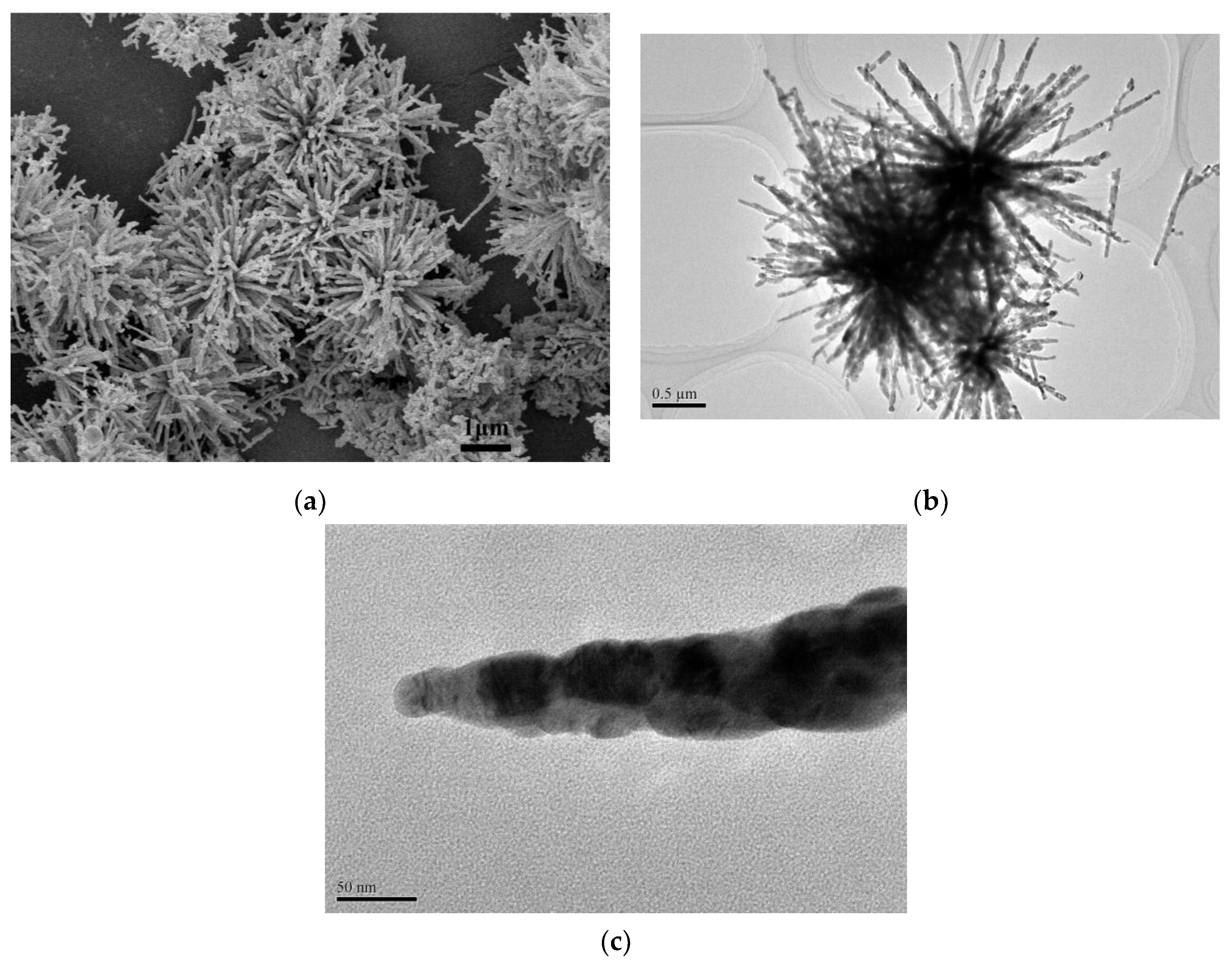
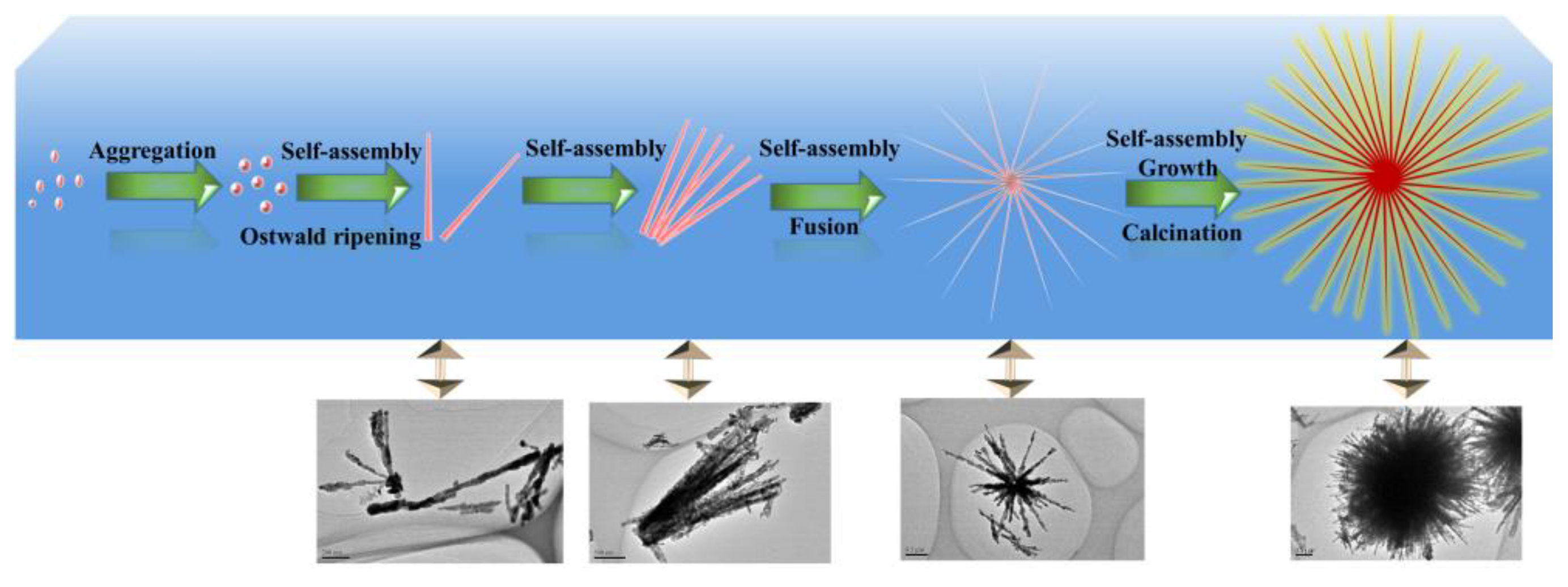
3.2. Formation Mechanism of Urchin-Like CuO
- (i)
- Formation of the primary nanocrystals: In initial reaction time, the Cu(OH)2 nucleation was formed by the equilibria of complex ions in solution. In addition, the chemical process for formation of CuO microsphere is ascribed in Equations (1)–(5) as followsCu2+ + 6H2O ⇋ [Cu(H2O)6]2+[Cu(H2O)6]2+ + 4NH3 ⇋ [Cu(NH3)4]2+ + 6H2O[Cu(NH3)4]2+ + 4OH− ⇋ [Cu(OH)4]2− + 4NH3[Cu(OH)4]2− → Cu(OH)2↓ + 2OH−Cu(OH)2 → CuO + H2O
- (ii)
- Growth of the secondary structure: In highly alkaline conditions, it was easy to form the pristine nanorod via the self-assembly of Cu(OH)2 nanoparticles [37] and Ostwald ripening effect [43,44]. This should be attributed to the Van der Waals forces for minimizing the overall surface free energy [45]. In addition, TOAB also could facilitate the formation of nanorods via oriented attachment.
