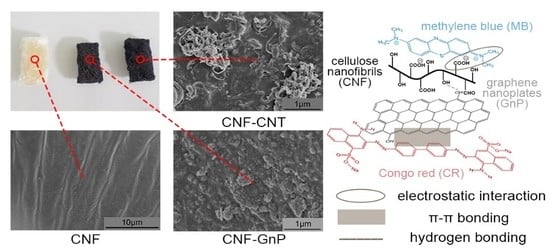
Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Nanofibrils
2.3. Preparation of CNF, CNF–CNT and CNF–GnP Aerogels
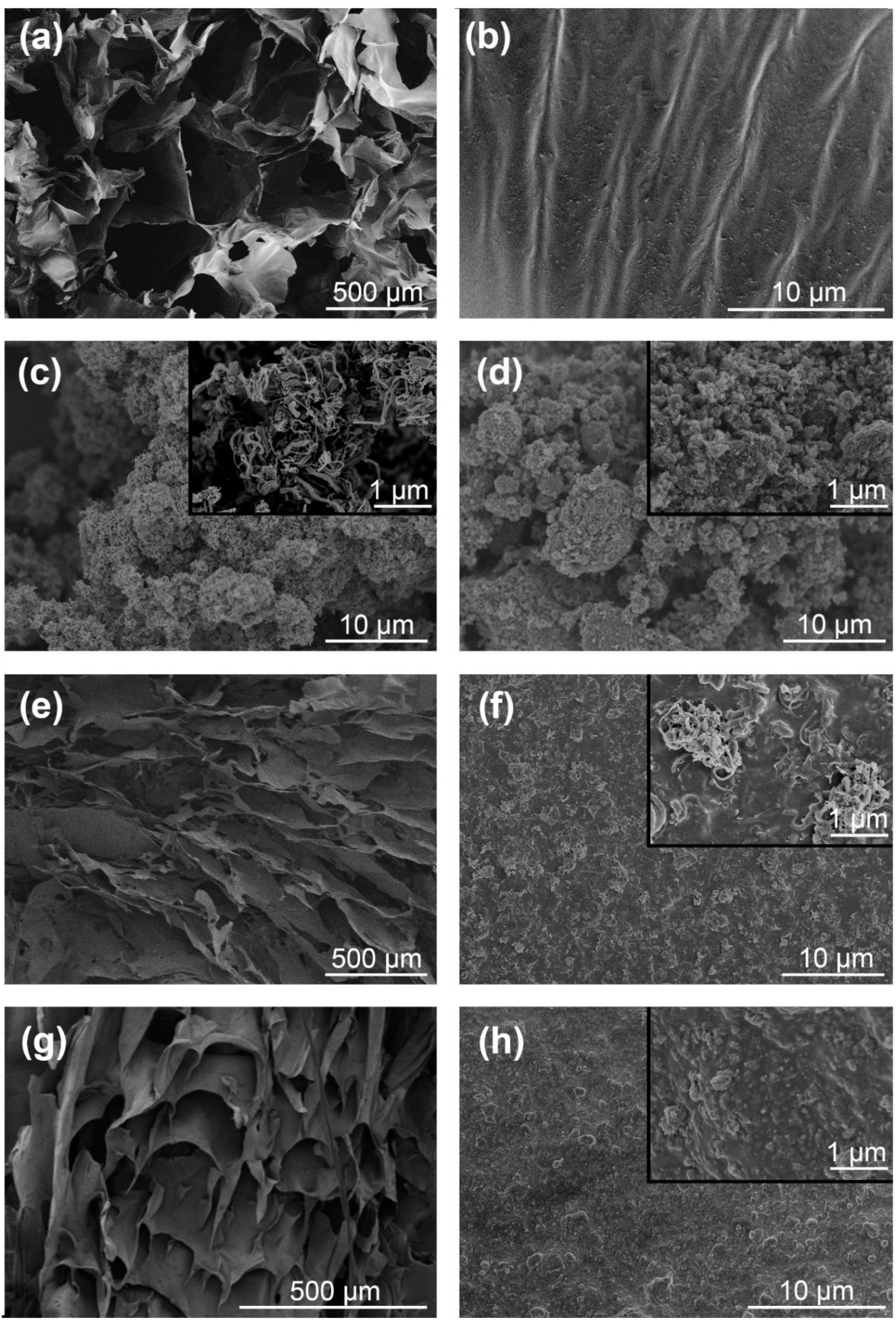
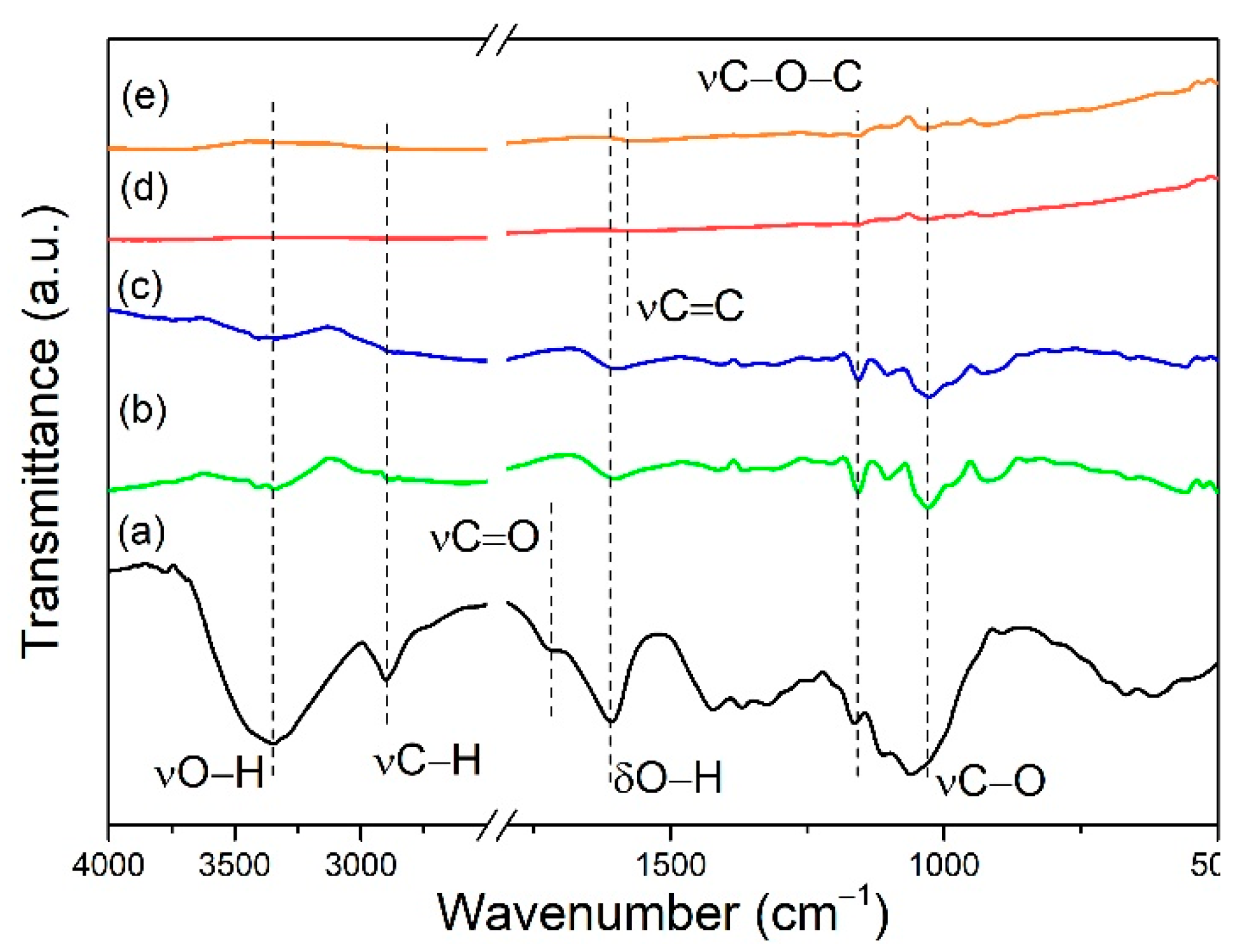
2.4. Characterization
2.5. Adsorption of Dyes in Single Systems
2.6. Adsorption of Dyes in Binary Systems
2.7. Desorption of Dyes
3. Results and Discussion
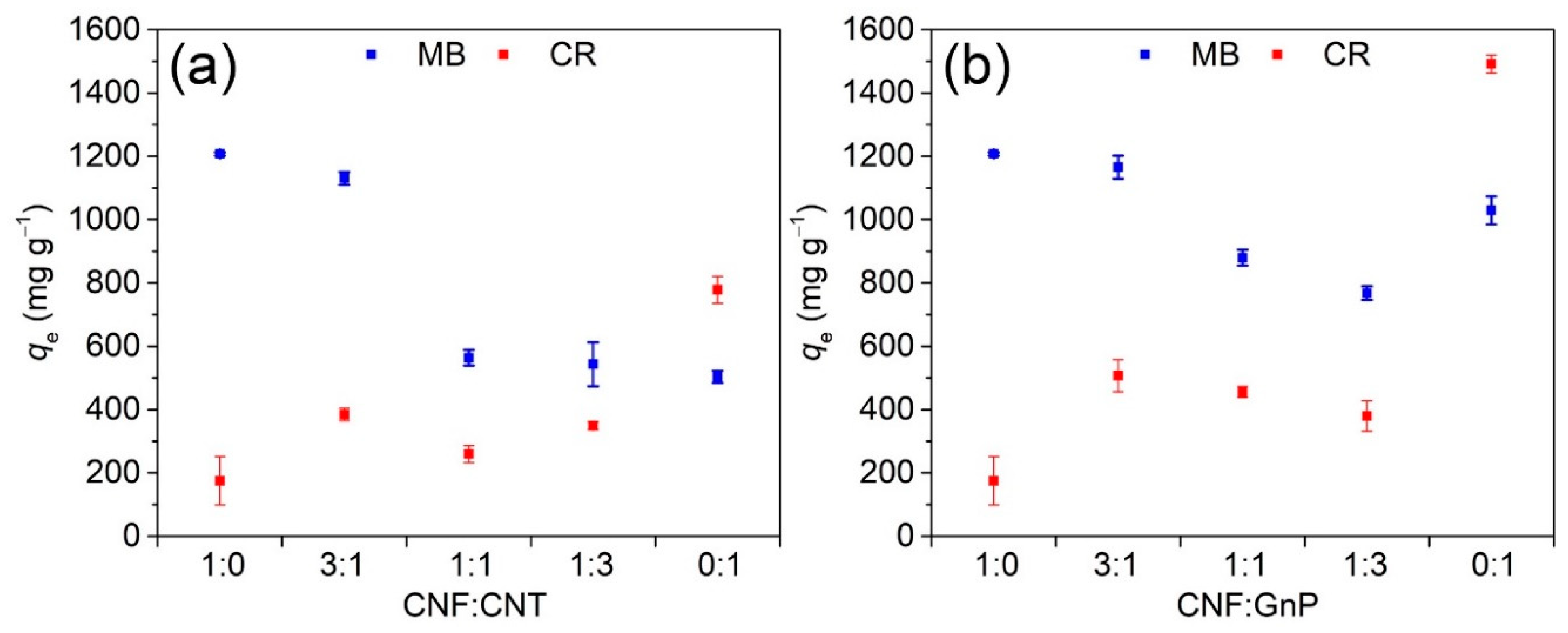
3.1. Effect of Adsorbent Structure
3.2. Effect of Contact Time and Adsorption Kinetics
3.3. Effect of Initial Dye Concentration and Adsorption Isotherm
3.4. Adsorption of Dyes in Binary Systems
3.5. Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, M.J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: Review. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye using activated carbon prepared from oil palm shell: Batch and fixed bed studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; DasGupta, S.; De, S. Removal of congo red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Gu, J.; Hu, C.; Zhang, W.; Dichiara, A. Reagentless preparation of shape memory cellulose nanofibril aerogels decorated with Pd nanoparticles and their application in dye discoloration. Appl. Catal. B Environ. 2018, 237, 482–490. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Chamorro-Posada, P.; Meejoo-Smith, S.; Vázquez-Cabo, J.; Rubiños-López, Ó.; Lartundo-Rojas, L.; Sánchez-Árevalo, F.M.; Trakulmututa, J.; Rutto, D.; et al. Comparison of the activities of C2N and BCNO towards Congo red degradation. Mater. Chem. Phys. 2019, 221, 397–408. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Zhou, C.; Zhang, S.; Chen, J. Novel and High-Performance Magnetic Carbon Composite Prepared from Waste Hydrochar for Dye Removal. ACS Sustain. Chem. Eng. 2014, 2, 969–977. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution by Alkali-Activated Multiwalled Carbon Nanotubes. ACS Appl. Mat. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef]
