Propolis as a Natural Remedy in Reducing Dental Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
2.5.1. Identification of Influential Studies
2.5.2. Pooled Effect Estimation and Between-Study Heterogeneity
2.5.3. Assessment of Publication Bias
2.5.4. Statistical Environment
3. Results
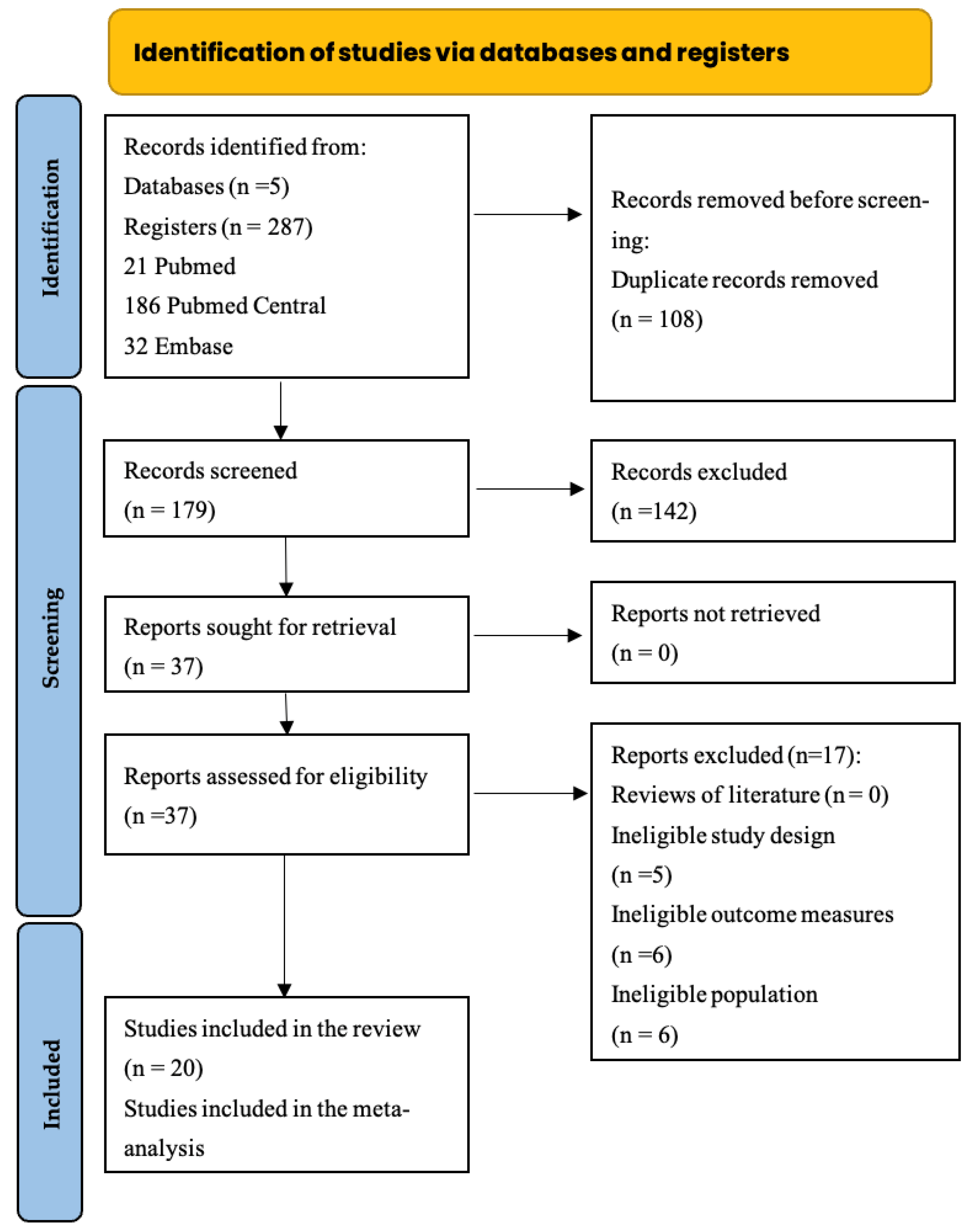
3.1. Search Strategy and Study Selection
3.2. Mouthwash Results
3.3. Toothpaste Results
3.4. Quality Assessment
| N | Author, Year | Randomization Process | Deviations from the Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Results | Overall |
|---|---|---|---|---|---|---|---|
| 1 | Amano et al., 2025 [40] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| 2 | Bapat et al., 2021 [28] | Low risk | Low risk | Some concerns | Low risk | Low risk | Low risk |
| 3 | Biria et al., 2019 [42] | Some concerns | Some concerns | Low risk | Some concerns | Low risk | Some concerns |
| 4 | Dehghani et al., 2019 [29] | Low risk | Low risk | Some concerns | Low risk | Low risk | Low risk |
| 5 | Dodwad et al., 2011 [20] | Low risk | Some concerns | Low risk | Some concerns | Some concerns | Some concerns |
| 6 | Ercan et al., 2015 [30] | Low risk | Some concerns | Low risk | Low risk | Some concerns | Low risk |
