Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
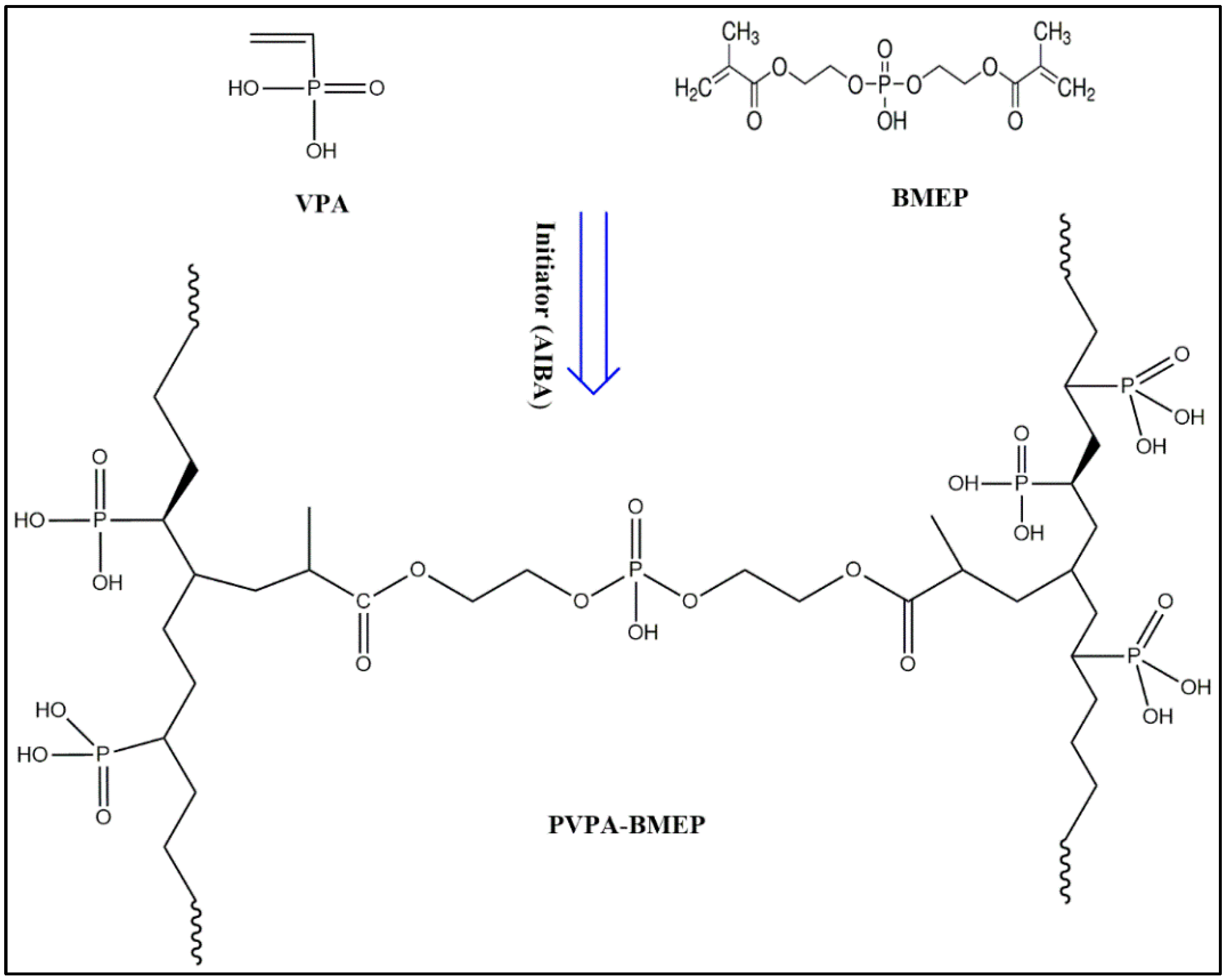
2.2. Preparation of Poly(Vinylphosphonic Acid)-Bis[2-(methacryloyloxy)ethyl] Phosphate (PVPA-BMEP) Hydrogels
2.3. Characterization of PVPA-BMEP Hydrogels
2.4. Adsorption Studies
2.5. Adsorption Kinetics
2.6. Adsorption Isotherms
2.7. Adsorption Thermodynamics
3. Results and Discussion
3.1. Characterization of PVPA-BMEP Hydrogels
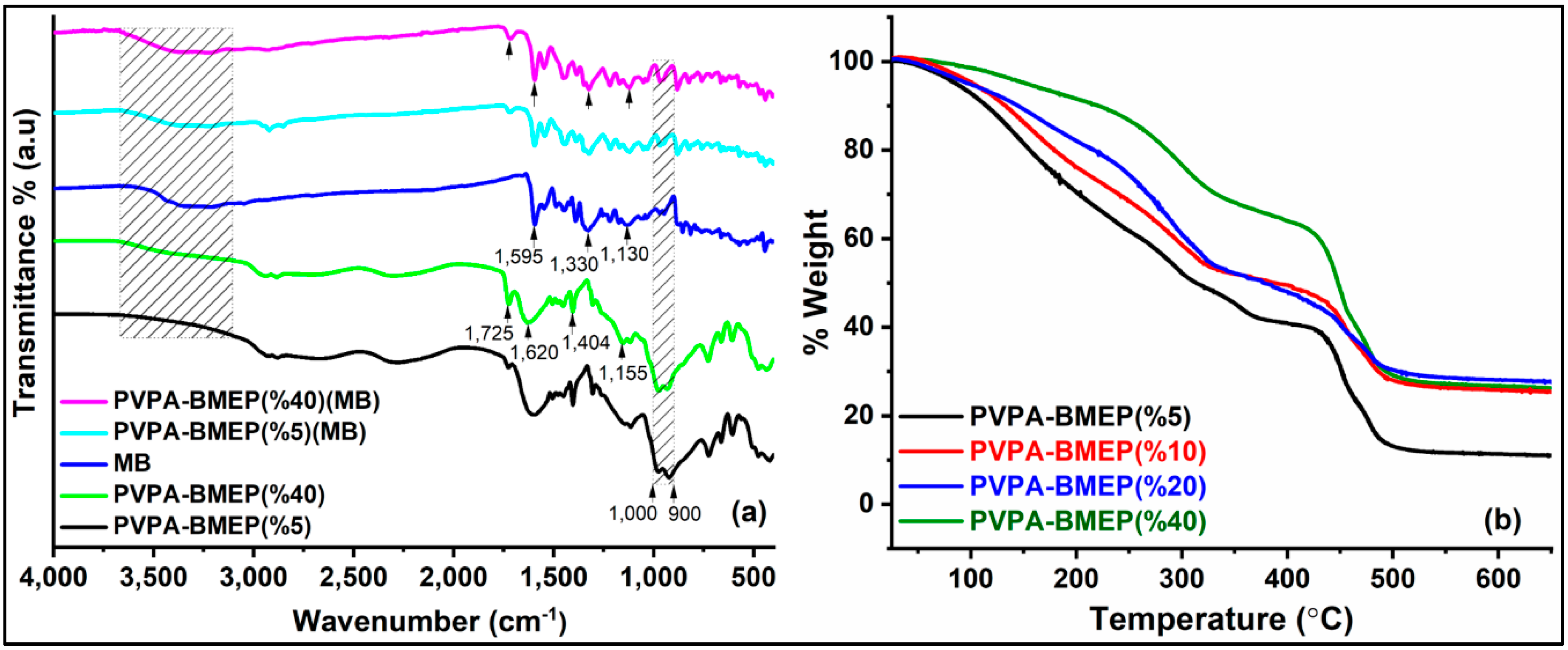
3.1.1. Fourier Transform Infrared (FT-IR) Spectra
3.1.2. Thermogravimetric Analysis (TGA) Study
3.1.3. Scanning Electron Microscope (SEM) Images
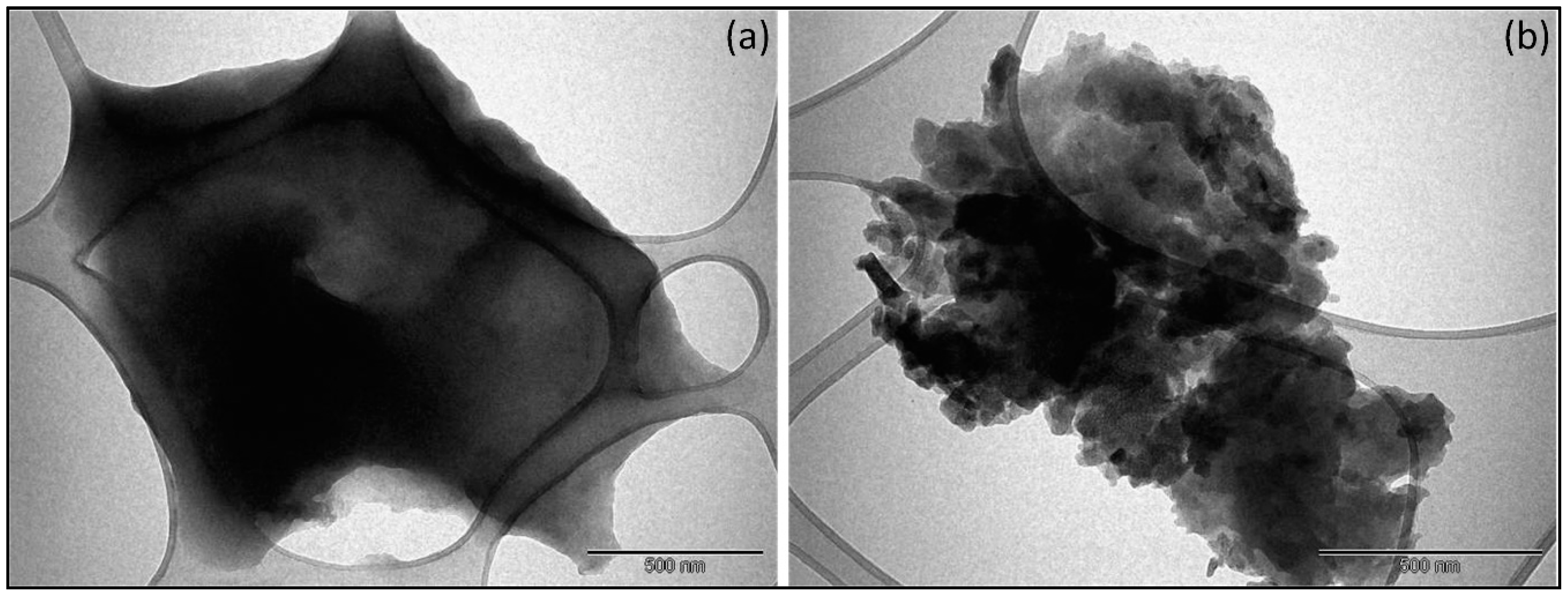
3.1.4. Transmission Electron Microscope (TEM) Images
3.2. Effect of Adsorption Parameters
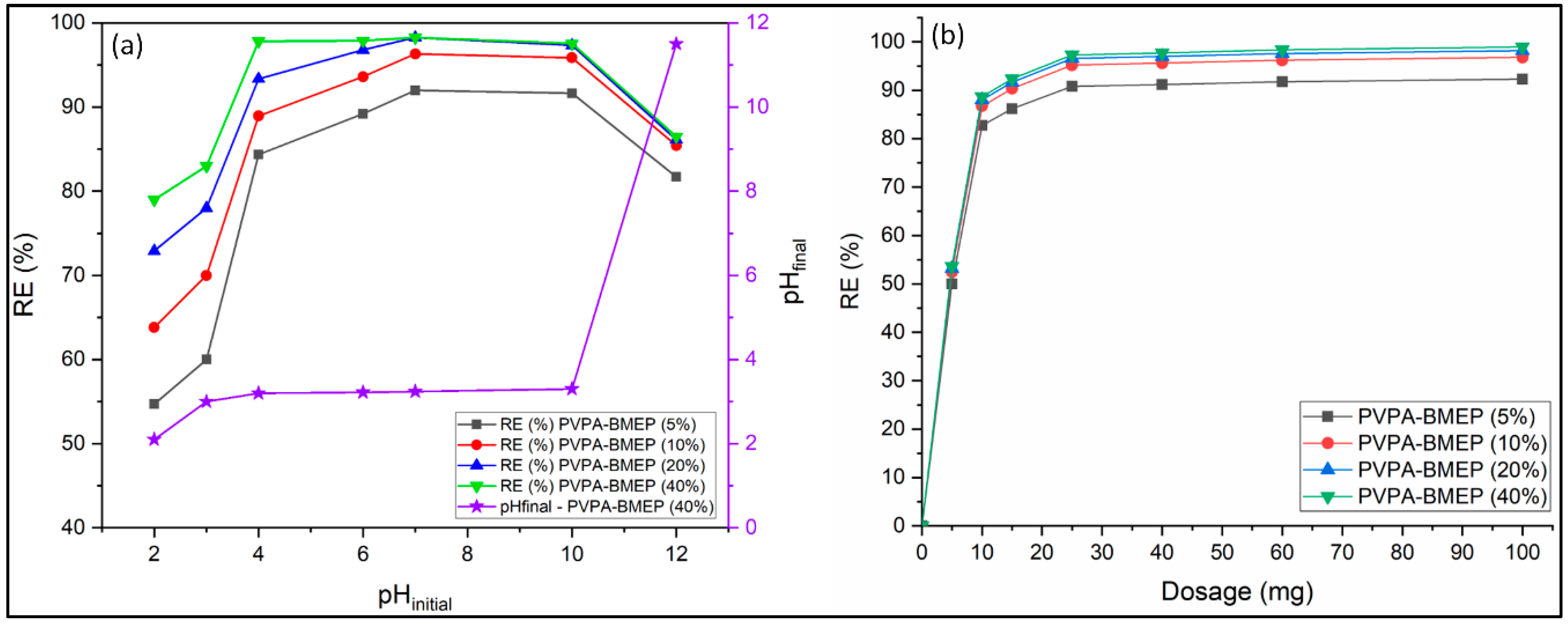
3.2.1. Initial Solution pH
3.2.2. Adsorbent Dose
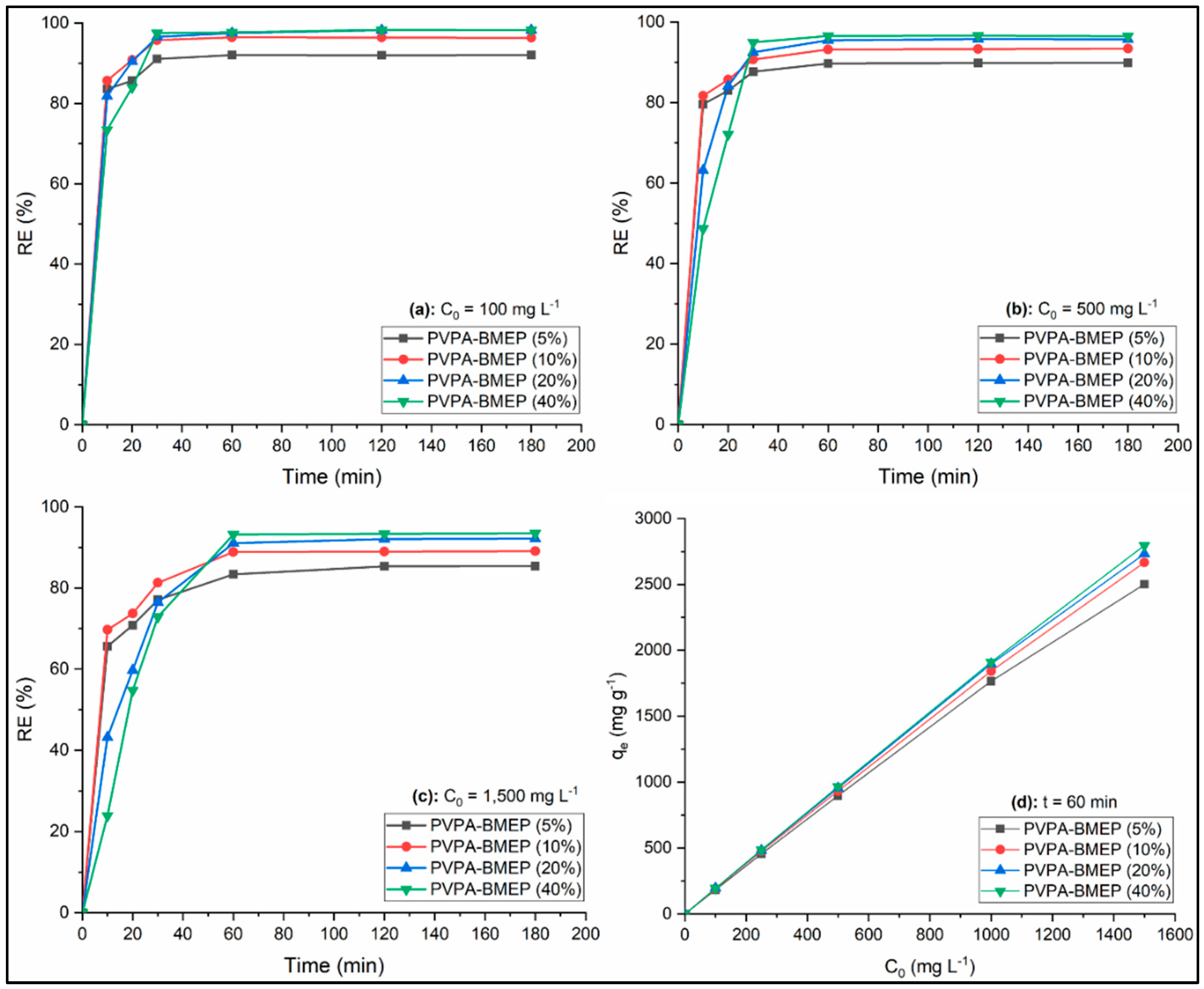
3.2.3. Contact Time
3.2.4. Initial Dye Concentration
3.3. Adsorption Kinetics
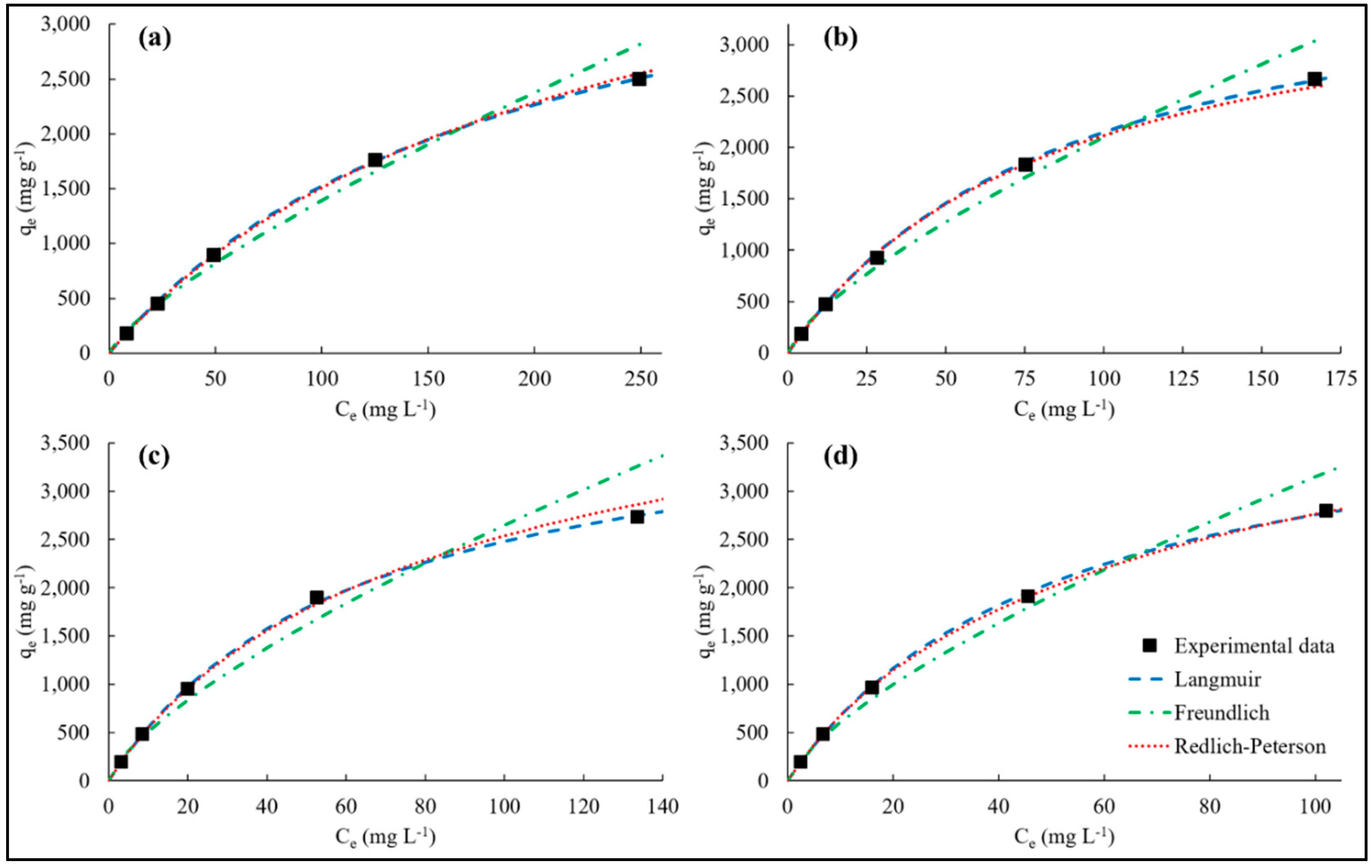
3.4. Adsorption Isotherms
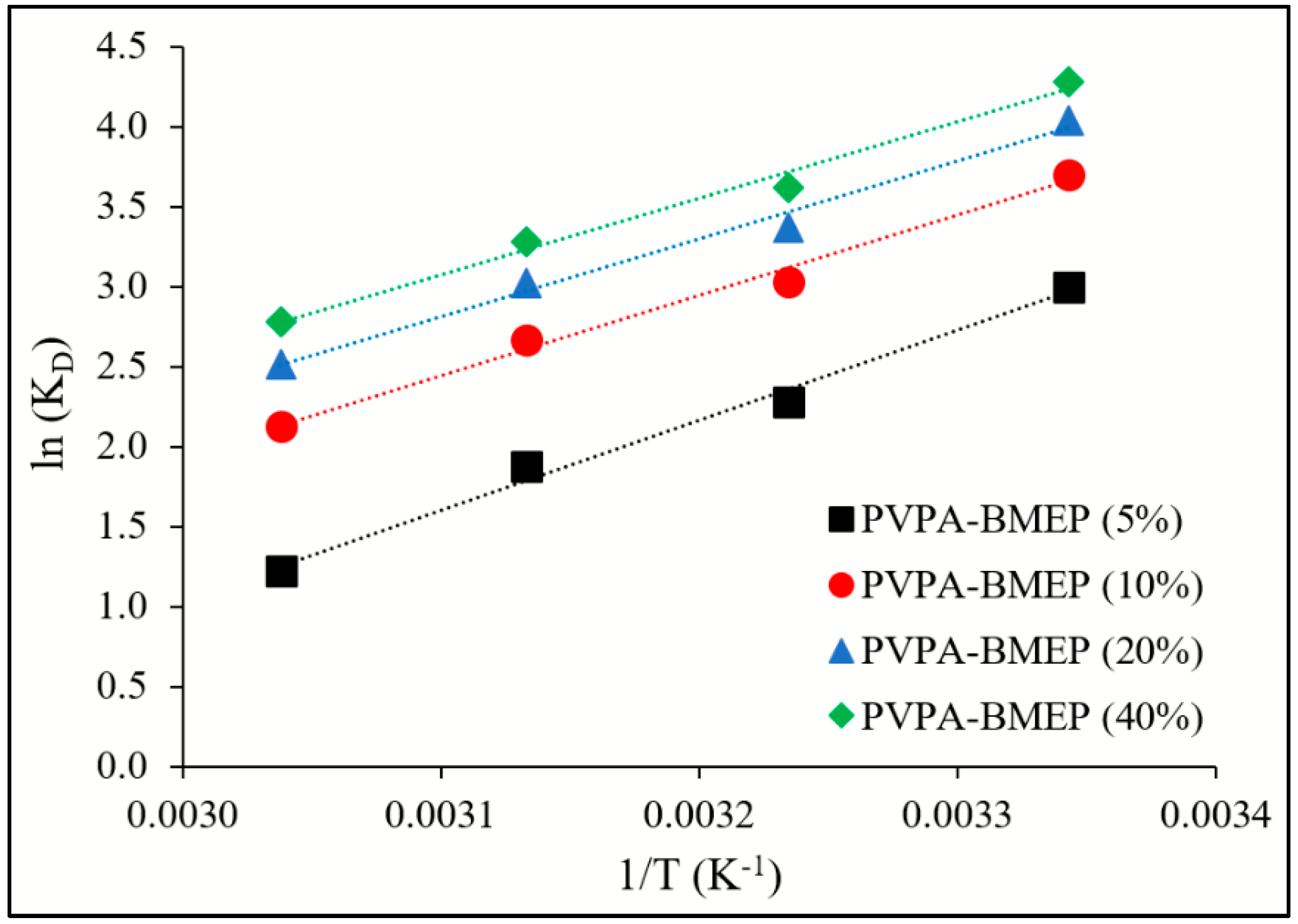
3.5. Adsorption Thermodynamics
3.6. Literature Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zargar, B.; Parham, H.; Rezazade, M. Fast removal and recovery of methylene blue by activated carbon modified with magnetic iron oxide nanoparticles. J. Chin. Chem. Soc. 2011, 58, 694–699. [Google Scholar] [CrossRef]
- Sakin Omer, O.; Hussein, M.A.; Hussein, B.H.M.; Mgaidi, A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]
- Ramezani, S.; Zahedi, P.; Bahrami, S.-H.; Nemati, Y. Microfluidic fabrication of nanoparticles based on ethyl acrylate-functionalized chitosan for adsorption of methylene blue from aqueous solutions. J. Polym. Environ. 2019, 27, 1653–1665. [Google Scholar] [CrossRef]
- Yao, T.; Guo, S.; Zeng, C.; Wang, C.; Zhang, L. Investigation on efficient adsorption of cationic dyes on porous magnetic polyacrylamide microspheres. J. Hazard. Mater. 2015, 292, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, H.; Li, Y.; Lu, Q.; Wang, Q.; Yang, W. Synthesis, characterization, and property testing of PGS/P(AMPS-co-AM) superabsorbent hydrogel initiated by glow-discharge electrolysis plasma. Colloid Polym. Sci. 2015, 294, 257–270. [Google Scholar] [CrossRef]
- Blaisi, N.I.; Zubair, M.; Ihsanullah; Ali, S.; Kazeem, T.S.; Manzar, M.S.; Al-Kutti, W.; Al Harthi, M.A. Date palm ash-MgAl-layered double hydroxide composite: Sustainable adsorbent for effective removal of methyl orange and eriochrome black-T from aqueous phase. Environ. Sci. Pollut. Res. Int. 2018, 25, 34319–34331. [Google Scholar] [CrossRef]