- Moradi, O. Adsorption Behavior of Basic Red 46 by Single-Walled Carbon Nanotubes Surfaces. Fuller. Nanotub. Carbon Nanostruct. 2013, 21, 286–301. [Google Scholar] [CrossRef]
- Zare, K.; Sadegh, H.; Shahryari-Ghoshekandi, R.; Maazinejad, B.; Ali, V.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Enhanced removal of toxic Congo red dye using multi walled carbon nanotubes: Kinetic, equilibrium studies and its comparison with other adsorbents. J. Mol. Liq. 2015, 212, 266–271. [Google Scholar] [CrossRef]
- Yu, J.; Yu, L.; Yang, H.; Liu, Q.; Chen, X.; Jiang, X.; Chen, X.; Jiao, F. Graphene nanosheets as novel adsorbents in adsorption, preconcentration and removal of gases, organic compounds and metal ions. Sci. Total Environ. 2015, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Chen, X.; Zhao, Q.; Ghaffar, A.; Chen, B. Application of graphene-based materials in water purification: From the nanoscale to specific devices. Environ. Sci. Nano 2018, 5, 1264–1297. [Google Scholar] [CrossRef]
- Pan, X.; Du, Q.; Zhou, Y.; Liu, L.; Xu, G.; Yan, C. Ammonia Borane Promoted Synthesis of Graphene Aerogels as High Efficient Dye Adsorbent. J. Nanosci. Nanotechnol. 2018, 18, 7231–7240. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.-O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Benton-Smith, J.; Rogers, R.E. Enhanced adsorption of carbon nanocomposites exhausted with 2, 4-dichlorophenoxyacetic acid after regeneration by thermal oxidation and microwave irradiation. Environ. Sci. Nano 2014, 1, 113–116. [Google Scholar] [CrossRef]
