Characterization of Tensile Mechanical Behavior of MSCs/PLCL Hybrid Layered Sheet
Abstract
:1. Introduction
2. Results
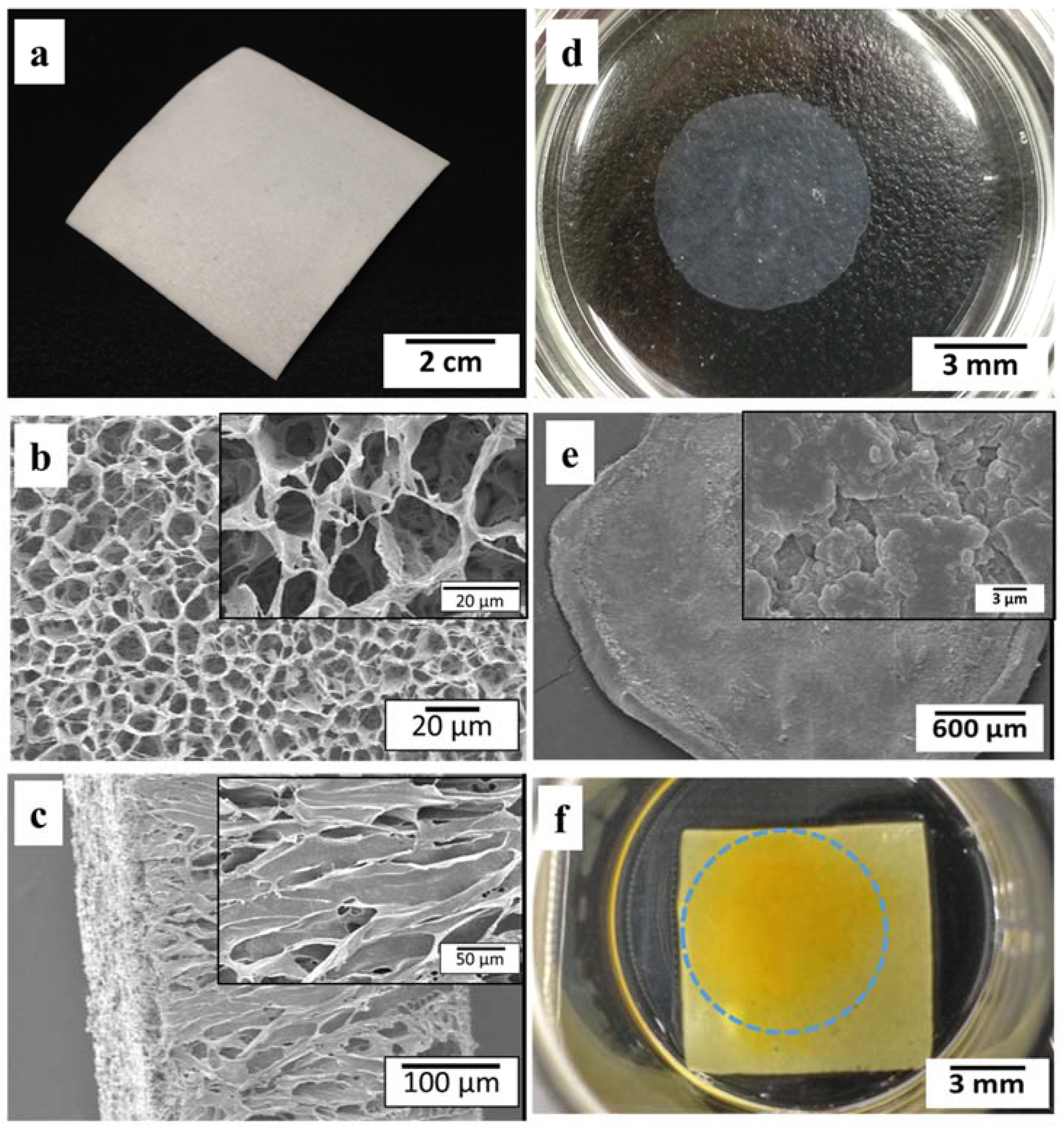
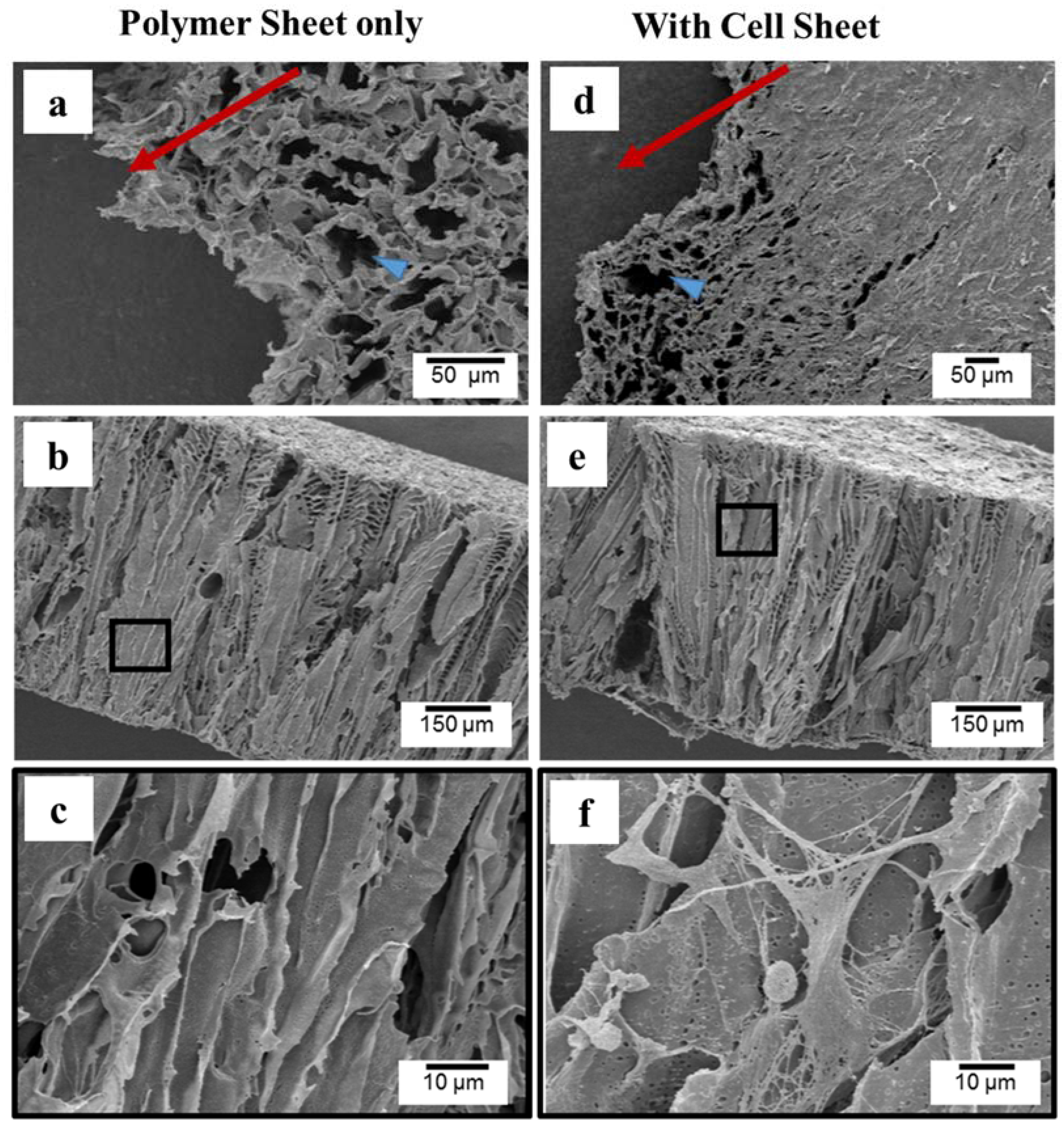
2.1. Morphology and Microstructure of PLCL Sheet and MSCs Sheet
