Metallic Nanoparticle Block Copoloymer Vesicles with Enhanced Optical Properties
Abstract
:1. Introduction
1.1. Block-Copolymer Vesicles
1.2. Contrast Agents and Metallic Nanoparticles
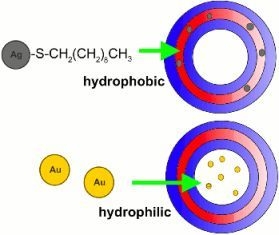
1.3. Polymerosme-Metallic Nanoparticle Constructions
2. Experimental Section
2.1. Vesicle Formation
2.2. Particle Encapsulation
2.3. Electron Microscopy Imaging
2.4. Transmission Spectrophotometry
2.5. Scattering Calculations
3. Results and Discussion
3.1. Transmission Electron Microscopy
3.2. Transmission Spectrophotometry
3.3. Optical Densities and Attenuation Coefficient
4. Conclusions






| Area per molecule | Volume per molecule | Membrane thickness |
| am = 1.130 nm2 | υm = 2.712 nm3 | t = 2.4 nm |
| Molecular weight | Avogadro's number | Polymer concentration |
| MW = 2300 | NA = 6.022 × 1023 mol−1 | C = 10 gL−1 |
Acknowledgments
References
- Discher, D.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar]
- Battaglia, G.; Ryan, A. Pathways of polymeric vesicle formation. J. Phys. Chem. B 2006, 110, 10272–10279. [Google Scholar]
- Battaglia, G.; Ryan, A. Neuron-like tubular membranes made of diblock copolymer amphiphiles. Angew. Chem. Int. Ed. 2006, 45, 2052–2056. [Google Scholar]
- Smart, T. Block copolymer nanostructures. Nanotoday 2008, 3, 38–46. [Google Scholar]
- Battaglia, G.; Ryan, A. The evolution of vesicles from bulk lamellar gels. Nat. Mater. 2005, 4, 869–876. [Google Scholar]
- Battaglia, G.; Ryan, A. Effect of amphiphile size on the transformation from a lyotropic gel to a vesicular dispersion. Macromolecules 2006, 39, 798–805. [Google Scholar]
- Battaglia, G.; Ryan, A. Bilayers and interdigitation in block copolymer vesicles. J. Am. Chem. Soc. 2005, 127, 8757–8764. [Google Scholar]
- Lomas, H. Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discuss. 2008, 139, 1–18. [Google Scholar]
- Photos, P.J.; Bacakovaa, L.; Dischera, B.; Batesb, F.S.; Discher, D.E. Polymer vesicles in vivo: Correlations with PEG molecular weight. J. Control. Release 2003, 90, 323–334. [Google Scholar]
- Hearnden, V. Diffusion studies of nanometer polymersomes across tissue engineered human oral mucosa. Pharm. Res. 2009, 26, 1718–1728. [Google Scholar]
- Discher, D. Emerging applications of polymersomes in delivery: From molecular dynamics to shrinkage of tumors. Prog. Polym. Sci. 2007, 32, 838–857. [Google Scholar]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomedicine 2005, 22–30. [Google Scholar]
- Jain, P.; Lee, K.; El-Sayed, I. Calculated absorption and scattering properties of gold nanoparticles of different size, shape and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. 2006, 110, 7238–7248. [Google Scholar]
- El-Sayed, I.; Huang, X.; El-Sayed, M. Surface plasmon resonance scattering and adsorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar]
- Rosi, N.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar]
- Ghoroghchian, P. Near-infrared-emissive polymerosomes: Self-assembled soft matter for in vivo optical imaging. Proc. Nat. Acad. Sci. USA 2005, 102, 2922–2927. [Google Scholar]
- Lee, T. Engineered microsphere contrast agents for optical coherence tomography. Optic. Lett. 2003, 28, 1546–1548. [Google Scholar]
- Izatt, J.; Kulkarni, M. Optical coherence tomography and microscopy in gastrointestinal tissues. IEEE J. Sel. Top. Quantum Electr. 1996, 2, 1017–1028. [Google Scholar]
- Masato, O.; Masamitsu, H. Ultra-high resolution optical coherence tomography (OCT) using a halogen lamp as the light source. Opt. Rev. 2003, 10, 478–481. [Google Scholar]
- Boppart, J.; Hoying, J.; Sullivan, C. Optical probes and techniques for molecular contrast agents for spectroscopic optical coherence tomography. Opt. Lett. 2005, 30, 3048–3050. [Google Scholar]