| 7 | Fereidooni et al., 2014 [43] | Some concerns | Low risk | Low risk | Some concerns | Low risk | Some concerns |
| 8 | Gunjal et al., 2024 [31] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| 9 | Khabazian et al., 2025 [32] | Low risk | Low risk | Low risk | Some concerns | Some concerns | Some concerns |
| 10 | Kiani et al., 2022 [33] | Low risk | Low risk | Low risk | Low risk | Some concerns | Low risk |
| 11 | Koo et al., 2002 [34] | Low risk | Low risk | Low risk | Some concerns | Low risk | Low risk |
| 12 | Kripal et al., 2019 [35] | Low risk | Some concerns | Low risk | High risk | Some concerns | Some concerns |
| 13 | Mallikarjun et al., 2022 [36] | Some concerns | High risk | Low risk | High risk | Low risk | High risk |
| 14 | Murray et al., 1997 [37] | Some concerns | Some concerns | Low risk | High risk | Low risk | Some concerns |
| 15 | Penmetsa et al., 2023 [44] | Some concerns | Low risk | Some concerns | High risk | Some concerns | Some concerns |
| 16 | Porwal et al., 2018 [39] | Some concerns | High risk | Low risk | High risk | Low risk | High risk |
| 17 | Suriamah et al., 2019 [46] | Some concerns | Some concerns | Low risk | High risk | Low risk | Some concerns |
3.5. Characteristics of the Studies Included in the Meta-Analysis
