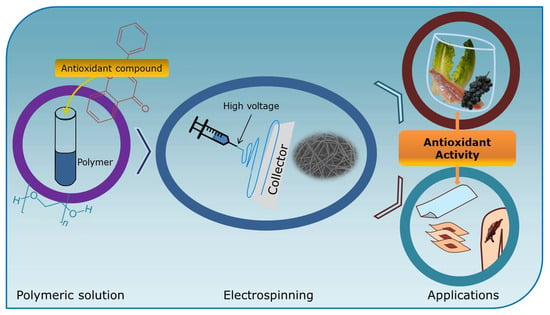
Applications of Electrospun Nanofibers with Antioxidant Properties: A Review
Abstract
1. Introduction
2. Reviewed Literature
3. Electrospun Nanofibers: Development and Applications
3.1. Polymeric Matrices of Electrospun Nanofibers
3.2. Antioxidants
3.2.1. Tannic Acid (TA)
3.2.2. Rutin
3.2.3. Poryphyrin
3.2.4. Green Tea Extract
3.2.5. Rice Extract
3.2.6. Garcinia mangostana Extract
3.3. Wound Dressings
3.4. Tissue Engineering
3.5. Nanoencapsulation
3.6. Food
3.7. Stem Cells
3.8. Polymer-Free Electrospun Fibers (Special Section)
3.9. Others
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Chaushu, L.; Weinreb, M.; Beitlitum, I.; Moses, O.; Nemcovsky, C.E. Evaluation of a topical herbal patch for soft tissue wound healing: An animal study. J. Clin. Periodontol. 2015, 42, 288–293. [Google Scholar] [CrossRef]
- Talekar, Y.P.; Apte, K.G.; Paygude, S.V.; Tondare, P.R.; Parab, P.B. Journal of Ayurveda and Integrative Medicine Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J. Ayurveda Integr. Med. 2017, 8, 73–81. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sakravarthi, C.; Kritika, T.; Arul Kirubakaran, M.; Sarada, D.V.L. Evaluation of antioxidant and wound healing potentials of Sphaeranthus amaranthoides Burm.f. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Vrignaud, S.; Benoit, J.P.; Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Meinel, A.J.; Germershaus, O.; Luhmann, T.; Merkle, H.P.; Meinel, L. Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications. Eur. J. Pharm. Biopharm. 2012, 81, 1–13. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surf. B Biointerfaces 2015, 139, 156–163. [Google Scholar] [CrossRef]
- Acevedo, F.; Villegas, P.; Urtuvia, V.; Hermosilla, J.; Navia, R.; Seeger, M. Bacterial polyhydroxybutyrate for electrospun fiber production. Int. J. Biol. Macromol. 2018, 106, 692–697. [Google Scholar] [CrossRef]
- Kim, J.I.; Pant, H.R.; Sim, H.-J.; Lee, K.M.; Kim, C.S. Electrospun propolis/polyurethane composite nanofibers for biomedical applications. Mater. Sci. Eng. C 2014, 44, 52–57. [Google Scholar] [CrossRef]
- Pant, H.R.; Bajgai, M.P.; Yi, C.; Nirmala, R.; Nam, K.T.; Baek, W.; Kim, H.Y. Effect of successive electrospinning and the strength of hydrogen bond on the morphology of electrospun nylon-6 nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 87–94. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Sukumaran, S.K.; Sugimoto, M.; Hara, T.; Takatsuka, Y.; Koyama, K. Towards novel wound dressings: Antibacterial properties of zinc oxide nanoparticles and electrospun fiber mats of zinc oxide nanoparticle/poly(vinyl alcohol) hybrids. J. Polym. Eng. 2015, 35, 575–586. [Google Scholar] [CrossRef]
- Pusporini, P.; Edikresnha, D.; Sriyanti, I.; Suciati, T.; Munir, M.M.; Khairurrijal, K. Electrospun polyvinylpyrrolidone (PVP)/green tea extract composite nanofiber mats and their antioxidant activities. Mater. Res. Express 2018, 5, 54001. [Google Scholar] [CrossRef]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Correlation between Structures and Antioxidant Activities of Polyvinylpyrrolidone/Garcinia mangostana L. Extract Composite Nanofiber Mats Prepared Using Electrospinning. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Zhou, B.; Hu, X.; Zhu, J.; Wang, Z.; Wang, X.; Wang, M. Release properties of tannic acid from hydrogen bond driven antioxidative cellulose nanofibrous films. Int. J. Biol. Macromol. 2016, 91, 68–74. [Google Scholar] [CrossRef]
