Engineering Metal-Organic Frameworks for Enhanced Antimicrobial Efficacy: Synthesis Methodologies, Mechanistic Perspectives, and Versatile Applications
Abstract
1. Introduction
2. Synthesis Methods for MOFs
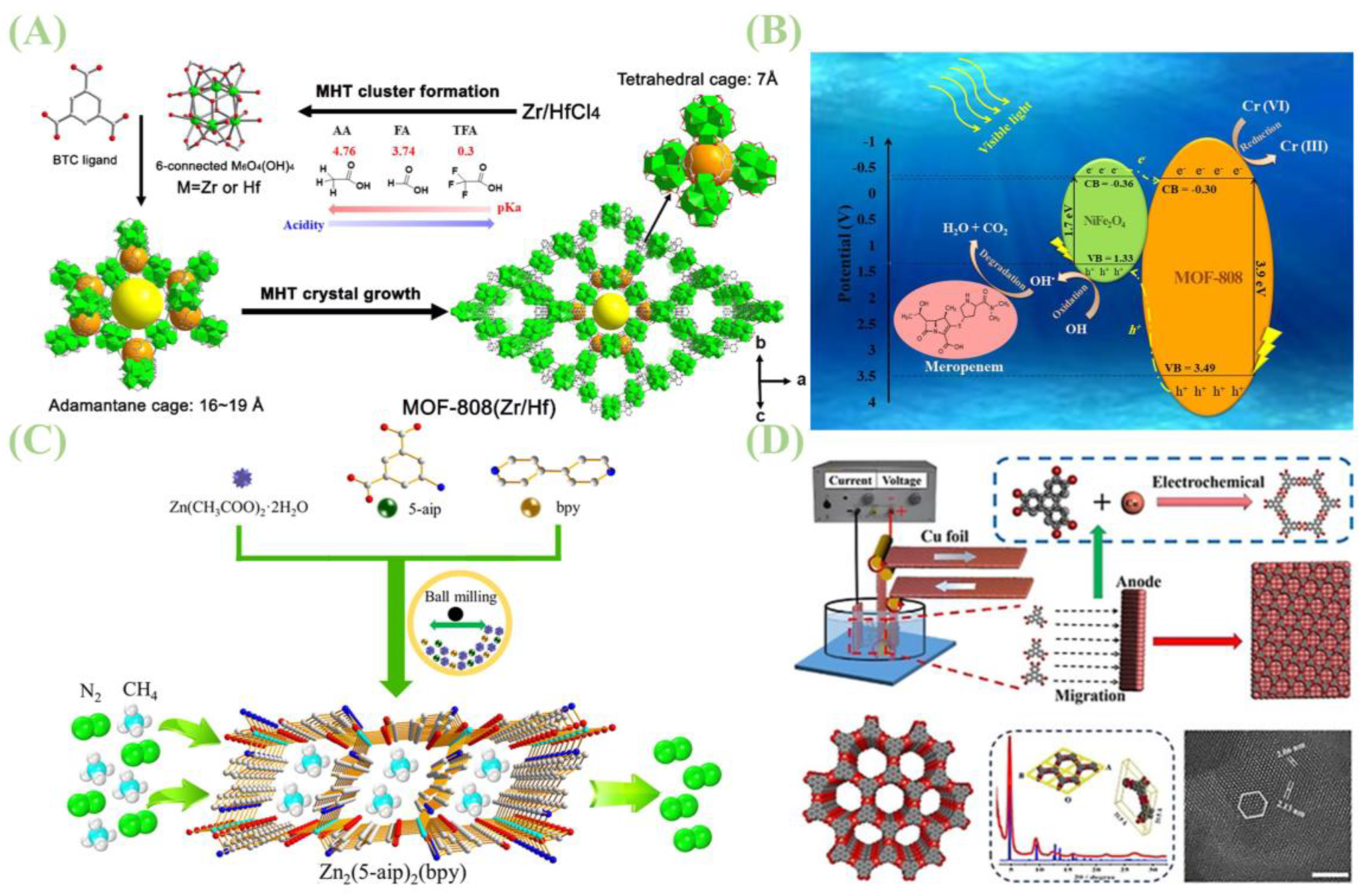
2.1. Hydrothermal Synthesis
2.2. Microwave-Assisted Synthesis
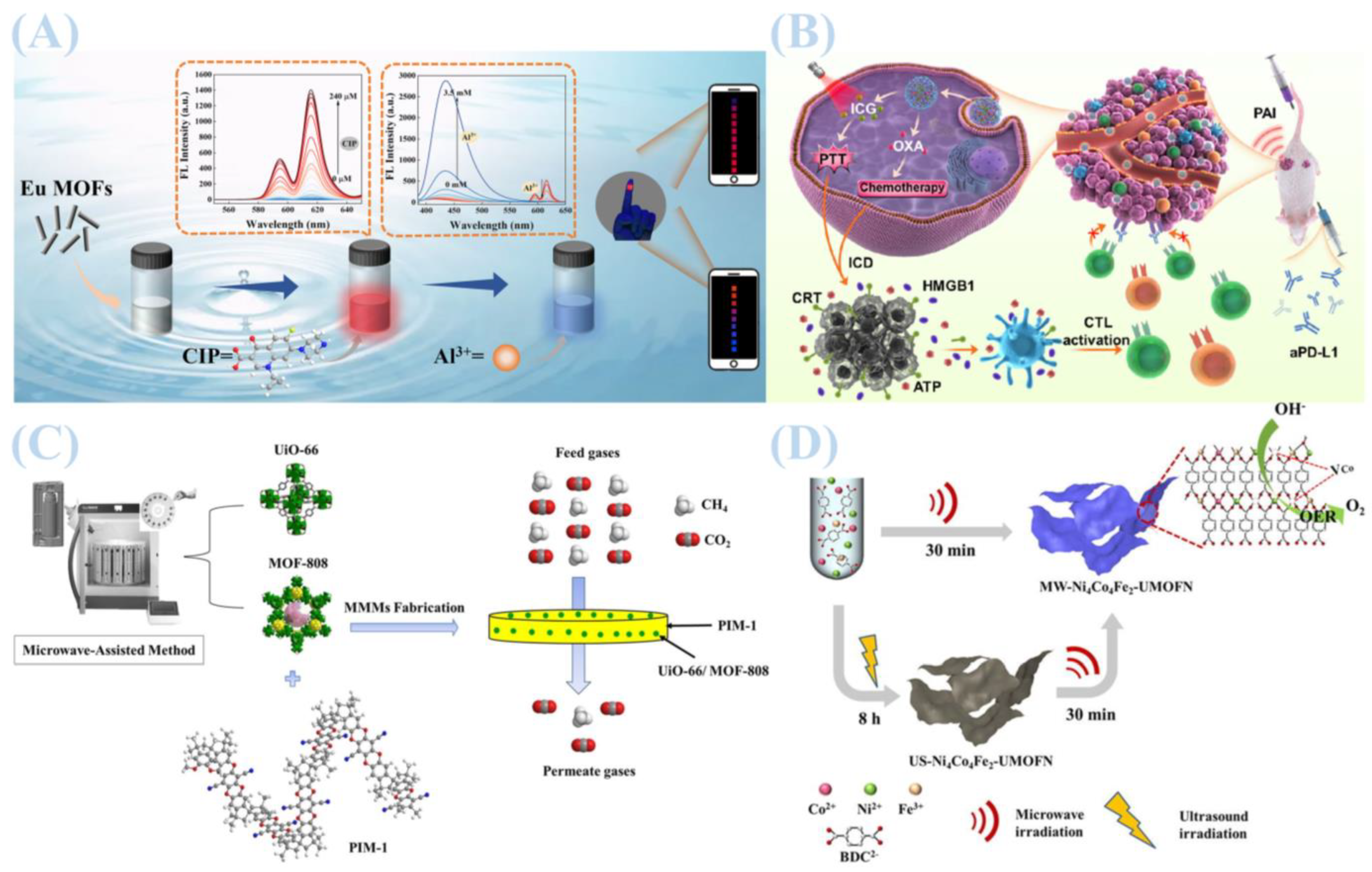
2.3. Chemical Synthesis
3. Compositions of MOFs
3.1. Single-Metal–Organic Frameworks
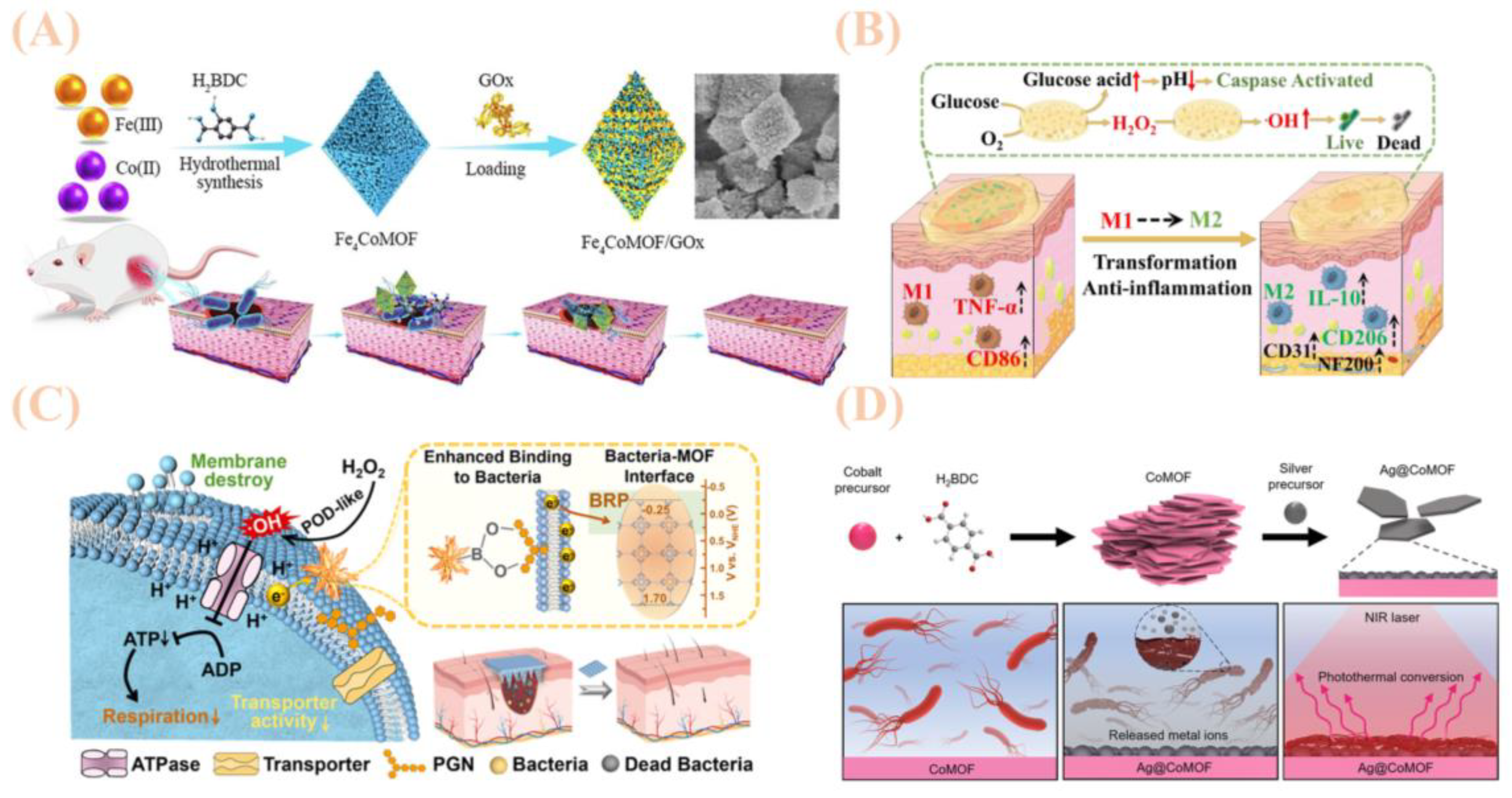
3.2. Bimetallic Metal–Organic Framework Materials
3.3. Multimetallic Metal–Organic Framework Materials
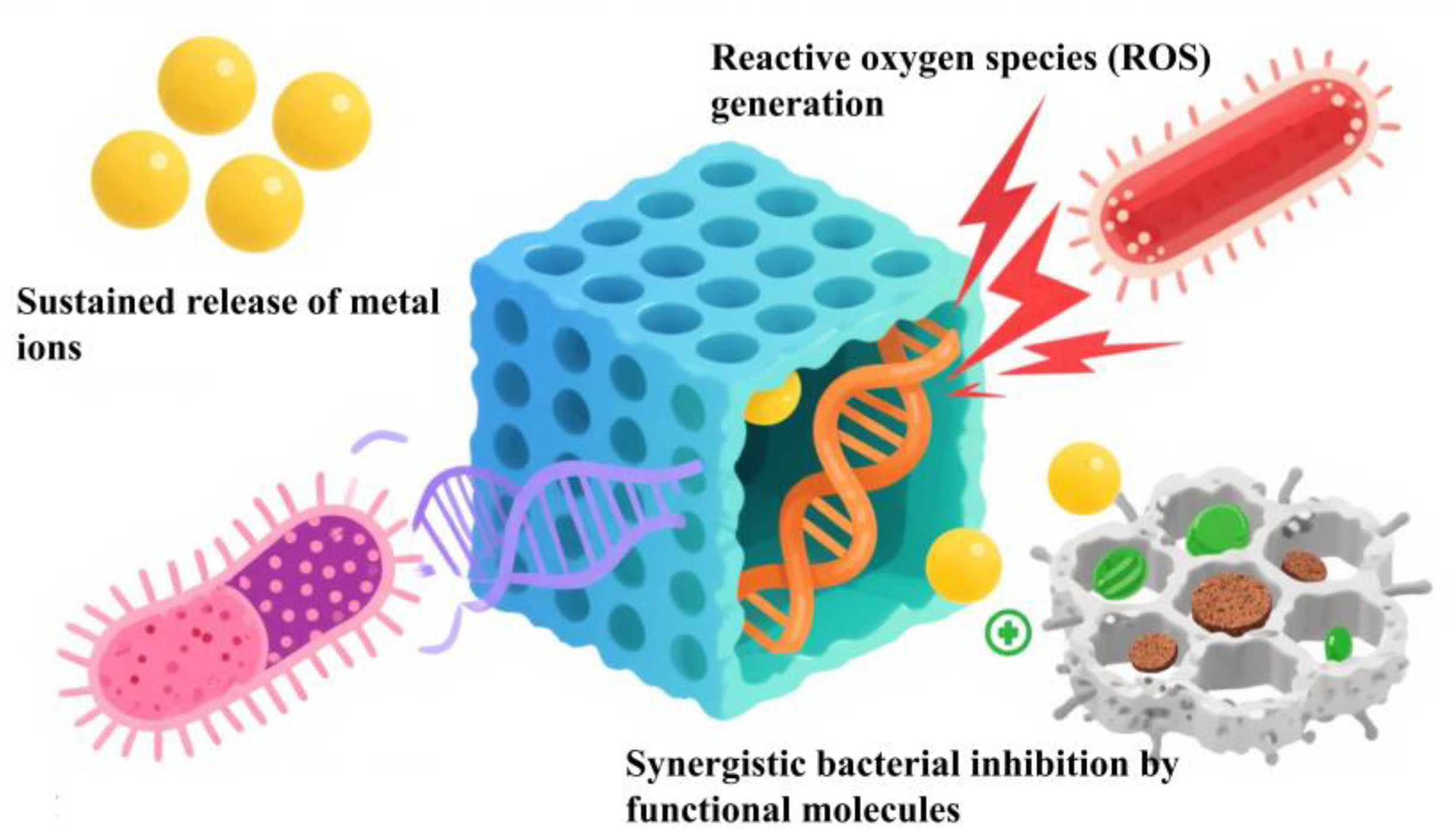
4. Antimicrobial Mechanisms of Metal–Organic Framework Materials
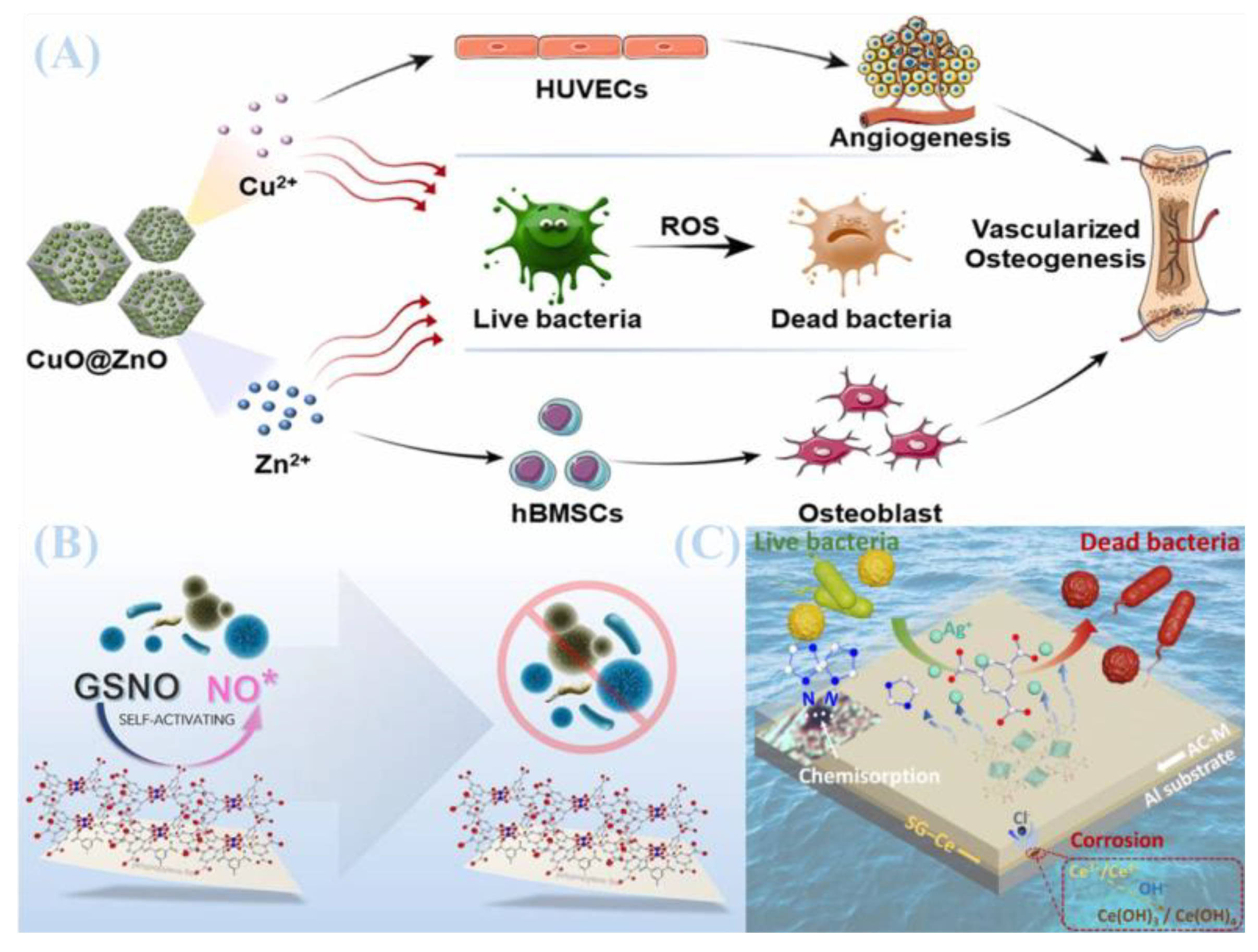
4.1. Sustained Release of Metal Ions
4.2. Reactive Oxygen Species (ROS) Generation
4.3. Synergistic Bacterial Inhibition by Functional Molecules
5. Antimicrobial Applications of MOFs
5.1. Wound Dressings and Medical Coatings
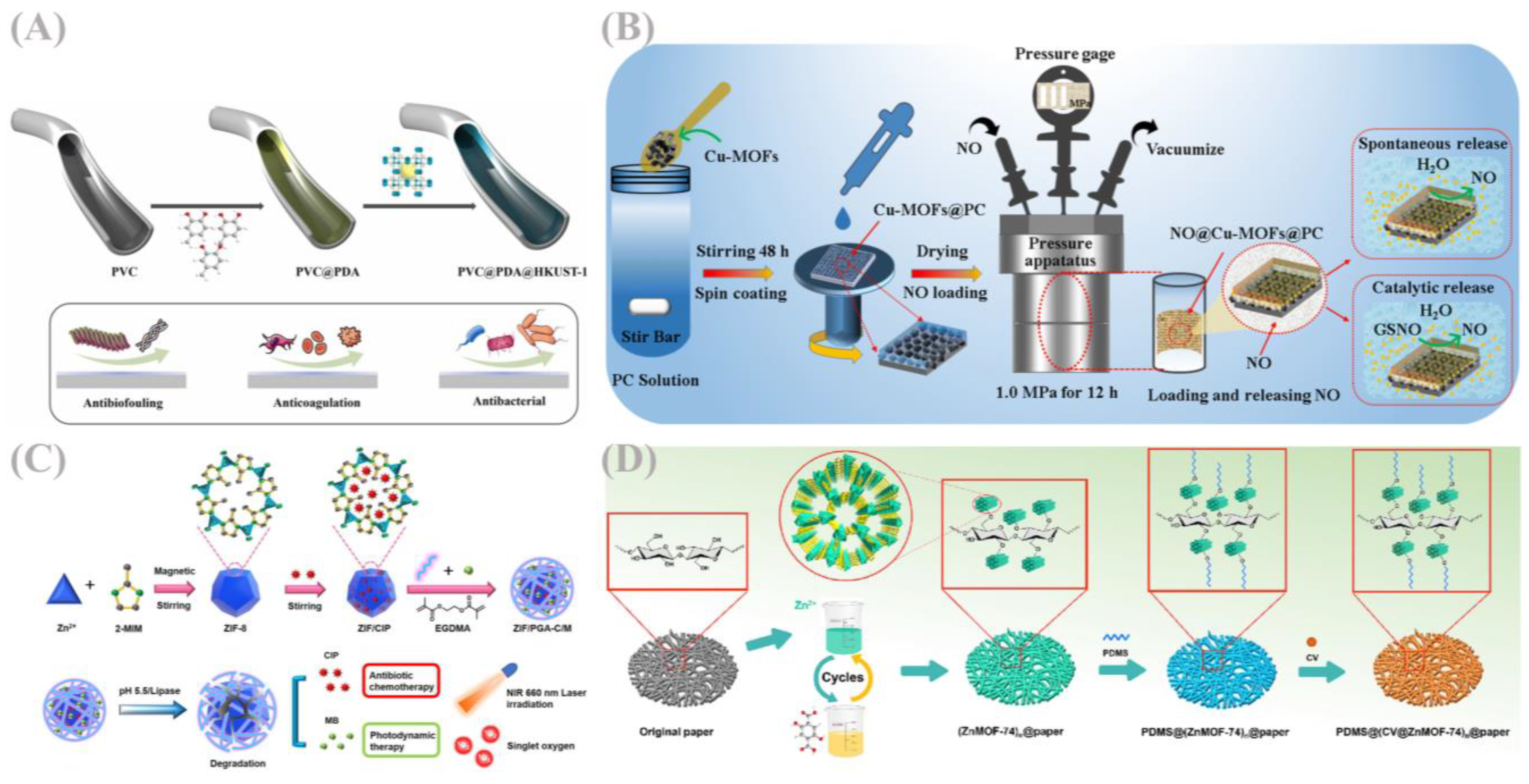
5.2. Treatment of Drug-Resistant Bacterial Infections
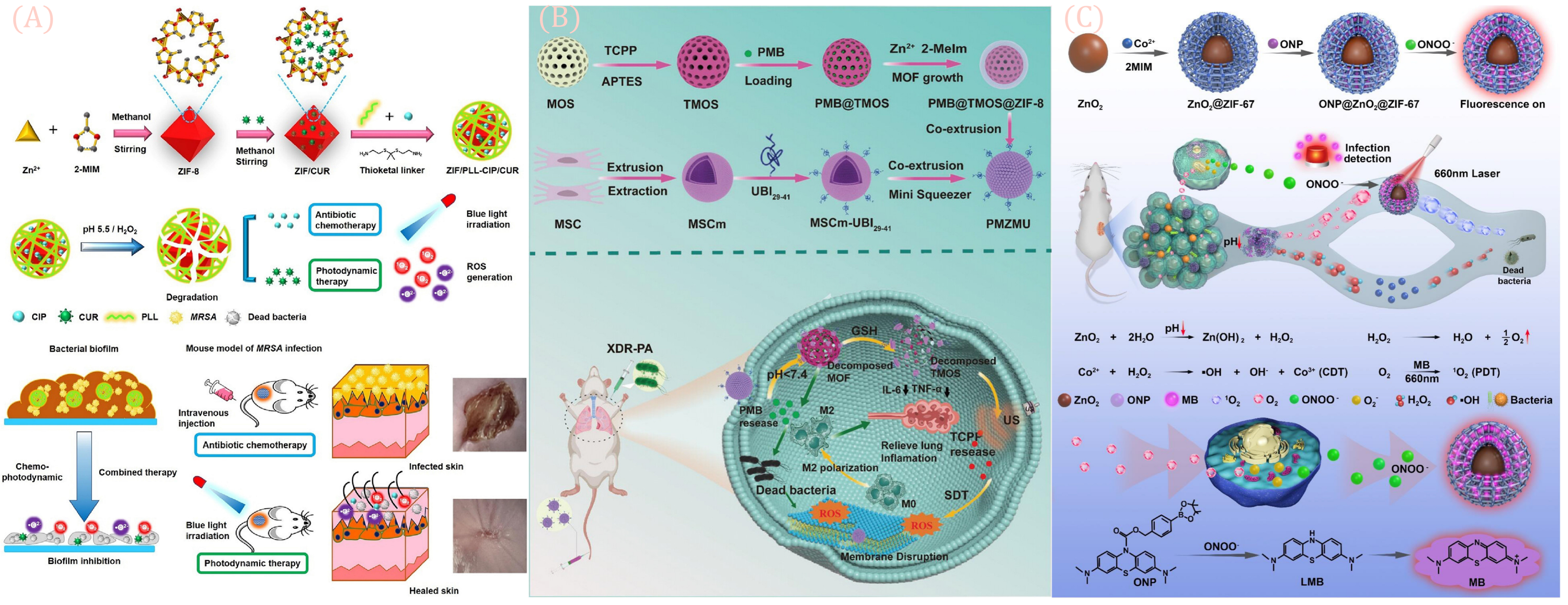
5.3. Other Antimicrobial Applications
| Application Category | MOF Material | Core Design/Strategy | Key Function & Outcome | Reference |
|---|---|---|---|---|
| 5.1 Wound dressings and medical coatings | Cu-MOF (HKUST-1) | Electrostatic spinning of fibers with chitosan/PVA blends. | Excellent physicochemical properties, biocompatibility, and antimicrobial activity for full-layer skin repair. | [79] |
| Ag@MOF | Dual-layer dressing: silver-loaded MOF/chitosan nanoparticles in the upper layer and PACS hydrogel in the lower layer. | Significantly accelerates wound healing, achieving more complete epithelialization and reducing inflammatory cells. | [79] | |
| Zn-MOF | Chitosan-based nanofiber scaffolds doped with tannic acid (TA). | Great potential in hemostatic wound care as a new antibacterial hemostatic wound dressing | [104] | |
| TA@ZIF-8 (TZ) | Complexed with oxidized bacterial cellulose (TBC) and MXene to form a hydrogel (TTZM) with a strong antimicrobial effect under 808 nm NIR irradiation. | Strong antimicrobial efficacy under 808 nm NIR irradiation for effective treatment of bacterial-infected wounds. | [80] | |
| Amino-functionalized nano-MOFs | MOFs were used as carriers and dynamic cross-linkers to form self-healing hydrogels with aldolylated alginates loaded with Cu NPs and curcumin. | Achieve synergistic anti-inflammatory and antimicrobial effects with good self-healing properties. | [81] | |
| CuBTC | Covalently immobilized on the surface of medical polypropylene (PP), releasing nitric oxide (NO). | Endowing surfaces with anti-fouling properties to inhibit bacterial adhesion on polypropylene. | [83] | |
| Cu-MOFs | Bionic coating with layer-by-layer self-assembly technology anchored to the inner surface of the PVC conduit. | Reduces non-specific adsorption of model proteins (anti-biofouling) and significantly inhibits platelet adhesion/activation (anti-platelet). | [84] | |
| Amino-functionalized Cu-MOF | Modification of thermoplastic polyurethane (TPU) by spin-coating and polyurethane prepolymer (PC) coating. | The release of NO demonstrates >96% antibacterial efficacy against Escherichia coli and Staphylococcus epidermidis. | [85] | |
| MOF-derived CuO@ZnO | Grafted on polydopamine (PDA) modified titanium alloy surface. | Controlled release of Zn2+/Cu2+ generates reactive oxygen species (ROS), effectively inhibiting bacterial biofilm formation and achieving a 99% kill rate against Staphylococcus aureus. | [86] | |
| Ag-MOF | Synthesized by a mild liquid phase method and integrated into acrylic coatings. | Continuous release of Ag+ achieves kill rates of 95.9% against Escherichia coli and 87.2% against Staphylococcus aureus. | [87] | |
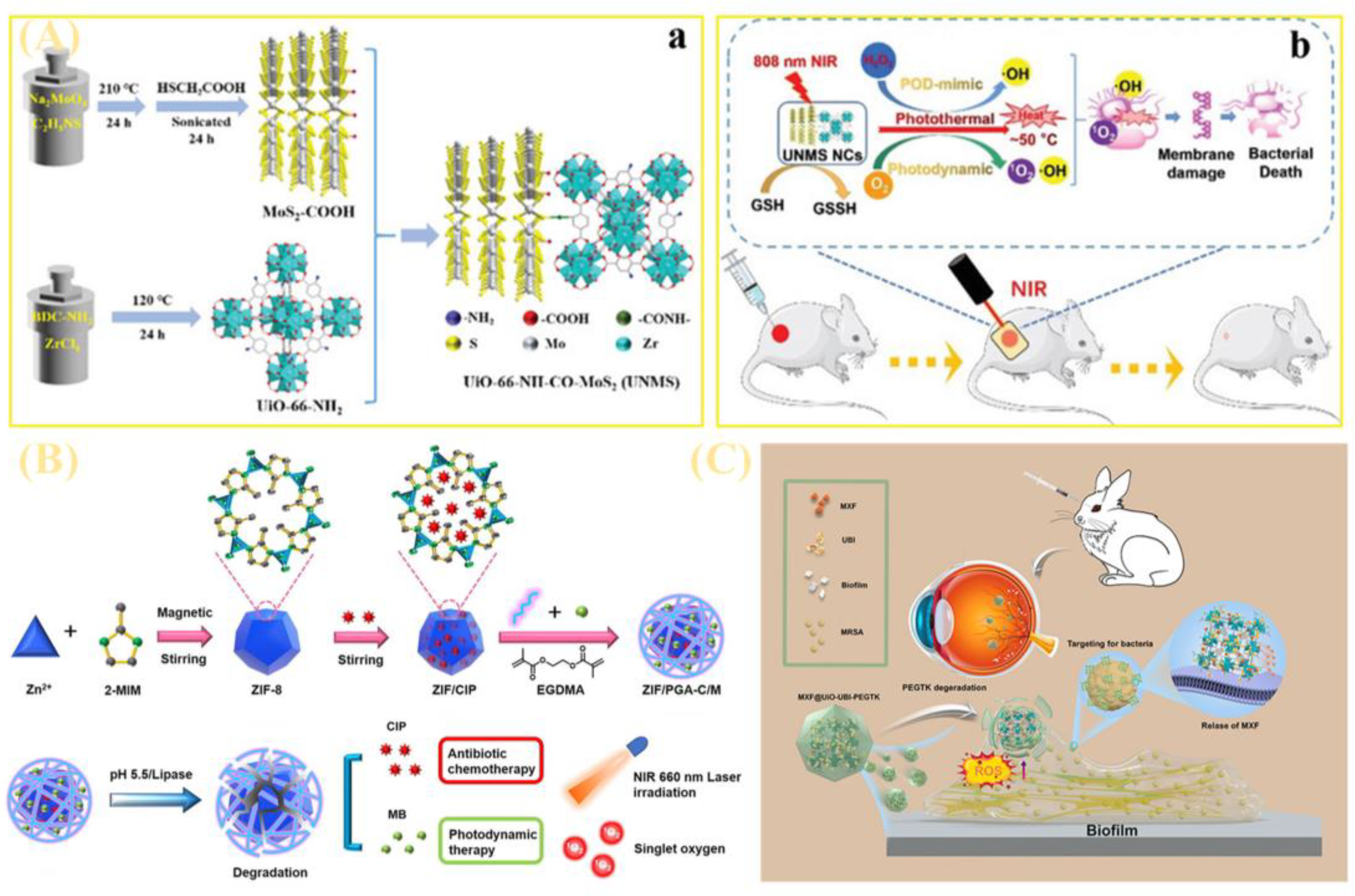
| 5.2 Treatment of drug-resistant bacterial infections | UiO-66 | Construction of the MXF@UiO-UBI-PEGTK nanosystem: loaded with antibiotic (moxifloxacin), targeted peptide (UBI), and ROS-responsive shell. | Possesses excellent biocompatibility, targeting capability, and synergistic bactericidal efficacy, specifically targeting biofilms and endophthalmitis. | [90] |
| UiO-66-NH2 | Forming UNMS nanocrystals with MoS2 via amidation yields positively charged particles. | Integrating photothermal, photodynamic, and peroxidase-mimetic activities, it generates reactive oxygen species under near-infrared light and exhibits broad-spectrum pH catalytic activity. | [91] | |
| Ga-MOF | Synthesized at room temperature, in situ loaded with the antimicrobial peptide (Melittin). | Achieves a synergistic antibacterial effect where the whole is greater than the sum of its parts, exhibits excellent biocompatibility, and accelerates the healing of MRSA-infected wounds. | [92] | |
| ZIF | The PLL modifies the ZIF via the ROS sensitive key, with internal loads CIP and CUR (ZIF/PLL-CIP/CUR). | Under blue light, ROS-responsive drug release generates 1O2/O2−, combining chemotherapy with photodynamic therapy to eliminate MRSA and biofilms. | [93] | |
| Zn-MOF | PDA-coated Zn-MOFs for enhancing the antibacterial efficacy of curcumin. | Small-sized MOFs effectively load and release curcumin, with PDA-Cur-Zn-MOFs inducing complete morphological distortion in bacteria. | [94] | |
| ZIF | Formation of the ZIF/PGA-C/M complex, featuring an enzyme-crosslinked polypeptide shell. | Effectively inhibits planktonic and biofilm MRSA, showing synergistic efficacy in a mouse skin infection model. | [88] | |
