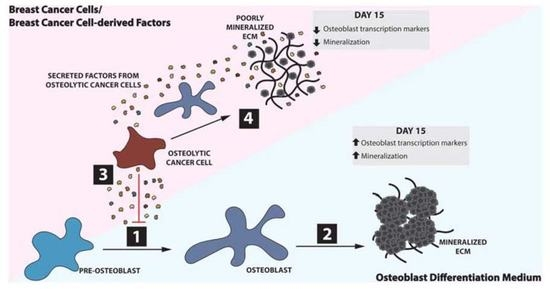
A Three-Dimensional Dense Collagen Hydrogel to Model Cancer Cell/Osteoblast Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
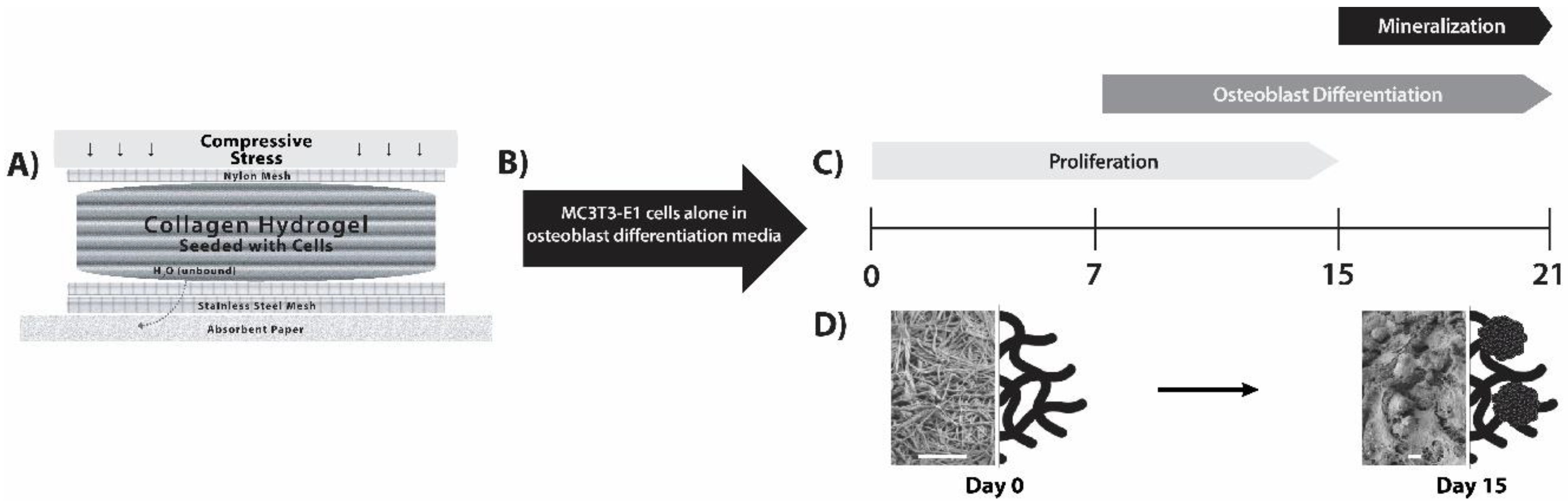
2.2. Construct Preparation and Cellularization
2.3. Assessment of Cell-Seeded Constructs with Time in Culture
2.3.1. Confocal Laser Scanning Microscopy (CLSM)
2.3.2. Scanning Electron Microscopy (SEM)
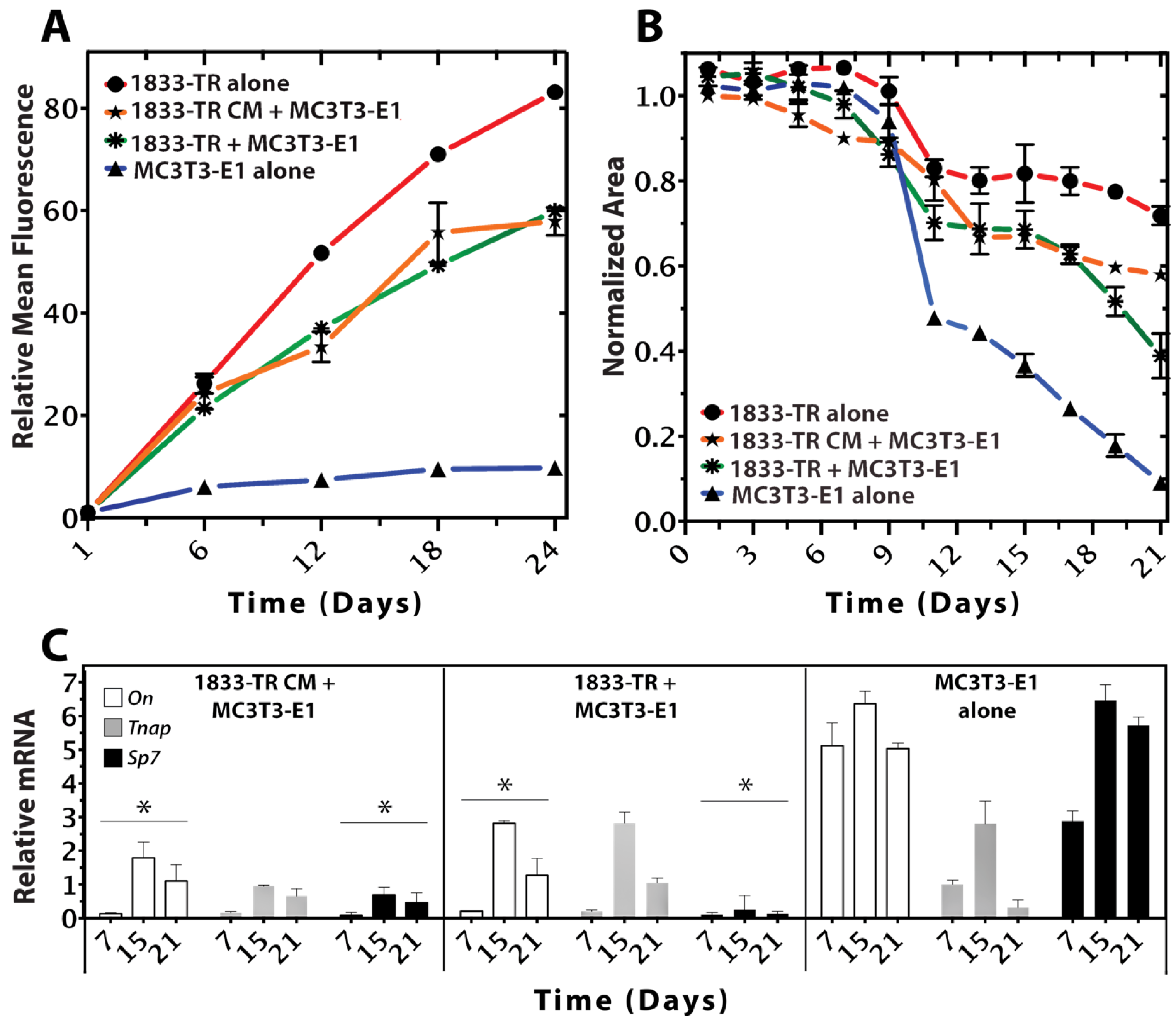
2.3.3. Resorufin Quantification Assay
2.3.4. Cell-Mediated Gel Contractility Assay
2.3.5. Quantitative RT-PCR (qPCR)
2.4. Analysis of Construct Mineralization
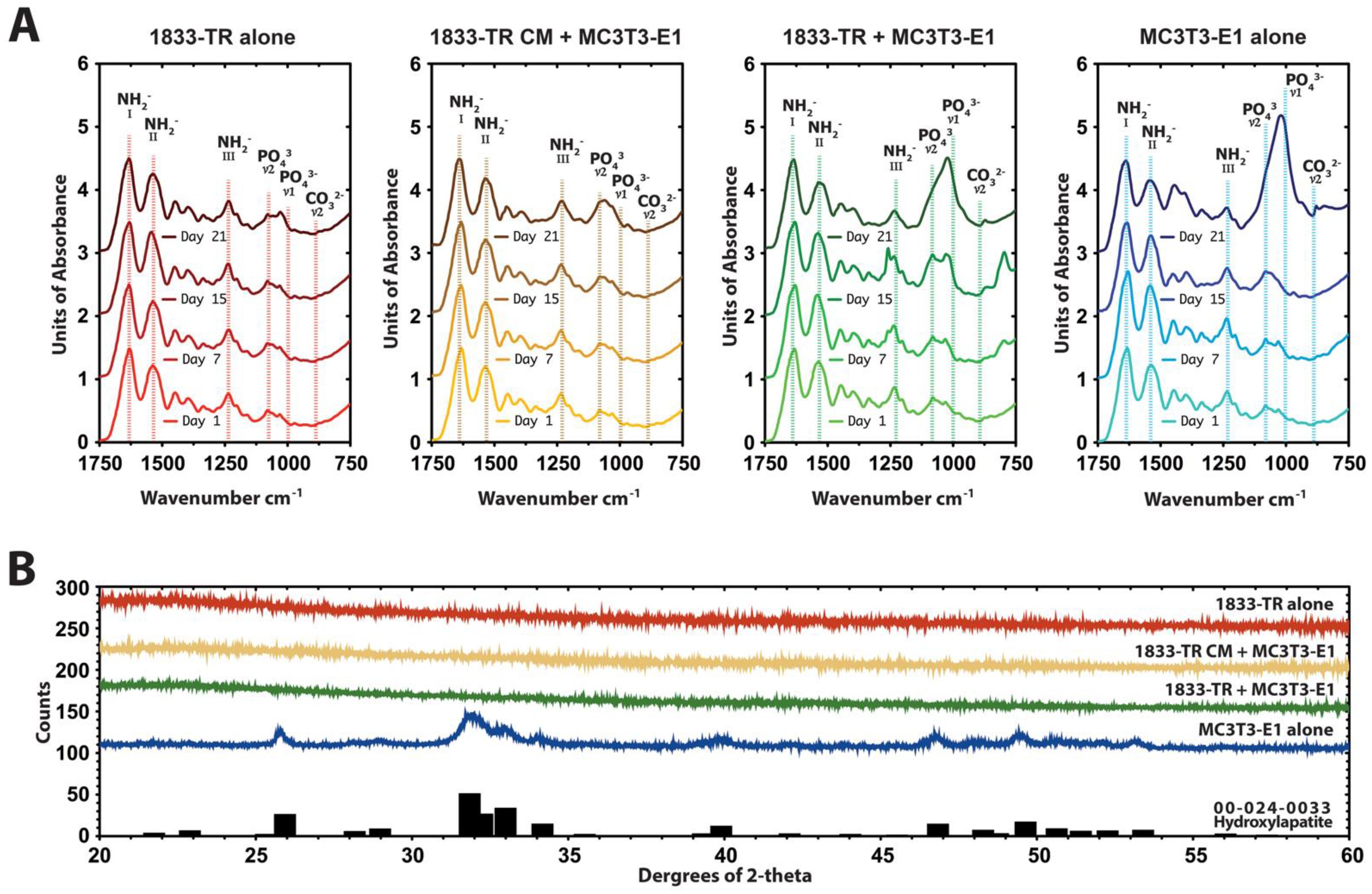
2.4.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) and Microscopy
2.4.2. X-Ray Diffraction (XRD)
2.4.3. Construct Reaction with Silver Nitrate
2.5. Statistical Analysis
3. Results
3.1. Matrix Mineralization by MC3T3-E1 Pre-Osteoblast Cells
3.2. Construct Dynamism and Molecular Biology
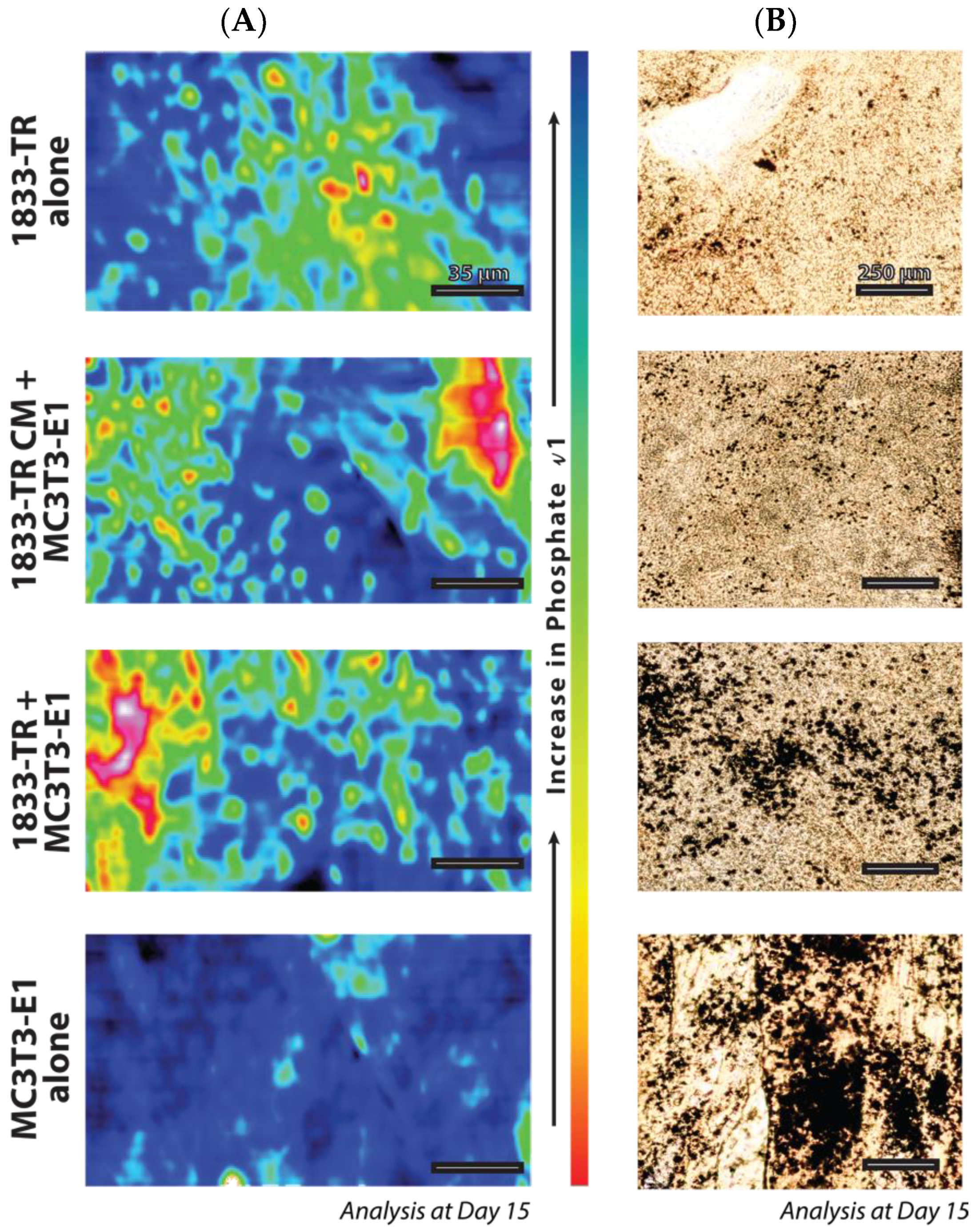
3.3. Construct Mineralization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Tolle, S.W.; Back, A.L.; Meier, D.E. End-of-life advance directive. N. Engl. J. Med. 2015, 372, 667–670. [Google Scholar]
- Hortobagyi, G.N.; Theriault, R.L.; Porter, L.; Blayney, D.; Lipton, A.; Sinoff, C.; Wheeler, H.; Simeone, J.F.; Seaman, J.; Knight, R.D.; et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N. Engl. J. Med. 1996, 335, 1785–1791. [Google Scholar] [CrossRef]
- Koch, L. Cancer: Bisphosphonate use and breast cancer. Nat. Rev. Endocrinol. 2010, 6, 532. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. 3D scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Jong, B.K.; Stein, R.; O’Hare, M.J. Three-dimensional in vitro tissue culture models of breast cancer—A review. Breast Cancer Res. Treat. 2004, 85, 281–291. [Google Scholar]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Wiseman, M.; Chuo, C.-B.; Cheema, U.; Nazhat, S.N. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Bitar, M.; Brown, R.A.; Salih, V.; Kidane, A.G.; Knowles, J.C.; Nazhat, S.N. Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules 2008, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.; Salih, V.; Brown, R.A.; Nazhat, S.N. Effect of multiple unconfined compression on cellular dense collagen scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 237–244. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Barralet, J.E.; Nazhat, S.N. Collagen gel fibrillar density dictates the extent of mineralization in vitro. Soft Matter 2011, 7, 9898–9907. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; James-Bhasin, M.; Nazhat, S.N. Fabrication of injectable, cellular, anisotropic collagen tissue equivalents with modular fibrillar densities. Biomaterials 2015, 37, 183–193. [Google Scholar] [CrossRef]
- Pedraza, C.E.; Marelli, B.; Chicatun, F.; McKee, M.D.; Nazhat, S.N. An in vitro assessment of a cell-containing collagenous extracellular matrix-like scaffold for bone tissue engineering. Tissue Eng. Part A 2010, 16, 781–793. [Google Scholar] [CrossRef]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Characterization and evaluation of chitosan matrix for in vitro growth of MCF-7 breast cancer cell lines. Biomaterials 2004, 25, 5147–5154. [Google Scholar] [CrossRef]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials 2005, 26, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Dhurjati, R.; Liu, X.; Gay, C.V.; Mastro, A.M.; Vogler, E.A. Extended-term culture of bone cells in a compartmentalized bioreactor. Tissue Eng. 2006, 12, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, C.; Kong, H.J.; Hsiong, S.X.; Evangelista, M.B.; Yuen, W.; Mooney, D.J. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc. Natl. Acad. Sci. USA 2009, 106, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Pathi, S.P.; Lin, D.D.W.; Dorvee, J.R.; Estroff, L.A.; Fischbach, C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 2011, 32, 5112–5122. [Google Scholar] [CrossRef] [PubMed]
- Kamranpour, N.O.; Miri, A.K.; James-Bhasin, M.; Nazhat, S.N. A gel aspiration-ejection system for the controlled production and delivery of injectable dense collagen scaffolds. Biofabrication 2016, 8, 015018. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef]