- Slokar, Y.M.; Majcen Le Marechal, A. Methods of decoloration of textile wastewaters. Dyes Pigment. 1998, 37, 335–356. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Nadeem, K.; Guyer, G.T.; Keskinler, B.; Dizge, N. Investigation of segregated wastewater streams reusability with membrane process for textile industry. J. Clean. Prod. 2019, 228, 1437–1445. [Google Scholar] [CrossRef]
- Tanveer, M.; Tezcanli Guyer, G. Solar assisted photo degradation of wastewater by compound parabolic collectors: Review of design and operational parameters. Renew. Sustain. Energy Rev. 2013, 24, 534–543. [Google Scholar] [CrossRef]
- Naushad, M.; Alothman, Z.A. Separation of toxic Pb2+ metal from aqueous solution using strongly acidic cation-exchange resin: Analytical applications for the removal of metal ions from pharmaceutical formulation. Desalin. Water Treat. 2013, 53, 2158–2166. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Naushad, M. Efficient photocatalytic degradation of toxic dyes using nanostructured TiO2/polyaniline nanocomposite. Desalin. Water Treat. 2018, 108, 322–328. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly (acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Kumar, A.; Al-Muhtaseb, A.A.H.; Dhiman, P.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots based nanocomposites. J. Clean. Prod. 2018, 172, 2919–2930. [Google Scholar] [CrossRef]
- Naushad, M.; Abdullah Alothman, Z.; Rabiul Awual, M.; Alfadul, S.M.; Ahamad, T. Adsorption of rose Bengal dye from aqueous solution by amberlite Ira-938 resin: Kinetics, isotherms, and thermodynamic studies. Desalin. Water Treat. 2015, 57, 13527–13533. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Duman, O.; Tunc, S.; Polat, T.G.; Bozoglan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-kappa-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef]
- Vutskits, L.; Briner, A.; Klauser, P.; Gascon, E.; Dayer, A.G.; Kiss, J.Z.; Muller, D.; Licker, M.J.; Morel, D.R. Adverse effects of methylene blue on the central nervous system. Anesthesiology 2008, 108, 684–692. [Google Scholar] [CrossRef]
- Bedin, K.C.; Souza, I.P.A.F.; Cazetta, A.L.; Spessato, L.; Ronix, A.; Almeida, V.C. CO2-spherical activated carbon as a new adsorbent for Methylene Blue removal: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq. 2018, 269, 132–139. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef]
