Organo-Clay Nanomaterials Based on Halloysite and Cyclodextrin as Carriers for Polyphenolic Compounds
Abstract
1. Introduction
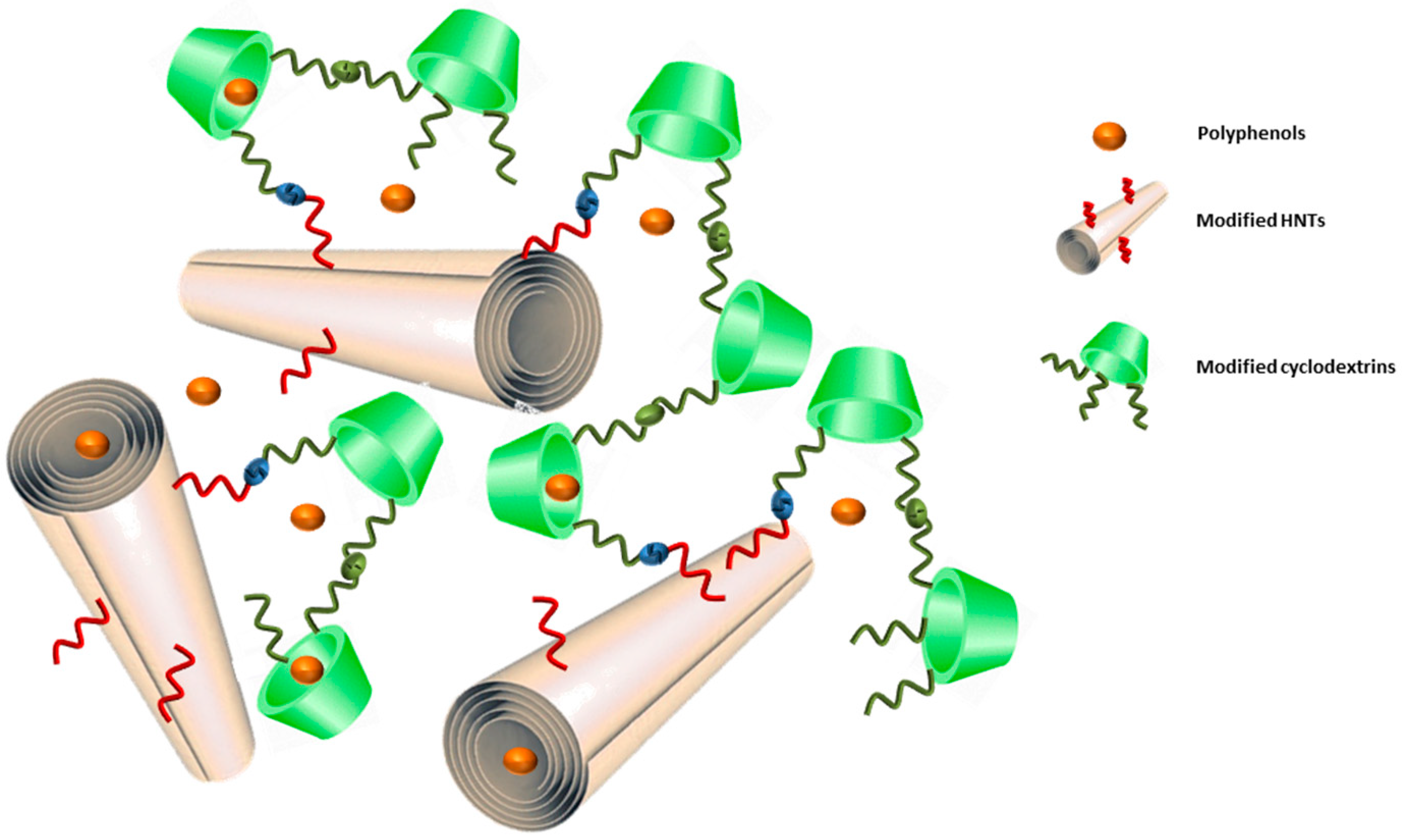
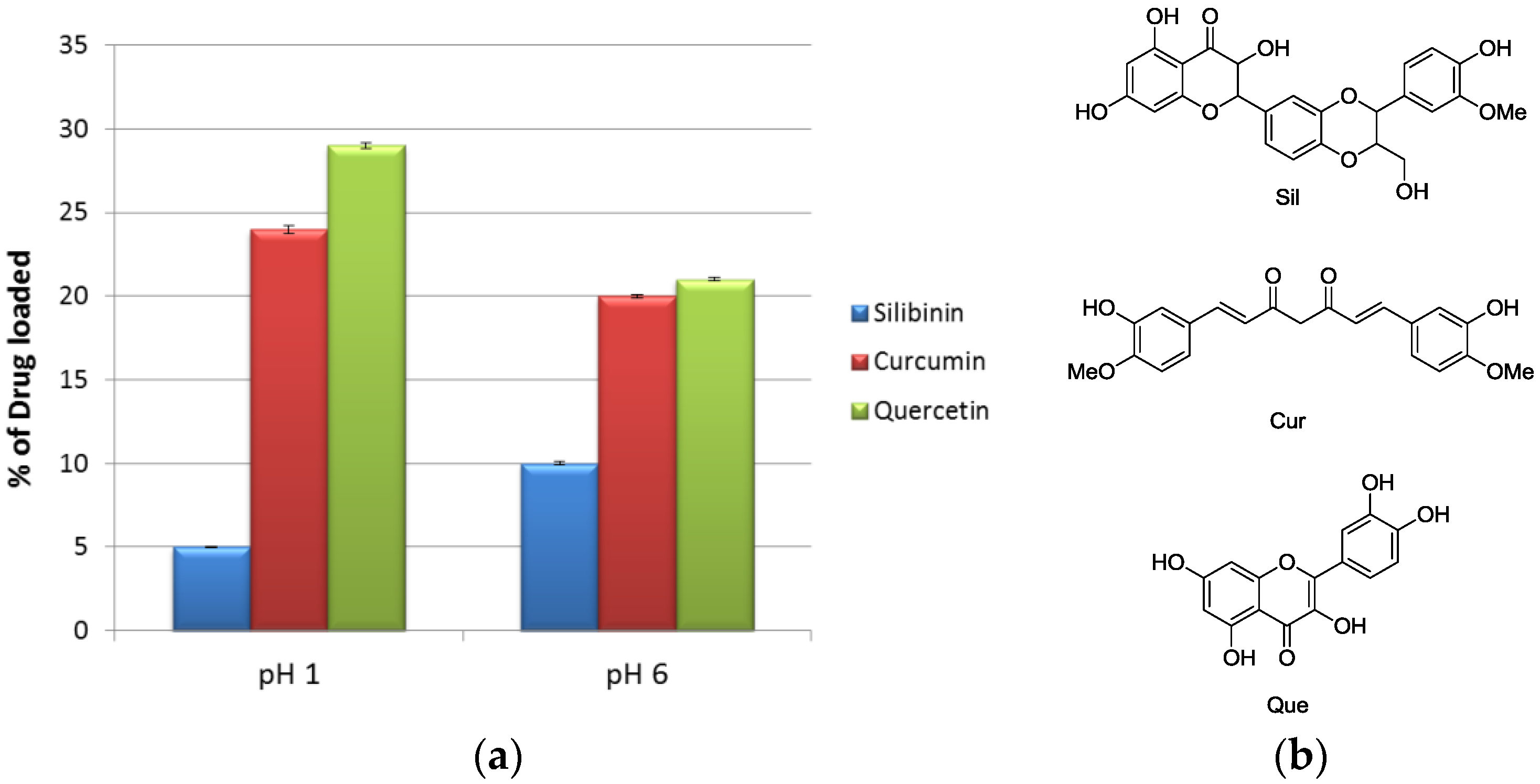
2. Results and Discussion
3. Materials and Methods
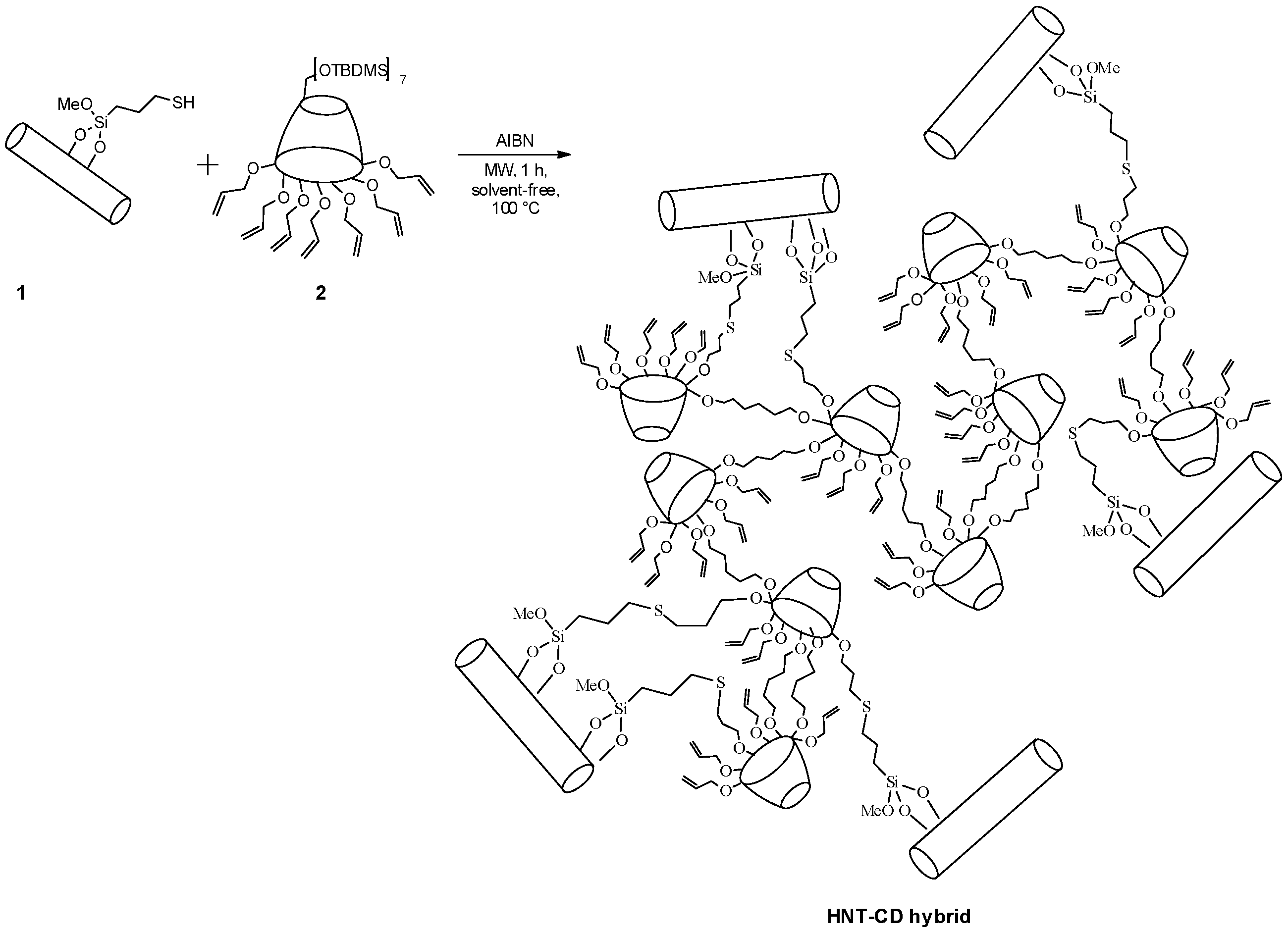
3.1. Synthesis of the HNT-CD Hybrid
3.2. Batch Adsorption Experiments
3.3. Loading of Curcumin in the HNT-CD Hybrid
3.4. Drug Release Study
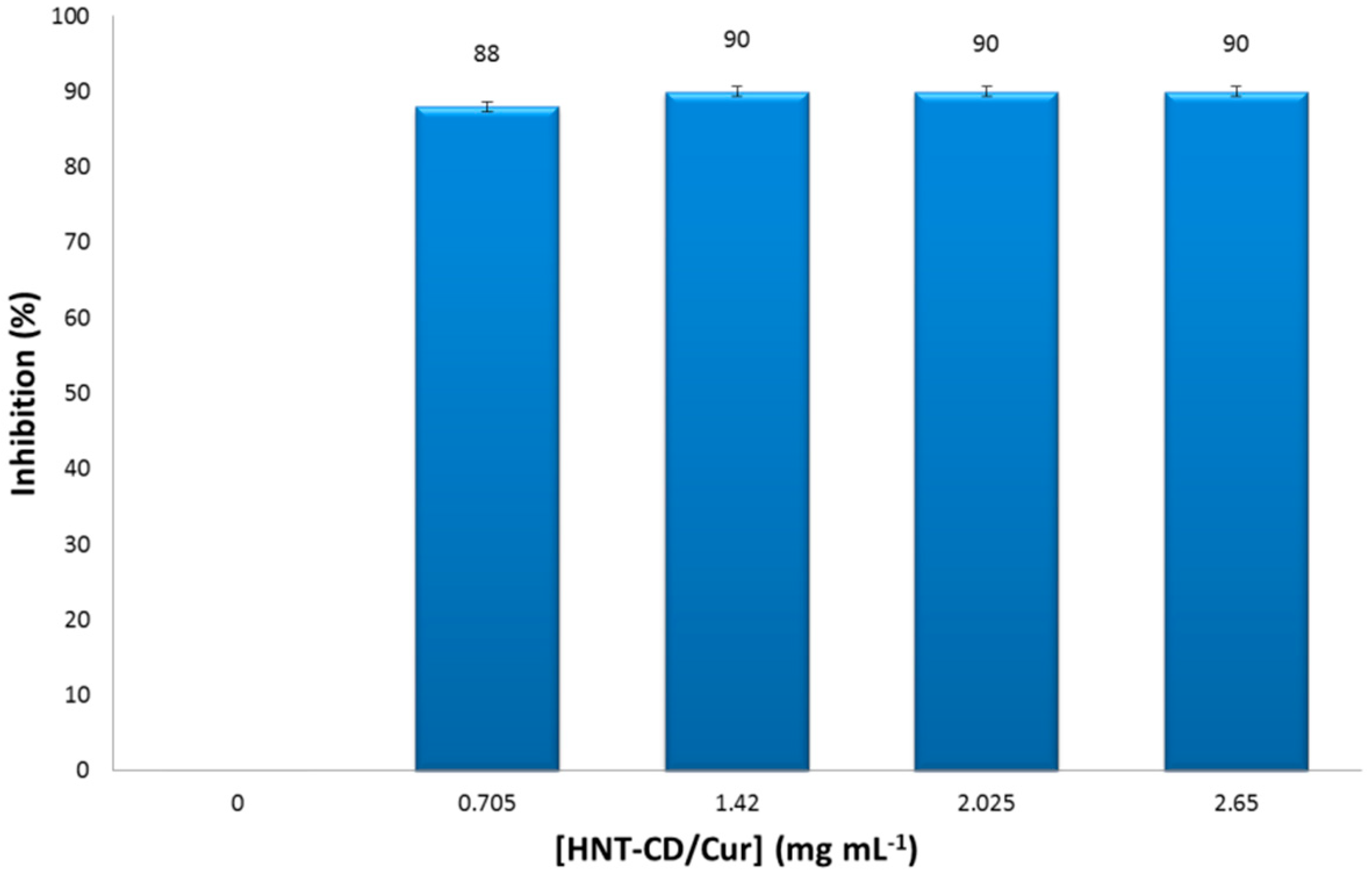
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of Toxicity of Nanoclays and Graphene Oxide in vivo: A Paramecium Caudatum Study. Environ. Sci. Nano 2016, 3, 442–452. [Google Scholar] [CrossRef]
- Bellani, L.; Giorgetti, L.; Riela, S.; Lazzara, G.; Scialabba, A.; Massaro, M. Ecotoxicity of Halloysite Nanotube-Supported Palladium Nanoparticles in Raphanus sativus L. Environ. Toxicol. Chem. 2016, 35, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent Advance in Research on Halloysite Nanotubes-Polymer Nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Bretti, C.; Cataldo, S.; Gianguzza, A.; Lando, G.; Lazzara, G.; Pettignano, A.; Sammartano, S. Thermodynamics of Proton Binding of Halloysite Nanotubes. J. Phys. Chem. C 2016, 120, 7849–7859. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite Nanotubes as Support for Metal-Based Catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Chemical Modification of Halloysite Nanotubes for Controlled Loading and Release. J. Mater. Chem. B 2018, 6, 3415–3433. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized Halloysite Nanotube by Chitosan Grafting for Drug Delivery of Curcumin to Achieve Enhanced Anticancer Efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Rizzo, C.; Arrigo, R.; D’Anna, F.; Di Blasi, F.; Dintcheva, N.T.; Lazzara, G.; Parisi, F.; Riela, S.; Spinelli, G.; Massaro, M. Hybrid Supramolecular Gels of Fmoc-F/Halloysite Nanotubes: Systems for Sustained Release of Camptothecin. J. Mater. Chem. B 2017, 5, 3217–3229. [Google Scholar] [CrossRef]
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; D’Azzo, G.; Guernelli, S.; Lazzara, G.; Pieraccini, S.; Riela, S. Halloysite Nanotubes for Efficient Loading, Stabilization and Controlled Release of Insulin. J. Colloid Interface Sci. 2018, 524, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, M.; Zhou, C. Cellulose–Halloysite Nanotube Composite Hydrogels for Curcumin Delivery. Cellulose 2017, 24, 2861–2875. [Google Scholar] [CrossRef]
- Yendluri, R.; Lvov, Y.; de Villiers, M.M.; Vinokurov, V.; Naumenko, E.; Tarasova, E.; Fakhrullin, R. Paclitaxel Encapsulated in Halloysite Clay Nanotubes for Intestinal and Intracellular Delivery. J. Pharm. Sci. 