| ZIF-8 | Constructing PMZMU nanoparticles: stem cell membrane encapsulation, incorporation of photosensitizer, acid-responsive ZIF-8 loading of polymyxin B. | Synergistic sonodynamic-nano-antimicrobial therapy under ultrasound targets Gram-negative drug-resistant bacteria, reduces inflammation, and improves survival rates. | [95] | |
| Multimetallic MOF | Integrating bacterial binding ligands (boric acid) and photosensitizers (porphyrins) into a single metal–organic framework. | Enhanced antimicrobial activity through synergistic action and tight physical gaps, effective elimination of drug-resistant bacteria. | [96] | |
| ZIF-67 | Constructing an ONP@ZnO2@ZIF-67 (ONP@ZZ) core–shell structure. | As a “ROS factory”, it enables image-guided, in situ activation of photodynamic therapy (PDT) to eliminate drug-resistant bacteria. | [97] | |
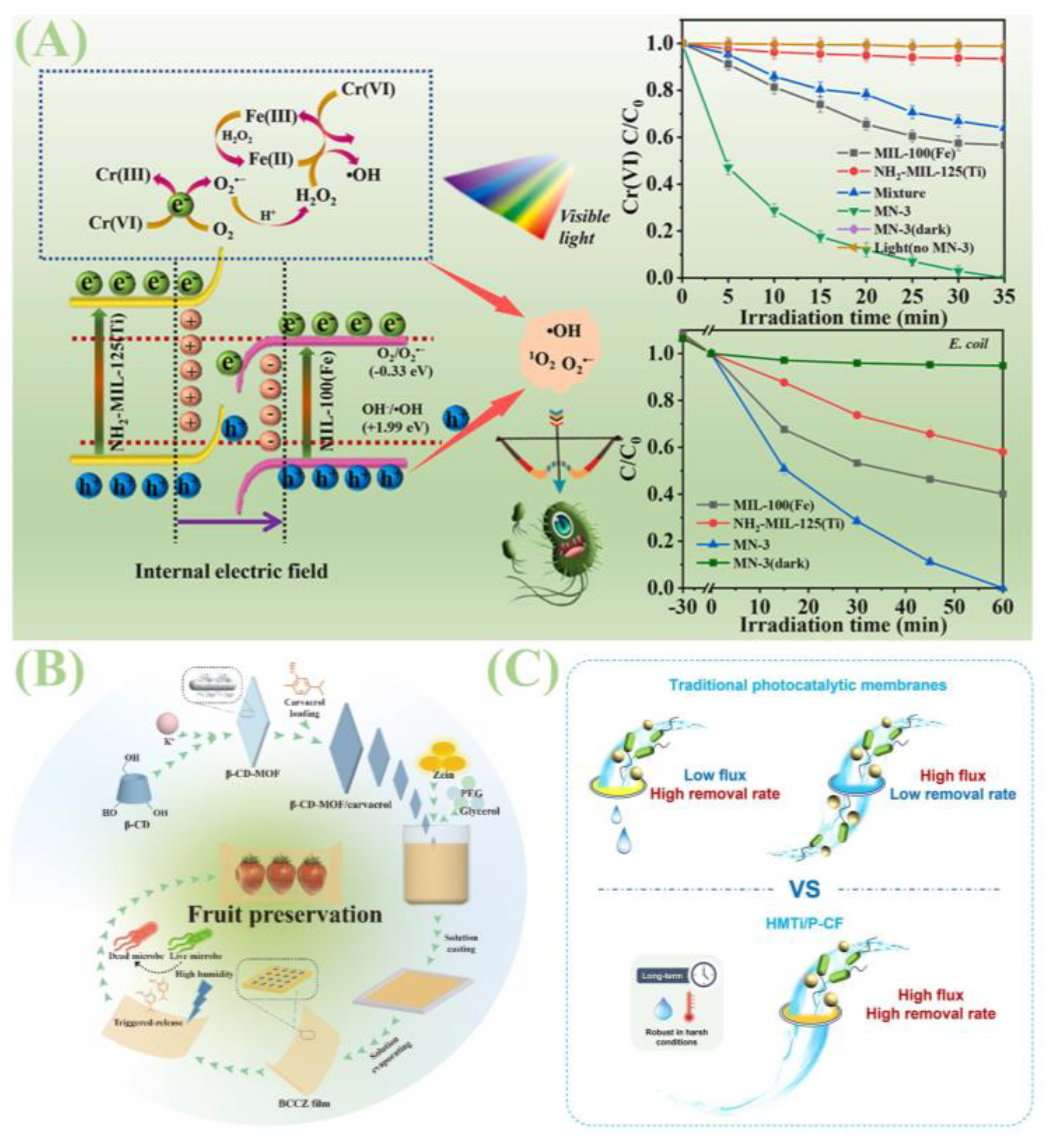
| 5.3 Other antimicrobial applications | β-CD-MOF | Loaded with apigenin and corn protein (zein), a BCCZ composite membrane was fabricated. | Effectively inhibits Gram-positive and Gram-negative bacteria and fungi under high humidity conditions; Suitable for food packaging. | [98] |
| MOFs | In situ formation of MOF-doped coatings on cellulose paper substrates. | Enhance hydrophobicity and provide long-lasting antibacterial effects; Active superhydrophobic paper-based packaging. | [89] | |
| Zn@MOF | As a carrier for volatile antibacterial essential oils (thymol). | An effective antimicrobial agent with potential for indirect application in the food sector. | [105] | |
| MOF-on-MOF (MN) | MOF-on-MOF heterojunctions prepared via simple ball milling. | For visible light-driven photoreduction of Cr(VI) and antimicrobial activity, treating wastewater containing Cr(VI) and bacteria. | [99] | |
| MIL-125(Ti)-NH2 | Following modification, it is immobilized within the non-woven fabric via phase inversion to form a photocatalytic membrane. | Electrostatic capture of pathogens, photocatalytic inactivation, and maintaining long-lasting antibacterial efficacy even in darkness, for water purification. | [100] | |
| MIL-125(Ti) | Derivative synthesis of carbon-MIL-125 materials. | New perspectives for the design of efficient photocatalysts for water disinfection. | [101] | |
| TCPP/UiO-66-NH2 | Multifunctional membranes fabricated by in situ growth on stainless steel mesh (SSM). | Achieving gravity-driven oil-water/seawater separation and visible light-driven bacterial inactivation. | [102] | |
| Anthracene-based metal–organic frameworks (GXNU-1/2/3) | Design and synthesis of porous anthracene-based metal–organic frameworks. | Possesses excellent ROS generation capacity, effectively inhibiting Gram-negative and Gram-positive bacteria, and can be used for treating items such as face masks and laboratory coats. | [103] |

6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monaghan, T.M.; Sloan, T.J.; Stockdale, S.R.; Blanchard, A.M.; Emes, R.D.; Wilcox, M.; Biswas, R.; Nashine, R.; Manke, S.; Gandhi, J.; et al. Metagenomics reveals impact of geography and acute diarrheal disease on the Central Indian human gut microbiome. Gut Microbes 2020, 12, 1752605. [Google Scholar] [CrossRef]
- Klümper, U.; Recker, M.; Zhang, L.; Yin, X.; Zhang, T.; Buckling, A.; Gaze, W.H. Selection for antimicrobial resistance is reduced when embedded in a natural microbial community. ISME J. 2019, 13, 2927–2937. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ito, K.; Nishimura, T.; Miyata, H.; Miyakawa, K.; Morita, T.; Ryo, A.; Kobayashi, H.; Mizunoe, Y.; Iwase, T. Antimicrobial strategy for targeted elimination of different microbes, including bacterial, fungal and viral pathogens. Commun. Biol. 2022, 5, 647. [Google Scholar] [CrossRef]
- Medina-Pizzali, M.L.; Venkatesh, A.; Riveros, M.; Cuicapuza, D.; Salmon-Mulanovich, G.; Mäusezahl, D.; Hartinger, S.M. Whole-Genome Characterisation of ESBL-Producing E. coli Isolated from Drinking Water and Dog Faeces from Rural Andean Households in Peru. Antibiotics 2022, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Cravo Oliveira Hashiguchi, T.; Ait Ouakrim, D.; Padget, M.; Cassini, A.; Cecchini, M. Resistance proportions for eight priority antibiotic-bacterium combinations in OECD, EU/EEA and G20 countries 2000 to 2030: A modelling study. Euro Surveill. 2019, 24, 1800445. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, G.; Yao, H.; Zhu, Y.; Shi, C.; Fu, Q.; Yang, F.; Wang, X. Formulation of pH-responsive PEGylated nanoparticles with high drug loading capacity and programmable drug release for enhanced antibacterial activity. Bioact. Mater. 2022, 16, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lyousfi, N.; Letrib, C.; Legrifi, I.; Blenzar, A.; El Khetabi, A.; El Hamss, H.; Belabess, Z.; Barka, E.A.; Lahlali, R. Combination of Sodium Bicarbonate (SBC) with Bacterial Antagonists for the Control of Brown Rot Disease of Fruit. J. Fungi 2022, 8, 636. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.-K.; Wong, T.W.; Thomas, S.; Grohens, Y. Natural Polymer/Inorganic Material Based Hybrid Scaffolds for Skin Wound Healing. Polym. Rev. 2015, 55, 453–490. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Jia, S. Silica encapsulated catalase@metal-organic framework composite: A highly stable and recyclable biocatalyst. Chem. Eng. J. 2018, 351, 506–514. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Wang, B. Shaping of metal-organic frameworks, a critical step toward industrial applications. Matter 2022, 5, 1070–1091. [Google Scholar] [CrossRef]