| Plaque Index Studies | |||||||
|---|---|---|---|---|---|---|---|
| Study ID | Citation | Pre-Treatment | Post-Treatment | ||||
| Mean | SD | N | Mean | SD | N | ||
| 1 | Gunjal, 2024 [31] | 1.37 | 0.27 | 45 | 0.70 | 0.25 | 45 |
| 2 | Bapat, 2021 [28] | 0.99 | 0.12 | 30 | 0.46 | 0.09 | 30 |
| 3 | Dehghani, 2019 [29] | 1.40 | 0.60 | 18 | 0.70 | 0.50 | 18 |
| 4 | Kripal, 2019 [35] | 1.95 | 0.07 | 15 | 1.47 | 0.21 | 15 |
| 5 | Pereira, 2011 [38] | 2.39 | 0.69 | 22 | 1.82 | 0.62 | 21 |
| 6 | Koo, 2002 [34] | 1.13 | 0.14 | 6 | 0.78 | 0.17 | 6 |
| Gingival Index Studies | |||||||
| Study ID | Citation | Pre-Treatment | Post-Treatment | ||||
| Mean | SD | N | Mean | SD | N | ||
| 1 | Pereira, 2011 [38] | 1.17 | 0.20 | 22 | 0.70 | 0.18 | 21 |
| 2 | Khabazian, 2025 [32] | 2.25 | 0.37 | 10 | 2.00 | 0.19 | 10 |
| 3 | Gunjal, 2024 [31] | 1.31 | 0.24 | 45 | 0.65 | 0.26 | 45 |
| 4 | Bapat, 2021 [28] | 1.03 | 0.13 | 30 | 0.52 | 0.16 | 30 |
| 5 | Dehghani, 2019 [29] | 1.70 | 0.30 | 18 | 1.30 | 0.60 | 18 |
| 6 | Kripal, 2019 [35] | 1.86 | 0.19 | 15 | 1.42 | 0.20 | 15 |
3.6. Plaque Index Meta-Analysis
3.6.1. Influence Diagnostics Analysis
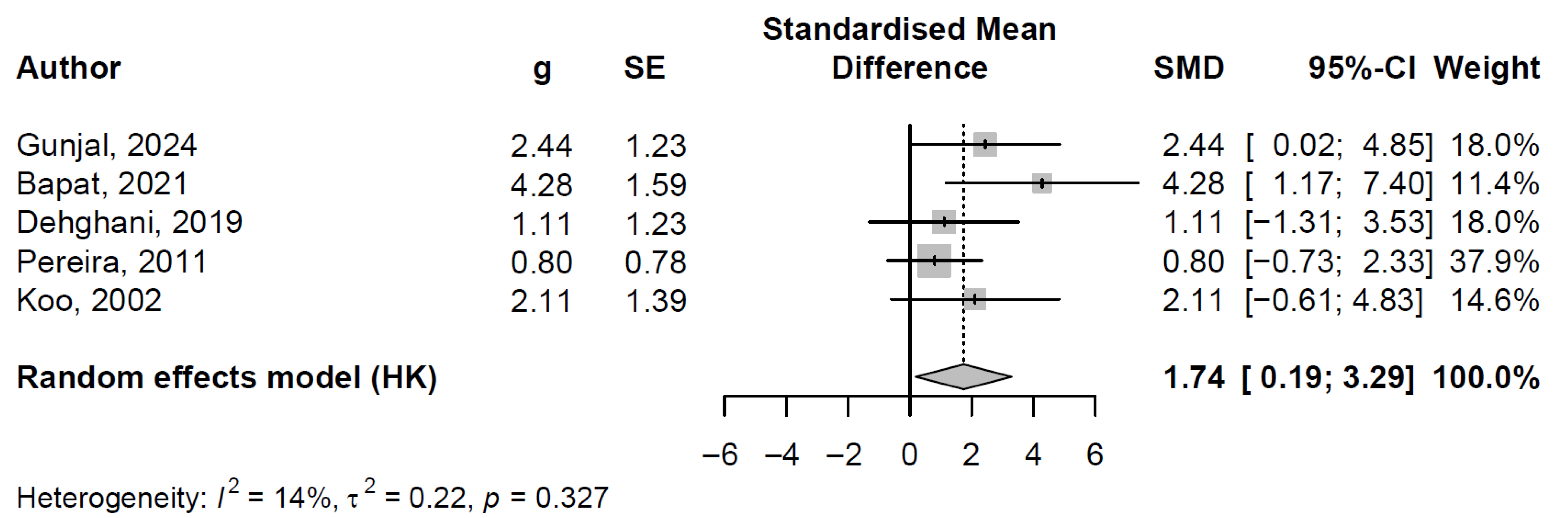
3.6.2. Pooled Effect and Between-Study Heterogeneity
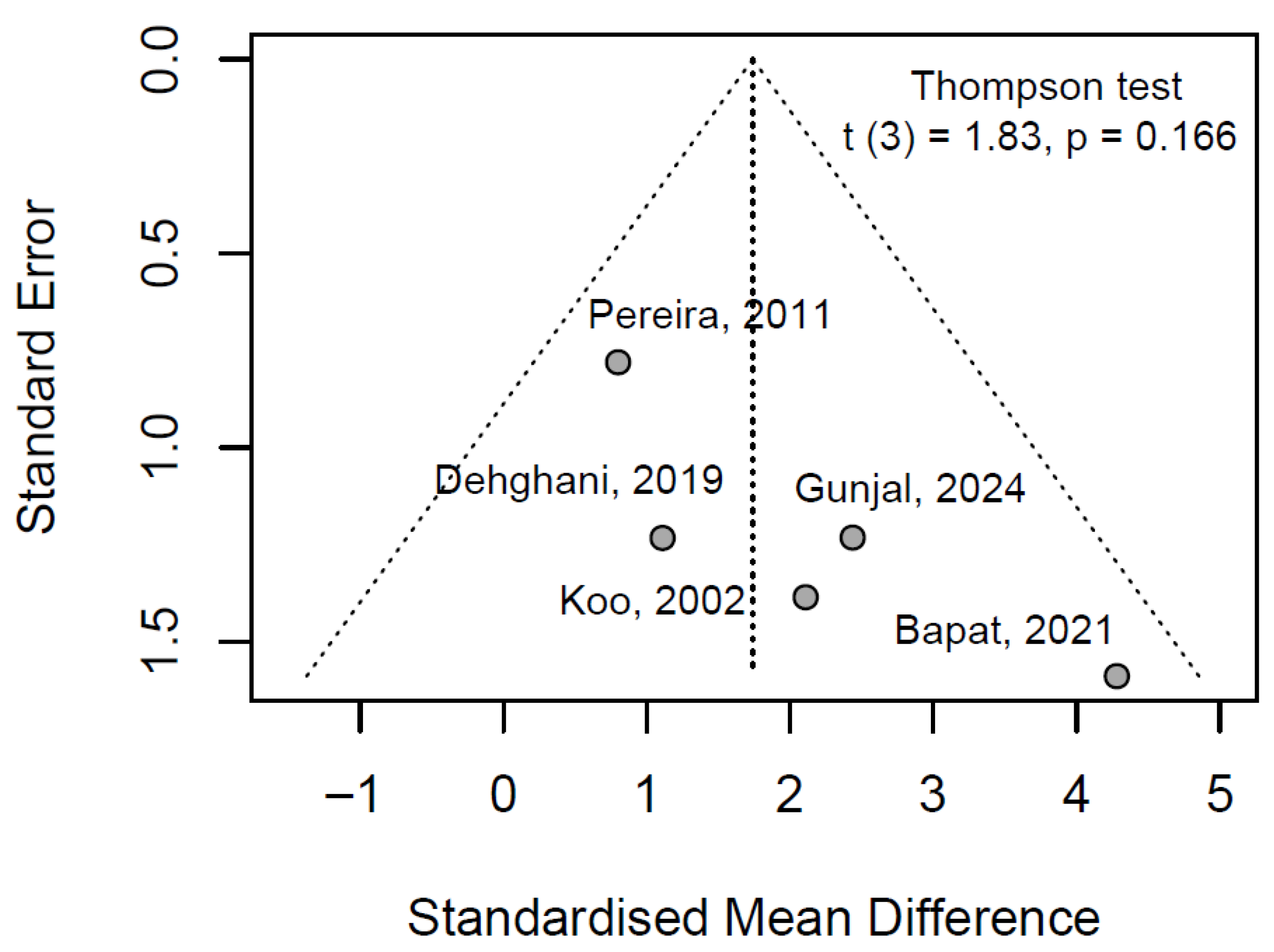