- Benhouria, A.; Islam, M.A.; Zaghouane-Boudiaf, H.; Boutahala, M.; Hameed, B.H. Calcium alginate-bentonite-activated carbon composite beads as highly effective adsorbent for methylene blue. Chem. Eng. J. 2015, 270, 621–630. [Google Scholar] [CrossRef]
- Mukherjee, K.; Kedia, A.; Jagajjanani Rao, K.; Dhir, S.; Paria, S. Adsorption enhancement of methylene blue dye at kaolinite clay–water interface influenced by electrolyte solutions. RSC Adv. 2015, 5, 30654–30659. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Choi, H.-J.; Yu, S.-W. Biosorption of methylene blue from aqueous solution by agricultural bioadsorbent corncob. Environ. Eng. Res. 2018, 24, 99–106. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Z. Citrus pectin-derived carbon microspheres with superior adsorption ability for methylene blue. Nanomaterials (Basel) 2017, 7, 161. [Google Scholar] [CrossRef]
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef]
- Ulucan-Altuntas, K.; Kuzu, S.L. Modelling and optimization of dye removal by Fe/Cu bimetallic nanoparticles coated with different Cu ratios. Mater. Res. Express 2019, 6, 1150a4. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Paliychuk, N.; Babu Bitra, R.; Shyichuk, A.; Naushad, M.; Mironyuk, I.; Ziolkovska, D. Adsorptive removal of toxic Methylene Blue and Acid Orange 7 dyes from aqueous medium using cobalt-zinc ferrite nanoadsorbents. Desalin. Water Treat. 2019, 150, 374–385. [Google Scholar] [CrossRef]
- Tang, Y.; He, T.; Liu, Y.; Zhou, B.; Yang, R.; Zhu, L. Sorption behavior of methylene blue and rhodamine B mixed dyes onto chitosan graft poly (acrylic acid-co-2-acrylamide-2-methyl propane sulfonic acid) hydrogel. Adv. Polym. Technol. 2018, 37, 2568–2578. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave assisted synthesis of xanthan gum-cl-poly (acrylic acid) based-reduced graphene oxide hydrogel composite for adsorption of methylene blue and methyl violet from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Makhado, E.; Pandey, S.; Nomngongo, P.N.; Ramontja, J. Preparation and characterization of xanthan gum-cl-poly (acrylic acid)/o-MWCNTs hydrogel nanocomposite as highly effective re-usable adsorbent for removal of methylene blue from aqueous solutions. J. Colloid Interface Sci. 2018, 513, 700–714. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, A. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination 2011, 266, 33–39. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, M.; Wang, L.; Liu, X. The adsorption of methylene blue by an amphiphilic block co-poly (arylene ether nitrile) microsphere-based adsorbent: Kinetic, isotherm, thermodynamic and mechanistic studies. Nanomaterials (Basel) 2019, 9, 1356. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, T.; Hu, W.; He, X.; Xie, J.; Wang, P.; Jia, K.; Liu, X. Scalable fabrication of metallopolymeric superstructures for highly efficient removal of methylene blue. Nanomaterials (Basel) 2019, 9, 1. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Pathania, D.; Mittal, A.; El-desoky, G.E. Modification ofHibiscus cannabinusfiber by graft copolymerization: Application for dye removal. Desalin. Water Treat. 2014, 54, 3114–3121. [Google Scholar] [CrossRef]
- Macarie, L.; Ilia, G. Poly (vinylphosphonic acid) and its derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092. [Google Scholar] [CrossRef]
- Çelik, S.Ü.; Bozkurt, A. Novel inorganic proton-conducting graft copolymers based on 4-vinyl benzene boronic acid and vinyl phosphonic acid. Macromol. Chem. Phys. 2013, 214, 486–491. [Google Scholar] [CrossRef]
- Bozkurt, A. PAA/imidazol-based proton conducting polymer electrolytes. J. Power Sources 2003, 123, 126–131. [Google Scholar] [CrossRef]
- Bozkurt, A.; Meyer, W.H.; Gutmann, J.; Wegner, G. Proton conducting copolymers on the basis of vinylphosphonic acid and 4-vinylimidazole. Solid State Ion. 2003, 164, 169–176. [Google Scholar] [CrossRef]
- Celik, S.U.; Akbey, U.; Graf, R.; Bozkurt, A.; Spiess, H.W. Anhydrous proton-conducting properties of triazole-phosphonic acid copolymers: A combined study with MAS NMR. Phys. Chem. Chem. Phys. 2008, 10, 6058–6066. [Google Scholar] [CrossRef] [PubMed]
- Göktepe, F.; Çelik, S.Ü.; Bozkurt, A. Preparation and the proton conductivity of chitosan/poly(vinyl phosphonic acid) complex polymer electrolytes. J. Non Cryst. Solids 2008, 354, 3637–3642. [Google Scholar] [CrossRef]
- Hussain, T.; Ansari, M.; Ranjha, N.M.; Khan, I.U.; Shahzad, Y. Chemically cross-linked poly(acrylic-co-vinylsulfonic) acid hydrogel for the delivery of isosorbide mononitrate. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, K.; Tay, F.R.; Nishiyama, N.; Pashley, D.H.; Endo, T. Design of new phosphonic acid monomers for dental adhesives-synthesis of (Meth)acryloxyalkyl 3-phosphonopropionates and evaluation of their adhesion-promoting functions. Dent. Mater. J. 2006, 25, 566–575. [Google Scholar] [CrossRef]
- Zhao, R.; Rupper, P.; Gaan, S. Recent development in phosphonic acid-based organic coatings on aluminum. Coatings 2017, 7, 133. [Google Scholar] [CrossRef]
