Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity
Abstract
:1. Introduction
2. Experimental
2.1. Material
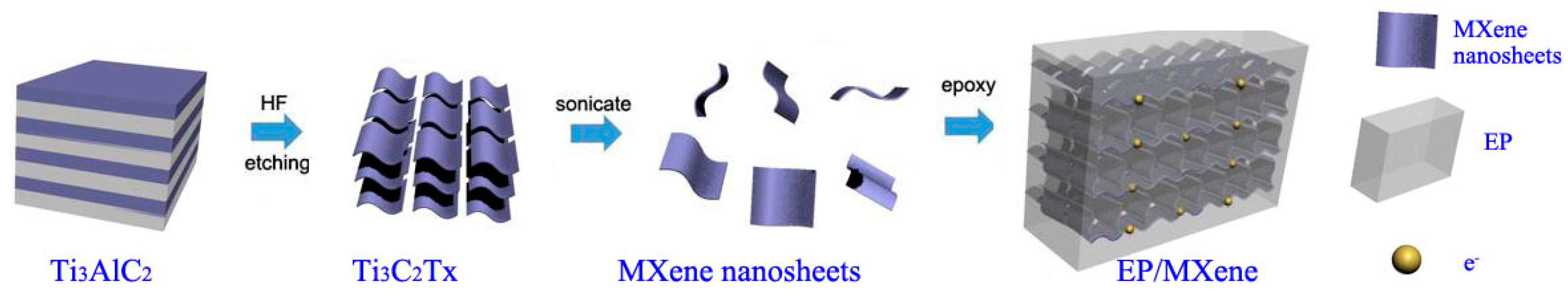
2.2. Preparation of EP/Ti3C2Tx MXene Nanosheets ECAs
2.3. Characterization and Measurement
3. Results and Discussion
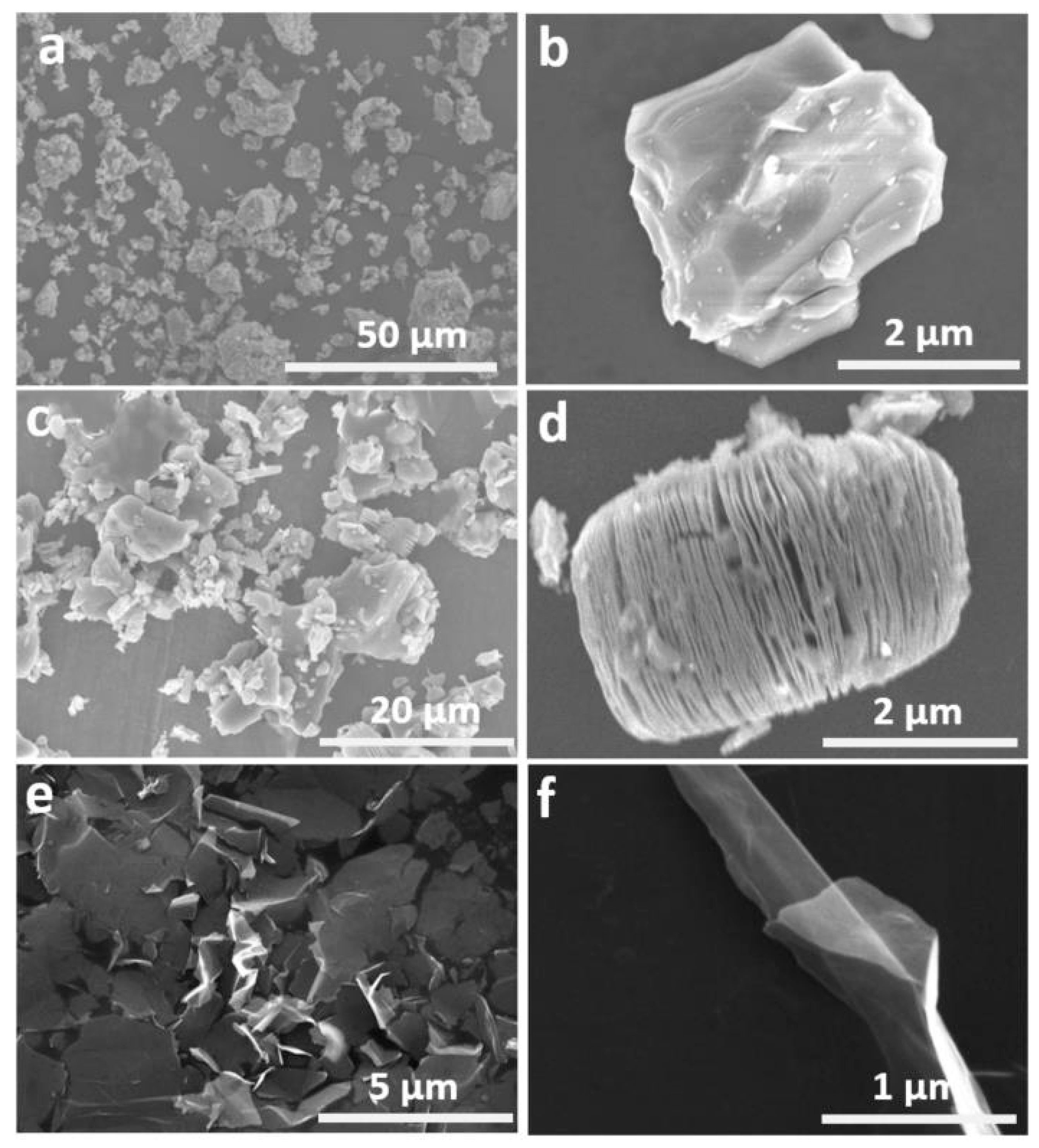
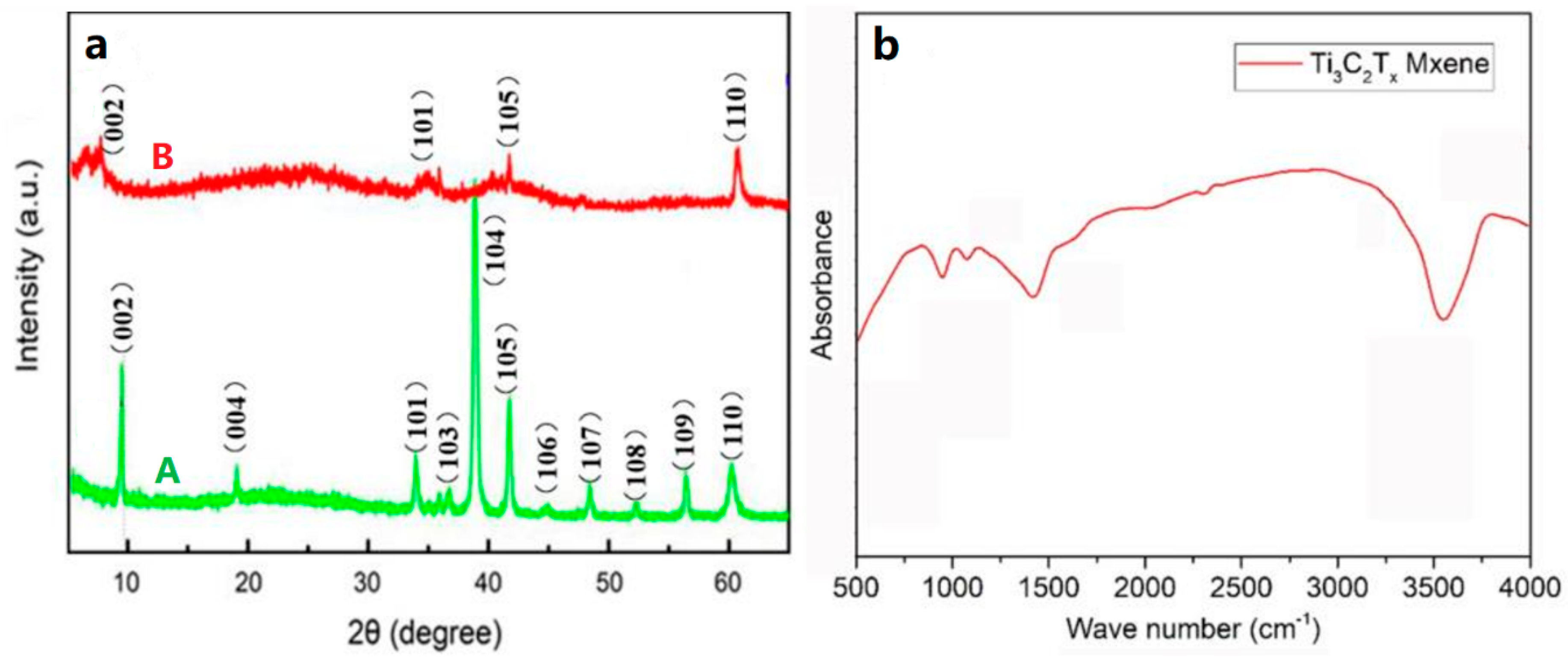
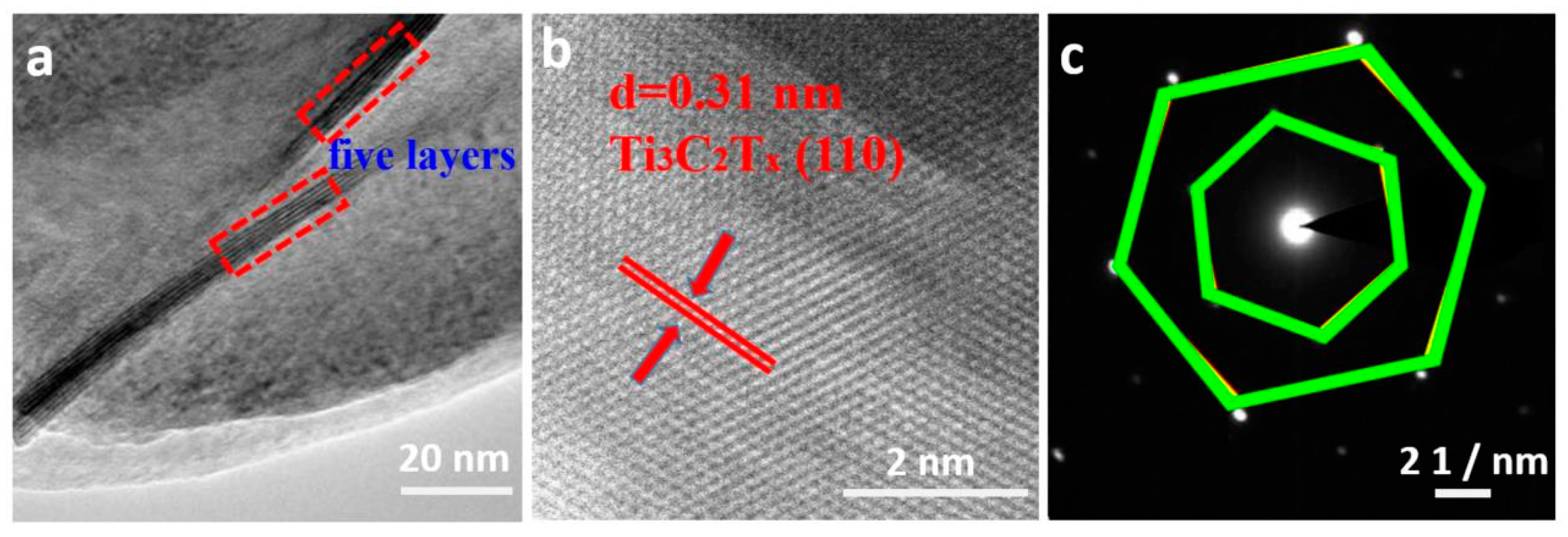
3.1. The Structure and Morphology of Ti3AlC2 and Ti3C2Tx MXene
3.2. The Structure of EP/Ti3C2Tx MXene Nanosheets ECAs
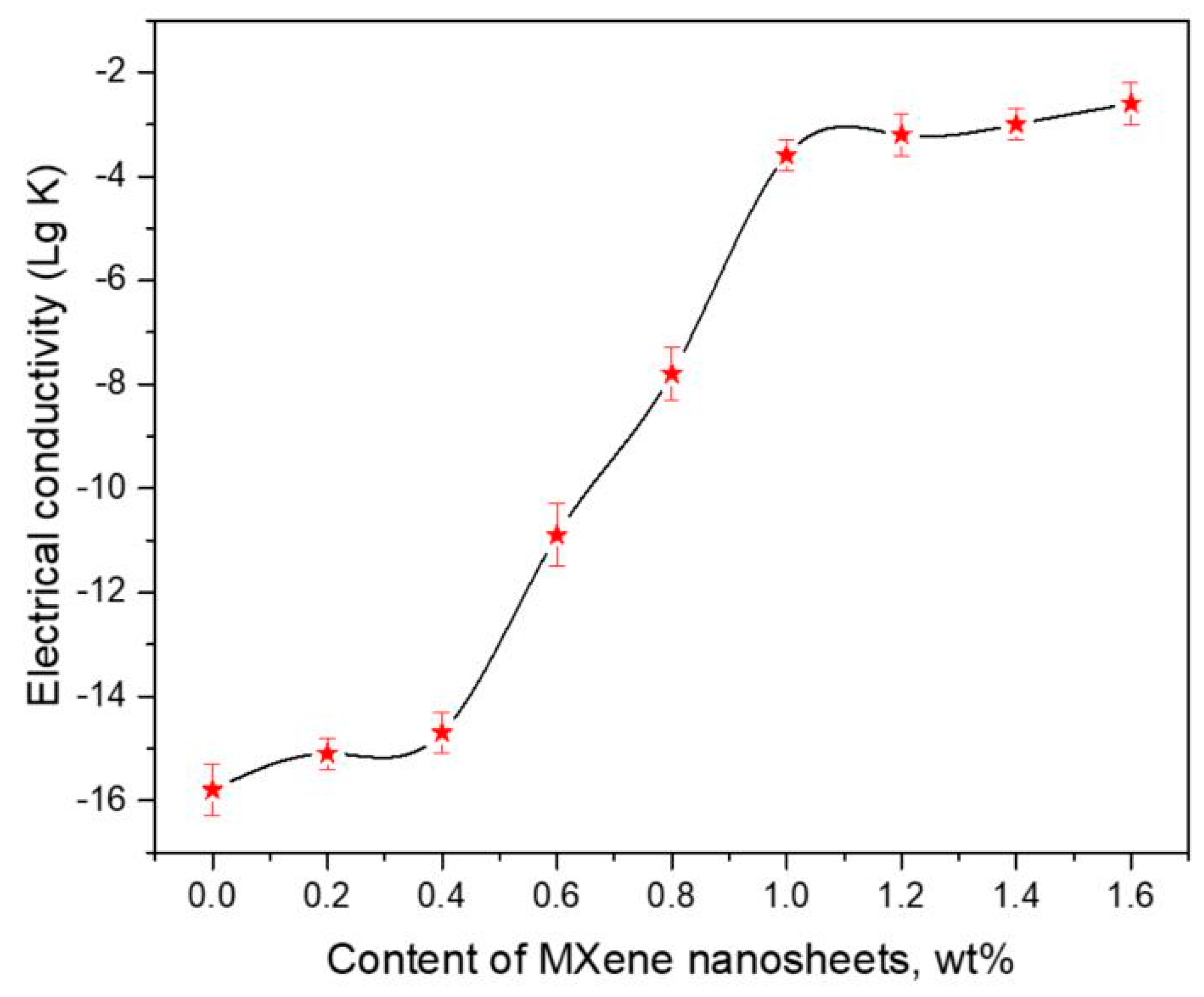
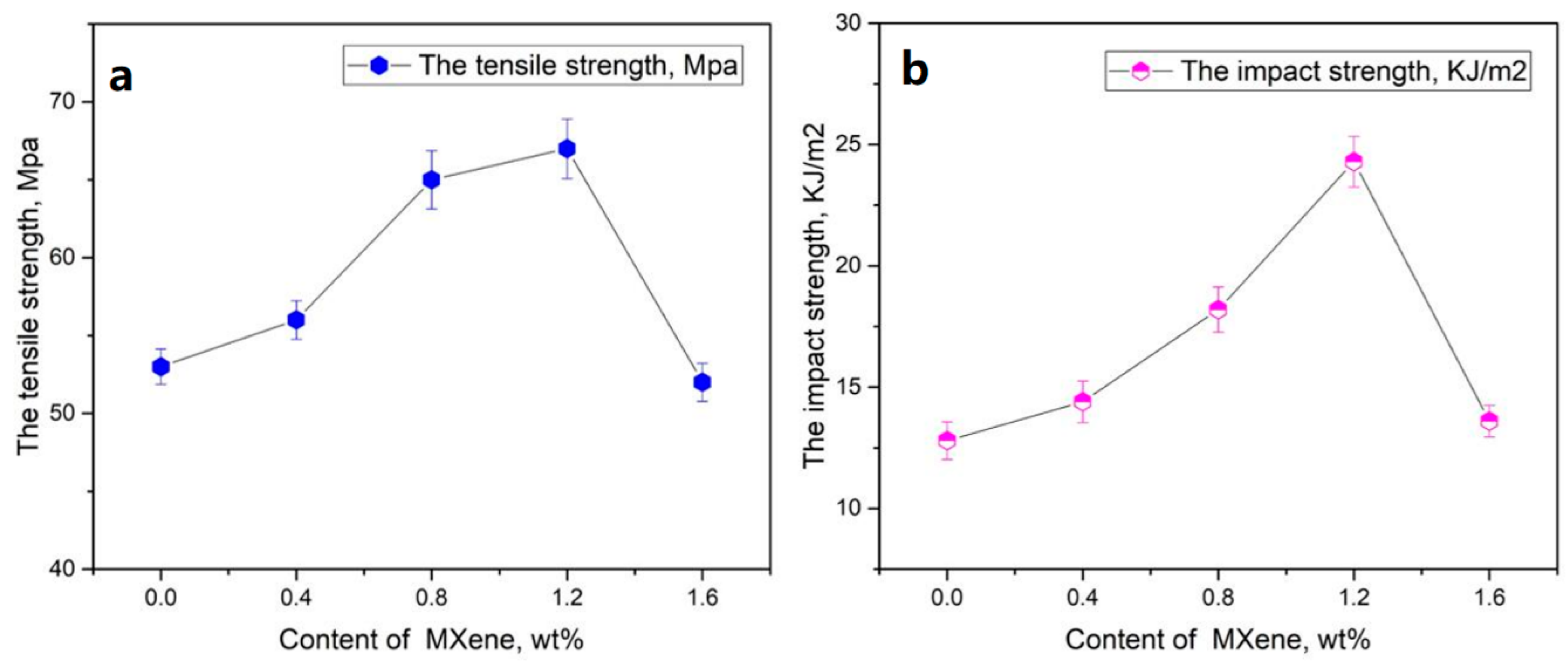
3.3. Electrical Conductivity and Mechanical Performance
3.4. The Morphology of EP/Ti3C2Tx MXene Nanosheets Composite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jakubinek, M.B.; Ashrafi, B.; Zhang, Y.; Martinez-Rubi, Y.; Kingston, C.T.; Johnston, A.; Simard, B. Single-walled carbon nanotube—Epoxy composites for structural and conductive aerospace adhesives. Compos. Part B 2015, 69, 87–93. [Google Scholar] [CrossRef]