- Lee, I.W.; Li, J.; Chen, X.; Park, H.J. Fabrication of electrospun antioxidant nanofibers by rutin-pluronic solid dispersions for enhanced solubility. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Fabrication and characterization of electrospun gelatin nanofibers crosslinked with oxidized phenolic compounds. Int. J. Biol. Macromol. 2017, 103, 1062–1068. [Google Scholar] [CrossRef]
- Yang, W.; Sousa, A.; Thomas-Gahring, A.; Fan, X.; Jin, T.; Li, X.; Tomasula, P.; Liu, L. Electrospun Polymer Nanofibers Reinforced by Tannic Acid/Fe+++ Complexes. Materials 2016, 9, 757. [Google Scholar] [CrossRef]
- Arai, T.; Tanaka, M.; Kawakami, H. Porphyrin-containing electrospun nanofibers: Positional control of porphyrin molecules in nanofibers and their catalytic application. ACS Appl. Mater. Interfaces 2012, 4, 5453–5457. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chimnoi, N.; Pattani, L.; Khlaychan, P.; Nooeaid, P.; Techasakul, S. Poly(lactic acid) (PLA) electrospun fibers containing rice extract: Release characteristics and their antioxidant activity. Key Eng. Mater. 2017, 757, 83–87. [Google Scholar] [CrossRef]
- Berthet, M.; Gauthier, Y.; Lacroix, C.; Verrier, B.; Monge, C. Nanoparticle-Based Dressing: The Future of Wound Treatment? Trends Biotechnol. 2017, 35, 770–784. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Xiao, Q.; Lim, L.-T. Pullulan-alginate fibers produced using free surface electrospinning. Int. J. Biol. Macromol. 2018, 112, 809–817. [Google Scholar] [CrossRef]
- Garcia-orue, I.; Gainza, G.; Borja, F.; Javier, J.; Evora, C.; Luis, J.; Maria, R.; Delgado, A.; Igartua, M. Novel nano fi brous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Basal, G.; Tetik, G.D.; Kurkcu, G.; Bayraktar, O.; Gurhan, I.D.; Atabey, A. Olive leaf extract loaded silk fibroin/hyaluronic acid nanofiber webs for wound dressing applications. Dig. J. Nanomater. Biostruct. 2016, 11, 1113–1123. [Google Scholar]
- Ajmal, G.; Bonde, G.V.; Thokala, S.; Mittal, P.; Khan, G.; Singh, J.; Pandey, V.K.; Mishra, B. Ciprofloxacin HCl and quercetin functionalized electrospun nanofiber membrane: Fabrication and its evaluation in full thickness wound healing. Artif. Cells Nanomed. Biotechnol. 2019, 47, 228–240. [Google Scholar] [CrossRef]
- Ravichandran, S.; Radhakrishnan, J.; Jayabal, P.; Venkatasubbu, G.D. Antibacterial Screening Studies of Electrospun Polycaprolactone Nano Fibrous Mat Containing Clerodendrum Phlomidis Leaves Extract; Elsevier: Amsterdam, The Netherlands, 2019; Volume 484. [Google Scholar]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Deldar, Y.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Montazer Saheb, S.; Rahmati-Yamchi, M.; Zarghami, N. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Luna, G.L.F.; Brassolatti, P.; Anibal, F.d.F.; Correa, D.S. Biocompatible and biodegradable electrospun nanofibrous membranes loaded with grape seed extract for wound dressing application. J. Nanomater. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Manolova, N.; Rashkov, I.; Kussovski, V.; Danchev, D. Curcumin-loaded poly(l-lactide-co-D,l-lactide) electrospun fibers: Preparation and antioxidant, anticoagulant, and antibacterial properties. J. Bioact. Compat. Polym. 2014, 29, 607–627. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The use of electrospun curcumin-loaded poly(L-lactic acid) fiber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Peršin, Z.; Ravber, M.; Stana Kleinschek, K.; Knez, Ž.; Škerget, M.; Kurečič, M. Bio-nanofibrous mats as potential delivering systems of natural substances. Text. Res. J. 2017, 87, 444–459. [Google Scholar] [CrossRef]