- Li, D.; Mueller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M.; Kaiser, E.Q.; Archibald, D.D. Quantification of cellulose nanowhiskers sulfate esterification levels. Carbohydr. Polym. 2013, 92, 1809–1816. [Google Scholar] [CrossRef]
- Gu, J.; Hsieh, Y.-L. Surface and Structure Characteristics, Self-Assembling, and Solvent Compatibility of Holocellulose Nanofibrils. ACS Appl. Mater. Interfaces 2015, 7, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Dinh, D.M.; Hsieh, Y.-L. Adsorption and desorption of cationic malachite green dye on cellulose nanofibril aerogels. Carbohydr. Polym. 2017, 173, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Sun, Q.; Xu, Q.; Xu, Y. Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Bioresour. Technol. 2015, 197, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.M.; Hajian, A.; Fall, A.B.; Håkansson, K.; Salajkova, M.; Lundell, F.; Wågberg, L.; Berglund, L.A. Highly Conducting, Strong Nanocomposites Based on Nanocellulose-Assisted Aqueous Dispersions of Single-Wall Carbon Nanotubes. ACS Nano 2014, 8, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Jing-Quan, H.; Kai-Yue, L.; Yi-Ying, Y.; Chang-Tong, M.; Hui-Xiang, W.; Peng-Bin, Y.; Xin-Wu, X. Synthesis and electrochemical performance of flexible cellulose nanofiber-carbon nanotube/natural rubber composite elastomers as supercapacitor electrodes. New Carbon Mater. 2018, 33, 341–350. [Google Scholar]
- Hajian, A.; Lindström, S.B.; Pettersson, T.; Hamedi, M.M.; Wågberg, L. Understanding the Dispersive Action of Nanocellulose for Carbon Nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Yang, J.-H.; Zhang, N.; Wang, Y.; Zhou, Z.-W. Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J. Hazard. Mater. 2017, 335, 28–38. [Google Scholar] [CrossRef]