- El Kassaoui, M.; Lakhal, M.; Benyoussef, A.; El Kenz, A.; Loulidi, M. Effect of zinc substitution by magnesium and cadmium on hydrogen storage properties of connector-metal-organic framework-5. J. Alloys Compd. 2021, 874, 159902. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, G.; Yan, A.; Liu, Y.; Niu, Y.; Qu, R.; Ji, C. Preparation of metal organic framework materials with defects via a mixed-metallic centers strategy for enhanced removal of organic dye. J. Mol. Liq. 2023, 370, 121016. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.R.; Arafat, Y.; Khiadani, M.; Wang, S.; Shao, Z. Water-stable MOFs-based core-shell nanostructures for advanced oxidation towards environmental remediation. Compos. Part B Eng. 2020, 192, 107985. [Google Scholar] [CrossRef]
- Maya, F.; Palomino Cabello, C.; Frizzarin, R.M.; Estela, J.M.; Turnes Palomino, G.; Cerdà, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TrAC Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Sun, X.; Zhang, H.; Zhu, C.; Yin, X.; Li, Z.; Ma, X. MOFs assisted construction of Ni@NiOx/C nanosheets with tunable porous structure for high performance supercapacitors. J. Alloys Compd. 2022, 903, 163993. [Google Scholar] [CrossRef]
- Kolbe, F.; Krause, S.; Bon, V.; Senkovska, I.; Kaskel, S.; Brunner, E. High-Pressure in situ (129)Xe NMR Spectroscopy: Insights into Switching Mechanisms of Flexible Metal-Organic Frameworks Isoreticular to DUT-49. Chem. Mater. 2019, 31, 6193–6201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Wang, R. Functionally decorated metal–organic frameworks in environmental remediation. Chem. Eng. J. 2023, 455, 140741. [Google Scholar] [CrossRef]
- Choi, J.; Ingsel, T.; Neupane, D.; Mishra, S.R.; Kumar, A.; Gupta, R.K. Metal-organic framework-derived cobalt oxide and sulfide having nanoflowers architecture for efficient energy conversion and storage. J. Energy Storage 2022, 50, 104145. [Google Scholar] [CrossRef]
- Sozeri, H.; Kurtan, U.; Topkaya, R.; Baykal, A.; Toprak, M.S. Polyaniline (PANI)–Co0.5Mn0.5Fe2O4 nanocomposite: Synthesis, characterization and magnetic properties evaluation. Ceram. Int. 2013, 39, 5137–5143. [Google Scholar] [CrossRef]
- He, Y.; Xu, J.; Sun, X.; Ren, X.; Maharjan, A.; York, P.; Su, Y.; Li, H.; Zhang, J. Cuboidal tethered cyclodextrin frameworks tailored for hemostasis and injured vessel targeting. Theranostics 2019, 9, 2489–2504. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Zhao, H.; Cao, L.; Si, X.; Jiang, Y.; Cheng, P.; Zuo, Y.; Zhang, Y.; Su, L.; Wang, Y.; et al. A direct mechanochemical conversion of Pt-doped metal-organic framework-74 from doped metal oxides for CO oxidation. Mater. Today Nano 2022, 17, 100158. [Google Scholar] [CrossRef]
- Roy, R.N.; Lomakin, I.B.; Gagnon, M.G.; Steitz, T.A. The mechanism of inhibition of protein synthesis by the proline-rich peptide oncocin. Nat. Struct. Mol. Biol. 2015, 22, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, Y.; Yin, F.; Zhang, X.-F.; Qiu, J.; Yao, J. Integration of thermoresponsive MIL-121 into alginate beads for efficient heavy metal ion removal. J. Clean. Prod. 2022, 333, 130229. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Zhu, Y.; Chen, Y.; Gao, M.; Gao, H.; Liu, L.; Wang, L.; Mao, C.; Wang, Y. On-demand storage and release of antimicrobial peptides using Pandora’s box-like nanotubes gated with a bacterial infection-responsive polymer. Theranostics 2020, 10, 109–122. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, W.; Han, X.; Chen, Y.; da Silva, I.; Lee, D.; Sheveleva, A.M.; Wang, Z.; Li, J.; Li, W.; et al. Direct Observation of Ammonia Storage in UiO-66 Incorporating Cu(II) Binding Sites. J. Am. Chem. Soc. 2022, 144, 8624–8632. [Google Scholar] [CrossRef]
- Ruijin, L.; Danlian, H.; Lei, L.; Sha, C.; Yashi, C.; Guangfu, W.; Li, D.; Wei, Z.; Jiaxi, T.; Haojie, C. Lignin and metal–organic frameworks: Mutual partners on the road to sustainability. J. Mater. Chem. A 2023, 11, 2595–2617. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Chen, Y.; Jones, M.M.; Vanyo, S.T.; Li, C.; Visser, M.B.; Mahajan, S.D.; Sharma, R.K.; Swihart, M.T. Copper@ZIF-8 Core-Shell Nanowires for Reusable Antimicrobial Face Masks. Adv. Funct. Mater. 2021, 31, 2008054. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Q.; Chen, P.; Chen, L.; Ding, F.; Tang, J.; Li, Y.-J.; Au, C.-T.; Yin, S.-F. Copper-mediated metal-organic framework as efficient photocatalyst for the partial oxidation of aromatic alcohols under visible-light irradiation: Synergism of plasmonic effect and schottky junction. Appl. Catal. B-Environ. 2019, 248, 380–387. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Guo, J.; Tang, Z. Engineering Nanoscale Metal-Organic Frameworks for Heterogeneous Catalysis. Small Struct. 2021, 2, 2000141. [Google Scholar] [CrossRef]
- Meng, S.; Liu, J.; Yang, Y.; Mao, S.; Li, Z. Lanthanide MOFs based portable fluorescence sensing platform: Quantitative and visual detection of ciprofloxacin and Al3+. Sci. Total Environ. 2024, 922, 171115. [Google Scholar] [CrossRef]