2.2. Layered Construct of PLCL Sheet and MSCs Sheet
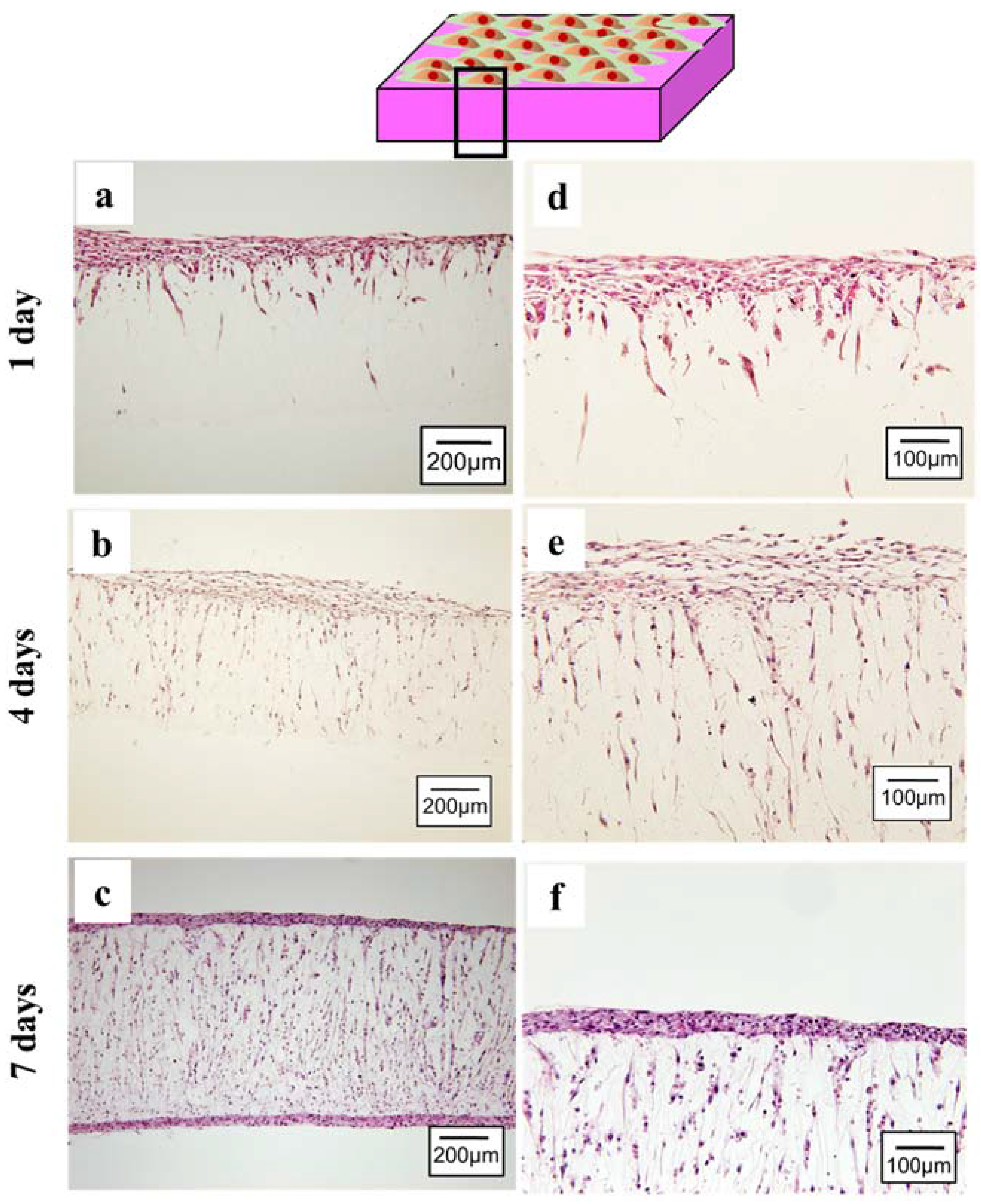
2.2.1. Cell Spreading and Infiltration
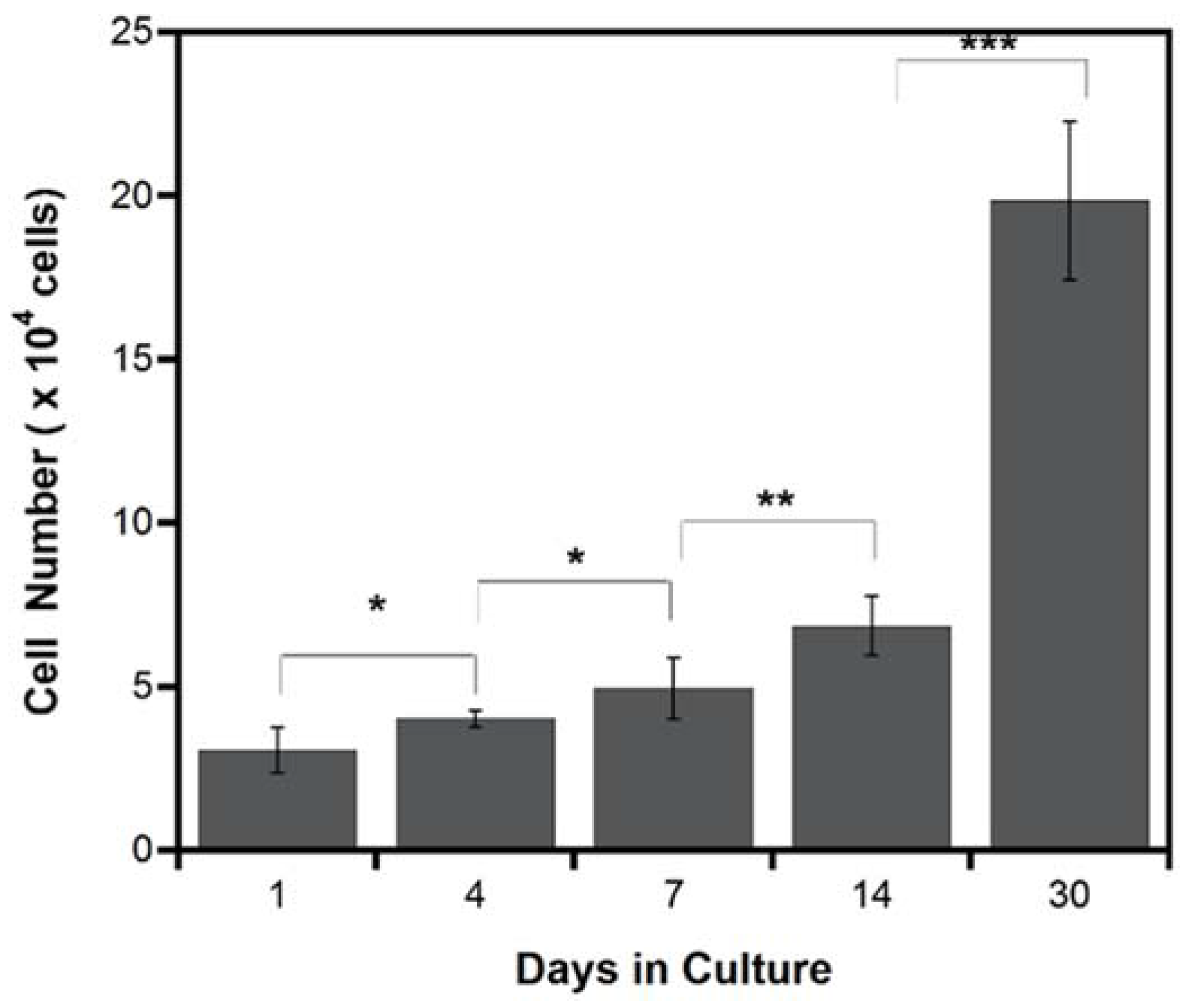
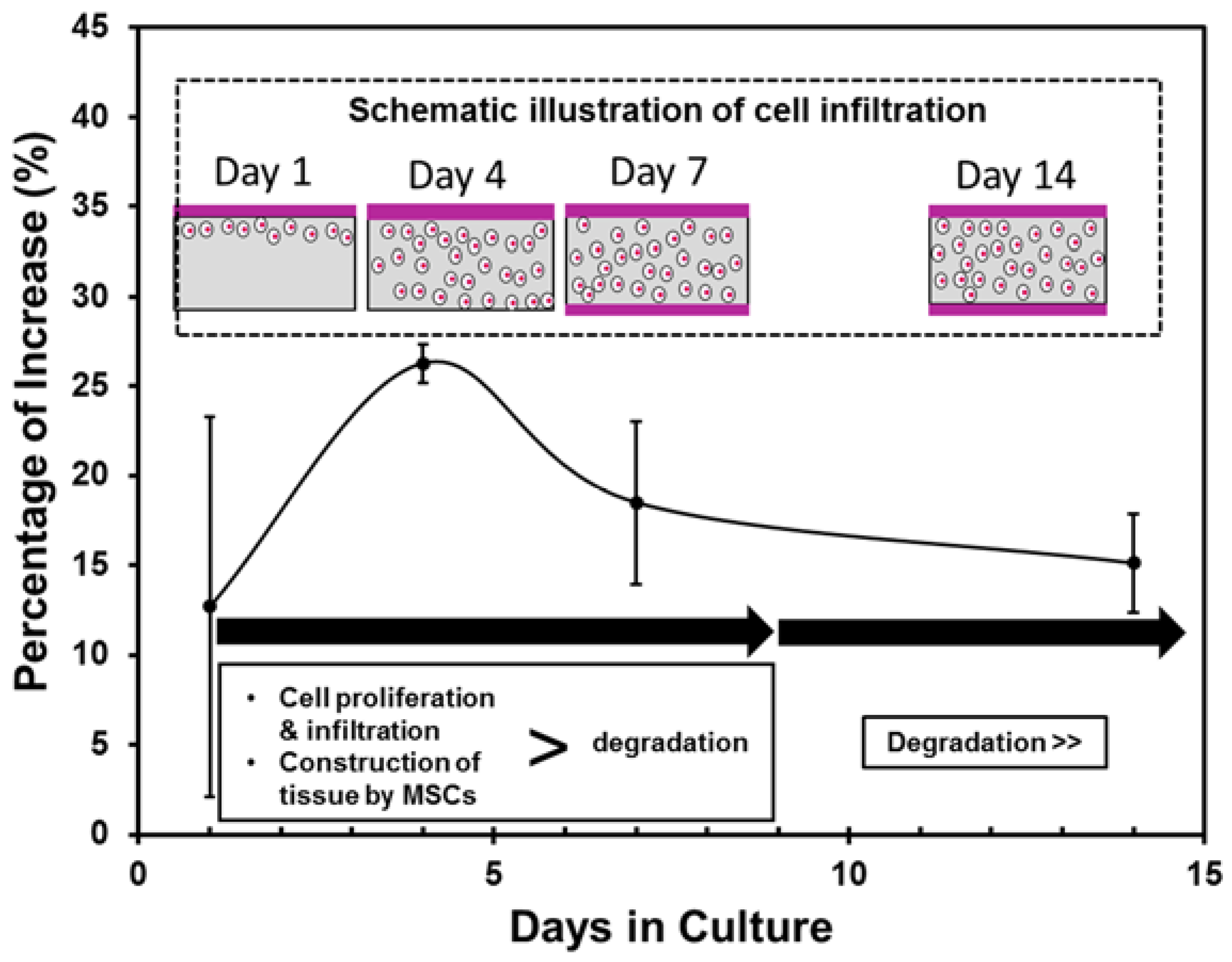