- Hussain, A.; Li, J.; Wang, J.; Xue, F.; Chen, Y.; Bin Aftab, T.; Li, D. Hybrid Monolith of Graphene/TEMPO-Oxidized Cellulose Nanofiber as Mechanically Robust, Highly Functional, and Recyclable Adsorbent of Methylene Blue Dye. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.-M.; Kumagai, A.; Endo, T. Amphiphilic cellulose nanofiber-interwoven graphene aerogel monolith for dyes and silicon oil removal. Compos. Sci. Technol. 2019, 171, 190–198. [Google Scholar] [CrossRef]
- Goodman, S.M.; Ferguson, N.; Dichiara, A.B. Lignin-assisted double acoustic irradiation for concentrated aqueous dispersions of carbon nanotubes. RSC Adv. 2017, 7, 5488–5496. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Gu, J.; Hsieh, Y.-L. Alkaline cellulose nanofibrils from streamlined alkali treated rice straw. ACS Sustain. Chem. Eng. 2017, 5, 1730–1737. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhang, R.; Li, W.; Liu, H.; Lin, Y.; Li, L.; Zhou, Y. Graphene—Carbon nanotube aerogel as an ultra-light, compressible and recyclable highly efficient absorbent for oil and dyes. Environ. Sci. Nano 2016, 3, 107–113. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Mazaheri, F. Single and Binary System Dye Removal from Colored Textile Wastewater by a Dendrimer as a Polymeric Nanoarchitecture: Equilibrium and Kinetics. J. Chem. Eng. Data 2010, 55, 4660–4668. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M. Textile Dye Removal from Single and Ternary Systems Using Date Stones: Kinetic, Isotherm, and Thermodynamic Studies. J. Chem. Eng. Data 2010, 55, 4638–4649. [Google Scholar] [CrossRef]
- Chen, L.; Han, Q.; Li, W.; Zhou, Z.; Fang, Z.; Xu, Z.; Wang, Z.; Qian, X. Three-Dimensional graphene-Based adsorbents in sewage disposal: A review. Environ. Sci. Pollut. Res. 2018, 25, 25840–25861. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Sui, G. Superamphiphilic Polyurethane Foams Synergized from Cellulose Nanowhiskers and Graphene Nanoplatelets. Adv. Mater. Interfaces 2018, 5, 1701094. [Google Scholar] [CrossRef]