- Zheng, H.; Hou, Y.; Li, S.; Ma, J.; Nan, J.; Li, T. Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation. Chin. Chem. Lett. 2022, 33, 5013–5022. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Zhao, K.; Wang, L.; Gao, X. Microwave-assisted synthesis of MOFs: Rational design via numerical simulation. Chem. Eng. J. 2021, 428, 131006. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Niu, S.; Li, C.; Chen, C.; Liu, Q.; Huo, J. Microwave-assisted rapid synthesis and activation of ultrathin trimetal–organic framework nanosheets for efficient electrocatalytic oxygen evolution. J. Colloid Interface Sci. 2021, 603, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yahia, M.; Lozano, L.A.; Zamaro, J.M.; Téllez, C.; Coronas, J. Microwave-assisted synthesis of metal–organic frameworks UiO-66 and MOF-808 for enhanced CO2/CH4 separation in PIM-1 mixed matrix membranes. Sep. Purif. Technol. 2024, 330, 125558. [Google Scholar] [CrossRef]
- Chongdar, S.; Ghosh, A.; Bal, R.; Bhaumik, A. Microwave-assisted synthesis of ZIF-9@xGO composites as cooperative electrocatalysts for electro-oxidation of benzyl alcohols coupled with H2 production. J. Mater. Chem. A 2024, 12, 233–246. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Meng, M.; Li, S.; Sheng, S.; Zhang, S.; Ni, W.; Tian, H.; Wang, Q. Metal-organic framework-mediated multifunctional nanoparticles for combined chemo-photothermal therapy and enhanced immunotherapy against colorectal cancer. Acta Biomater. 2022, 144, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Sun, Y.; Sun, Z.; Yang, M.; Gui, R. Zero-dimensional sulfur nanomaterials: Synthesis, modifications and applications. Coord. Chem. Rev. 2021, 438, 213913. [Google Scholar] [CrossRef]
- Hu, Z.; Kundu, T.; Wang, Y.; Sun, Y.; Zeng, K.; Zhao, D. Modulated Hydrothermal Synthesis of Highly Stable MOF-808(Hf) for Methane Storage. ACS Sustain. Chem. Eng. 2020, 8, 17042–17053. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Min, X.; Cheng, D.; Zhou, J.; Li, Y.; Xie, Z.; Liu, P.; Cai, W.; Zhang, C. Determination of catechol and hydroquinone with high sensitivity using MOF-graphene composites modified electrode. J. Electroanal. Chem. 2017, 789, 114–122. [Google Scholar] [CrossRef]
- Kubota, K.; Ito, H. Mechanochemical Cross-Coupling Reactions. Trends Chem. 2020, 2, 1066–1081. [Google Scholar] [CrossRef]
- Arul, P.; Gowthaman, N.S.K.; John, S.A.; Tominaga, M. Tunable electrochemical synthesis of 3D nucleated microparticles like Cu-BTC MOF-carbon nanotubes composite: Enzyme free ultrasensitive determination of glucose in a complex biological fluid. Electrochim. Acta 2020, 354, 136673. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Liu, M.; Bai, Y.; Wang, X.; Shang, S.; Chen, J.; Liu, Y. Electrochemical Synthesis of Large Area Two-Dimensional Metal–Organic Framework Films on Copper Anodes. Angew. Chem. Int. Ed. Engl. 2021, 60, 2887–2891. [Google Scholar] [CrossRef]
- Khosroshahi, N.; Bakhtian, M.; Safarifard, V. Mechanochemical synthesis of ferrite/MOF nanocomposite: Efficient photocatalyst for the removal of meropenem and hexavalent chromium from water. J. Photochem. Photobiol. A Chem. 2022, 431, 114033. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Yuan, Y.; Lv, D.; Qiao, Z.; An, D.; Wu, X.; Liang, H.; Li, Z.; Xia, Q. Highly rapid mechanochemical synthesis of a pillar-layer metal-organic framework for efficient CH4/N2 separation. Chem. Eng. J. 2020, 385, 123836. [Google Scholar] [CrossRef]
- Martín-Perales, A.I.; Peña-Ortiz, M.; Resende, J.A.; Malpartida, I.; Luque, R.; Balu, A.M. Continuous-flow mechanochemical preparation of Ag-Cu@ZIF-8 bimetallic system for antimicrobial applications. Results Eng. 2025, 25, 103581. [Google Scholar] [CrossRef]
- Sala, A.; Faye Diouf, M.D.; Marchetti, D.; Pasquale, L.; Gemmi, M. Mechanochemical Synthesis and Three-Dimensional Electron Diffraction Structure Solution of a Novel Cu-Based Protocatechuate Metal–Organic Framework. Cryst. Growth Des. 2024, 24, 3246–3255. [Google Scholar] [CrossRef]
- Carretero-Cerdán, A.; Carrasco, S.; Sanz-Marco, A.; Jaworski, A.; Martín-Matute, B. One-step microwave-assisted synthesis of amino-functionalized chromium(III) terephthalate MIL-101-NH2. Mater. Today Chem. 2023, 31, 101618. [Google Scholar] [CrossRef]
- Nazir, A.; Le, H.T.; Kasbe, A.; Park, C.-J. Si nanoparticles confined within a conductive 2D porous Cu-based metal–organic framework (Cu3(HITP)2) as potential anodes for high-capacity Li-ion batteries. Chem. Eng. J. 2020, 405, 126963. [Google Scholar] [CrossRef]
- Qiu, Q.; Chen, H.; Wang, Y.; Ying, Y. Recent advances in the rational synthesis and sensing applications of metal-organic framework biocomposites. Coord. Chem. Rev. 2019, 387, 60–78. [Google Scholar] [CrossRef]
- Rinawati, M.; Wang, Y.-X.; Chen, K.-Y.; Yeh, M.-H. Designing a spontaneously deriving NiFe-LDH from bimetallic MOF-74 as an electrocatalyst for oxygen evolution reaction in alkaline solution. Chem. Eng. J. 2021, 423, 130204. [Google Scholar] [CrossRef]
- Li, Y.-X.; Shen, J.-X.; Diao, Z.-J.; Qi, S.-C.; Liu, X.-Q.; Sun, L.-B. Loosening metal nodes in metal-organic frameworks to facilitate the regulation of valence. Fundam. Res. 2022, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Gu, G.; Wang, F.; Ren, J.; Zhang, H.; Zhao, Y. Microfluidic Electrospray Niacin Metal-Organic Frameworks Encapsulated Microcapsules for Wound Healing. Research 2019, 2019, 6175398. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, V.; Ragon, F.; Dan-Hardi, M.; Devic, T.; Vishnuvarthan, M.; Campo, B.; Vimont, A.; Clet, G.; Yang, Q.; Maurin, G.; et al. Inside Cover: A Series of Isoreticular, Highly Stable, Porous Zirconium Oxide Based Metal–Organic Frameworks (Angew. Chem. Int. Ed. 37/2012). Angew. Chem. Int. Ed. Engl. 2012, 51, 9188. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Li, H.; Xia, Z.; Yu, S.; Wang, S.; Sun, G. Iron-based binary metal-organic framework nanorods as an efficient catalyst for the oxygen evolution reaction. Chin. J. Catal. 2020, 42, 637–647. [Google Scholar] [CrossRef]