- Hou, T.; Wang, B.; Jia, Z.; Wu, H.; Lan, D.; Huang, Z.; Feng, A.; Ma, M.; Wu, G. A review of metal oxide-related microwave absorbing materials from the dimension and morphology perspective. J. Mater. Sci. 2019, 30, 10961–10984. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford university press: New York, NY, USA, 2003; pp. 88–89. [Google Scholar]
- Feng, A.; Wu, G.; Pan, C.; Wang, Y. Synthesis, preparation and mechanical property of wood fiber-reinforced poly (vinyl chloride) composites. J. Nanosci. Nanotechnol. 2017, 17, 3859–3863. [Google Scholar] [CrossRef]
- Tyson, W.R.; Miller, W.A. Surface free energies of solid metals: Estimation from liquid surface tension measurements. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- Wu, G.L.; Cheng, Y.H.; Yang, Z.H.; Jia, Z.R.; Wu, H.J.; Yang, L.J.; Li, H.L.; Guo, P.Z.; Lv, H.L. Design of Carbon Sphere/Magnetic Quantum Dots with Tunable Phase Compositions and Boost Dielectric Loss Behavior. Chem. Eng. J. 2018, 333, 519–528. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Safronov, A.P.; Terzian, T.V.; Volodina, N.S.; Beketov, I.V.; Lezama, L.; Prieto, L.M. Fe45Ni55 magnetic nanoparticles obtained by electric explosion of wire for the development of functional composites. IEEE Magn. Lett. 2015, 6, 1–4. [Google Scholar] [CrossRef]