2017, 106, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Wu, Y.P.; Gao, H.Y.; Li, Y.F.; He, R.R.; Liu, M. Functionalization of Halloysite Nanotubes via Grafting of Dendrimer for Efficient Intracellular Delivery of Sirna. Bioconj. Chem. 2018, 29, 2606–2618. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.; Yendluri, R.; Lvov, Y. Halloysite Clay Nanotubes for Enzyme Immobilization. Biomacromolecules 2016, 17, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Liu, M.; Zheng, J.; Zhou, C. Adsorption of Dyes in Aqueous Solutions by Chitosan-Halloysite Nanotubes Composite Hydrogel Beads. Microporous Mesoporous Mater. 2015, 201, 190–201. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, B.; Mei, D.; Zhang, H.; Liu, J. Adsorption of Methyl Violet from Aqueous Solution by Halloysite Nanotubes. Desalination 2011, 268, 111–116. [Google Scholar] [CrossRef]
- Cataldo, S.; Lazzara, G.; Massaro, M.; Muratore, N.; Pettignano, A.; Riela, S. Functionalized Halloysite Nanotubes for Enhanced Removal of Lead(Ii) Ions from Aqueous Solutions. Appl. Clay Sci. 2018, 156, 87–95. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Cavallaro, G.; Colletti, C.G.; Milioto, S.; Noto, R.; Lazzara, G. Ecocompatible Halloysite/Cucurbit[8]uril Hybrid as Efficient Nanosponge for Pollutants Removal. Chem. Sel. 2016, 1, 1773–1779. [Google Scholar] [CrossRef]