- Wutticharoenmongkol, P.; Hannirojram, P.; Nuthong, P. Gallic acid-loaded electrospun cellulose acetate nanofibers as potential wound dressing materials. Polym. Adv. Technol. 2019, 30, 1135–1147. [Google Scholar] [CrossRef]
- Sarkar, R.; Ghosh, A.; Barui, A.; Datta, P. Repositing honey incorporated electrospun nanofiber membranes to provide anti-oxidant, anti-bacterial and anti-inflammatory microenvironment for wound regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 31. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Amna, T.; Gharsan, F.N.; Shang, K.; Shamshi Hassan, M.; Khil, M.S.; Hwang, I. Electrospun Twin Fibers Encumbered with Intrinsic Antioxidant Activity as Prospective Bandage. Macromol. Res. 2019, 27, 663–669. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Kyzioł, A.; Michna, J.; Moreno, I.; Gamez, E.; Irusta, S. Preparation and characterization of electrospun alginate nanofibers loaded with ciprofloxacin hydrochloride. Eur. Polym. J. 2017, 96, 350–360. [Google Scholar] [CrossRef]
- Manchineella, S.; Thrivikraman, G.; Khanum, K.K.; Ramamurthy, P.C.; Basu, B.; Govindaraju, T. Pigmented Silk Nanofibrous Composite for Skeletal Muscle Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1222–1232. [Google Scholar] [PubMed]
- Nune, M.; Manchineella, S.; Govindaraju, T.; Narayan, K.S. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater. Sci. Eng. C 2019, 94, 17–25. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Arthi, N.; Arun, R.P.; Verma, R.S. Synthesis and fabrication of novel quinone-based chromenopyrazole antioxidant-laden silk fibroin nanofibers scaffold for tissue engineering applications. Mater. Sci. Eng. C 2019, 102, 773–787. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, D.S.; Ju, J.H.; Shin, H.S. Assessment of Spirulina-PCL nanofiber for the regeneration of dermal fibroblast layers. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 27–33. [Google Scholar] [CrossRef]
- Kepekçi, R.A.; İçoğlu, H.İ.; Kireçci, A. Assessment of antioxidant activity and phycocyanin release of Spirulina loaded poly(ε-caprolactone) electrospun nanofibers. J. Text. Inst. 2017, 108, 1840–1846. [Google Scholar] [CrossRef]
- Da Silva Uebel, L.; Angelica Schmatz, D.; Goettems Kuntzler, S.; Lima Dora, C.; Luiza Muccillo-Baisch, A.; Alberto Vieira Costa, J.; Greque De Morais, M. Quercetin and curcumin in nanofibers of polycaprolactone and poly(hydroxybutyrate-co-hydroxyvalerate): Assessment of in vitro antioxidant activity. J. Appl. Polym. Sci. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, B.; Li, J.; Pei, Y.; Li, B. Correction: Coordination-driven multilayer of phosvitin-polyphenol functional nanofibrous membranes: Antioxidant and biomineralization applications for tissue engineering (RSC Adv. (2016) 6 (98935-98944) DO: 10.1039/C6RA20996C). RSC Adv. 2016, 6, 108151. [Google Scholar] [CrossRef]
- Llorens, E.; Del Valle, L.J.; Díaz, A.; Casas, M.T.; Puiggalí, J. Polylactide nanofibers loaded with vitamin B6 and polyphenols as bioactive platform for tissue engineering. Macromol. Res. 2013, 21, 775–787. [Google Scholar] [CrossRef]
- Urtuvia, V.; Villegas, P.; González, M.; Seeger, M. Bacterial production of the biodegradable plastics polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 70, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Durgun, E.; Uyar, T. Antioxidant electrospun zein nanofibrous web encapsulating quercetin/cyclodextrin inclusion complex. J. Mater. Sci. 2018, 53, 1527–1539. [Google Scholar] [CrossRef]