- Zhou, X.F.; Jia, Z.R.; Feng, A.L.; Wang, X.X.; Liu, J.J.; Zhang, M.; Cao, H.J.; Wu, G.L. Synthesis of fish skin -derived 3D carbon foams with broadened bandwidth and excellent electromagnetic wave absorption performance. Carbon 2019, 152, 827–836. [Google Scholar] [CrossRef]
- Gao, Z.G.; Xu, B.H.; Ma, M.L.; Feng, A.L.; Zhang, Y.; Liu, X.H.; Jia, Z.R.; Wu, G.L. Electrostatic self-assembly synthesis of ZnFe2O4 quantum dots (ZnFe2O4@C) and electromagnetic microwave absorption. Compos. Part B 2019, 179, 107417. [Google Scholar] [CrossRef]
- Li, Z.; Le, T.; Wu, Z.; Yao, Y.; Li, L.; Tentzeris, M.; Moon, K.; Wong, C.P. Rational Design of a Printable, Highly Conductive Silicone-based Electrically Conductive Adhesive for Stretchable Radio-Frequency Antennas. Adv. Funct. Mater. 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Du, Z.X.; Li, J.X. The synthesis, structure and magnetic properties of a mononuclear cobalt compound with dipyrimidine sulfane ligand derived from 2-thio-barbituric acid. Inorg. Chim. Acta 2015, 436, 159–162. [Google Scholar] [CrossRef]