- Li, E.; Zhang, J.; Yuan, T.; Ma, B. MiR-143 suppresses osteogenic differentiation by targeting Osterix. Mol. Cell. Biochem. 2014, 390, 69–74. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Sudo, H.; Kodama, H.A.; Amagai, Y.; Yamamoto, S.; Kasai, S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 1983, 96, 191–198. [Google Scholar] [CrossRef]
- Wang, D.; Christensen, K.; Chawla, K.; Xiao, G.; Krebsbach, P.H.; Franceschi, R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999, 14, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Zhou, H.Y.; Yamada, H.; Kawamura, M.; Hirano, H.; Kuboki, Y. An osteonectin-like protein in the matrix of cultured osteogenic cell-line MC3T3-E1, which is associated with calcification. Calcif. Tissue Int. 1992, 51, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R.; et al. Engineered in vitro disease models. Ann. Rev. Pathol. Mech. Dis. 2015, 10, 195–262. [Google Scholar] [CrossRef] [PubMed]
- Bouet, G.; Marchat, D.; Cruel, M.; Malaval, L.; Vico, L. In vitro three-dimensional bone tissue models: From cells to controlled and dynamic environment. Tissue Eng. Part B Rev. 2015, 21, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.S.; Martinez, M.; Yang, J.; Mikos, A.G.; Navone, N.M.; Harrington, D.A.; Farach-Carson, M.C. Hydrogel-based 3D model of patient-derived prostate xenograft tumors suitable for drug screening. Mol. Pharm. 2014, 11, 2040–2050. [Google Scholar] [CrossRef]
- Fong, E.L.S.; Wan, X.; Yang, J.; Morgado, M.; Mikos, A.G.; Harrington, D.A.; Navone, N.M.; Farach-Carson, M.C. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 2016, 77, 164–172. [Google Scholar] [CrossRef]
- Rong, Z.-J.; Yang, L.-J.; Cai, B.-T.; Zhu, L.-X.; Cao, Y.-L.; Wu, G.-F.; Zhang, Z.-J. Porous nano-hydroxyapatite/collagen scaffold containing drug-loaded ADM–PLGA microspheres for bone cancer treatment. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef]