- Kim, D.; Park, K.W.; Park, J.T.; Choi, I. Photoactive MOF-Derived Bimetallic Silver and Cobalt Nanocomposite with Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2023, 15, 22903–22914. [Google Scholar] [CrossRef]
- Sun, S.; Liu, X.; Meng, X.; Yang, Z.; Zhang, X.; Dong, H. Bimetallic Metal–Organic Framework Microneedle Array for Wound Healing through Targeted Reactive Oxygen Species Generation and Electron Transfer Disruption. ACS Nano 2025, 19, 15109–15119. [Google Scholar] [CrossRef]
- Van Phuc, T.; Kang, S.G.; Chung, J.S.; Hur, S.H. Highly selective metal-organic framework-based electrocatalyst for the electrochemical reduction of CO2 to CO. Mater. Res. Bull. 2021, 138, 111228. [Google Scholar] [CrossRef]
- Tian, M.; Zhou, L.; Fan, C.; Wang, L.; Lin, X.; Wen, Y.; Su, L.; Dong, H. Bimetal-organic framework/GOx-based hydrogel dressings with antibacterial and inflammatory modulation for wound healing. Acta Biomater. 2023, 158, 252–265. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, W.; Li, Y.; Wang, D.; Li, X.; Nie, J.; Cui, H. Bimetallic FeCo metal–organic framework based cascade reaction system: Enhanced peroxidase activity for antibacterial performance. Arab. J. Chem. 2024, 17, 106032. [Google Scholar] [CrossRef]
- Ma, X.; Xiong, Y.; Liu, Y.; Han, J.; Duan, G.; Chen, Y.; He, S.; Mei, C.; Jiang, S.; Zhang, K. When MOFs meet wood: From opportunities toward applications. Chem 2022, 8, 2342–2361. [Google Scholar] [CrossRef]
- Wan, S.; Ou, M.; Zhong, Q.; Wang, X. Perovskite-type CsPbBr3 quantum dots/UiO-66(NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 2019, 358, 1287–1295. [Google Scholar] [CrossRef]
- Shen, X.; Yie, K.H.R.; Wu, X.; Zhou, Z.; Sun, A.; Al-Bishari, A.M.; Fang, K.; Baadani, M.A.A.; Deng, Z.; Ma, P.; et al. Improvement of aqueous stability and anti-osteoporosis properties of Zn-MOF coatings on titanium implants by hydrophobic raloxifene. Chem. Eng. J. 2022, 430, 133094. [Google Scholar] [CrossRef]
- Lin, C.; Guo, X.; Chen, L.; You, T.; Lu, J.; Sun, D. Ultrathin trimetallic metal–organic framework nanosheets for accelerating bacteria-infected wound healing. J. Colloid Interface Sci. 2022, 628, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.-A.; Li, Y.; Wang, J. The progress of electrochromic materials based on metal–organic frameworks. Coord. Chem. Rev. 2022, 475, 214891. [Google Scholar] [CrossRef]
- Li, W.-Q.; Wang, Y.-X.; Chen, J.-Q.; Hou, N.-N.; Li, Y.-M.; Liu, X.-C.; Ding, R.-R.; Zhou, G.-N.; Li, Q.; Zhou, X.-G.; et al. Boosting photo-Fenton process enabled by ligand-to-cluster charge transfer excitations in iron-based metal organic framework. Appl. Catal. B-Environ. 2021, 302, 120882. [Google Scholar] [CrossRef]
- Xie, T.; Qi, Y.; Li, Y.; Zhang, F.; Li, W.; Zhong, D.; Tang, Z.; Zhou, M. Ultrasmall Ga-ICG nanoparticles based gallium ion/photodynamic synergistic therapy to eradicate biofilms and against drug-resistant bacterial liver abscess. Bioact. Mater. 2021, 6, 3812–3823. [Google Scholar] [CrossRef]
- Erum, J.K.E.; Zhao, T.; Yu, Y.; Gao, J. MOF-derived tri-metallic nanoparticle-embedded cellulose aerogels for tetracycline degradation. Sustain. Mater. Technol. 2025, 45, e01441. [Google Scholar] [CrossRef]
- Hu, M.; Liu, J.; Song, S.; Wang, W.; Yao, J.; Gong, Y.; Li, C.; Li, H.; Li, Y.; Yuan, X.; et al. Ultra-thin Two-Dimensional Trimetallic Metal–Organic Framework for Photocatalytic Reduction of CO2. ACS Catal. 2022, 12, 3238–3248. [Google Scholar] [CrossRef]
- Huang, L.; Lin, H.; Zheng, C.Y.; Kluender, E.J.; Golnabi, R.; Shen, B.; Mirkin, C.A. Multimetallic High-Index Faceted Heterostructured Nanoparticles. J. Am. Chem. Soc. 2020, 142, 4570–4575. [Google Scholar] [CrossRef]
- Jian, M.; Liu, J.X.; Li, W.X. Hydroxyl improving the activity, selectivity and stability of supported Ni single atoms for selective semi-hydrogenation. Chem. Sci. 2021, 12, 10290–10298. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.Q.; Shu, G.F.; Jiang, S.P.; Xu, X.L.; Qi, J.; Jin, F.Y.; Liu, D.; Xiao, Y.H.; Lu, X.Y.; Du, Y.Z. Effective targeted therapy for drug-resistant infection by ICAM-1 antibody-conjugated TPGS modified β-Ga2O3:Cr3+ nanoparticles. Theranostics 2019, 9, 2739–2753. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Lin, Y.; Li, Z. Ultrasonic-assisted preparation of eucalyptus oil nanoemulsion: Process optimization, in vitro digestive stability, and anti-Escherichia coli activity. Ultrason. Sonochem. 2022, 82, 105904. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Q.; Hu, T.-L. Reverse-selective metal–organic framework materials for the efficient separation and purification of light hydrocarbons. Coord. Chem. Rev. 2022, 468, 214628. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Shen, E.; Liu, B.; Tian, Y.; Liang, L.; Yan, X.; Wu, H. Highly water-stable bimetallic organic framework MgCu-MOF74 for inhibiting bacterial infection and promoting bone regeneration. Biomed. Mater. 2022, 17, 065026. [Google Scholar] [CrossRef]