- Deka, J.R.; Liu, C.L.; Wang, T.H.; Chang, W.C.; Kao, H.M. Synthesis of highly phosphonic acid functionalized benzene-bridged periodic mesoporous organosilicas for use as efficient dye adsorbents. J. Hazard. Mater. 2014, 278, 539–550. [Google Scholar] [CrossRef]
- Sengel, S.B.; Sahiner, N. Poly (vinyl phosphonic acid) nanogels with tailored properties and their use for biomedical and environmental applications. Eur. Polym. J. 2016, 75, 264–275. [Google Scholar] [CrossRef]
- Nakhjiri, M.T.; Marandi, G.B.; Kurdtabar, M. Poly (AA-co-VPA) hydrogel cross-linked with N-maleyl chitosan as dye adsorbent: Isotherms, kinetics and thermodynamic investigation. Int. J. Biol. Macromol. 2018, 117, 152–166. [Google Scholar] [CrossRef]
- Herrera-Gonzalez, A.M.; Caldera-Villalobos, M.; Pelaez-Cid, A.A. Adsorption of textile dyes using an activated carbon and crosslinked polyvinyl phosphonic acid composite. J. Environ. Manag. 2019, 234, 237–244. [Google Scholar] [CrossRef]
- Melo, B.C.; Paulino, F.A.A.; Cardoso, V.A.; Pereira, A.G.B.; Fajardo, A.R.; Rodrigues, F.H.A. Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly(acrylic acid) hydrogel. Carbohydr. Polym. 2018, 181, 358–367. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. BioChem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Low, M.J.D. Kinetics of chemisorption of gases on solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Kapillarchemie, Eine Darstellung der Chemie der Kolloide und Verwandter Gebiete; Akademische Verlagsgesellschaft: Leipzig, Germany, 1909. [Google Scholar]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Van’t Hoff, J.H. Studies in Chemical Dynamics; Kessinger Publishing, LLC: Whitefish, MT, USA, 1896. [Google Scholar]
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H. Removal of methylene blue by mesoporous CMK-3: Kinetics, isotherms and thermodynamics. J. Mol. Liq. 2016, 223, 763–770. [Google Scholar] [CrossRef]
- Sinirlioglu, D.; Çelik, S.Ü.; Muftuoglu, A.E.; Bozkurt, A. Novel composite polymer electrolyte membranes based on poly (vinyl phosphonic acid) and poly (5-(methacrylamido)tetrazole). Polym. Eng. Sci. 2015, 55, 260–269. [Google Scholar] [CrossRef]
- Vilela, C.; Martins, A.; Sousa, N.; Silvestre, A.; Figueiredo, F.; Freire, C. Poly (bis [2-(methacryloyloxy) ethyl] phosphate)/bacterial cellulose nanocomposites: Preparation, characterization and application as polymer electrolyte membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, Q.; Zhang, W.; Zhang, Y.; Zhao, M. Comparative adsorption of methylene blue by magnetic baker’s yeast and EDTAD-modified magnetic baker’s yeast: Equilibrium and kinetic study. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Feng, D.; Bai, B.; Wang, H.; Suo, Y. Novel fabrication of PAA/PVA/yeast superabsorbent with interpenetrating polymer network for pH-dependent selective adsorption of dyes. J. Polym. Environ. 2017, 26, 567–588. [Google Scholar] [CrossRef]