2.2.2. Cell Proliferation
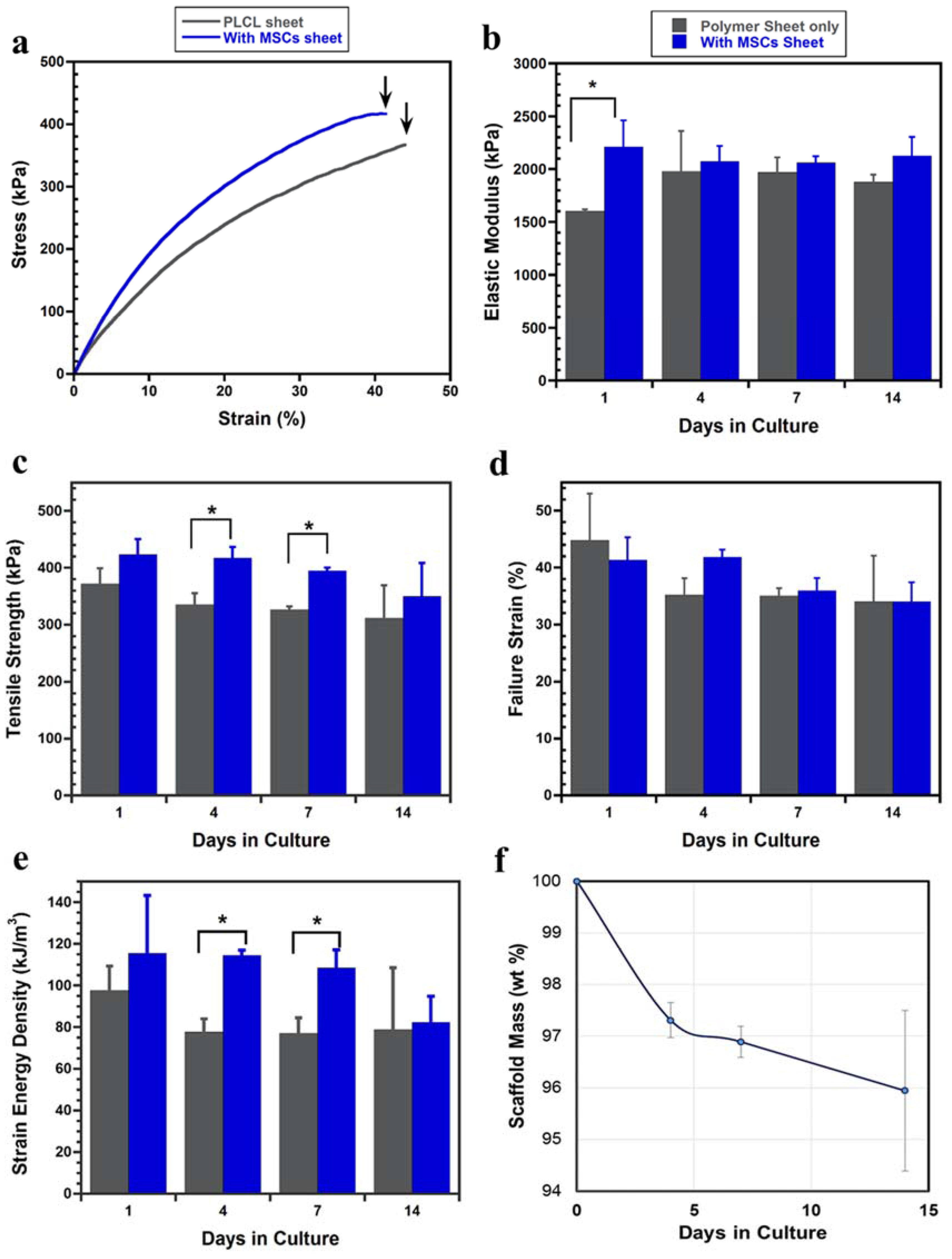
2.2.3. Tensile Mechanical Evaluation
3. Discussion
4. Conclusions
- MSCs sheet was able to survive (even in a thicker density) and actively proliferate on the porous PLCL sheet, suggesting that the porous structure is important in transporting nutrient and oxygen supply while removing metabolic waste.
- Variations in the tensile strength of the layered constructs were affected by two factors, the cell proliferation and infiltration and the degradation of the PLCL sheet. Once the cell sheet proliferated entirely into the pores of the PLCL sheet and formed a new layer on the opposite surface, the layered construct exhibited significantly higher tensile strength than that without MSCs. However, after 14 days of culture the effect of the degradation of the PLCL sheet overtook that of cell proliferation, resulting the decrease of the tensile strength.