- Seib, F.P.; Berry, J.E.; Shiozawa, Y.; Taichman, R.S.; Kaplan, D.L. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 2015, 51, 313–319. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zeng, D.F.; Kong, P.Y.; Ma, Y.Y.; Zhang, X. AMD3100 and G-CSF disrupt the cross-talk between leukemia cells and the endosteal niche and enhance their sensitivity to chemotherapeutic drugs in biomimetic polystyrene scaffolds. Blood Cells Mol. Dis. 2016, 59, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Parajuli, H.; Sun, Y.; Johannessen, A.C.; Finne-Wistrand, A.; McCormack, E.; Mustafa, K.; Costea, D.E. Establishment of a bioluminescence model for microenvironmentally induced oral carcinogenesis with implications for screening bioengineered scaffolds. Head Neck 2016, 38, E1177–E1187. [Google Scholar] [CrossRef] [PubMed]
- Villasante, A.; Vunjak-Novakovic, G. Bioengineered tumors. Bioengineered 2015, 6, 73–76. [Google Scholar] [CrossRef]
- Cox, R.F.; Jenkinson, A.; Pohl, K.; O’Brien, F.J.; Morgan, M.P. Osteomimicry of mammary adenocarcinoma cells in vitro; increased expression of bone matrix proteins and proliferation within a 3D collagen environment. PLoS ONE 2012, 7, e41679. [Google Scholar] [CrossRef]
- Hsiao, A.Y.; Torisawa, Y.; Tung, Y.-C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 2009, 30, 3020–3027. [Google Scholar] [CrossRef]
- Rajagopalan, P.; Kasif, S.; Murali, T.M. Systems biology characterization of engineered tissues. Ann. Rev. Biomed. Eng. 2013, 15, 55–70. [Google Scholar] [CrossRef]
- Sieh, S.; Taubenberger, A.V.; Lehman, M.L.; Clements, J.A.; Nelson, C.C.; Hutmacher, D.W. Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone 2014, 63, 121–131. [Google Scholar] [CrossRef]
- Villasante, A.; Marturano-Kruik, A.; Vunjak-Novakovic, G. Bioengineered human tumor within a bone niche. Biomaterials 2014, 35, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Szot, C.S.; Buchanan, C.F.; Freeman, J.W.; Rylander, M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011, 32, 7905–7912. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Cheema, U.; Knowles, J.C.; Brown, R.A.; Nazhat, S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter 2006, 2, 986–992. [Google Scholar] [CrossRef]