- (iii)
- Formation of the three dimensional structure: With the assistance of TOAB, the nanorods self-assembled into urchin-like structures through the Van der Waals force and driving force of hydrogen bond between the OH− groups of Cu(OH)2 [37,46], followed by the growth and crystallographic fusion into bigger urchin-like structures for the decrease of Gibbs free energies of whole system [38,47]. Finally, the reaction system was inclined to be the thermodynamically stable state. During the process of the nanostructure formation, TOAB molecules, acted as a growth-directing agent, could modulate spontaneous self-assembling from nanocrystals to urchin-like structures.
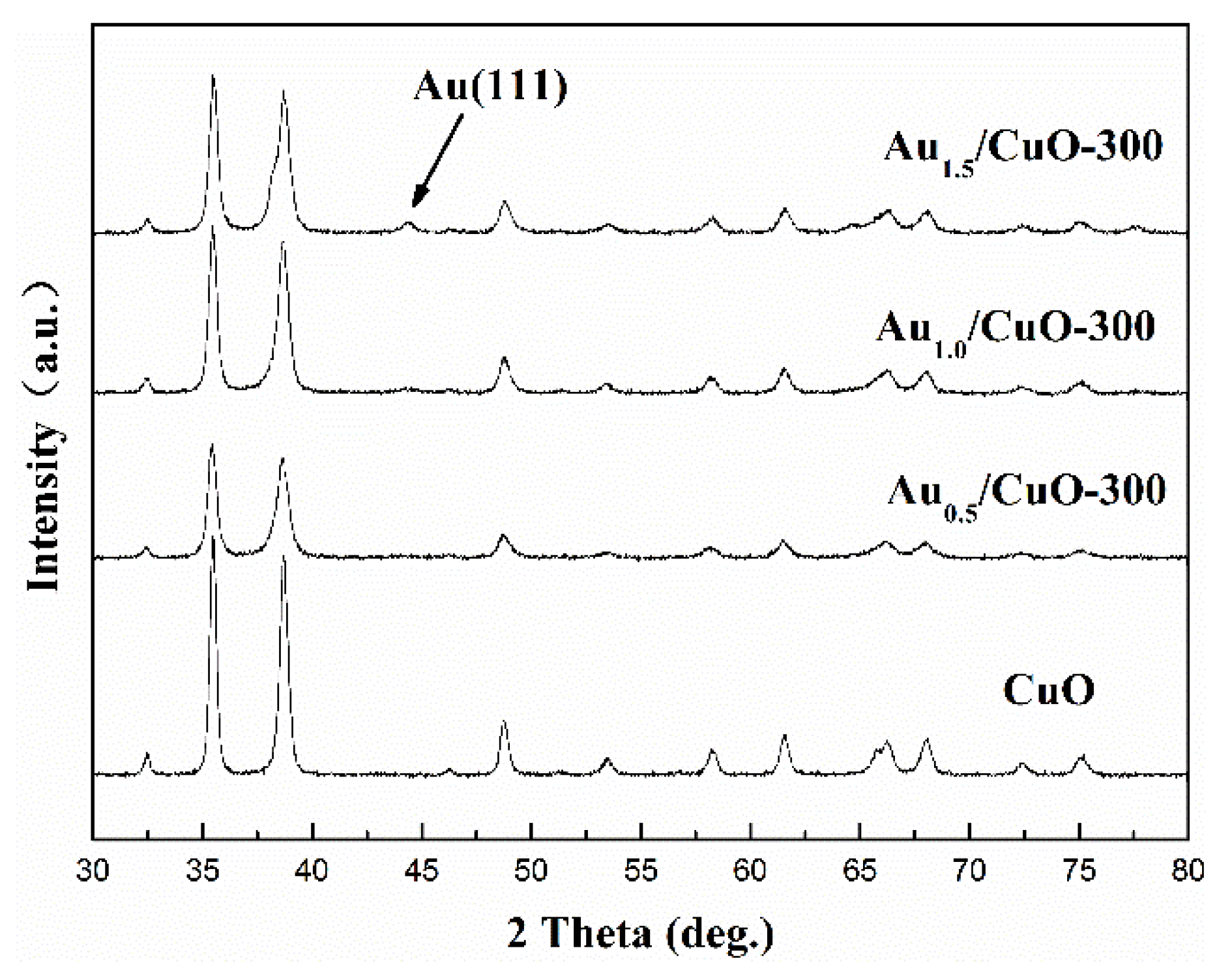
3.3. XRD
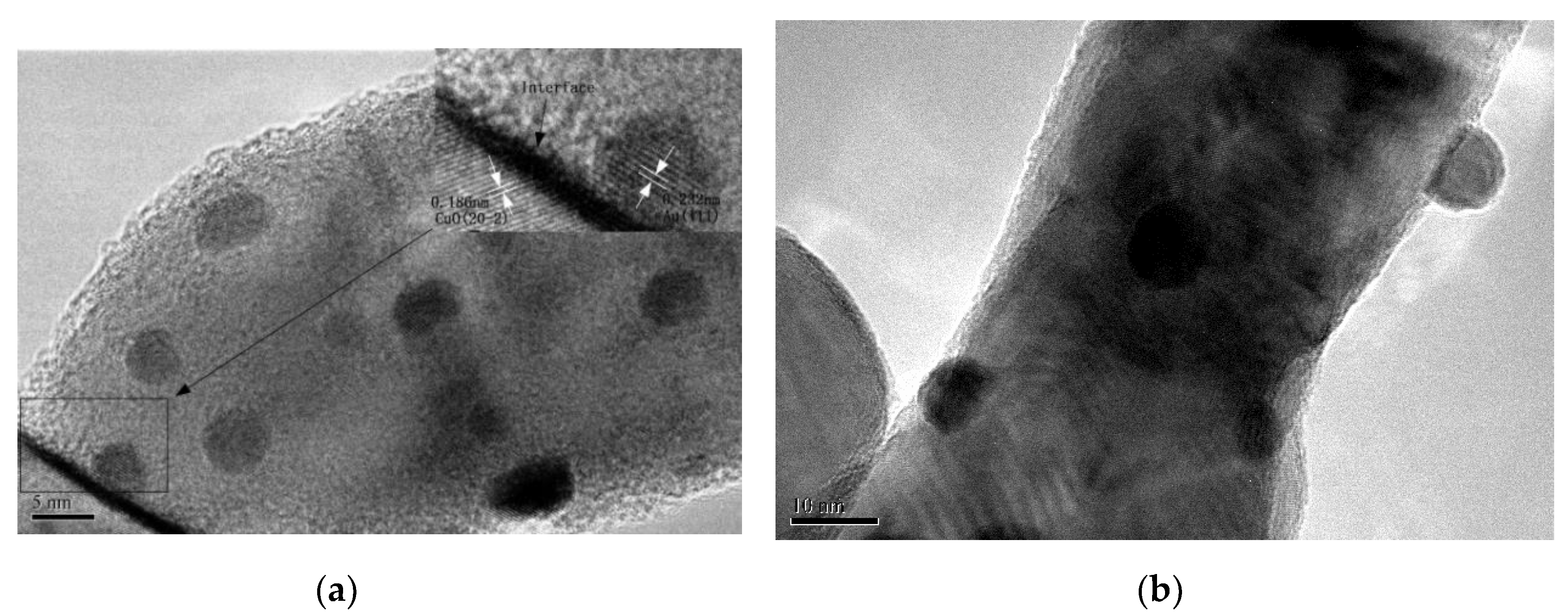
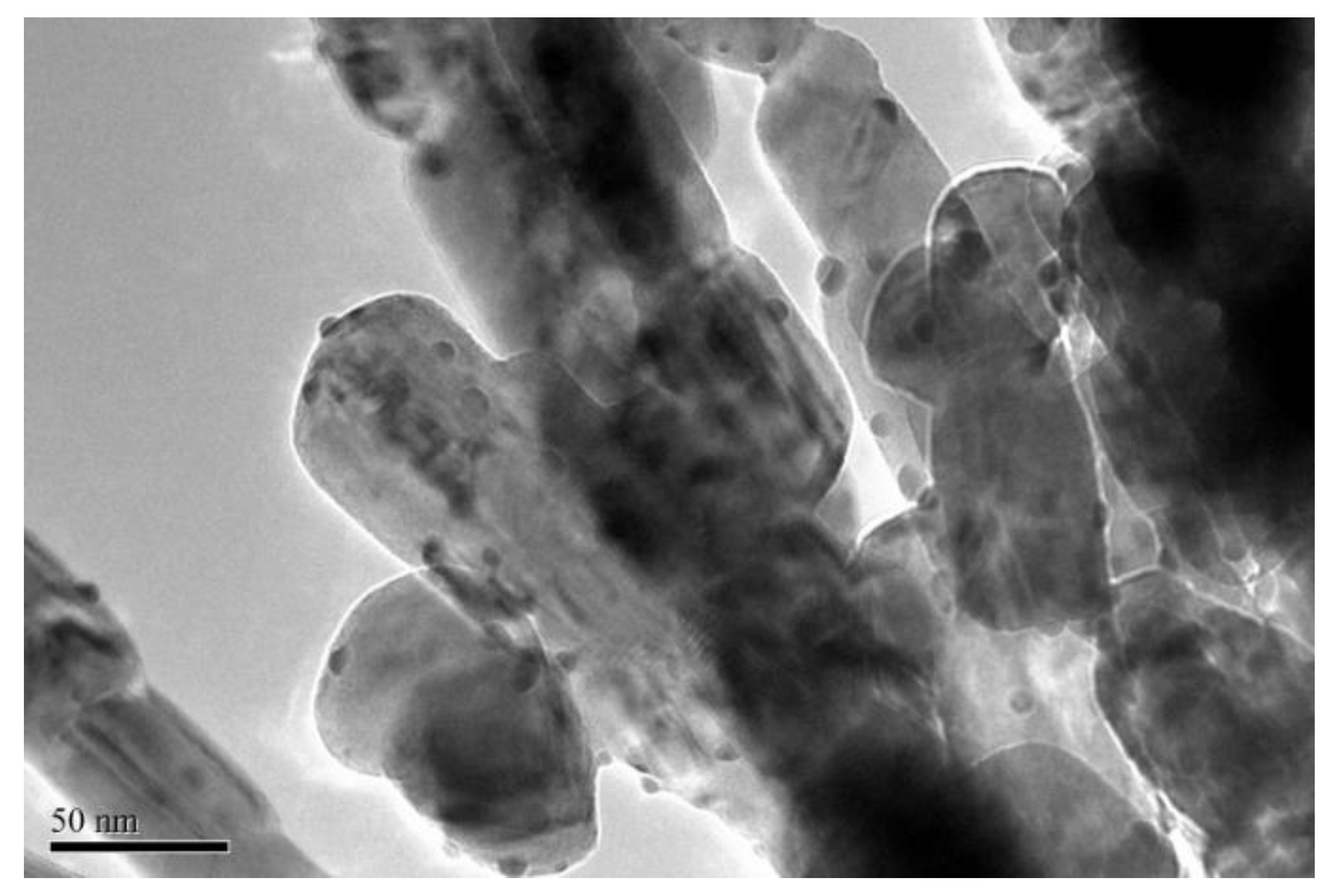
3.4. TEM
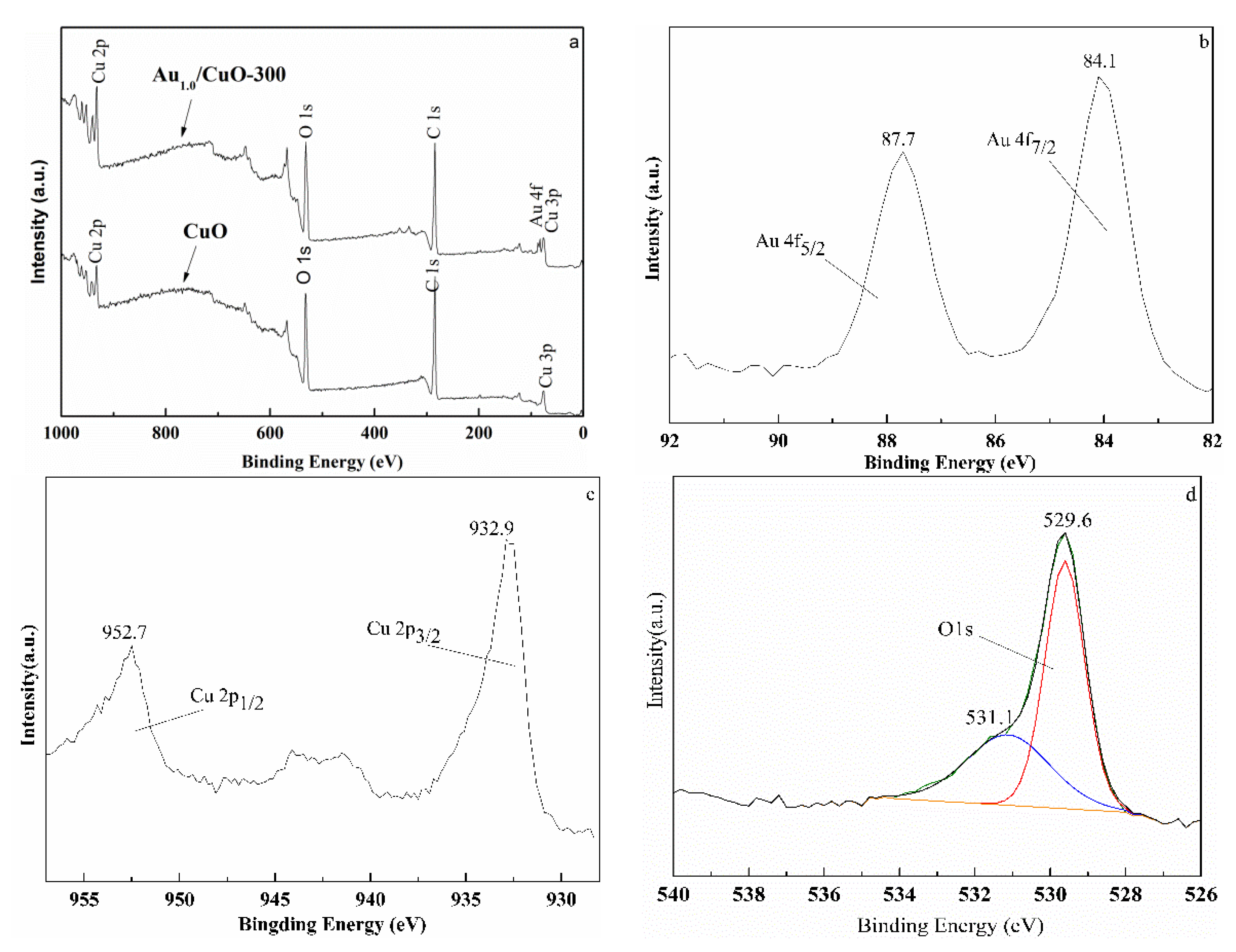
3.5. XPS
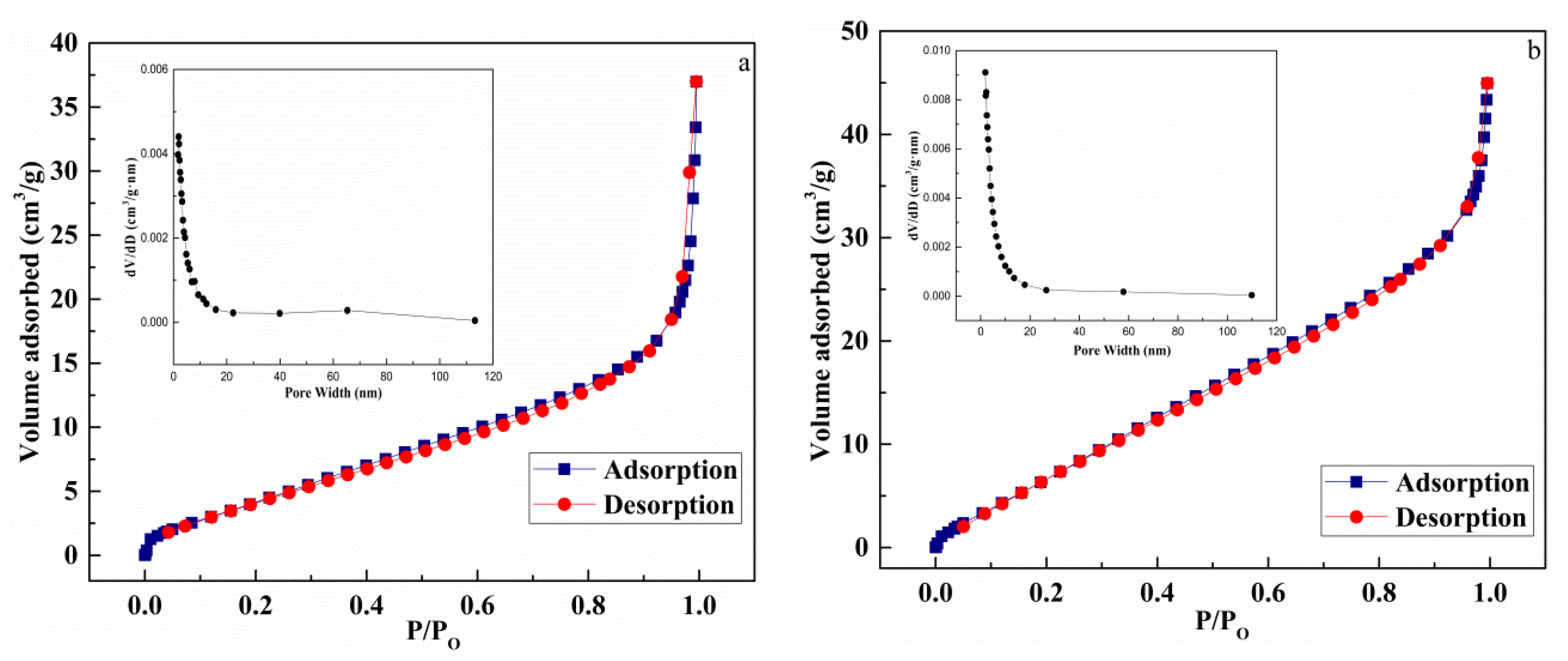
3.6. BET
3.7. Catalytic Activity
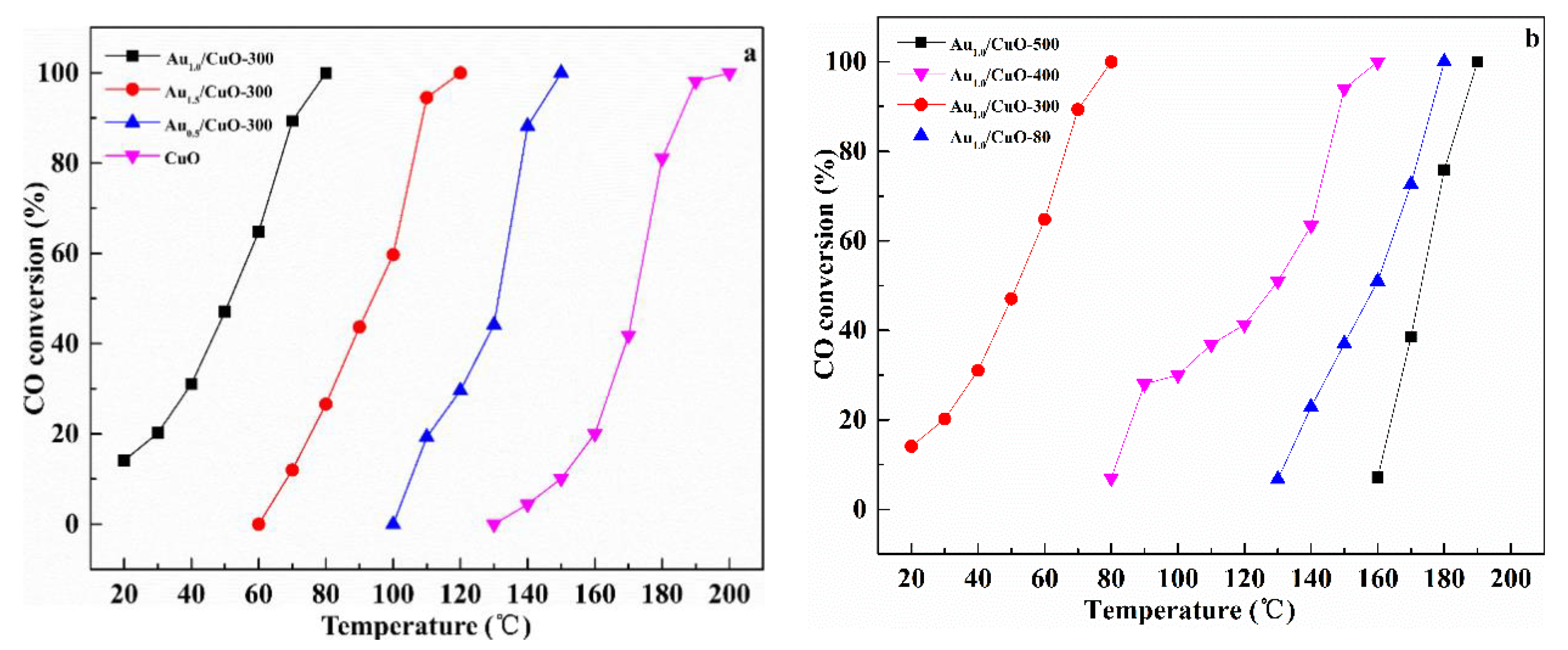
3.7.1. Effect of Au Contents
3.7.2. Effect of Calcination Temperatures
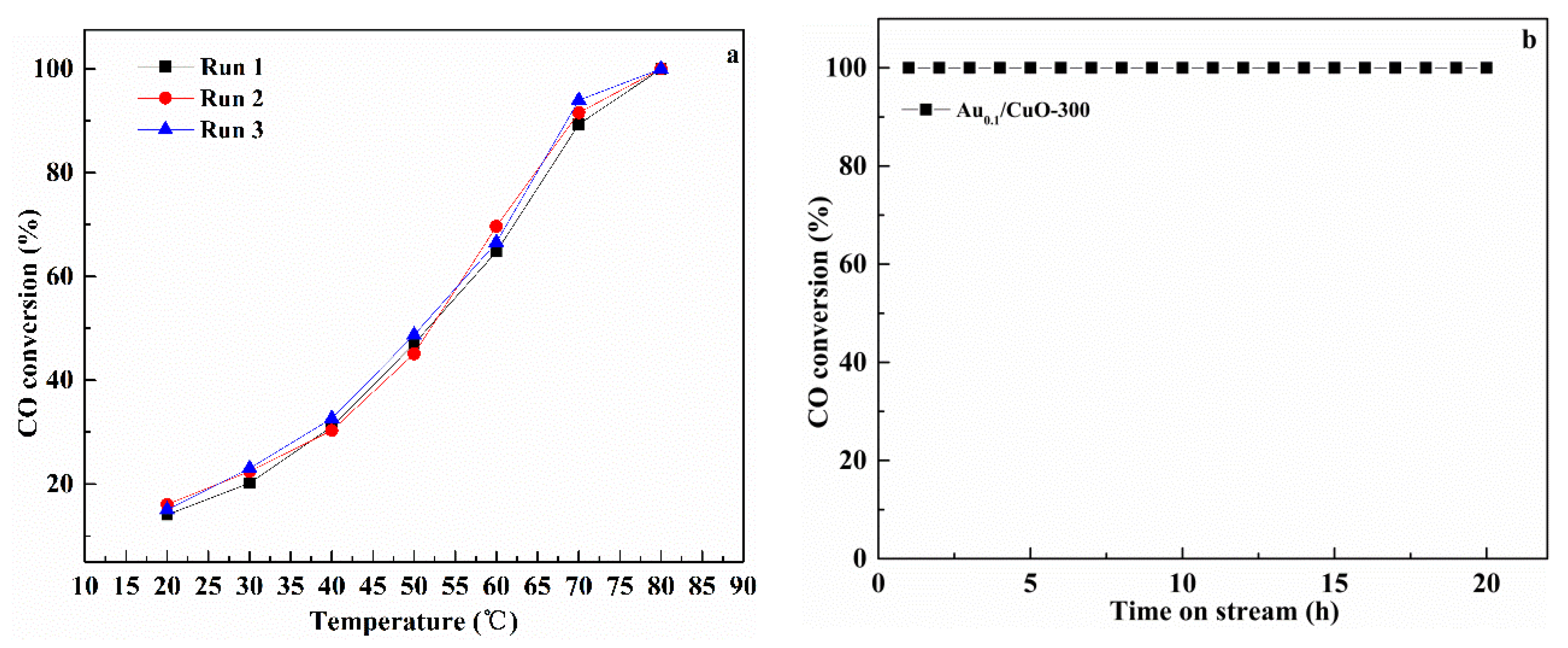
3.7.3. Reusability and Stability Test
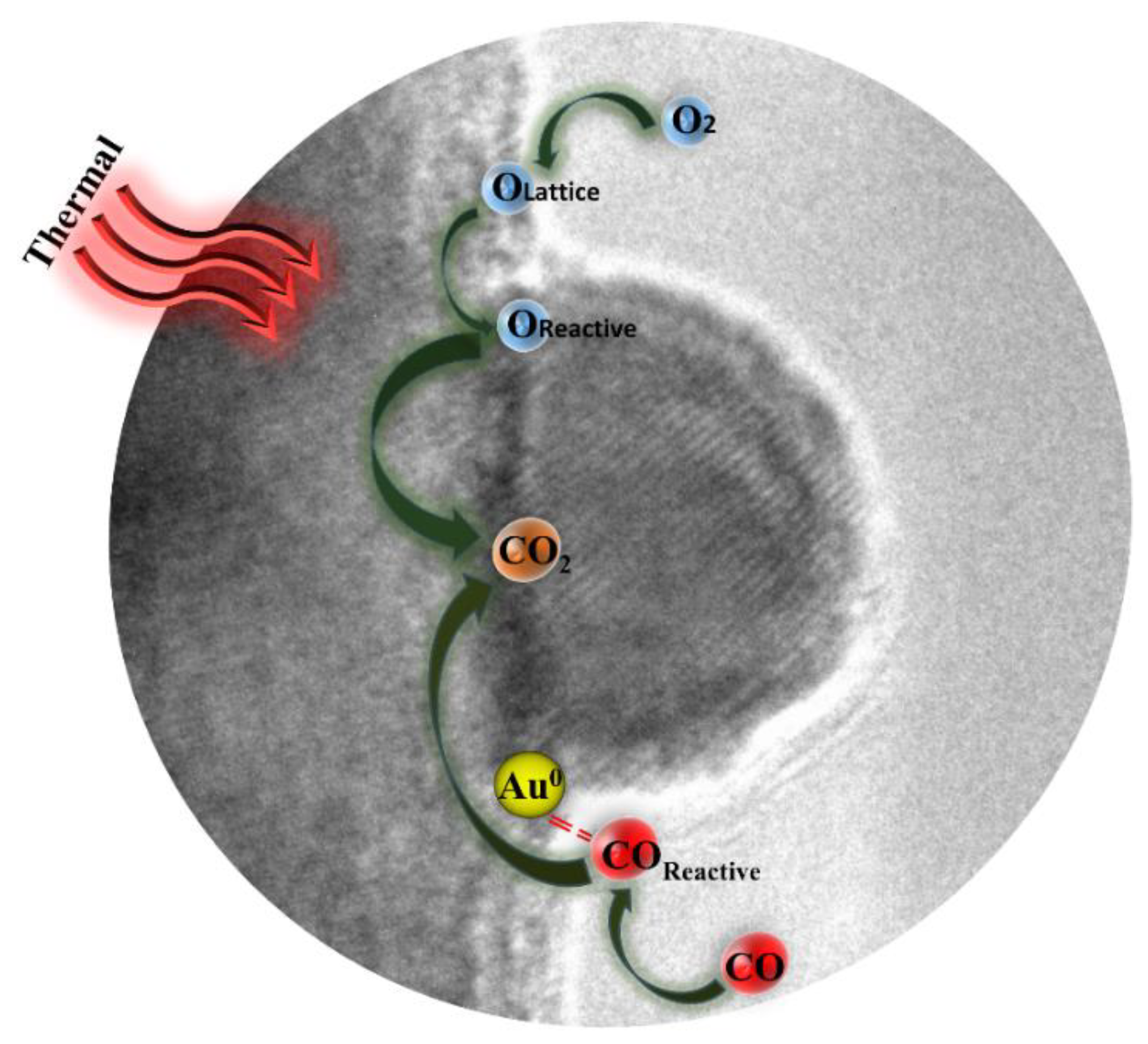
3.7.4. Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, R.; Gao, N.; Zhen, F. Doping effect of Al2O3 and CeO2 on Fe2O3 support for gold catalyst in CO oxidation at low temperature. Chem. Eng. J. 2013, 225, 245–253. [Google Scholar] [CrossRef]
- Yan, W.F.; Brown, S.; Pan, Z.W.; Mahurin, S.M.; Overbury, S.H.; Dai, S. Ultrastable Gold Nanocatalyst Supported by Nanosized Non-Oxide Substrate. Angew. Chem. Int. Ed. 2006, 45, 3614–3618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Choi, H.B.; Kamat, P.V. Metal-Cluster-Sensitized Solar Cells. A New Class of Thiolated Gold Sensitizers Delivering Efficiency Greater Than 2%. J. Am. Chem. Soc. 2013, 135, 8822–8825. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Ijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A 2001, 222, 427–437. [Google Scholar] [CrossRef]
- DelRío, E.; Hungría, A.B.; Tinoco, M. CeO2-modified Au/TiO2 catalysts with outstanding stability under harsh CO oxidation conditions. Appl. Catal. B Environ. 2016, 197, 86–94. [Google Scholar] [CrossRef]