5. Materials and Methods
5.1. Fabrication of PLCL Sheet
5.2. Fabrication of Layered Construct
5.3. SEM Imaging
5.4. Cell Proliferation
5.5. H&E Staining
5.6. Mechanical Testing
5.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, R.; Liu, M.; Lu, L.; Zheng, Y.; Zhang, P. Congenital heart disease: Causes, diagnosis, symptoms, and treatments. Cell Biochem. Biophys. 2015, 72, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Caputo, M.; Madeddu, P. Stem cell therapy and tissue engineering for correction of congenital heart disease. Front. Cell Dev. Biol. 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Mickle, D.A.G.; Weisel, R.D.; Koyama, N.; Ozawa, S.; Li, R.-K. Optimal biomaterial for creation of autologous cardiac grafts. Circulation 2002, 106, 176–182. [Google Scholar]
- Ozawa, T.; Mickle, D.A.G.; Weisel, R.D.; Koyama, N.; Wong, H.; Ozawa, S.; Li, R.-K. Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J. Thorac. Cardiovasc. Surg. 2002, 124, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Mirensky, T.L.; Breuer, C.K. The development of tissue-engineered grafts for reconstructive cardiothoracic surgical applications. Pediatr. Res. 2008, 63, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Park, K.D.; Kim, Y.H.; Suh, H.; Park, J.C.; Lee, J.E.; Sun, K.; Baek, M.J.; Kim, H.-M.; Kim, S.H. Improved calcification resistance and biocompatibility of tissue patch grafted with sulfonated peo or heparin after glutaraldehyde fixation. J. Biomed. Mater. Res. 2001, 58, 27–35. [Google Scholar] [CrossRef]
- Said, S.M.; Burkhart, H.M. When repair is not feasible: Prosthesis selection in children and adults with congenital heart disease. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2014, 17, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Okwulehie, V.; Dharmapuram, A.K.; Swain, S.K.; Ramdoss, N.; Sundararaghavan, S.; Kona, S.M. Experience with autologous pericardial patch closure of ventricular septal defect. Indian J. Thorac. Cardiovasc. Surg. 2006, 22, 212–214. [Google Scholar] [CrossRef]
- Pok, S.; Jacot, J.G. Biomaterials advances in patches for congenital heart defect repair. J. Cardiovasc. Transl. Res. 2011, 4, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Mol, A.; Neuenschwander, S.; Breymann, C.; Gössi, M.; Zund, G.; Turina, M.; Hoerstrup, S.P. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur. J. Cardiothorac. Surg. 2005, 27, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Hoerstrup, S.P.; Kadner, A.; Breymann, C.; Maurus, C.F.; Guenter, C.I.; Sodian, R.; Visjager, J.F.; Zund, G.; Turina, M.I. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann. Thorac. Surg. 2002, 74, 46–52. [Google Scholar] [CrossRef]
- Cho, S.-W.; Jeon, O.; Lim, J.E.; Gwak, S.-J.; Kim, S.-S.; Choi, C.Y.; Kim, D.-I.; Kim, B.-S. Preliminary experience with tissue engineering of a venous vascular patch by using bone marrow–derived cells and a hybrid biodegradable polymer scaffold. J. Vasc. Surg. 2006, 44, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Yeong, W.Y.; Sudarmadji, N.; Yu, H.Y.; Chua, C.K.; Leong, K.F.; Venkatraman, S.S.; Boey, Y.C.F.; Tan, L.P. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Pagani, R.; Ameer, G.A.; Vallet-Regi, M.; Portoles, M.T. Endothelial cells derived from circulating progenitors as an effective source to functional endothelialization of naoh-treated poly(epsilon-caprolactone) films. J. Biomed. Mater. Res. A 2008, 87, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, J.; Chen, L.; Geng, X.; Zhang, A.Y.; Guo, L.R.; Gu, Y.Q.; Feng, Z.G. The fabrication of double layer tubular vascular tissue engineering scaffold via coaxial electrospinning and its 3d cell coculture. J. Biomed. Mater. Res. A 2015, 103, 3863–3871. [Google Scholar] [CrossRef] [PubMed]