- Dai, J.; Huang, T.; Tian, S.-Q.; Xiao, Y.-J.; Yang, J.-H.; Zhang, N.; Wang, Y.; Zhou, Z.-W. High structure stability and outstanding adsorption performance of graphene oxide aerogel supported by polyvinyl alcohol for waste water treatment. Mater. Des. 2016, 107, 187–197. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Song, A.; Goodman, S.M.; He, D.; Bai, J. Smart papers comprising carbon nanotubes and cellulose microfibers for multifunctional sensing applications. J. Mater. Chem. A 2017, 5, 20161–20169. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelosterstoffeKungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 5, 451–465. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B 2012, 90, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Kumar, R.; Ansari, S.A.; Ansari, S.P.; Barakat, M.A.; Alshahrie, A.; Cho, M.H. Anion selective pTSA doped polyaniline@graphene oxide-multiwalled carbon nanotube composite for Cr(VI) and Congo red adsorption. J. Colloid Interface Sci. 2017, 496, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Dichiara, A.B.; Sherwood, T.J.; Rogers, R.E. Binder free graphene-single-wall carbon nanotube hybrid papers for the removal of polyaromatic compounds from aqueous systems. J. Mater. Chem. A 2013, 1, 14480–14483. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica andplatinum. J. Am. Chem. Soc. 1918, 40, 42. [Google Scholar] [CrossRef]
- Freundlich, H. Veber die adsorption in loesungen (Adsorption in solution). Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Khanday, W.A.; Marrakchi, F.; Asif, M.; Hameed, B.H. Mesoporous zeolite—Activated carbon composite from oil palm ash as an effective adsorbent for methylene blue. J. Taiwan Inst. Chem. Eng. 2017, 70, 32–41. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid Interface Sci. 2017, 509, S0021979717310226. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 2010, 101, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Yang, L.; Zhou, L.; You, T. Self-assembled three-dimensional graphene-based materials for dye adsorption and catalysis. J. Mater. Chem. A 2015, 3, 10031–10037. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Peng, H.; Wu, J.; Liu, Z.; Guo, X. Removal of Anionic Dyes from Aqueous Solutions by Cellulose-Based Adsorbents: Equilibrium, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2016, 61, 3266–3276. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; Xia, L. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 2013, 95, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Chook, S.W.; Khiew, P.S. CaCO3-decorated cellulose aerogel for removal of Congo Red from aqueous solution. Cellulose 2015, 22, 2683–2691. [Google Scholar] [CrossRef]

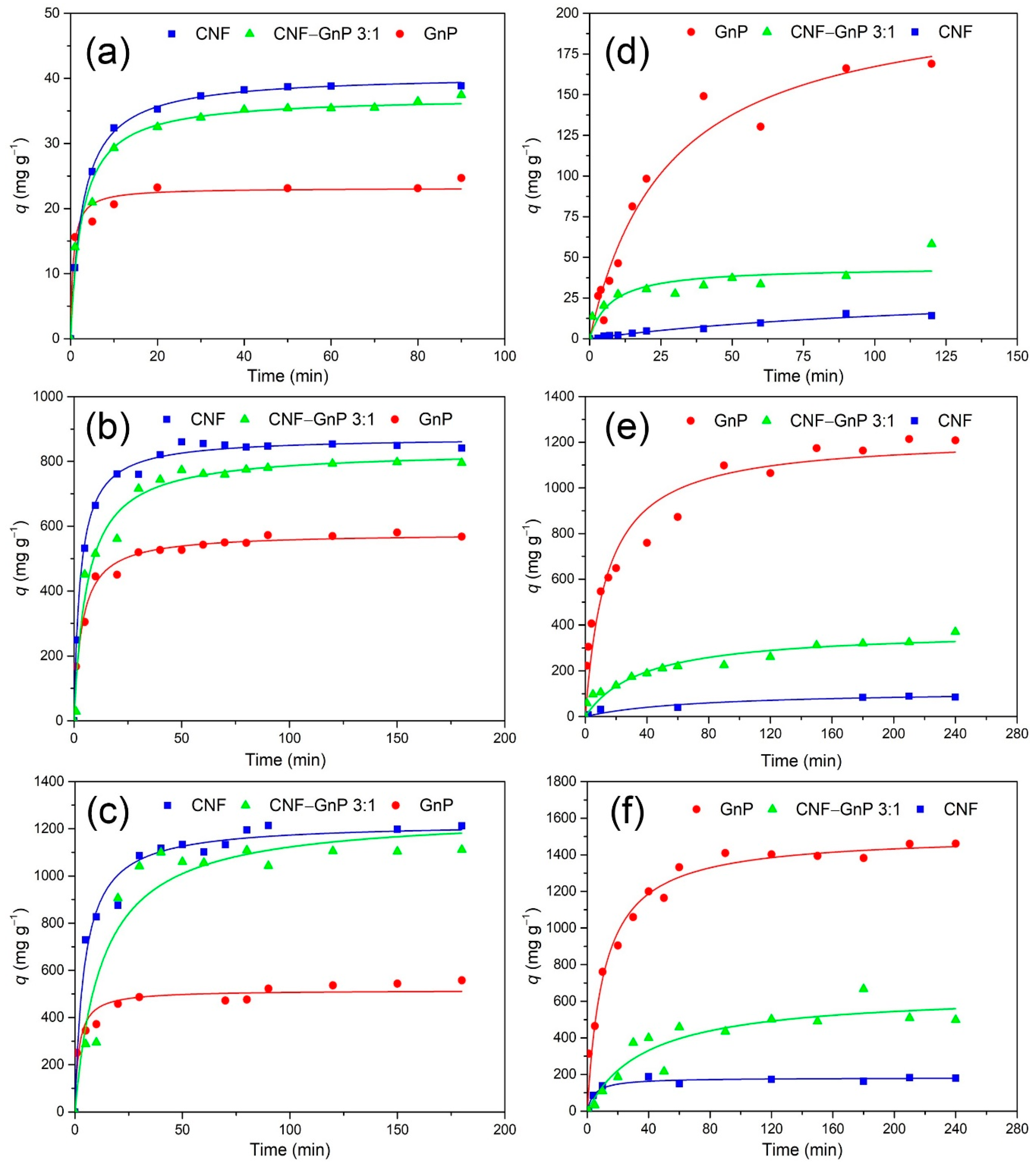

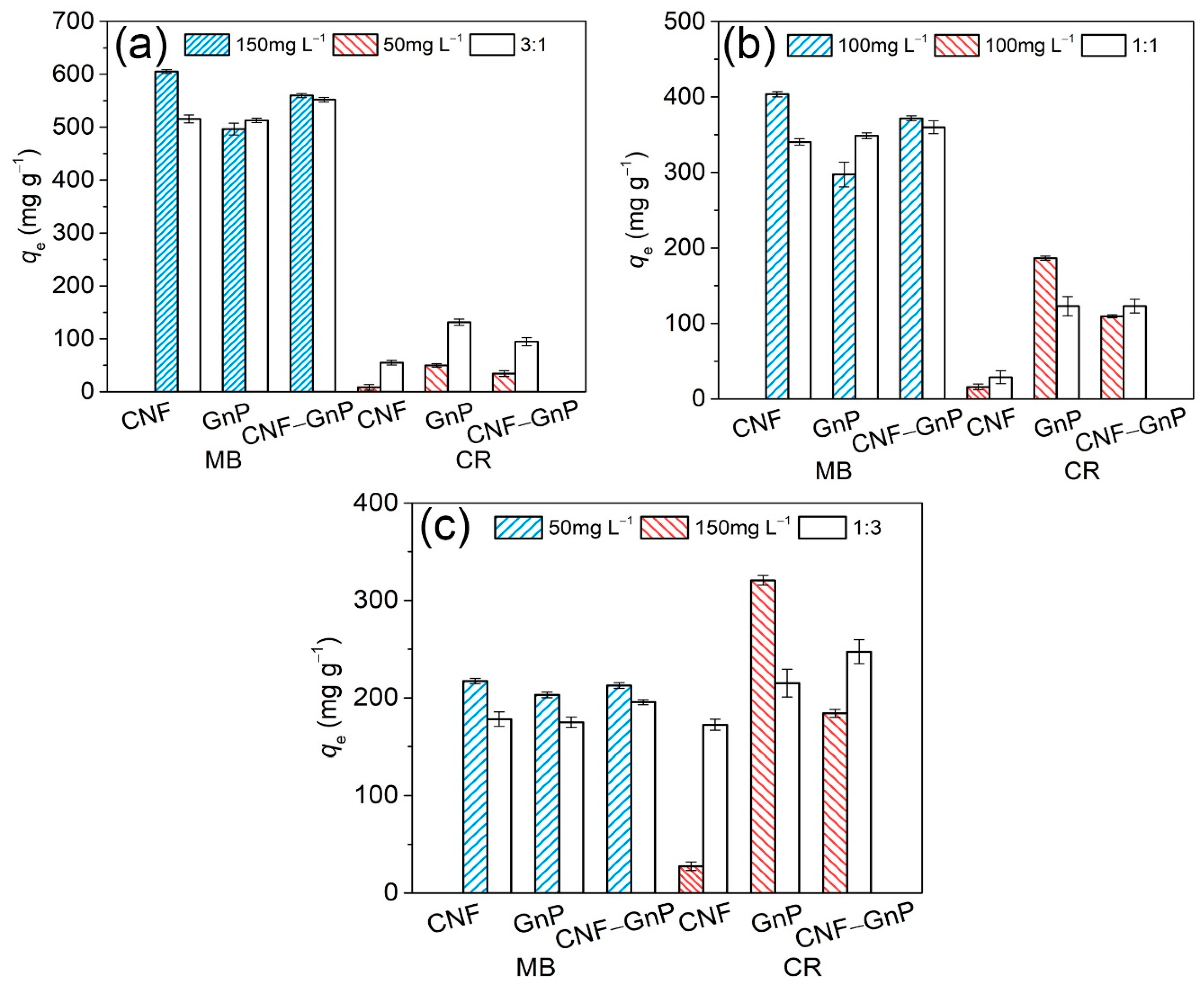
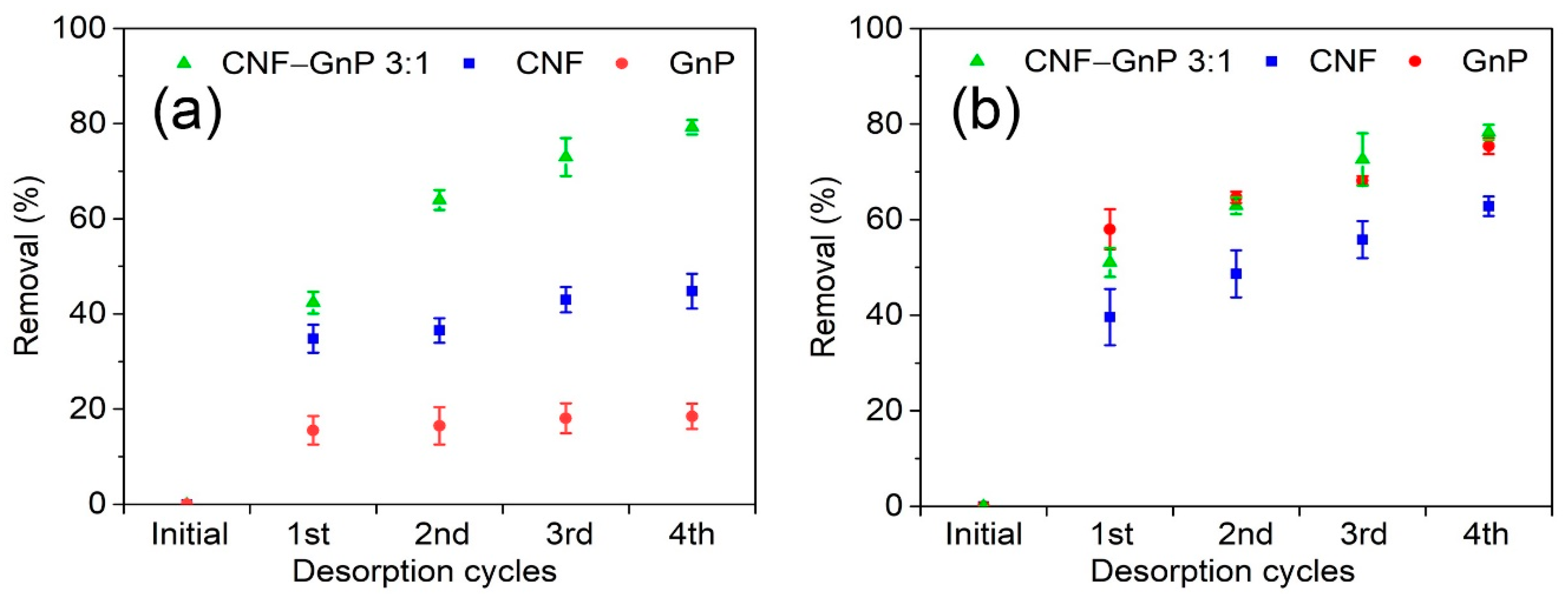
| Sample | MB Concentration (mg L−1) | CR Concentration (mg L−1) | |||||
|---|---|---|---|---|---|---|---|
| GnP | Param. | 10 | 250 | 500 | 100 | 600 | 2000 |
| pseudo-first order | qe (mg g−1) | 22.2 | 544.2 | 496.7 | 167.0 | 1113.7 | 1383.5 |
| k1 (min−1) | 1.192 | 0.170 | 0.282 | 0.040 | 0.049 | 0.059 | |
| R2 | 0.927 | 0.955 | 0.856 | 0.971 | 0.912 | 0.960 | |
| pseudo-second order | qe (mg g−1) | 23.2 | 578.3 | 515.9 | 213.2 | 1223.5 | 1510.4 |
| k2 (g mg−1 min−1) | 7.1 × 10−2 | 4.7 × 10−4 | 1.1 × 10−3 | 1.7 × 10−4 | 5.9 × 10−5 | 6.1 × 10−5 | |
| v0 (mg g−1 min−1) | 38.0 | 157.1 | 282.2 | 7.76 | 87.9 | 138.4 | |
| R2 | 0.966 | 0.985 | 0.937 | 0.968 | 0.954 | 0.981 | |
| CNF | |||||||