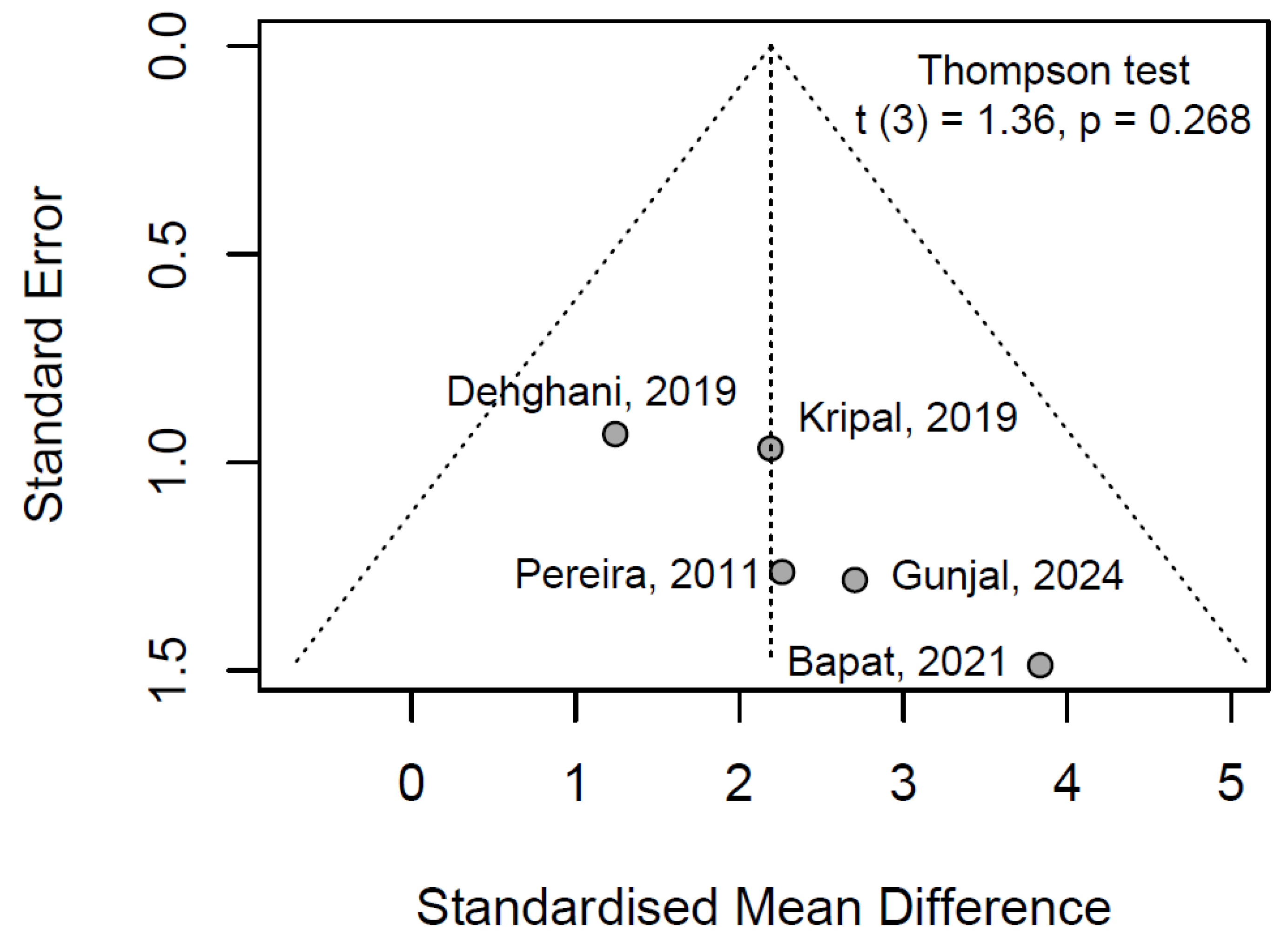
3.6.3. Publication Bayes
3.7. Gingival Index Meta-Analysis
3.7.1. Influence Diagnostics Analysis
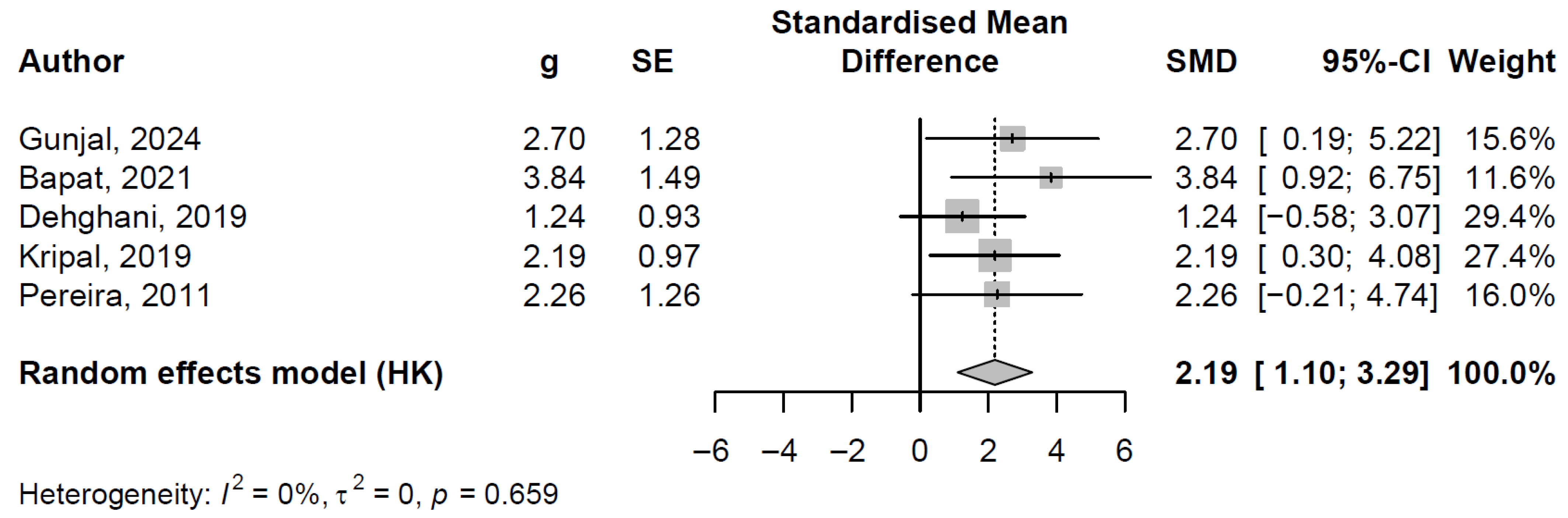
3.7.2. Pooled Effect and Between-Study Heterogeneity
3.7.3. Publication Bayes
4. Discussion
4.1. Mouthwash
4.2. Toothpaste
4.3. Formulation Variability and Clinical Implications
4.4. Practical Relevance for Clinicians
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D. Dental Plaque as a Biofilm and a Microbial Community—Implications for Health and Disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental Plaque as a Microbial Biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Khan, J.H.; Brand-Miller, J.C.; Eberhard, J. The Impact of Carbohydrate Quality on Dental Plaque pH: Does the Glycemic Index of Starchy Foods Matter for Dental Health? Nutrients 2021, 13, 2711. [Google Scholar] [CrossRef]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental Plaque–Induced Gingival Conditions. J. Periodontol. 2018, 89, S17–S27. [Google Scholar] [CrossRef]
- Halboub, E.; Al-Maweri, S.A.; Al-Wesabi, M.; Al-Kamel, A.; Shamala, A.; Al-Sharani, A.; Koppolu, P. Efficacy of Propolis-Based Mouthwashes on Dental Plaque and Gingival Inflammation: A Systematic Review. BMC Oral Health 2020, 20, 198. [Google Scholar] [CrossRef]