- Garkhal, K.; Verma, S.; Tikoo, K.; Kumar, N. Surface modified poly(l-lactide-co-epsilon-caprolactone) microspheres as scaffold for tissue engineering. J. Biomed. Mater. Res. A 2007, 82, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Krishnan, U.M.; Sethuraman, S. Living cardiac patch: The elixir for cardiac regeneration. Expert Opin. Biol. Ther. 2012, 12, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Shin’oka, T.; Matsumura, G.; Hibino, N.; Naito, Y.; Watanabe, M.; Konuma, T.; Sakamoto, T.; Nagatsu, M.; Kurosawa, H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 2005, 129, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Mescher, A.L.; Mescher, A.L.; Junqueira, L.C.U.A. Junqueira’s Basic Histology: Text and Atlas, 14th ed.; McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Cho, S.-W.; Park, H.J.; Ryu, J.H.; Kim, S.H.; Kim, Y.H.; Choi, C.Y.; Lee, M.-J.; Kim, J.-S.; Jang, I.-S.; Kim, D.-I.; et al. Vascular patches tissue-engineered with autologous bone marrow-derived cells and decellularized tissue matrices. Biomaterials 2005, 26, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(n-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardail infarction. Nature 2006, 12, 459–465. [Google Scholar]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Sekine, H.; Yang, J.; Isoi, Y.; Yamato, M.; Kikuchi, A.; Kobayashi, E.; Okano, T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006, 20, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Sekine, W.; Haraguchi, Y.; Shimizu, T.; Umezawa, A.; Okano, T. Thickness limitation and cell viability of multilayered cell sheets and overcoming the diffusion limit by a porous-membrane culture insert. J. Biochip Tissue Chip 2011, 1–9. [Google Scholar] [CrossRef]
- Suzuki, R.; Hattori, F.; Itabashi, Y.; Yoshioka, M.; Yuasa, S.; Manabe-Kawaguchi, H.; Murata, M.; Makino, S.; Kokaji, K.; Yozu, R.; et al. Omentopexy enhances graft function in myocardial cell sheet transplantation. Biochem. Biophys. Res. Commun. 2009, 387, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A. Tissue engineering of vascular grafts. Matrix Biol. 2000, 19, 353–357. [Google Scholar] [CrossRef]
- Cleary, M.A.; Geiger, E.; Grady, C.; Best, C.; Naito, Y.; Breuer, C. Vascular tissue engineering: The next generation. Trends Mol. Med. 2012, 18, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Ju, Y.M.; Takahashi, H.; Williams, D.F.; Yoo, J.J.; Lee, S.J.; Okano, T.; Atala, A. Engineered small diameter vascular grafts by combining cell sheet engineering and electrospinning technology. Acta Biomater. 2015, 16, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: An in vitro study. Arch. Med. Res. 2013, 44, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Tondreau, M.Y.; Laterreur, V.R.; Gauvin, R.; ValliAres, K.; Bourget, J.-M.; Lacroix, D.; Tremblay, C.; Germain, L.; Ruel, J.; Auger, F.O.A. Mechanical properties of endothelialized fibroblast-derived vascular scaffolds stimulated in a bioreactor. Acta Biomater. 2015, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sekine, W.; Haraguchi, Y.; Shimizu, T.; Yamato, M.; Umezawa, A.; Okano, T. Chondrocyte differentiation of human endometrial gland-derived mscs in layered cell sheets. Sci. World J. 2013, 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Mun, C.H.; Jung, Y.; Kim, S.-H.; Kim, H.C.; Kim, S.H. Effects of pulsatile bioreactor culture on vascular smooth muscle cells seeded on electrospun poly(lactide-co-ε-caprolactone) scaffold. Artif. Organs 2013, 37, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Sang, B.S.; Jung, Y.; Kwon, I.K.; Hay, M.C.; Kim, S.H. Fibroblast culture on poly(l-lactide-co-ε-caprolactone) an electrospun nanofiber sheet. Macromol. Res. 2012, 20, 1234–1242. [Google Scholar]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pangesty, A.I.; Arahira, T.; Todo, M. Characterization of Tensile Mechanical Behavior of MSCs/PLCL Hybrid Layered Sheet. J. Funct. Biomater. 2016, 7, 14. https://doi.org/10.3390/jfb7020014
Pangesty AI, Arahira T, Todo M. Characterization of Tensile Mechanical Behavior of MSCs/PLCL Hybrid Layered Sheet. Journal of Functional Biomaterials. 2016; 7(2):14. https://doi.org/10.3390/jfb7020014
Chicago/Turabian StylePangesty, Azizah Intan, Takaaki Arahira, and Mitsugu Todo. 2016. "Characterization of Tensile Mechanical Behavior of MSCs/PLCL Hybrid Layered Sheet" Journal of Functional Biomaterials 7, no. 2: 14. https://doi.org/10.3390/jfb7020014
APA StylePangesty, A. I., Arahira, T., & Todo, M. (2016). Characterization of Tensile Mechanical Behavior of MSCs/PLCL Hybrid Layered Sheet. Journal of Functional Biomaterials, 7(2), 14. https://doi.org/10.3390/jfb7020014