- Sumalinog, D.A.G.; Capareda, S.C.; De Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef]
- Wang, Y.; Lopez-Valdivieso, A.; Zhang, T.; Mwamulima, T.; Zhang, X.; Song, S.; Peng, C. Preparation of microscale zero-valent iron-fly ash-bentonite composite and evaluation of its adsorption performance of crystal violet and methylene blue dyes. Environ. Sci. Pollut. Res. Int. 2017, 24, 20050–20062. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Wang, A. Enhanced adsorption of Methylene Blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J. Environ. Sci. 2010, 22, 486–493. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Mittal, H.; Mishra, S.B.; Mishra, A.K. Gum ghatti and acrylic acid based biodegradable hydrogels for the effective adsorption of cationic dyes. J. Ind. Eng. Chem. 2015, 22, 171–178. [Google Scholar] [CrossRef]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Kazeem, T.S.; Zubair, M.; Al-Harthi, M. Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of Eriochrome Black T. Appl. Clay Sci. 2018, 161, 23–34. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Zubair, M.; Jarrah, N.; Ihsanullah; Khalid, A.; Manzar, M.S.; Kazeem, T.S.; Al-Harthi, M.A. Starch-NiFe-layered double hydroxide composites: Efficient removal of methyl orange from aqueous phase. J. Mol. Liq. 2018, 249, 254–264. [Google Scholar] [CrossRef]
- Ma, T.; Chang, P.R.; Zheng, P.; Zhao, F.; Ma, X. Fabrication of ultra-light graphene-based gels and their adsorption of methylene blue. Chem. Eng. J. 2014, 240, 595–600. [Google Scholar] [CrossRef]
- Naushad, M.; Alqadami, A.A.; AlOthman, Z.A.; Alsohaimi, I.H.; Algamdi, M.S.; Aldawsari, A.M. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 2019, 293. [Google Scholar] [CrossRef]
- Naushad, M.; Ali Khan, M.; Abdullah Alothman, Z.; Rizwan Khan, M.; Kumar, M. Adsorption of methylene blue on chemically modified pine nut shells in single and binary systems: Isotherms, kinetics, and thermodynamic studies. Desalin. Water Treat. 2015, 57, 15848–15861. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Shao, Z.J.; Huang, X.L.; Yang, F.; Zhao, W.F.; Zhou, X.Z.; Zhao, C.S. Engineering sodium alginate-based cross-linked beads with high removal ability of toxic metal ions and cationic dyes. Carbohydr. Polym. 2018, 187, 85–93. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]