- Tang, H.L.; Wei, J.; Liu, F. Strong metal–support interactions between gold nanoparticles and nonoxides. J. Am. Chem. Soc. 2016, 138, 56–59. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.C.; Liu, Y.M. Gold nanoparticles supported on hydroxylapatite as high performance catalysts for low temperature CO oxidation. Appl. Catal. B 2011, 101, 560–569. [Google Scholar] [CrossRef]
- El-Moemen, A.A.; Abdel-Mageed, A.M.; Bansmann, J. Deactivation of Au/CeO2 catalysts during CO oxidation: Influence of pretreatment and reaction conditions. J. Catal. 2016, 341, 160–179. [Google Scholar] [CrossRef]
- Liu, Z.P.; Hu, P.; Alavi, A. Catalytic Role of Gold in Gold-Based Catalysts: A Density Functional Theory Study on the CO Oxidation on Gold. J. Am. Chem. Soc. 2002, 124, 14770. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mavrikakis, M. Adsorption and Dissociation of O2 on Gold Surfaces: Effect of Steps and Strain. J. Phys. Chem. B 2003, 107, 9298–9307. [Google Scholar] [CrossRef]
- Overbury, S.H.; Ortiz-Soto, L.; Zhu, H.G.; Lee, M.; Amiridis, D.M.; Dai, S. Comparison of Au Catalysts Supported on Mesoporous Titania and Silica: Investigation of Au Particle Size Effects and Metal-Support Interactions. Catal. Lett. 2004, 95, 99–106. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO Oxidation over Supported Gold Catalysts—“Inert” and “Active” Support Materials and Their Role for the Oxygen Supply during Reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Lei, J.H.; Liu, Y.; Wang, X.Y.; Hua, P.; Peng, X.S. Au/CuO nanosheets composite for glucose sensor and CO oxidation. RSC Adv. 2015, 5, 9130. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, G.J.; Yang, S.; Song, X.P.; Sun, Z.B. Nanoporous CuO ribbons modified by Au nanoparticles through chemical dealloying and calcination for CO oxidation. Microporous Mesoporous Mater. 2016, 226, 61–70. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Q.; Yuan, K.D.; Yang, W.S.; Chen, Q.W.; Geng, Z.H.; Zhang, J.L.; Shao, X.; Chen, W.; Xu, G.Q.; et al. Unraveling Charge State of Supported Au Single-Atoms during CO Oxidation. J. Am. Chem. Soc. 2018, 140, 554–557. [Google Scholar] [CrossRef]
- Solsona, B.E.; Garcia, T.; Jones, C.; Taylor, S.H.; Carley, A.F.; Hutchings, G.J. Supported gold catalysts for the total oxidation of alkanes and carbon monoxide. Appl. Catal. A Gen. 2006, 312, 67–76. [Google Scholar] [CrossRef]