- Luo, A.; Wu, F.; Han, R.; Huang, S.; Zhang, Y.; Jing, X.; Zhao, X. Clinicopathological features and prognostic evaluation of bone metastasis in triple-negative breast cancer. J. Cancer Res. Ther. 2017, 13, 778–784. [Google Scholar] [PubMed]
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996. [Google Scholar] [CrossRef] [PubMed]
- Bollen, L.; Wibmer, C.; Wang, M.; van der Linden, Y.M.; Leithner, A.; Bünger, C.E.; Jensen, A.B.; Fiocco, M.; Bratschitsch, G.; Pondaag, W.; et al. Molecular phenotype is associated with survival in breast cancer patients with spinal bone metastases. Clin. Exp. Metastasis 2015, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Kang, Y.; Serganova, I.; Gupta, G.P.; Giri, D.D.; Doubrovin, M.; Ponomarev, V.; Gerald, W.L.; Blasberg, R.; Massagué, J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Investig. 2005, 115, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; He, W.; Tulley, S.; Gupta, G.P.; Serganova, I.; Chen, C.R.; Manova-Todorova, K.; Blasberg, R.; Gerald, W.L.; Massagué, J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 13909–13914. [Google Scholar] [CrossRef] [PubMed]
- Serganova, I.; Moroz, E.; Vider, J.; Gogiberidze, G.; Moroz, M.; Pillarsetty, N.; Doubrovin, M.; Minn, A.; Thaler, H.T.; Massague, J.; et al. Multimodality imaging of TGFbeta signaling in breast cancer metastases. FASEB J. 2009, 23, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.-F.; Wang, Q.; Gerald, W.; Hudis, C.A.; Norton, L.; Smid, M.; Foekens, J.A.; Massagué, J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009, 16, 67–78. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Q.; Hu, G.; Van Poznak, C.; Fleisher, M.; Reiss, M.; Massagué, J.; Kang, Y. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009, 23, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mu, E.; Wei, Y.; Riethdorf, S.; Yang, Q.; Yuan, M.; Yan, J.; Hua, Y.; Tiede, B.J.; Lu, X.; et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 2011, 20, 701–714. [Google Scholar] [CrossRef]