- Isik, B.S.; Altay, F.; Capanoglu, E. The uniaxial and coaxial encapsulations of sour cherry (Prunus cerasus L.) concentrate by electrospinning and their in vitro bioaccessibility. Food Chem. 2018, 265, 260–273. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Amariei, G.; Boltes, K.; Letón, P.; Iriepa, I.; Moraleda, I.; Rosal, R. Poly(amidoamine) dendrimers grafted on electrospun poly(acrylic acid)/poly(vinyl alcohol) membranes for host-guest encapsulation of antioxidant thymol. J. Mater. Chem. B 2017, 5, 6776–6785. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Kumar, N.; Sharma, M.; Pruthi, V. Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biotechnol. Rep. 2015, 8, 36–44. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid encapsulation structures based on β-carotene-loaded nanoliposomes within electrospun fibers. Colloids Surf. B Biointerfaces 2015, 134, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ghitescu, R.E.; Popa, A.M.; Popa, V.I.; Rossi, R.M.; Fortunato, G. Encapsulation of polyphenols into pHEMA e-spun fibers and determination of their antioxidant activities. Int. J. Pharm. 2015, 494, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Ng, S.F.; Khan, S.; Katas, H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf. B Biointerfaces 2017, 150, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Gámez, E.; Mendoza, G.; Salido, S.; Arruebo, M.; Irusta, S. Antimicrobial Electrospun Polycaprolactone-Based Wound Dressings: An in Vitro Study about the Importance of the Direct Contact to Elicit Bactericidal Activity. Adv. Wound Care 2019, 8, 438–451. [Google Scholar] [CrossRef]
- Akman, P.K.; Bozkurt, F.; Balubaid, M.; Yilmaz, M.T. Fabrication of Curcumin-loaded Gliadin Electrospun Nanofibrous Structures and Bioactive Properties. Fibers Polym. 2019, 20, 1187–1199. [Google Scholar] [CrossRef]
- Sharif, N.; Golmakani, M.T.; Niakousari, M.; Hosseini, S.M.H.; Ghorani, B.; Lopez-Rubio, A. Active food packaging coatings based on hybrid electrospun gliadin nanofibers containing ferulic acid/hydroxypropyl-beta-cyclodextrin inclusion complexes. Nanomaterials 2018, 8, 919. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and antioxidant performance of various essential oils and natural extracts and their incorporation into biowaste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) layers made from electrospun ultrathin fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Cruxen, C.E.d.S.; Bruni, G.P.; Fiorentini, Â.M.; Zavareze, E.d.R.; Lim, L.T.; Dias, A.R.G. Development of antimicrobial and antioxidant electrospun soluble potato starch nanofibers loaded with carvacrol. Int. J. Biol. Macromol. 2019, 139, 1182–1190. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Nahvi, Z.; Zandi, M. Antioxidant peptide-loaded electrospun chitosan/poly(vinyl alcohol) nanofibrous mat intended for food biopackaging purposes. Food Hydrocoll. 2019, 89, 637–648. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Sacarescu, L.; Vasiliu, A.L.; Hitruc, G.E.; Pricope, G.M.; Sivertsvik, M.; Rosnes, J.T.; Vasile, C. Antioxidant/antibacterial electrospun nanocoatings applied onto PLA films. Materials 2018, 11, 1973. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Sheng, F.; Yan, X.; Zhu, Y.; Jin, W.; Li, J.; Li, B. Enhancement of antioxidant and antibacterial properties for tannin acid/chitosan/tripolyphosphate nanoparticles filled electrospinning films: Surface modification of sliver nanoparticles. Int. J. Biol. Macromol. 