- Liang, W.T.; Zhu, L.Q.; Li, W.P.; Liu, H.C. Facile fabrication of a flower-like CuO/Cu(OH)2 nanorod film with tunable wetting transition and excellent stability. RSC Adv. 2015, 5, 38100. [Google Scholar] [CrossRef]
- Sun, S.D.; Sun, Y.X.; Chen, A.R.; Zhang, X.Z.; Yang, Z.M. Nanoporous copper oxide ribbon assembly of free-standing nanoneedles as biosensors for glucose. Analyst 2015, 140, 5205. [Google Scholar] [CrossRef] [PubMed]
- Konar, S.; Kalita, H.; Puvvada, N.; Tantubay, S.; Mahto, M.K.; Biswas, S.; Pathak, A. Shape-dependent catalytic activity of CuO nanostructures. J. Catal. 2016, 336, 11–22. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Jiang, T.T.; Meng, D.W.; Kong, J.H.; Jia, H.X.; Yu, M.H. Controllable fabrication of nanostructured copper compound on a Cu substrate by a one-step route. RSC Adv. 2015, 5, 16277. [Google Scholar] [CrossRef]
- Fan, Z.J.; Liu, B.; Li, Z.P.; Ma, L.M.; Wang, J.Q.; Yang, S.R. One-pot hydrothermal synthesis of CuO with tunable morphologies on Ni foam as a hybrid electrode for sensing glucose. RSC Adv. 2014, 4, 23319. [Google Scholar] [CrossRef]
- Chang, T.H.; Hsu, C.Y.; Lin, H.C.; Chang, K.H.; Li, Y.Y. Formation of urchin-like CuO structure through thermal oxidation and its field-emission lighting application. J. Alloy. Compd. 2015, 644, 324–333. [Google Scholar] [CrossRef]
- Zou, Y.L.; Li, Y.; Guo, Y.; Zhou, Q.J.; An, D.M. Ultrasound-assisted synthesis of CuO nanostructures templated by cotton fibers. Mater. Res. Bull. 2012, 47, 3135–3140. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Huang, M.; Li, F.; Wen, Z.Q. Controlled Synthesis of Hierarchical CuO Nanostructures for Electrochemical Capacitor Electrodes. Int. J. Electrochem. Sci. 2013, 8, 8645–8661. [Google Scholar]
- Qiu, G.H.; Saminda, D.; Zhang, Y.; Naftali, O.; Huang, H.; Steven, L.S. Facile Microwave-Assisted Hydrothermal Synthesis of CuO Nanomaterials and Their Catalytic and Electrochemical Properties. J. Phys. Chem. 2012, 116, 468–477. [Google Scholar] [CrossRef]
- Liu, M.; Jin, H.Y.; Liu, M.Y.; Dong, J.; Hou, P.; Ji, Z.J.; Hou, S.E. Synthesis and self-assembled mechanism of CuO peony-flowers by a composite hydroxide-mediated approach at low temperature. Cryst. Res. Technol. 2014, 49, 820–828. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Tu, J.P.; Zhang, L.; Zhou, Y.; Wang, X.L.; Shi, S.J. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. J. Power Sources 2010, 195, 313–319. [Google Scholar] [CrossRef]