- Massaro, M.; Schembri, V.; Campisciano, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Noto, R.; Parisi, F.; Riela, S. Design of Pnipaam Covalently Grafted on Halloysite Nanotubes as a Support for Metal-Based Catalysts. RSC Adv. 2016, 6, 55312–55318. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Buscemi, G.; Cataldo, S.; Guernelli, S.; Lazzara, G.; Liotta, L.F.; Parisi, F.; Pettignano, A.; Riela, S. Palladium Nanoparticles Immobilized on Halloysite Nanotubes Covered by a Multilayer Network for Catalytic Applications. New J. Chem. 2018, 42, 13938–13947. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Lazzara, G.; Gruttadauria, M.; Milioto, S.; Noto, R. Green Conditions for the Suzuki Reaction Using Microwave Irradiation and a New Hnt-Supported Ionic Liquid-Like Phase (Hnt-Sillp) Catalyst. Appl. Organomet. Chem. 2014, 28, 234–238. [Google Scholar] [CrossRef]
- Lo Meo, P.; D’Anna, F.; Riela, S.; Gruttadauria, M.; Noto, R. Host-Guest Interactions Involving Cyclodextrins: Useful Complementary Insights Achieved by Polarimetry. Tetrahedron 2007, 63, 9163–9171. [Google Scholar] [CrossRef]
- Lo Meo, P.; D’Anna, F.; Riela, S.; Gruttadauria, M.; Noto, R. Spectrophotometric Determination of Binding Constants between Some Aminocyclodextrins and Nitrobenzene Derivatives at Various Ph Values. Tetrahedron 2002, 58, 6039–6045. [Google Scholar] [CrossRef]