2017, 104, 813–820. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Effects of surfactants on the formation of gelatin nanofibres for controlled release of curcumin. Food Chem. 2017, 231, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Lan, X.; Huang, D.; Luo, T.; Ji, J.; Mafang, Z.; Miao, X.; Wang, H.; Wang, W. Electrospun Gelatin Nanofibers Encapsulated with Peppermint and Chamomile Essential Oils as Potential Edible Packaging. J. Agric. Food Chem. 2019, 67, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Erbay, E.A.; Dağtekin, B.B. (Gözü); Türe, M.; Yeşilsu, A.F.; Torres-Giner, S. Quality improvement of rainbow trout fillets by whey protein isolate coatings containing electrospun poly(ε-caprolactone) nanofibers with Urtica dioica L. extract during storage. LWT—Food Sci. Technol. 2017, 78, 340–351. [Google Scholar] [CrossRef]
- Salević, A.; Prieto, C.; Cabedo, L.; Nedović, V.; Lagaron, J. Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials 2019, 9, 270. [Google Scholar] [CrossRef]
- Aytac, Z.; Keskin, N.O.S.; Tekinay, T.; Uyar, T. Antioxidant α-tocopherol/γ-cyclodextrin–inclusion complex encapsulated poly(lactic acid) electrospun nanofibrous web for food packaging. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silver-NP/VitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanoparticle Res. 2014, 16, 2643. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun antimicrobial polylactic acid/tea polyphenol nanofibers for food-packaging applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Mohammadi, S.A.; Zarghami, N.; Pourhassan-Moghaddam, M.; Alizadeh, E.; Jafar Maleki, M.; Firouzi-Amandi, A.; Nouri, M. Watercress-based electrospun nanofibrous scaffolds enhance proliferation and stemness preservation of human adipose-derived stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Deldar, Y.; Zarghami, F.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Antioxidant effects of chrysin-loaded electrospun nanofibrous mats on proliferation and stemness preservation of human adipose-derived stem cells. Cell Tissue Bank. 2017, 18, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Kayaci-Senirmak, F.; Ipek, S.; Durgun, E.; Uyar, T. Polymer-free nanofibers from vanillin/cyclodextrin inclusion complexes: High thermal stability, enhanced solubility and antioxidant property. Food Funct. 2016, 7, 3141–3153. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; San Keskin, N.O.; Tekinay, T.; Uyar, T. Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: Enhanced shelf-life of geraniol with antibacterial and antioxidant properties. RSC Adv. 2016, 6, 46089–46099. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef]
- Jørgensen, L.; Qvortrup, K.; Chronakis, I.S. Phospholipid electrospun nanofibers: Effect of solvents and co-axial processing on morphology and fiber diameter. RSC Adv. 2015, 5, 53644–53652. [Google Scholar] [CrossRef]
- Mendes, A.C.; Nikogeorgos, N.; Lee, S.; Chronakis, I.S. Nanomechanics of electrospun phospholipid fiber. Appl. Phys. Lett. 2015, 106, 223108. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Mendes, A.C.; Baj, V.; Beeren, S.R.; Chronakis, I.S. Electrospun phospholipid fibers as micro-encapsulation and antioxidant matrices. Molecules 2017, 22, 1708. [Google Scholar] [CrossRef]