- Gamboa-Rosalesa, N.K.; Ayastuya, J.L.; Boukhaa, Z.; Bionb, N.; Duprezb, D.; Pérez-Omilc, J.A.; delRíoc, E.; Gutiérrez-Ortiz, M.A. Ceria-supported Au–CuO and Au–Co3O4 catalysts for CO oxidation: An 18O/16O isotopic exchange. Appl. Catal. B Environ. 2015, 168–169, 87–97. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Sun, N.N.; Lin, W.W.; Ma, Y.; Lai, C.; Wang, Q.H. Facile fabrication of three-dimensional hierarchical CuO nanostructures with enhanced lithium storage Capability. RSC Adv. 2015, 5, 68061. [Google Scholar] [CrossRef]
- Diogo, P.V.; Anderson, A.F.; Marcelo, O.O.; George, W.; Yang, D.; Elson, L.; Harry, L.T.; José, A.V. The Role of Hierarchical Morphologies in the Superior Gas Sensing Performance of CuO-Based Chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar]
- Guo, Y.; Xu, Y.T.; Zhao, B.; Wang, T.; Zhang, K.; Matthew, M.F.; Fu, X.Z.; Sun, R.; Wong, C. Urchin-like Pd@CuO–Pd yolk–shell nanostructures: Synthesis, characterization and Electrocatalysis. J. Mater. Chem. A 2015, 3, 13653. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Zhang, X.B.; Kong, R.M.; Qu, F.L. Enhanced biosensing platform constructed using urchin-like ZnO-Au@CdS microspheres based on the combination of photoelectrochemical and bioetching strategies. Sens. Actuator B Chem. 2018, 255, 1753–1761. [Google Scholar] [CrossRef]
- Feng, Y.J.; Wang, J.P.; Liu, L.L.; Wang, X.D. Self-Conversion from ZnO Nanorod Arrays to Tubular Structures and Their Applications in Nanoencapsulated Phase-Change Materials. Acta Phys. Chim. Sin. 2019, 35, 644–650. [Google Scholar]
- Lu, C.H.; Qi, L.M.; Yang, J.H.; Zhang, D.Y.; Wu, N.Z.; Ma, J.M. Simple Template-Free Solution Route for the Controlled Synthesis of Cu(OH)2 and CuO Nanostructures. J. Phys. Chem. B 2004, 108, 17825–17831. [Google Scholar] [CrossRef]
- Zhu, J.W.; Bi, H.P.; Wang, Y.P.; Wang, X.; Yang, X.J. Synthesis of flower-like CuO nanostructures via a simple hydrolysis route. Mater. Lett. 2007, 61, 5236–5238. [Google Scholar] [CrossRef]
- Beena, B.; Chudasama, U. A comparative study of the catalytic activity of [Cu(NH3)4]2+ sorbed on zirconium phosphate and zirconium phenylphosphonate. Transit. Met. Chem. 1995, 20, 166–169. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Huang, M.; Kuang, M.; Liu, C.P.; Tan, J.L.; Dong, M.; Yuan, Y.; Zhao, X.L.; Wen, Z.Q. Facile synthesis of mesoporous CuO nanoribbons for electrochemical capacitors applications. Int. J. Electrochem. Sci. 2013, 8, 1366. [Google Scholar]