- Riela, S.; D’Anna, F.; Lo Meo, P.; Gruttadauria, M.; Giacalone, R.; Noto, R. Chiral Recognition of Protected Amino Acids by Means of Fluorescent Binary Complex Pyrene/Heptakis-(6-Amino)-(6-Deoxy)-Β-Cyclodextrin. Tetrahedron 2006, 62, 4323–4330. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-Based Host-Guest Supramolecular Hydrogel and Its Application in Biomedical Fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of Water Pollution with Native Cyclodextrins and Modified Cyclodextrins: A Comparative Overview and Perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Massaro, M.; Piana, S.; Colletti, C.G.; Noto, R.; Riela, S.; Baiamonte, C.; Giordano, C.; Pizzolanti, G.; Cavallaro, G.; Milioto, S.; et al. Multicavity Halloysite-Amphiphilic Cyclodextrin Hybrids for Co-Delivery of Natural Drugs into Thyroid Cancer Cells. J. Mater. Chem. B 2015, 3, 4074–4081. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Lo Meo, P.; Noto, R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Functionalized Halloysite Multivalent Glycocluster as a New Drug Delivery System. J. Mater. Chem. B 2014, 2, 7732–7738. [Google Scholar] [CrossRef]
- Lo Meo, P.; Lazzara, G.; Liotta, L.; Riela, S.; Noto, R. Cyclodextrin-Calixarene Co-Polymers as a New Class of Nanosponges. Polym. Chem. 2014, 5, 4499–4510. [Google Scholar] [CrossRef]