| pseudo-first order | qe (mg g−1) | 37.8 | 831.0 | 1140.0 | 20.7 | 92.5 | 173.4 |
| k1 (min−1) | 0.232 | 0.198 | 0.145 | 0.011 | 0.012 | 0.153 | |
| R2 | 0.986 | 0.972 | 0.933 | 0.982 | 0.942 | 0.960 | |
| pseudo-second order | qe (mg g−1) | 40.6 | 874.8 | 1225.0 | 33.0 | 114.5 | 182.4 |
| k2 (g mg−1 min−1) | 8.9 × 10−3 | 3.8 × 10−4 | 1.9 × 10−4 | 2.2 × 10−4 | 1.20 × 10−4 | 1.2 × 10−3 | |
| v0 (mg g−1 min−1) | 14.7 | 294.0 | 278.1 | 0.241 | 1.57 | 38.6 | |
| R2 | 0.999 | 0.994 | 0.976 | 0.978 | 0.948 | 0.952 | |
| CNF–GnP 3:1 | |||||||
| pseudo-first order | qe (mg g−1) | 35.2 | 767.2 | 1114.0 | 37.9 | 321.4 | 538.8 |
| k1 (min−1) | 0.210 | 0.112 | 0.063 | 0.128 | 0.022 | 0.024 | |
| R2 | 0.944 | 0.961 | 0.951 | 0.915 | 0.931 | 0.992 | |
| pseudo-second order | qe (mg g−1) | 37.2 | 834.3 | 1264.5 | 44.1 | 379.4 | 648.5 |
| k2 (g mg−1 min−1) | 1.0 × 10−2 | 2.0 × 10−4 | 6.3 × 10−5 | 3.0 × 10−3 | 7.07 × 10−5 | 4.0 × 10−5 | |
| v0 (mg g−1 min−1) | 14.1 | 137.2 | 100.2 | 5.92 | 10.2 | 16.9 | |
| R2 | 0.978 | 0.977 | 0.910 | 0.940 | 0.955 | 0.972 | |
| Langmuir Adsorption Model | GnP | CNF | CNF–GnP 3:1 | ||||
|---|---|---|---|---|---|---|---|
| MB | CR | MB | CR | MB | CR | ||
| qe = (qmaxKLCe)/(1 + KLCe) | qmax (mg g−1) | 567.1 | 1787.3 | 1387.2 | 351.7 | 1178.5 | 585.3 |
| KL (L g−1) | 7.0 × 10−2 | 7.1 × 10−3 | 8.3 × 10−2 | 8.6 × 10−4 | 1.1 × 10−1 | 3.8 × 10−3 | |
| R2 | 0.879 | 0.858 | 0.984 | 0.960 | 0.985 | 0.980 | |
| Freundlich adsorption model | GnP | CNF | CNF–GnP 3:1 | ||||
| MB | CR | MB | CR | MB | CR | ||
| qe = KF × Ce1/n | n | 5.431 | 2.995 | 4.512 | 1.428 | 4.984 | 1.944 |
| KF (mg g−1) | 181.3 | 156.1 | 376.5 | 0.966 | 364.2 | 29.9 | |
| R2 | 0.773 | 0.719 | 0.902 | 0.935 | 0.892 | 0.864 | |
| Sips adsorption model | GnP | CNF | CNF–GnP 3:1 | ||||
| MB | CR | MB | CR | MB | CR | ||