- Dinesh, P.S.; Animesh, K.O.; Onkar, N.S. Synthesis of Different Cu(OH)2 and CuO (Nanowires, Rectangles, Seed-, Belt-, and Sheetlike) Nanostructures by Simple Wet Chemical Route. J. Phys. Chem. C 2009, 113, 3409–3418. [Google Scholar]
- Liu, S.; Xing, R.; Lu, F.; Rana, R.K.; Zhu, J.J. One-Pot Template-Free Fabrication of Hollow Magnetite Nanospheres and Their Application as Potential Drug Carriers. J. Phys. Chem. C 2009, 113, 21042–21047. [Google Scholar] [CrossRef]
- Luo, Y.S.; Dai, X.J.; Zhang, W.D.; Yang, Y.; Sun, C.Q.; Fu, S.Y. Controllable synthesis and luminescent properties of novel erythrocyte-like CaMoO4 hierarchical nanostructures via a simple surfactant-free hydrothermal route. Dalton Trans. 2010, 39, 2226–2231. [Google Scholar] [CrossRef]
- Behrouz, S.; Ebrahim, A.G.; Yashar, A.K.; Ali, K. Preparation of CuO nanopowders and their catalytic activity in photodegradation of Rhodamine-B. Adv. Powder Technol. 2015, 25, 1043–1052. [Google Scholar]
- Ni, Y.H.; Li, H.; Jin, L.N.; Hong, J.M. Synthesis of 1D Cu(OH)2 nanowires and transition to 3D CuO microstructures under ultrasonic irradiation, and their electrochemical property. Cryst. Growth Des. 2009, 9, 3868–3873. [Google Scholar] [CrossRef]
- Wang, J.H.; Xu, Z.; Zhu, J.J.; Chen, H.Y. Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth 2002, 244, 88–94. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Umar, A. Ethanol chemi-sensor: Evaluation of structural, optical and sensing properties of CuO nanosheets. Mater. Lett. 2011, 65, 1400–1403. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A Gen. 2011, 391, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.Y.; Luo, J.J.; Chu, W. Non-thermal plasma-treated gold catalyst for CO oxidation. RSC Adv. 2014, 4, 25729–25735. [Google Scholar] [CrossRef]
- Lim, D.C.; Lopez, S.I.; Dietsche, R.; Bubek, M.; Kim, Y.D. Size-Selectivity in the Oxidation Behaviors of Au Nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 2413–2415. [Google Scholar] [CrossRef] [PubMed]