- Cinà, V.; Russo, M.; Lazzara, G.; Chillura Martino, D.; Lo Meo, P. Pre- and Post-Modification of Mixed Cyclodextrin-Calixarene Co-Polymers: A Route Towards Tunability. Carbohydr. Polym. 2017, 157, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Cinà, V.; Labbozzetta, M.; Lazzara, G.; Lo Meo, P.; Poma, P.; Riela, S.; Noto, R. Chemical and Pharmaceutical Evaluation of the Relationship between Triazole Linkers and Pore Size on Cyclodextrin-Calixarene Nanosponges Used as Carriers for Natural Drugs. RSC Adv. 2016, 6, 50858–50866. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Guernelli, S.; Noto, R.; Riela, S. Synthesis and Characterization of Halloysite-Cyclodextrin Nanosponges for Enhanced Dyes Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 3346–3352. [Google Scholar] [CrossRef]
- Massaro, M.; Campofelice, A.; Colletti, C.G.; Lazzara, G.; Noto, R.; Riela, S. Functionalized Halloysite Nanotubes: Efficient Carrier Systems for Antifungine Drugs. Appl. Clay Sci. 2018, 160, 186–192. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Baiamonte, C.; Blanco, J.L.J.; Giordano, C.; Lo Meo, P.; Milioto, S.; Noto, R.; Parisi, F.; Pizzolanti, G.; et al. Dual Drug-Loaded Halloysite Hybrid-Based Glycocluster for Sustained Release of Hydrophobic Molecules. RSC Adv. 2016, 6, 87935–87944. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Scialabba, C.; Licciardi, M.; Merli, M.; Sciascia, L.; Turco Liveri, M.L. Montmorillonite Nanodevices for the Colon Metronidazole Delivery. Int. J. Pharm. 2013, 457, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Amorati, R.; Cavallaro, G.; Guernelli, S.; Lazzara, G.; Milioto, S.; Noto, R.; Poma, P.; Riela, S. Direct Chemical Grafted Curcumin on Halloysite Nanotubes as Dual-Responsive Prodrug for Pharmacological Applications. Colloids Surf. B. Biointerfaces 2016, 140, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A Synergic Nanoantioxidant Based on Covalently Modified Halloysite-Trolox Nanotubes with Intra-Lumen Loaded Quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-Based Nanosponges of Curcumin: Formulation and Physicochemical Characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-Linked, Cyclodextrin-Based Nanosponges for Curcumin Delivery—Physicochemical Characterization, Drug Release, Stability and Cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Wang, T.; Bae, M.; Lee, J.-Y.; Luo, Y. Solid Lipid-Polymer Hybrid Nanoparticles Prepared with Natural Biomaterials: A New Platform for Oral Delivery of Lipophilic Bioactives. Food Hydrocoll. 2018, 84, 581–592. [Google Scholar] [CrossRef]
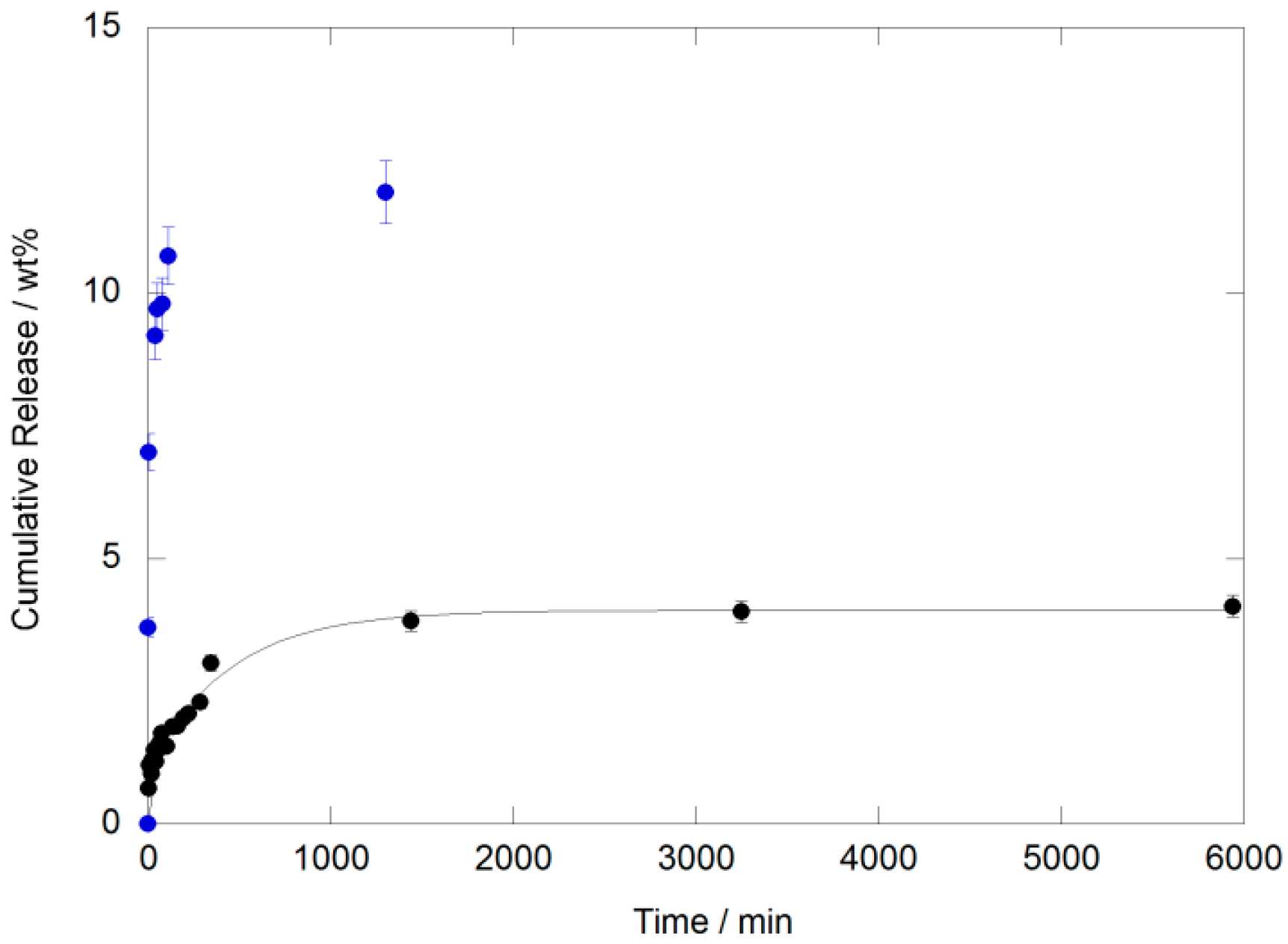