- Zhang, X.H.-F.; Jin, X.; Malladi, S.; Zou, Y.; Wen, Y.H.; Brogi, E.; Smid, M.; Foekens, J.A.; Massagué, J. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 2013, 154, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Villanueva, D.; Zeef, L.; Shore, P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFβ-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011, 13, R106. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.R.; Miyasaka, C.; Mastro, A.M. Metastatic breast cancer cells suppress osteoblast adhesion and differentiation. Clin. Exp. Metastasis 2004, 21, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Dhurjati, R.; Krishnan, V.; Shuman, L.A.; Mastro, A.M.; Vogler, E.A. Metastatic breast cancer cells colonize and degrade three-dimensional osteoblastic tissue in vitro. Clin. Exp. Metastasis 2008, 25, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. A compartmentalized device for the culture of animal cells. Biomater. Artif. Cells Artif. Organs 1989, 17, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Ghezzi, C.E.; Mohn, D.; Stark, W.J.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 2011, 32, 8915–8926. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Le Nihouannen, D.; Hacking, S.A.; Tran, S.; Li, J.; Murshed, M.; Doillon, C.J.; Ghezzi, C.E.; Zhang, Y.L.; Nazhat, S.N.; et al. Newly identified interfibrillar collagen crosslinking suppresses cell proliferation and remodelling. Biomaterials 2015, 54, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Muja, N.; Kamranpour, N.O.; Lepry, W.C.; Boccaccini, A.R.; Clarke, S.A.; Nazhat, S.N. Ectopic bone formation in rapidly fabricated acellular injectable dense collagen-Bioglass hybrid scaffolds via gel aspiration-ejection. Biomaterials 2016, 85, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Wang, H.; Yu, C.; Gao, X.; Welte, T.; Muscarella, A.M.; Tian, L.; Zhao, H.; Zhao, Z.; Du, S.; Tao, J.; et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 2015, 27, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Mastro, A.M.; Vogler, E.A. A three-dimensional osteogenic tissue model for the study of metastatic tumor cell interactions with bone. Cancer Res. 2009, 69, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Siegel, P.M. CCN3 modulates bone turnover and is a novel regulator of skeletal metastasis. J. Cell Commun. Signal. 