- Vatankhah, E. Rosmarinic acid-loaded electrospun nanofibers: In vitro release kinetic study and bioactivity assessment. Eng. Life Sci. 2018, 18, 732–742. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Singh, R.P.; Pruthi, V. A controlled release system for quercetin from biodegradable poly(lactide-co-glycolide)-polycaprolactone nanofibers and its in vitro antitumor activity. J. Bioact. Compat. Polym. 2015, 31, 260–272. [Google Scholar] [CrossRef]
- Guo, M.; Bi, S.; Liu, J.; Xu, W.; Zhou, G.; Liu, Y.; Chen, C. C60(OH)n-loaded nanofibrous membranes protect HaCaT cells from ROS-associated damage. Chin. Chem. Lett. 2017, 28, 1889–1892. [Google Scholar] [CrossRef]
- Shin, Y.C.; Yang, W.J.; Lee, J.H.; Oh, J.W.; Kim, T.W.; Park, J.C.; Hyon, S.H.; Han, D.W. PLGA nanofiber membranes loaded with epigallocatechin-3-O-gallate are beneficial to prevention of postsurgical adhesions. Int. J. Nanomed. 2014, 9, 4067–4078. [Google Scholar]
- Llorens, E.; Del Valle, L.J.; Puiggalí, J. Inhibition of Radical-Induced Oxidative DNA Damaage by Antioxidants Loaded in Electrospun Polyactide Nanofibers. Macromoecular Res. 2014, 22, 338–396. [Google Scholar]
- Miletić, A.; Pavlić, B.; Ristić, I.; Zeković, Z.; Pilić, B. Encapsulation of Fatty Oils into Electrospun Nanofibers for Cosmetic Products with Antioxidant Activity. Appl. Sci. 2019, 9, 2955. [Google Scholar] [CrossRef]
- Chu, L.L.; Kang, X.J.; Wang, Y. Extraction of Onion (Allium Cepa) Essential Oil by Polystyrene Nanofibrous Membranes. J. Food Process Eng. 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Scampicchio, M.; Bulbarello, A.; Arecchi, A.; Mannino, S. Electrospun nanofibers as selective barrier to the electrochemical polyphenol oxidation. Electrochem. Commun. 2008, 10, 991–994. [Google Scholar] [CrossRef]
| Polymeric Matrix | Active Compound | Electrospinning Parameters | Average Diameter of Fibers (nm) | Reference |
|---|---|---|---|---|
| Polyvinylpyrrolidone (PVP) | Green tea extract | 12.5 kV; 0.5 mL/h; 10 cm | 344–386 | [17] |
| PVP | Garcinia mangostana extract | 15 kV; 0.5 mL/h | 217–421 | [18] |
| Poly(ethylene glycol) (PEG) | Tannic acid | 17 kV; 20 cm | - | [19] |
| Pluronic and pullulan | Rutin | 20 kV; 0.125 mL/h; 13 cm | 100–102 | [20] |
| Gelatin (GE) | Tannic, gallic, ferulic, and caffeic acids | 25 kV; 0.3 mL/h; 13 cm | 145–280 | [21] |
| Polyvinyl alcohol (PVA) | Tannic acid/Fe3+ complexes | 15 kV; 0.5 mL/h; 15 cm | 144–337 | [22] |
| Polyacrylonitrile (PAN) | Porphyrin | 20 kV; 0.12 mL/h; 10 cm | Around 200 | [23] |
| Poly(lactic acid) (PLA) | Rice extract | 20 kV; 0.5 mL/h; 18 cm | 450–656 | [24] |
| Polymeric Matrix | Active Compound | Electrospinning Parameters | Diameter of Fibers (nm) | Fiber Characteristics | Reference |
|---|---|---|---|---|---|
| Chitosan–ethylenediaminetetraacetic acid/PVA | Garcinia mangostana extract (GME) | 15 kV; 0.25 mL/h; 20 cm | 205–251 | Rapid GME release regarding matrix erosion | [30] |
| Silk fibroin (SF) | Grape seed extract (GSE) | 14 kV; 0.3 mL/h; 15 cm | 414–427 | Antioxidant activity improved with the increase of GSE concentration | [12] |
| SF | Fenugreek seed extract | 25 kV; 0.5 mL/h; 10 cm | 438–640 | The higher the fenugreek concentration, the more time-extended the inhibition of radical damage is | [31] |
| SF/hyaluronic acid (HA) | Olive leaf extract | 20.46 kV; 0.12 or 0.36 mL/h; 10 cm | 123 | Antioxidant activity similar to original extract | [32] |
| Polycaprolactone (PCL) | Quercetin (Que) | 16 kV; 0.6 mL/h; 10 cm | 101 | Antioxidant activity increases from 12% to 40% with incorporation of Que | [33] |
| PCL | Clerodendrum phlomidis leaf extract | 12 kV; 12 cm | 293 | Antioxidant activity of resulting mats equivalent to C. phlomidis extract (between 68.4 and 69.4 μg/mL) | [34] |