- Boyen, H.G.; Ethirajan, A.; Stle, G.K.; Weigl, F.; Ziemann, P. Alloy Formation of Supported Gold Nanoparticles at Their Transition from Clusters to Solids: Does Size Matter. Phys. Rev. Lett. 2005, 94, 016804. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liu, Y.; Zhang, X.G. Bimetallic synergistic Au/CuO-hydroxyapatite catalyst for aerobic oxidation of alcohols. Chin. J. Catal. 2015, 36, 1358–1364. [Google Scholar] [CrossRef]
- Gamboa-Rosales, N.K.; Ayastuy, J.L.; González-Marcos, M.P.; Gutiérrez-Ortiz, M.A. Oxygen-enhanced water gas shift over ceria-supported Au-Cu bimetallic catalysts prepared by wet impregnation and deposition-precipitation. Int. J. Hydrog. Energ. 2012, 37, 7005–7016. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Beni, V.; Liu, X.; Willander, M. Synthesis of Novel CuO Nanosheets and Their Non-Enzymatic Glucose Sensing Applications. Sensors 2013, 13, 7926–7938. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.L.; Wang, Y.; Yu, X.L.; Wang, S.R.; Wu, S.H.; Yuan, Z.Y. Mesoporous CuO–Fe2O3 composite catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. B Environ. 2008, 79, 26–34. [Google Scholar] [CrossRef]
- Zhou, C.J.; Jiang, X.; Yang, L.N.; Yin, Y.D.; Jin, M.S. Low-Temperature Carbon Monoxide Oxidation with Au–Cu Meatball-Like Cages Prepared by Galvanic Replacement. ChemSusChem 2013, 6, 1883–1887. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Gold-catalysed oxidation of carbon monoxide. Gold Bull. 2000, 33, 41–50. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, B.L.; Wang, G.C.; Liu, Z.F.; Huang, W.P.; Zhang, S.M. Enhanced CO catalytic oxidation over an Au–Pt alloy supported on TiO2 nanotubes: Investigation of the hydroxyl and Au/Pt ratio influences. Catal. Sci. Technol. 2018, 8, 6109. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, B.L.; Wang, G.C.; Liu, Z.F.; Huang, W.P.; Zhang, S.M. Role of Hydroxyl Groups in Low-Temperature CO Catalytic Oxidation over Zn4Si2O7(OH)2 Nanowire-Supported Gold Nanoparticles. J. Phys. Chem. C 2018, 122, 25456–25466. [Google Scholar] [CrossRef]
- Luo, J.J.; Liu, Y.F.; Niu, Y.M.; Jiang, Q.; Huang, R.; Zhang, B.S.; Su, D.S. Insight into the chemical adsorption properties of CO molecules supported on Au or Cu and hybridized Au-CuO nanoparticles. Nanoscale 2017, 9, 15033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.J.; Liu, Y.F.; Zhang, L.Y.; Ren, Y.J.; Miao, S.; Zhang, B.S.; Su, D.S.; Liang, C.H. Atomic-scale observation of bimetallic Au-CuOx nanoparticles and their interfaces for activation of CO molecules. ACS Appl. Mater. Interfaces 2019, 11, 35468–35478. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, A.Q.; Li, L.; Zhang, T.; Mou, C.Y.; Lee, J.F. Structural changes of Au–Cu bimetallic catalysts in CO oxidation: In situ XRD, EPR, XANES, and FT-IR characterizations. J. Catal. 2011, 278, 288–296. [Google Scholar] [CrossRef]
- Bauer, J.C.; Mullins, D.R.; Oyola, Y.; Overbury, S.H.; Dai, S. Structure Activity Relationships of Silica Supported AuCu and AuCuPd Alloy Catalysts for the Oxidation of CO. Catal. Lett. 2013, 143, 926–935. [Google Scholar] [CrossRef]
- Ellert, O.G.; Tsodikov, M.V.; Nikolaev, S.A.; Novotortsev, V.M. Bimetallic nanoalloys in heterogeneous catalysis of industrially important reactions: Synergistic effects and structural organization of active components. Russ. Chem. Rev. 2014, 83, 718–732. [Google Scholar] [CrossRef] [Green Version]
- Valden, M.; Lai, X.; Goodman, D.W. Onset of Catalytic Activity of Gold Clusters on Titania with the Appearance of Nonmetallic Properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Goodman, D.W. Catalytically active gold on ordered titania supports. Chem. Soc. Rev. 2008, 37, 1860–1870. [Google Scholar] [CrossRef] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Guo, Y.; Zhang, D.; Zhu, B.; Huang, W.; Zhang, S. Gold Nanoparticles Supported on Urchin-Like CuO: Synthesis, Characterization, and Their Catalytic Performance for CO Oxidation. Nanomaterials 2020, 10, 67. https://doi.org/10.3390/nano10010067
Dong F, Guo Y, Zhang D, Zhu B, Huang W, Zhang S. Gold Nanoparticles Supported on Urchin-Like CuO: Synthesis, Characterization, and Their Catalytic Performance for CO Oxidation. Nanomaterials. 2020; 10(1):67. https://doi.org/10.3390/nano10010067
Chicago/Turabian StyleDong, Feng, Yuan Guo, Dongyang Zhang, Baolin Zhu, Weiping Huang, and Shoumin Zhang. 2020. "Gold Nanoparticles Supported on Urchin-Like CuO: Synthesis, Characterization, and Their Catalytic Performance for CO Oxidation" Nanomaterials 10, no. 1: 67. https://doi.org/10.3390/nano10010067
APA StyleDong, F., Guo, Y., Zhang, D., Zhu, B., Huang, W., & Zhang, S. (2020). Gold Nanoparticles Supported on Urchin-Like CuO: Synthesis, Characterization, and Their Catalytic Performance for CO Oxidation. Nanomaterials, 10(1), 67. https://doi.org/10.3390/nano10010067