- Li, J.X.; Du, Z.X.; Wang, J.G.; Wang, T.; Lv, J.N. Zinc and manganese coordination polymers constructed by a new coordination mode of 4,5-dicyanoimidazolate ligand: Syntheses, crystal structures, fluorescent and magnetic properties. Inorg. Chem. Commun. 2012, 15, 243–247. [Google Scholar] [CrossRef]
- Ren, H.; Guo, Y.; Huang, S.; Zhang, K.; Yuen, M.; Fu, X.; Yu, S.; Sun, R.; Wong, C. One-step preparation of silver hexagonal microsheets as electrically conductive adhesive fillers for printed electronics. ACS Appl. Mater. Int. 2015, 7, 13685–13692. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Jia, Z.R.; Zhou, X.F.; Nie, G.Z.; Lv, H.L. Interlayer Controllable of Hierarchical MWCNTs@C@FexOy Cross-linked Composite with Wideband Electromagnetic Absorption Performance. Compos. Part A 2020, 128, 105687. [Google Scholar] [CrossRef]
- Wu, W.; Yu, C.; Chen, J.; Yang, Q. Fluorometric detection of copper ions using click chemistry and the target-induced conjunction of split DNAzyme fragments. Int. J. Environ. Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Jia, Z.R.; Wang, C.; Feng, A.L.; Shi, P.B.; Zhang, C.H.; Liu, X.H.; Wang, K.K.; Wu, G.L. A low dielectric decoration strategy to achieve absorption dominated electromagnetic shielding material. Compos. Part B 2020, 183, 107690. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, J.Q.; Kou, K.C.; Zhang, Y.; Wu, G.L. Investigation of the through-plane thermal conductivity of polymer composites with in-plane oriented hexagonal boron nitride. Int. J. Heat Mass Tran. 2018, 120, 1–8. [Google Scholar] [CrossRef]
- Jia, Z.R.; Wang, B.B.; Feng, A.L.; Liu, J.J.; Zhang, C.H.; Zhang, M.; Wu, G.L. Fabrication of NixCo3−xS4 hollow nanosphere as wideband electromagnetic absorber at thin matched thickness. Ceram. Int. 2019, 45, 15854–15859. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D. Epoxy-based adhesives filled with flakes Ag-coated copper as conductive fillers. Polym. Compos. 2017, 38, 846–851. [Google Scholar] [CrossRef]
- Li, J.X.; Du, Z.X.; Feng, X. A new binuclear NiII complex with tetrafluorophthalate and 2,2′-bipyridine ligands: Synthesis, crystal structure and magnetic properties. Z. Naturforsch. 2019, 74, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Li, J.X.; Du, Z.X.; Wang, J.; Feng, X. Two mononuclear zinc(II) complexes constructed by two types of phenoxyacetic acid ligands: Syntheses, crystal structures and fluorescence properties. Z. Naturforsch. 2019, 74, 839–845. [Google Scholar] [CrossRef]