| PCL/GE | Cerium oxide | 1 kV/cm; 1 mL/h; 15 cm | 300–760 | PCL/GE/CeNPs nanofibers decrease by 30% the fluorescence intensity of DCF | [35] |
| PCL/PEG | Chrysin | 18–22 kV; 2 mL/h; 20 cm | 300–400 | Fiber with the highest amount of chrysin maintained its antioxidant activity longer | [36] |
| PLA/polyethylene oxide (PEO) | Grape seed extract | 17 kV; 1 mL/h; 9 cm | 130–270 | Near 85% 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging even after 45 days | [37] |
| Poly(l-lactide-co-d,l-lactide) (coPLA)/PEG | Curcumin | 17 kV; 3 mL/h; 10 cm | 1360–1480 | Curcumin reached 71–72% scavenging capacity | [38] |
| PLA | Curcumin | 24 kV; 15 cm | 333–380 | DPPH inhibition reached 42–53% and decreased over time due to curcumin instability | [39] |
| Carboxymethylcellulose (CMC)/sodium alginate/CaCl2/PEO | Olive leaf extract | 60 kV; 16 cm | 167 | 89% of olive leaf extract radical scavenging | [40] |
| Cellulose acetate | Gallic acid (GA) | 15, 18, or 21 kV; 1 mL/h; 15 cm | 295–787 | GA release was higher from the lowest concentrated fibers | [41] |
| PVA | Honey | 20 kV; 0.3 mL/h; 10 cm | 300–410 | Antioxidant activity maintained after electrospinning process | [42] |
| Alginate/PVA | Honey | 15 kV; 0.4 mL/h; 10 cm | 378–528 | DPPH scavenging resulted in 66% as the maximum obtained in 9 h | [43] |
| Polyurethane (PU) | Capsaicin | 15 kV | 150–500 | DPPH radical scavenging of encapsulated capsaicin results were higher than capsaicin by itself (78% and 70%, respectively) | [44] |
| Polymeric Matrix | Active Compound | Electrospinning Parameters | Average Diameter of Fibers (nm) | Proposed Technology | Reference |
|---|---|---|---|---|---|
| SF | Melanin | 1.5 kV/cm; 0.8 mL/h | 343 | Skeletal muscle tissue engineering (SMTE) | [47] |
| SF | Melanin | 12 kV; 1 mL/h; 8 cm | 800–840 | Nerve regeneration | [48] |
| SF | Quinone-based chromenopyrazole (QCP) | 15 kV; 0.5 mL/h; 10 cm | 1400 | Scaffolds for skin | [49] |
| PCL | Spirulina | 15 kV; 15 cm | 710 | Scaffolds | [50] |
| PCL | Spirulina | 12 kV; 0.3 mL/h; 15 cm | 160–315 | Scaffolds | [51] |
| PCL and poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) | Quercetin and curcumin | 25 kV; 0.2 or 2 mL/h; 12 cm | 332–556 | Scaffolds | [52] |
| Cellulose | Tannic acid and phosvitin | 17 kV; 20 cm | 528–538 | Scaffolds | [53] |
| Polylactide (PLA) | Vitamin B6, p-coumaric acid, and caffeic acid | 17, 17.5, or 19 kV; 0.5 or 1 mL/h; 12.5 cm | 81–101 | Scaffolds | [54] |
| Polymeric Matrix | Active Compound | Electrospinning Parameters | Average Diameter of Fibers (nm) | Possible Application Areas | Reference |
|---|---|---|---|---|---|
| Zein | Quercetin/gamma-cyclodextrin inclusion complex | 15 kV; 1 mL/h; 10 cm | 750 | Food and pharmaceutical | [58] |
| GE | Sour cherry concentrate | 25 kV; 0.4 and 0.1 mL/h; 10 cm | - | Functional foods | [59] |
| PCL | α-tocopherol/β-cyclodextrin inclusion complex | 15 kV; 0.5 mL/h; 8 cm | 205–345 | Topical drug delivery | [60] |
| Poly(amide-amine)/polyacrylic acid (PAA)/PVA | Thymol | 23 kV; 0.8 mL/h; 23 cm | 254–320 | Food packaging | [61] |
| PLA | Gallic acid/cyclodextrin inclusion complex | 15 kV; 1 mL/h; 10 cm | 235–495 | Food packaging | [62] |
| Poly (d,l-lactide-co-glycolide) (PLGA)/PEO | Ferulic acid | 18 kV; 0.5 mL/h; 12 cm | 150 | Biomedical | [63] |
| PVA/PEO | β-carotene | 10 kV; 0.1 mL/h; 10 cm | 195–408 | Industrial applications | [64] |
| Poly(2-hydroxyethyl methacrylate) (pHEMA) | Polyphenols | −2 and 10 kV; 0.23 mL/h; 15 cm | 470–1930 | Medical and biological | [65] |