- Yu, M.; Wang, H.; Zhou, X. One template synthesis of raspberry-like hierarchical siliceous hollow spheres. J. Am. Chem. Soc. 2007, 129, 14576–14577. [Google Scholar]
- Wang, R. Self-assembled gold nanoshells on biodegradable chitosan fibers. Biomacromolecules 2006, 7, 2719–2721. [Google Scholar]
- Li, Y. In Situ formation of Gold-“Decorated” vesicles from a RAFT-synthesized, thermally responsive block copolymer. Macromolecules 2007, 40, 8524–8526. [Google Scholar]
- Troutman, T.; Barton, J.; Romanowski, M. Biodegradable plasmon resonant nanoshells. Adv. Mater. 2008, 20, 2604–2608. [Google Scholar]
- Yuan, J.J. Facile synthesis of highly biocompatible poly(2-(methacryloyloxy)ethyl phosphorylchline)-coated gold nanoparticles in aqueous solution. Langmuir 2006, 22, 11022–11027. [Google Scholar]
- Booth, C.; Yu, G.; Nace, V. Self-assembly in simple and complex systems. In Amphiphilic Block Copolymers; Lindman, B., Alexandridis, P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.; Takeshita, K.; Subbarao, N.K.; Hu, L.R. Small-volume extrusion apparatus for preparation of large unilamellar vesicles. Biochim. Biophys. Acta 1991, 1061, 297–303. [Google Scholar]
- Lomas, H. Biomimetic pH sensitive polymersomes for efficient DNA encapsulation and delivery. Adv. Mater. 2007, 19, 4238–4243. [Google Scholar]
- Prahl, S. Mie Scattering Calculator; Oregon Medical Laser Center: Portland, OR, USA, 2007. Avalable online: http://omlc.ogi.edu/calc/mie_calc.html (accessed on 3 May 2011).
- Malynych, S.; Chumanov, G. Coupled planar silver nanoparticle arrays as refractive index sensors. J. Opt. A-Pure Appl. Opt. 2006, 8, S144–S147. [Google Scholar]
- Johnson, P.; Christy, R. Optical constants of the nobel metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar]
- Scaffardi, L.; Tocho, J. Size dependence of refractive index of gold nanoparticles. Nanotechnology 2006, 17, 1309–1315. [Google Scholar]
- Curry, A.; Nusz, G.; Chilkoti, A.; Wax, A. Substrate effect on refractive index dependence of plasmon resonance for individual silver nanoparticles observed using darkfield micro-spectroscopy. Opt. Express 2005, 13, 2668–2677. [Google Scholar]
- Chen, J.T.; Thomas, E.L. The use of force modulation microscopy to investigate block copolymer morphology. J. Mater. Sci. 1996, 31, 2531–2538. [Google Scholar]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonance. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar]
- Agrawal, A.; Pfefer, T.J.; Huang, S.; Lin, A.W.H.; Lee, M.-H.; Drezek, R.A.; Barton, J.K. Quantitative evaluation of optical coherence tomography signal enhancement with gold nanoshells. J. Biomed. Opt. 2006, 11, 041121. [Google Scholar]
- Lu, Q.; Gan, X.; Gu, M.; Luo, Q. Monte carlo modeling of optical coherence tomography imaging through turbid media. Appl. Opt. 2004, 44, 1628–1637. [Google Scholar]
- Faber, D.; van der Meer, F.; Aalders, M.; van Leeuwen, T. Quantitative measurement of attenuation coefficients of weakly scattering media using optical coherence tomography. Opt. Express 2004, 12, 4353–4365. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/.)
Share and Cite
Martinez-Hurtado, J.L. Metallic Nanoparticle Block Copoloymer Vesicles with Enhanced Optical Properties. Nanomaterials 2011, 1, 20-30. https://doi.org/10.3390/nano1010020
Martinez-Hurtado JL. Metallic Nanoparticle Block Copoloymer Vesicles with Enhanced Optical Properties. Nanomaterials. 2011; 1(1):20-30. https://doi.org/10.3390/nano1010020
Chicago/Turabian StyleMartinez-Hurtado, Juan Leonardo. 2011. "Metallic Nanoparticle Block Copoloymer Vesicles with Enhanced Optical Properties" Nanomaterials 1, no. 1: 20-30. https://doi.org/10.3390/nano1010020
APA StyleMartinez-Hurtado, J. L. (2011). Metallic Nanoparticle Block Copoloymer Vesicles with Enhanced Optical Properties. Nanomaterials, 1(1), 20-30. https://doi.org/10.3390/nano1010020