- Wang, S.; Yan, F.; Ren, P.; Li, Y.; Wu, Q.; Fang, X.; Chen, F.; Wang, C. Incorporation of metal-organic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. Int. J. Biol. Macromol. 2020, 158, 9–17. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Wang, D.; Zheng, Y.; Li, Y.; Meng, W.; Zhang, X.; Du, F.; Lee, S. Ag@MOF-loaded chitosan nanoparticle and polyvinyl alcohol/sodium alginate/chitosan bilayer dressing for wound healing applications. Int. J. Biol. Macromol. 2021, 175, 481–494. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, A.; Nitharwal, R.G.; Alam, J.; Mukhopadhyay, A.K.; Dasgupta, S.; Dhar, S.K. Modulation of the enzymatic activities of replicative helicase (DnaB) by interaction with Hp0897: A possible mechanism for helicase loading in Helicobacter pylori. Nucleic Acids Res. 2016, 44, 3288–3303. [Google Scholar] [CrossRef]
- Bigham, A.; Islami, N.; Khosravi, A.; Zarepour, A.; Iravani, S.; Zarrabi, A. MOFs and MOF-Based Composites as Next-Generation Materials for Wound Healing and Dressings. Small 2024, 20, 2311903. [Google Scholar] [CrossRef]
- Cheng, F.; Yi, X.; Dai, J.; Fan, Z.; He, J.; Huang, Y.; Li, H. Photothermal MXene@Zn-MOF-decorated bacterial cellulose-based hydrogel wound dressing for infectious wound healing. Cell Rep. Phys. Sci. 2023, 4, 101619. [Google Scholar] [CrossRef]
- Fan, Y.-L.; Liu, H.-J.; Wang, Z.-L.; Wang, Z.-L.; Zhu, L.-L.; Wang, Y.-Z.; Song, F. A One-Nano MOF-Two-Functions Strategy Toward Self-healing, Anti-inflammatory, and Antibacterial Hydrogels for Infected Wound Repair. Chem. Eng. J. 2024, 497, 155037. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Kazemzad, M. Recent advances in surface-mounted metal–organic framework thin film coatings for biomaterials and medical applications: A review. Biomater. Res. 2023, 27, 115. [Google Scholar] [CrossRef]
- Skvortsova, A.; Kocianova, A.; Guselnikova, O.; Elashnikov, R.; Burtsev, V.; Rimpelova, S.; Svobodova Pavlickova, V.; Fitl, P.; Lukesova, M.; Kolska, Z.; et al. Self-activated antibacterial MOF-based coating on medically relevant polypropylene. Appl. Surf. Sci. 2023, 623, 157048. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, X.; Gao, Y.; Long, J.; Bao, T.; Wang, S. Fabrication of metal-organic frameworks containing Cu2+ coated medical PVC catheters with durable antiplatelet and antibacterial activities. Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134779. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, X.; Huang, M.; Pei, X.; Gao, S.; Wu, D.; Chen, J.; Weng, Y. NO released via both a Cu-MOF-based donor and surface-catalyzed generation enhances anticoagulation and antibacterial surface effects. Biomater. Sci. 2023, 11, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Liu, H.; Yu, H.; Jiang, X.; Sun, D. MOF-derived CuO@ZnO modified titanium implant for synergistic antibacterial ability, osteogenesis and angiogenesis. Colloids Surf. B Biointerfaces 2022, 219, 112840. [Google Scholar] [CrossRef]
- Xie, X.; Wei, K.; Gao, D.; Zou, M.; Zeng, D.; Zhang, T.; Zhang, Y. Dual-functional Ag-MOF nanoparticle composite coatings for enhanced anticorrosion and antibacterial performance on aluminum alloys. Surf. Coat. Technol. 2025, 513, 132504. [Google Scholar] [CrossRef]
- Xiang, Y.-L.; Huang, S.-H.; Tang, D.-Y.; Zhang, P.-C.; Yong, Y.; Zhou, Q.-H. MOF/polypeptide hybrid nanocomposites with pH/lipase dual responsive drug release and photodynamic effect for combination treatment of drug-resistant bacterial infection. Mater. Des. 2023, 232, 112180. [Google Scholar] [CrossRef]
- Yang, R.; Liu, B.; Yu, F.; Li, H.; Zhuang, Y. Superhydrophobic cellulose paper with sustained antibacterial activity prepared by in-situ growth of carvacrol-loaded zinc-based metal organic framework nanorods for food packaging application. Int. J. Biol. Macromol. 2023, 234, 123712. [Google Scholar] [CrossRef]
- Yu, J.; Xu, H.; Wei, J.; Niu, L.; Zhu, H.; Jiang, C. Bacteria-Targeting Nanoparticles with ROS-Responsive Antibiotic Release to Eradicate Biofilms and Drug-Resistant Bacteria in Endophthalmitis. Int. J. Nanomed. 2024, 19, 2939–2956. [Google Scholar] [CrossRef]
- Liao, Z.-Y.; Xia, Y.-M.; Zuo, J.-M.; Wang, T.; Hu, D.-T.; Li, M.-Z.; Shao, N.-N.; Chen, D.; Song, K.-X.; Yu, X.; et al. Metal–Organic Framework Modified MoS2 Nanozyme for Synergetic Combating Drug-Resistant Bacterial Infections via Photothermal Effect and Photodynamic Modulated Peroxidase-Mimic Activity. Adv. Healthc. Mater. 2022, 11, 2101698. [Google Scholar] [CrossRef]
- Liu, S.; Ji, Y.; Zhu, H.; Shi, Z.; Li, M.; Yu, Q. Gallium-based metal–organic frameworks loaded with antimicrobial peptides for synergistic killing of drug-resistant bacteria. J. Mater. Chem. B 2023, 11, 10446–10454. [Google Scholar] [CrossRef]
- Xiang, Y.-L.; Tang, D.-Y.; Yan, L.-L.; Deng, L.-L.; Wang, X.-H.; Liu, X.-Y.; Zhou, Q.-H. Poly-l-lysine modified MOF nanoparticles with pH/ROS sensitive CIP release and CUR triggered photodynamic therapy against drug-resistant bacterial infection. Int. J. Biol. Macromol. 2024, 266, 131330. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Rehman, K.; Jabri, T.; Kanwal, T.; Perveen, S.; Rashid, M.A.; Kazi, M.; Ahmad Khan, S.; Saifullah, S.; Shah, M.R. Improving curcumin bactericidal potential against multi-drug resistant bacteria via its loading in polydopamine coated zinc-based metal–organic frameworks. Drug Deliv. 2023, 30, 2159587. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, X.; Chen, S.; He, Y.; Xie, L.; Gao, F.; Zhu, C.; Jin, X.; Yan, H.; Ye, Y.; et al. Biomimetic Metal–Organic Framework Gated Nanoplatform for Sonodynamic Therapy against Extensively Drug Resistant Bacterial Lung Infection. Adv. Sci. 2024, 11, 2402473. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Qi, J.; Dong, R.; Liu, H.; Wu, D.; Shao, H.; Jiang, X. Boronic Acid-Decorated Multivariate Photosensitive Metal–Organic Frameworks for Combating Multi-Drug-Resistant Bacteria. ACS Nano 2022, 16, 7732–7744. [Google Scholar] [CrossRef]