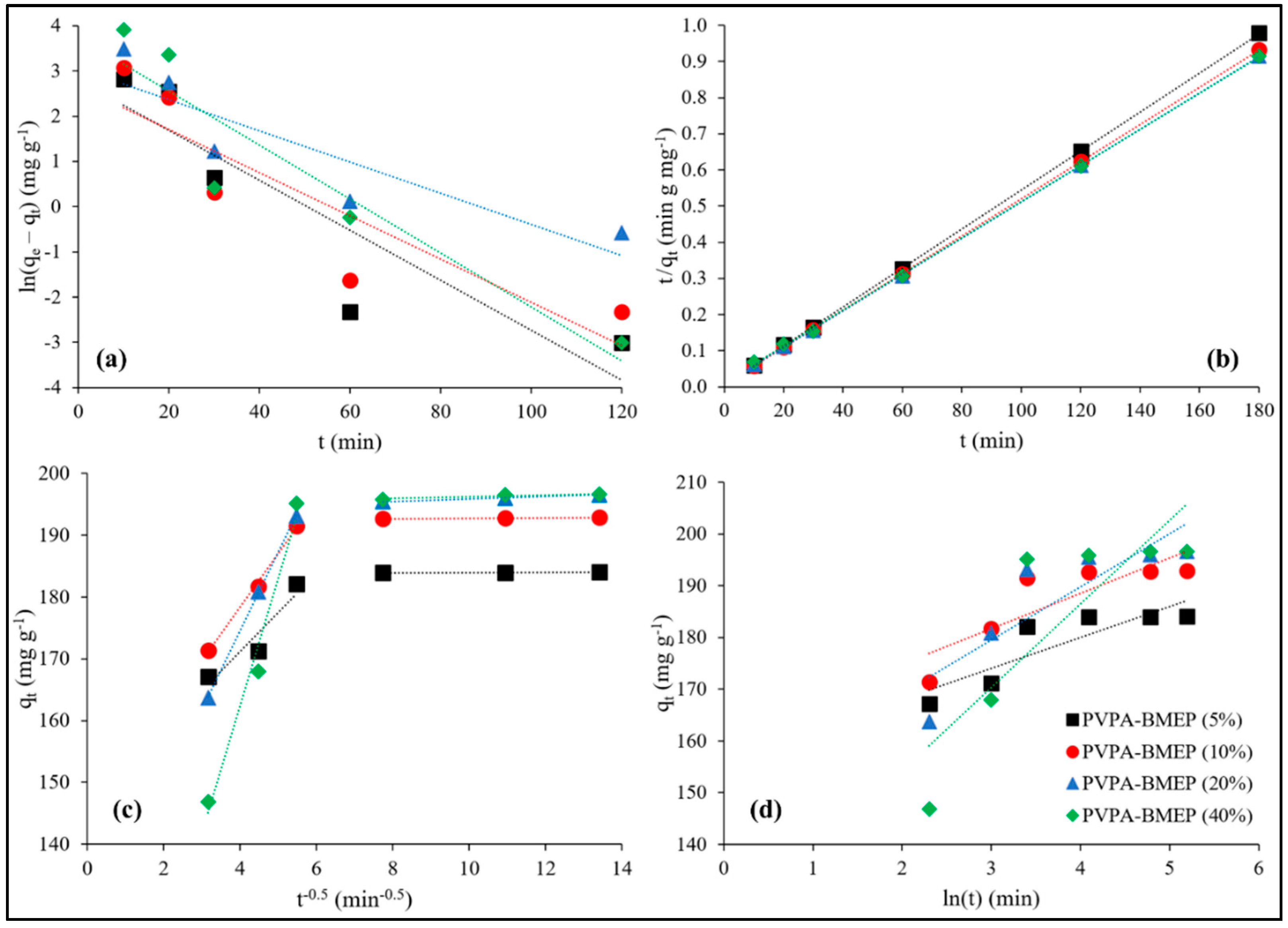
| Kinetic Model | Linear Form | Equation No | Plot |
|---|---|---|---|
| Pseudo first-order | (a) | ln(qe − qt) vs. t | |
| Pseudo second-order | (b) | ||
| Intraparticle diffusion | (c) | qt vs. t0.5 | |
| Elovich | (d) | qt vs. ln(t) |
| Adsorbent | C0 | qe,exp | Pseudo First-Order | Pseudo Second-Order | Intraparticle Diffusion | Elovich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe,cal | k1 | R2 | qe,cal | k2 × 10−3 | R2 | kd | C | R2 | α | β × 10−3 | R2 | |||
| PVPA-BMEP (5%) | 100 | 184 | 16.3 | 0.055 | 0.821 | 185 | 5.72 | 0.999 | 1.48 | 168 | 0.607 | 1.39 × 1012 | 168 | 0.758 |
| 500 | 899 | 101 | 0.043 | 0.852 | 909 | 0.877 | 0.999 | 8.90 | 799 | 0.667 | 2.73 × 1010 | 28.0 | 0.822 | |
| 1500 | 2562 | 1407 | 0.059 | 0.990 | 2632 | 0.102 | 0.999 | 56.2 | 1915 | 0.804 | 2.27 × 105 | 4.59 | 0.924 | |
| PVPA-BMEP (10%) | 100 | 193 | 14.4 | 0.048 | 0.793 | 192 | 5.63 | 0.999 | 1.66 | 175 | 0.552 | 1.10 × 1011 | 146 | 0.723 |
| 500 | 934 | 118 | 0.045 | 0.853 | 943 | 0.764 | 0.999 | 10.1 | 820 | 0.675 | 3.65 × 109 | 24.7 | 0.830 | |
| 1500 | 2673 | 792 | 0.052 | 0.844 | 2703 | 0.114 | 0.999 | 56.1 | 2036 | 0.751 | 3.66 × 105 | 4.56 | 0.878 | |
| PVPA-BMEP (20%) | 100 | 197 | 21.3 | 0.035 | 0.797 | 200 | 3.29 | 0.999 | 2.49 | 169 | 0.570 | 2.04 × 107 | 97.5 | 0.741 |
| 500 | 958 | 252 | 0.053 | 0.882 | 980 | 0.325 | 0.999 | 23.4 | 702 | 0.523 | 1.75 × 104 | 10.2 | 0.699 | |
| 1500 | 2766 | 2405 | 0.058 | 0.971 | 2941 | 0.034 | 0.998 | 131 | 1285 | 0.724 | 9.24 × 102 | 1.92 | 0.871 | |
| PVPA-BMEP (40%) | 100 | 197 | 42.4 | 0.060 | 0.878 | 200 | 2.03 | 0.999 | 3.90 | 154 | 0.543 | 3.10 × 104 | 62.0 | 0.711 |
| 500 | 966 | 282 | 0.058 | 0.750 | 1010 | 0.162 | 0.997 | 36.4 | 568 | 0.529 | 8.83 × 102 | 6.60 | 0.702 | |
| 1500 | 2805 | 2753 | 0.063 | 0.869 | 3226 | 0.016 | 0.982 | 177 | 827 | 0.688 | 3.35 × 102 | 1.41 | 0.847 | |
| Adsorbent | Langmuir | Freundlich | Redlich-Peterson | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm | kL × 10−3 | Δqe | R2 | kF | 1/n | Δqe | R2 | krp | αrp × 10−3 | β | Δqe | R2 | |
| PVPA-BMEP (5%) | 2593 | 5.24 | 2.66 | 0.998 | 40.4 | 0.769 | 9.66 | 0.992 | 22.7 | 5.91 | 0.966 | 2.33 | 0.999 |
| PVPA-BMEP (10%) | 2724 | 11.1 | 3.15 | 0.997 | 76.0 | 0.721 | 11.3 | 0.989 | 45.2 | 10.3 | 0.980 | 3.31 | 0.992 |
| PVPA-BMEP (20%) | 2787 | 16.0 | 1.85 | 0.999 | 98.0 | 0.716 | 15.2 | 0.981 | 65.6 | 24.5 | 0.906 | 2.50 | 0.997 |