| 1/qe = (1/qmaxKs) × (1/Ce)1/n + (1/qmax) | qmax (mg g−1) | 552.4 | 1515.0 | 1453.0 | 235.2 | 1231.1 | 517.9 |
| Ks (L g−1) | 4.1 × 10−2 | 2.3 × 10−4 | 1.2 × 10−1 | 1.0 × 10−4 | 1.5 × 10−1 | 6.8 × 10−4 | |
| n | 0.819 | 0.534 | 1.222 | 0.727 | 1.243 | 0.723 | |
| R2 | 0.869 | 0.885 | 0.991 | 0.960 | 0.988 | 0.969 | |
| Adsorbent | qmax (mg g−1) MB | Ref. | Adsorbent | qmax (mg g−1) CR | Ref. |
|---|---|---|---|---|---|
| CNF–GnP aerogel | 1178.5 | this study | CNF–GnP aerogel | 585.3 | this study |
| Zeolite—activated carbon composite from oil palm ash | 285.71 | [49] | Polyaniline@GO-multiwalled carbon nanotube nanocomposite | 66.67 | [44] |
| Nickel nanoparticles/porous carbon—carbon nanotube hybrids | 312 | [50] | Chitosan hydrogel beads impregnated with CNT | 450.4 | [51] |
| 3D rGO/L–Cys hydrogel | 660 | [52] | Functionalized multiwalled carbon nanotubes | 148 | [53] |
| Graphene/cellulose nanofibers | 227.27 | [28] | Polyacrylamide grafted quaternized cellulose | 349.28 | [54] |
| GO/calcium alginate composites | 181.81 | [55] | CaCO3−cellulose aerogel | 75.81 | [56] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Hu, C.; Dichiara, A.B.; Jiang, W.; Gu, J. Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials 2020, 10, 169. https://doi.org/10.3390/nano10010169
Yu Z, Hu C, Dichiara AB, Jiang W, Gu J. Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials. 2020; 10(1):169. https://doi.org/10.3390/nano10010169
Chicago/Turabian StyleYu, Zhencheng, Chuanshuang Hu, Anthony B. Dichiara, Weihui Jiang, and Jin Gu. 2020. "Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes" Nanomaterials 10, no. 1: 169. https://doi.org/10.3390/nano10010169
APA StyleYu, Z., Hu, C., Dichiara, A. B., Jiang, W., & Gu, J. (2020). Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials, 10(1), 169. https://doi.org/10.3390/nano10010169