- Mihai, C.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Chirilă, F.; Moritz, R.F.A.; Schlüns, H. Interactions among Flavonoids of Propolis Affect Antibacterial Activity against the Honeybee Pathogen Paenibacillus Larvae. J. Invertebr. Pathol. 2012, 110, 68–72. [Google Scholar] [CrossRef]
- Borba, R.S.; Spivak, M. Propolis Envelope in Apis Mellifera Colonies Supports Honey Bees against the Pathogen, Paenibacillus Larvae. Sci. Rep. 2017, 7, 11429. [Google Scholar] [CrossRef]
- Wilson, M.B.; Pawlus, A.D.; Brinkman, D.; Gardner, G.; Hegeman, A.D.; Spivak, M.; Cohen, J.D. 3-Acyl Dihydroflavonols from Poplar Resins Collected by Honey Bees Are Active against the Bee Pathogens Paenibacillus Larvae and Ascosphaera Apis. Phytochemistry 2017, 138, 83–92. [Google Scholar] [CrossRef]
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The Use of Propolis in Dentistry, Oral Health, and Medicine: A Review. J. Oral Biosci. 2021, 63, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An Update on Its Chemistry and Pharmacological Applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Więckiewicz, W.; Miernik, M.; Więckiewicz, M.; Morawiec, T. Does Propolis Help to Maintain Oral Health? Evid.