| PVPA-BMEP (40%) | 2841 | 19.2 | 2.45 | 0.998 | 116 | 0.710 | 12.3 | 0.987 | 85.9 | 35.4 | 0.888 | 0.32 | 0.999 |
| Adsorbent | T (K) | ΔG° (kJ moL−1) | ΔH° (kJ mL−1) | ΔS° (J mL−1 K−1) |
|---|---|---|---|---|
| PVPA-BMEP (5%) | 299 | −7.42 | −46.9 | −132 |
| 308 | −6.23 | |||
| 318 | −4.91 | |||
| 328 | −3.59 | |||
| PVPA-BMEP (10%) | 299 | −9.12 | −41.6 | −109 |
| 308 | −8.14 | |||
| 318 | −7.06 | |||
| 328 | −5.97 | |||
| PVPA-BMEP (20%) | 299 | −9.94 | −40.3 | −102 |
| 308 | −9.03 | |||
| 318 | −8.01 | |||
| 328 | −6.99 | |||
| PVPA-BMEP (40%) | 299 | −10.5 | −39.6 | −97.3 |
| 308 | −9.67 | |||
| 318 | −8.69 | |||
| 328 | −7.72 |
| Adsorbent | qm (mg g−1) | C0 (mg L−1) | te (min) | Reference |
|---|---|---|---|---|
| PVPA nanogels | 14 | 30 | 5 | [49] |
| Brown macroalga (N. zanardinii) | 35 | 200 | 120 | [80] |
| Activated lignin-chitosan extruded blends | 36 | 80 | 180 | [17] |
| Poly(acrylic acid-co-VPA) hydrogel cross-linked with N-maleyl chitosan | 67 | 50 | 100 | [50] |
| Chemically modified pine nut shells in single and binary systems | 137 | 200 | 90 | [79] |
| Arginine modified activated carbon | 220 | 250 | 120 | [78] |
| PVPA-co-triethyleneglycol dimethacrylate | 417 | 2100 | 360 | [51] |
| Phosphonic acid functionalized benzene-bridged periodic mesoporous organosilicas | 524 | 600 | 320 | [48] |
| Chitosan-g-poly(acrylic acid) hydrogels improved with cellulose nanowhiskers | 2074 | 2000 | 60 | [52] |
| Sodium alginate-based organic/inorganic superabsorbent composite hydrogel | 2257 | 600 | 400 | [71] |
| Sodium alginate-based cross-linked beads | 2977 | 160 | 1500 | [81] |
| PVPA-BMEP (5%) | 2593 | 1500 | 60 | This study |
| PVPA-BMEP (10%) | 2724 | |||
| PVPA-BMEP (20%) | 2787 | |||
| PVPA-BMEP (40%) | 2841 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anil, I.; Gunday, S.T.; Bozkurt, A.; Alagha, O. Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal. Nanomaterials 2020, 10, 131. https://doi.org/10.3390/nano10010131
Anil I, Gunday ST, Bozkurt A, Alagha O. Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal. Nanomaterials. 2020; 10(1):131. https://doi.org/10.3390/nano10010131
Chicago/Turabian StyleAnil, Ismail, Seyda Tugba Gunday, Ayhan Bozkurt, and Omar Alagha. 2020. "Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal" Nanomaterials 10, no. 1: 131. https://doi.org/10.3390/nano10010131
APA StyleAnil, I., Gunday, S. T., Bozkurt, A., & Alagha, O. (2020). Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal. Nanomaterials, 10(1), 131. https://doi.org/10.3390/nano10010131