- Li, J.X.; Du, Z.X.; Bai, R.F. Crystal structure of aqua-bis(5-bromo-6-methyl-picolinato-κ2N,O)zinc(II) dihydrate, C14H16Br2N2O7Zn. Z. Krist. New Cryst. Struct. 2019, 235, 63–65. [Google Scholar] [CrossRef] [Green Version]
- Li, J.X.; Du, Z.X. A Binuclear Cadmium (II) Cluster Based on π–π Stacking and Halogen-Halogen Interactions: Synthesis, Crystal Analysis and Fluorescent Properties. J. Clust. Sci. 2019, 22, 1–5. [Google Scholar] [CrossRef]
- Feng, A.; Ma, M.; Jia, Z.; Zhang, M.; Wu, G. Fabrication of NiFe2O4@carbon fiber coated with phytic acid-doped polyaniline composite and its application as an electromagnetic wave absorber. RSC Adv. 2019, 9, 25932–25941. [Google Scholar] [CrossRef] [Green Version]
- Simon, P. Two-dimensional MXene with controlled interlayer spacing for electrochemical energy storage. ACS Nano 2017, 11, 2393. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 155. [Google Scholar] [CrossRef]
- Nam, S.; Umrao, S.; Oh, S.; Shin, K.H.; Park, H.S.; Oh, I.K. Sonochemical self-growth of functionalized titanium carbide nanorods on Ti3C2 nanosheets for high capacity anode for lithium-ion batteries. Compos. Part B 2020, 181, 107583. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Wang, B.B.; Ma, M.L.; Feng, A.L.; Huang, Z.Y.; Zhang, Y.; Jia, Z.; Tan, G.X.; Cao, H.J.; Wu, G.L. Preparation of two-dimensional titanium carbide (Ti3C2Tx and NiCo2O4 composites to achieve excellent microwave absorption properties. Compos. Part B 2020, 180, 107577. [Google Scholar] [CrossRef]
- Wang, F.; Cao, M.; Qin, Y.; Zhu, J.; Wang, L.; Tang, Y. ZnO nanoparticle-decorated two-dimensional titanium carbide with enhanced supercapacitive performance. RSC Adv. 2016, 6, 88934–88942. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, B.; Hu, L.; Xu, Q.; Li, Y.; Liu, D.; Xu, M. Synthesis of SnS nanoparticle-modified MXene (Ti3C2Tx) composites for enhanced sodium storage. J. Alloys Compd. 2018, 732, 448–453. [Google Scholar] [CrossRef]
- Sandler, J.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Tjong, S.C.; Liang, G.D.; Bao, S.P. Electrical behavior of polypropylene/multiwalled carbon nanotube nanocomposites with low percolation threshold. Scr. Mater. 2007, 57, 461–464. [Google Scholar] [CrossRef]