2012, 6, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Tiedemann, K.; Mourskaia, A.; Fong, J.E.; Tran-Thanh, D.; Amir, E.; Clemons, M.; Perbal, B.; Komarova, S.V.; Siegel, P.M. CCN3 impairs osteoblast and stimulates osteoclast differentiation to favor breast cancer metastasis to bone. Am. J. Pathol. 2011, 178, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Coyac, B.R.; Chicatun, F.; Hoac, B.; Nelea, V.; Chaussain, C.; Nazhat, S.N.; McKee, M.D. Mineralization of dense collagen hydrogel scaffolds by human pulp cells. J. Dent. Res. 2013, 92, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Ghezzi, C.E.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Three-dimensional mineralization of dense nanofibrillar collagen−bioglass hybrid scaffolds. Biomacromolecules 2010, 11, 1470–1479. [Google Scholar] [CrossRef]
- Thula, T.T.; Rodriguez, D.E.; Lee, M.H.; Pendi, L.; Podschun, J.; Gower, L.B. In vitro mineralization of dense collagen substrates: A biomimetic approach toward the development of bone-graft materials. Acta Biomater. 2011, 7, 3158–3169. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
James-Bhasin, M.; Siegel, P.M.; Nazhat, S.N. A Three-Dimensional Dense Collagen Hydrogel to Model Cancer Cell/Osteoblast Interactions. J. Funct. Biomater. 2018, 9, 72. https://doi.org/10.3390/jfb9040072
James-Bhasin M, Siegel PM, Nazhat SN. A Three-Dimensional Dense Collagen Hydrogel to Model Cancer Cell/Osteoblast Interactions. Journal of Functional Biomaterials. 2018; 9(4):72. https://doi.org/10.3390/jfb9040072
Chicago/Turabian StyleJames-Bhasin, Mark, Peter M. Siegel, and Showan N. Nazhat. 2018. "A Three-Dimensional Dense Collagen Hydrogel to Model Cancer Cell/Osteoblast Interactions" Journal of Functional Biomaterials 9, no. 4: 72. https://doi.org/10.3390/jfb9040072
APA StyleJames-Bhasin, M., Siegel, P. M., & Nazhat, S. N. (2018). A Three-Dimensional Dense Collagen Hydrogel to Model Cancer Cell/Osteoblast Interactions. Journal of Functional Biomaterials, 9(4), 72. https://doi.org/10.3390/jfb9040072