| Various * | Curcumin | - | - | Wound healing | [66] |
| Polymeric Matrix | Active Compound | Electrospinning Parameters | Average Diameter of Fibers (nm) | Proposed Application | Reference |
|---|---|---|---|---|---|
| Gliadin | Curcumin | 15 kV; 0.5 mL/h; 10 cm | 375–410 | Food Industry | [68] |
| Gliadin | Inclusion complexes of ferulic acid with hydroxypropyl-beta-cyclodextrins | 18 kV; 1 mL/h; 10 cm | 269–279 | Food packaging | [69] |
| PHBV | Oregano essential oil, rosemary extract, and green tea extract | 38 kV; 4 mL/h; 20 cm | 800 | Food packaging | [70] |
| Potato starch | Carvacrol | −3 and 25 kV; 0.6 mL/h; 20 cm | 74–95 | Food packaging | [71] |
| Chitosan/PVA | Fish-purified antioxidant peptide | 15 kV; 0.2 mL/h; 15 cm | 158–195 | Food packaging | [72] |
| PLA/chitosan | Argan and clove oils | Food packaging | [73] | ||
| PVA/PAA/chitosan | Tannin acid/tripolyphosphate | Not reported (homemade) | 132–578 | Food packaging | [74] |
| GE | Curcumin | 15 kV; 0.5 mL/h; 10 cm | 295–368 | Nutraceutical carrier | [75] |
| GE | Peppermint and chamomile essentials oils | 15 kV; 0.3 mL/h; 10 cm | 293–462 | Edible food packaging | [76] |
| PCL | Urtica dioica L. extract | 15 kV; 1 mL/h; 12 cm | 575 | Food preservation | [77] |
| PCL | Sage extract | 19 kV; 3 mL/h; 15 cm | 3300–3800 | Food packaging | [78] |
| PLA | α-tocopherol/γ-cyclodextrin | Food packaging | [79] | ||
| PLA | Vitamin E | 15 kV; 1 mL/h; 10 cm | 140 | Preservative packaging | [80] |
| PLA | Tea polyphenol | 20 kV; 20 mL/h; 15 cm | 490–680 | Food packaging | [81] |
| PVA | Rosemary extract | 30 kV; 2.2 mL/h; 20 cm | 282 | Hydrophilic and acid food products packaging | [82] |
| Polymeric Matrix | Active Compound | Electrospinning Parameters | Average Diameter of Fibers (nm) | Application Area | Reference |
|---|---|---|---|---|---|
| Cellulose acetate | Rosmarinic acid | 20 kV; 0.25 mL/h; 12 cm | 314–331 | Transdermal patches | [91] |
| Cellulose acetate-based | Various * | - | - | Drug delivery | [92] |
| PLGA/PCL | Quercetin | 25–28 kV; 0.1 mL/h; 12 cm | 400–520 | Implantable anticancer drug | [93] |
| PLGA/PCL | Polyhydroxylated fullerene | 25 kV; 20 cm | 200 | Skin care | [94] |
| PLGA | Epigallocatechin-3-O-gallate | 18 kV; 1 mL/h; 12 cm | 300–500 | Nanomedicine (postoperative adhesion prevention) | [95] |
| PLA | Vitamin B6, pyridoxal, and hydroxycinnamic acids | 17–19 kV; 0.5–1 mL/h; 12.5 cm | 81–101 | Purification of DNA | [96] |
| PLA and PVP | Pomegranate seed oil (PSO), ethanolic extract of fermented pomegranate juice (EP), and cold-pressed sea-buckthorn oil (SB) | 11–15.5 kV; 0.75–2.5 mL/h; 10–15 cm | 820–1600 | Cosmetics | [97] |
| Polystyrene (PS) | -- | 13.5 kV; 1.5 mL/h; 12 cm | 300–600 | Extraction | [98] |
| Nylon-6 | -- | 24 kV; 0.03 mL/h | - | Electrochemistry | [99] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020, 10, 175. https://doi.org/10.3390/nano10010175
Vilchez A, Acevedo F, Cea M, Seeger M, Navia R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials. 2020; 10(1):175. https://doi.org/10.3390/nano10010175
Chicago/Turabian StyleVilchez, Ariel, Francisca Acevedo, Mara Cea, Michael Seeger, and Rodrigo Navia. 2020. "Applications of Electrospun Nanofibers with Antioxidant Properties: A Review" Nanomaterials 10, no. 1: 175. https://doi.org/10.3390/nano10010175
APA StyleVilchez, A., Acevedo, F., Cea, M., Seeger, M., & Navia, R. (2020). Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials, 10(1), 175. https://doi.org/10.3390/nano10010175