- Huang, K.; Li, F.; Yuan, K.; Yang, Y.; Chang, H.; Liang, Y.; Yan, X.; Zhao, J.; Tang, T.; Yang, S. A MOF-armored zinc-peroxide nanotheranostic platform for eradicating drug resistant bacteria via image-guided and in situ activated photodynamic therapy. Appl. Mater. Today 2022, 28, 101513. [Google Scholar] [CrossRef]
- Nong, W.; Luo, H.; Wang, G.; Chen, Q.; Zou, X.; Miao, W.; Wu, J.; Guan, W.; Qu, S. β-CD-MOF-based edible antimicrobial packaging film with humidity-controlled carvacrol release for preserving fresh strawberry. Carbohydr. Polym. 2025, 351, 123133. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Qian, J.; Qiu, M.; Li, N.; Du, H.; Hu, X.; Fu, Y.; Tan, M.; Hao, D.; et al. S-scheme MOF-on-MOF heterojunctions for enhanced photo-Fenton Cr(VI) reduction and antibacterial effects. Chemosphere 2023, 344, 140277. [Google Scholar] [CrossRef]
- Hou, F.; Wei, W.; Shen, R.; Hou, R.; Li, Y.; Guo, Z.; Wei, A. Leveraging the capture and disturbance effects to break the trade-off between high flux and high removal rate in photocatalytic membranes for continuous flow water disinfection. Sep. Purif. Technol. 2025, 358, 130334. [Google Scholar] [CrossRef]
- Sha, L.; Ji, X.; Si, H.; Zhang, L.; Li, C.; Wu, Q.; Zhao, X.; Chen, H. The facile fabrication and structural control of carbon-MIL-125 by coupling pre-hydrolysate and Ti-MOF for photocatalytic sterilization under visible light. J. Chem. Technol. Biotechnol. 2021, 96, 2579–2587. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, J.; Wu, Z.; Wang, M.; Lv, Y.; Chen, Z.; Ma, S. Superwetting stainless steel mesh decorated with TCPP-doped UiO-66-NH2 with enhanced gravity-driven oil–water separation and visible-light-driven sterilization performance. Appl. Surf. Sci. 2023, 638, 157993. [Google Scholar] [CrossRef]
- Li, Y.-L.; Ai, J.-F.; Chen, Z.-C.; Wang, H.-L.; Zhu, Z.-H.; Liang, F.-P.; Zou, H.-H. Multiple Strategies Enhance the ROS of Metal–Organic Frameworks for Energy-Efficient Photocatalytic Water Purification and Sterilization. ACS Mater. Lett. 2023, 5, 1317–1331. [Google Scholar] [CrossRef]
- Lamei, E.; Hasanzadeh, M. Fabrication of chitosan nanofibrous scaffolds based on tannic acid and metal-organic frameworks for hemostatic wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 409–420. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Zhou, B.; Mei, L.; Wang, Q.; Zhang, B. Porous metal-organic framework (MOF) Carrier for incorporation of volatile antimicrobial essential oil. Food Control 2019, 98, 174–178. [Google Scholar] [CrossRef]
- Wang, N.; Wei, C.; Wei, F.; Huang, H.; Huang, F.; Chen, D.; Yan, C.; Zhu, Y. 3D printing of MOF reinforced flame-retardant PA12 composites with customizable structures. J. Mater. Chem. A 2025, 13, 13230–13247. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wang, Z.; Chen, S.; Wang, F.; Zhang, J. Designing homochiral metal–organic frameworks with ultrahigh surface areas and stability for practical applications. Sci. Bull. 2025, 70, 1038–1041. [Google Scholar] [CrossRef]
- Thordstein, M. Transspinal direct current stimulation as treatment of polyneuropathy pain. Translation to clinical practice. Brain Stimul. 2025, 18, 576–577. [Google Scholar] [CrossRef]
- Eremina, J.A.; Ermakova, E.A.; Smirnova, K.S.; Klyushova, L.S.; Berezin, A.S.; Sukhikh, T.S.; Zubenko, A.A.; Fetisov, L.N.; Kononenko, K.N.; Lider, E.V. Cu(II), Co(II), Mn(II) complexes with 5-phenyltetrazole and polypyridyl ligands: Synthesis, characterization and evaluation of the cytotoxicity and antimicrobial activity. Polyhedron 2021, 206, 115352. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Zhao, J.; Heng, H.; Peng, M.; Sun, L.; Dai, L.; Chan, E.W.-C.; Chen, S. LTX-315 is a novel broad-spectrum antimicrobial peptide against clinical multidrug-resistant bacteria. J. Adv. Res. 2025, in press ISSN 2090-1232. [Google Scholar] [CrossRef]
- Marchenko, R.D.; Lysova, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Potapov, A.S. Synthesis, structural diversity, luminescent properties and antibacterial effects of cadmium(II) and silver(I) coordination compounds with bis(1,2,3-benzotriazol-1-yl)alkanes. Polyhedron 2020, 177, 114330. [Google Scholar] [CrossRef]

| Characteristic Dimension | Metal–Organic Frameworks (MOFs) [12,13,14,15,16,17,18,19,20,21,22] | Conventional Organic/Inorganic Compounds and Salts [1,2,3] | Single-Metal/Metal Oxide Nanoparticles (Such as nAg, nZnO, TiO2) [9,10,11] | Antibiotics [4,5,6,7,8] |
|---|---|---|---|---|
| Antimicrobial mechanism |
|
|
|
|
| Structural adjustability/design flexibility | Pore size, shape, and functional groups can be precisely controlled | Fixed chemical structure | Limited control of size and shape | Dependent on the inherent chemical structure |