- Lu, T.; Jiang, M.; Jiang, Z.; Hui, D.; Wang, Z.; Zhou, Z. Effect of surface modification of bamboo cellulose fibers on mechanical properties of cellulose/epoxy composites. Compos. Part B 2013, 51, 28–34. [Google Scholar] [CrossRef]
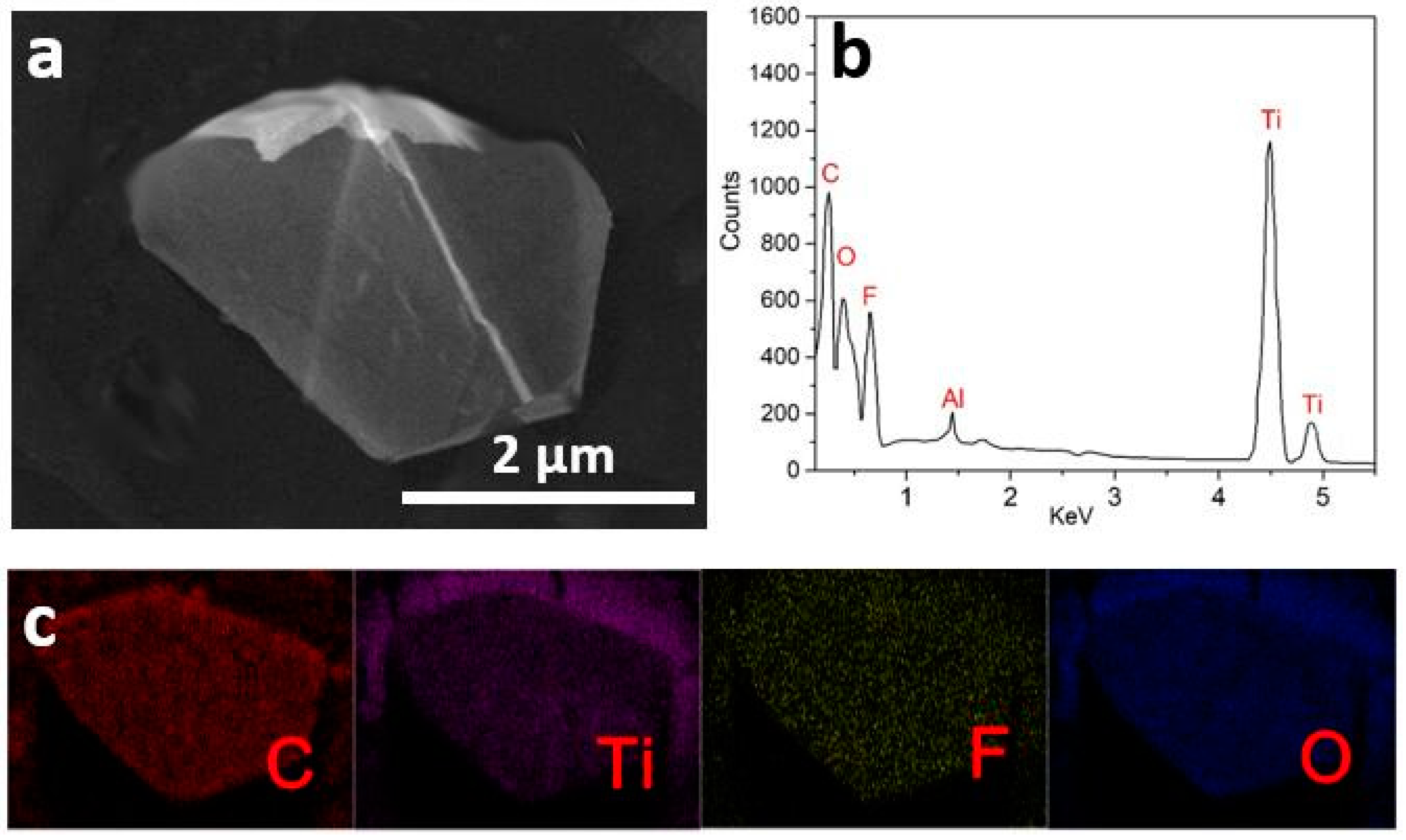


| Element | Atomic % | Weight % |
|---|---|---|
| C | 27.4 | 11.0 |
| Ti | 40.8 | 69.0 |
| O | 19.0 | 11.1 |
| F | 11.6 | 7.8 |
| Al | 1.2 | 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, A.; Hou, T.; Jia, Z.; Zhang, Y.; Zhang, F.; Wu, G. Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity. Nanomaterials 2020, 10, 162. https://doi.org/10.3390/nano10010162
Feng A, Hou T, Jia Z, Zhang Y, Zhang F, Wu G. Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity. Nanomaterials. 2020; 10(1):162. https://doi.org/10.3390/nano10010162
Chicago/Turabian StyleFeng, Ailing, Tianqi Hou, Zirui Jia, Yi Zhang, Fan Zhang, and Guanglei Wu. 2020. "Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity" Nanomaterials 10, no. 1: 162. https://doi.org/10.3390/nano10010162
APA StyleFeng, A., Hou, T., Jia, Z., Zhang, Y., Zhang, F., & Wu, G. (2020). Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity. Nanomaterials, 10(1), 162. https://doi.org/10.3390/nano10010162