| First Order | Korsemeyer Peppas | Hixon and Crowell | Higuchi | DEM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M∞ (wt %) | k (min−1) | R2 | k (min−1) | n | R2 | k (min−1) | R2 | k (min−1) | R2 | M′ (wt %) | k′ (min−1) | M″ (wt %) | k″ (min−1) | R2 |
| 3.8 ± 0.3 | 0.005 ± 0.001 | 0.7825 | 0.58 ± 0.05 | 0.23 ± 0.01 | 0.9441 | (4.9 ± 0.3) × 10−8 | 0.8379 | n.a. | 1.01 ± 0.08 | 0.2 ± 0.1 | 3.0 ± 0.1 | 0.0022 ± 0.0003 | 0.9824 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, M.; Riela, S. Organo-Clay Nanomaterials Based on Halloysite and Cyclodextrin as Carriers for Polyphenolic Compounds. J. Funct. Biomater. 2018, 9, 61. https://doi.org/10.3390/jfb9040061
Massaro M, Riela S. Organo-Clay Nanomaterials Based on Halloysite and Cyclodextrin as Carriers for Polyphenolic Compounds. Journal of Functional Biomaterials. 2018; 9(4):61. https://doi.org/10.3390/jfb9040061
Chicago/Turabian StyleMassaro, Marina, and Serena Riela. 2018. "Organo-Clay Nanomaterials Based on Halloysite and Cyclodextrin as Carriers for Polyphenolic Compounds" Journal of Functional Biomaterials 9, no. 4: 61. https://doi.org/10.3390/jfb9040061
APA StyleMassaro, M., & Riela, S. (2018). Organo-Clay Nanomaterials Based on Halloysite and Cyclodextrin as Carriers for Polyphenolic Compounds. Journal of Functional Biomaterials, 9(4), 61. https://doi.org/10.3390/jfb9040061