| Persistence of antimicrobial activity | Metal ions are slowly released through the framework degradation, providing a prolonged duration of action. | Prone to rapid depletion or deactivation | Inactivation due to rapid release or agglomeration of ions | Prone to degradation by drug-resistant enzymes, with a limited effective period. |
| Load and Synergy Capacity | High specific surface area/porosity, achieving synergistic enhancement | No load capacity | The surface may be minimally modified, but it has low load-bearing capacity and is prone to leakage. | No load required |
| Risk of bacterial resistance | Multi-Mechanism Synergy: Reducing the Risk of Single-Mechanism Induced Resistance | A single mechanism of action is prone to inducing drug resistance. | Dependent on ion release or photocatalysis, it may be risky for long-term use. | Misuse leads to a sharp rise in drug resistance. |
| Biocompatibility and Toxicity | Selection of biocompatible metals/ligands controls toxicity | Certain compounds exhibit relatively high toxicity. | High concentrations of metal ions may cause cytotoxicity and the risk of nanoparticle accumulation in the body. | Side effects such as allergic reactions, liver and kidney toxicity |
| Primary application areas | Antimicrobial Coatings & Implants; Targeted Drug Delivery Systems; Smart Wound Dressings; Food Active Packaging; Water Purification. | Surface disinfectant; Industrial preservative | Antibacterial Textiles; Antimicrobial Coatings; Personal Care Additives. | Clinical Infection Treatment; Livestock growth promotion. |
| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Hydrothermal/Solvothermal [29,30,31,32] | The process is simple; MOFs with homogeneous morphology and high structural stability can be prepared, and the particles are well dispersed. | Requires high temperature and high pressure environments, limiting scalability for mass production. |
| Microwave-Assisted [33,34,35,36,37,39,48] | Highly efficient; fast reaction kinetics; Rapid and uniform heating can significantly reduce reaction time. High spatiotemporal yield (e.g., >1200 kg/m3/day); Good crystallinity and phase selectivity. | There exists a risk of localized overheating, which may adversely affect the nucleation and crystal growth of MOFs. |
| Electrochemical [37,38,40,41,42,43,44] | The release of metal ions/ligands can be precisely controlled. device integration is facilitated, and the crystalline quality of the prepared films is high | Constrained by large-scale production capacity |
| Mechanochemical [45,47] | Green and efficient; Fast reaction rate; easy to operate; high stability; suitable for industrialization; High temporal and spatial yields; continuous flow production is possible. | Challenges in improving material crystallinity. |
| Type | Metal Center/Structural Features | Core Benefits | Main Limitations |
|---|---|---|---|
| Single-metal–organic frameworks [50,51,52,53,54] | Single metal ions (such as Ag+, Cu2+, Zn2+, Fe3+, Zr4+) | Simple structure, mature synthesis; various metal choices, can have both intrinsic antimicrobial properties; adjustable ligand function, easy to introduce specific functional groups. | Relatively monofunctional; stability may be inadequate; some metal ions are potentially cytotoxic. |
| Bimetallic metal–organic framework materials [56,57,58,59,60] | Synergistic interaction between two metal ions (e.g., Zn/Cu, Fe/Zr, Mg/Cu) | Significant synergistic effect, performance (e.g., catalytic activity) 1 + 1 > 2; strong designability of performance; easier integration of environmental response (e.g., photothermal, glucose response) and other smart functions. | Synthesis is more complex; synergistic mechanisms are poorly elucidated; biosafety still needs systematic assessment. |
| Multimetallic metal–organic framework materials [61,62,63,64,65,66,67,68,69,70,71] | Three or more metal ions (e.g., NiCoCu, PtPdRhAu) | Synergistic multifunctionality and extremely high performance in catalysis, antimicrobial, and other applications; a wide range of application areas (environment, energy, biomedicine). | Extremely challenging to synthesize; difficult to characterize and analyze; costly (especially with precious metal systems); lack of biosafety data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Cui, J.; Wu, S.; Cao, Z.; Cao, P. Engineering Metal-Organic Frameworks for Enhanced Antimicrobial Efficacy: Synthesis Methodologies, Mechanistic Perspectives, and Versatile Applications. J. Funct. Biomater. 2025, 16, 353. https://doi.org/10.3390/jfb16090353
Zheng Z, Cui J, Wu S, Cao Z, Cao P. Engineering Metal-Organic Frameworks for Enhanced Antimicrobial Efficacy: Synthesis Methodologies, Mechanistic Perspectives, and Versatile Applications. Journal of Functional Biomaterials. 2025; 16(9):353. https://doi.org/10.3390/jfb16090353
Chicago/Turabian StyleZheng, Zaixiang, Junnan Cui, Shutong Wu, Zhimin Cao, and Pan Cao. 2025. "Engineering Metal-Organic Frameworks for Enhanced Antimicrobial Efficacy: Synthesis Methodologies, Mechanistic Perspectives, and Versatile Applications" Journal of Functional Biomaterials 16, no. 9: 353. https://doi.org/10.3390/jfb16090353
APA StyleZheng, Z., Cui, J., Wu, S., Cao, Z., & Cao, P. (2025). Engineering Metal-Organic Frameworks for Enhanced Antimicrobial Efficacy: Synthesis Methodologies, Mechanistic Perspectives, and Versatile Applications. Journal of Functional Biomaterials, 16(9), 353. https://doi.org/10.3390/jfb16090353