-Based Complement. Altern. Med. 2013, 2013, 351062. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Chimshirova, R.; Antonova, D.; Gechovska, K.; Thanh, L.N.; Lien, N.T.P.; Phuong, D.T.L.; Bankova, V. Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules 2022, 27, 7834. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities. Molecules 2021, 26, 4589. [Google Scholar] [CrossRef] [PubMed]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef] [PubMed]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef]
- Altabbal, S.; Athamnah, K.; Rahma, A.; Wali, A.F.; Eid, A.H.; Iratni, R.; Al Dhaheri, Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 2023, 16, 450. [Google Scholar] [CrossRef]
- López-Valverde, N.; Pardal-Peláez, B.; López-Valverde, A.; Flores-Fraile, J.; Herrero-Hernández, S.; Macedo-de-Sousa, B.; Herrero-Payo, J.; Ramírez, J.M. Effectiveness of Propolis in the Treatment of Periodontal Disease: Updated Systematic Review with Meta-Analysis. Antioxidants 2021, 10, 269. [Google Scholar] [CrossRef]
- Dodwad, V.; Kukreja, B.J. Propolis Mouthwash: A New Beginning. J. Indian Soc. Periodontol. 2011, 15, 121–125. [Google Scholar] [CrossRef]
- Sparabombe, S.; Monterubbianesi, R.; Tosco, V.; Orilisi, G.; Hosein, A.; Ferrante, L.; Putignano, A.; Orsini, G. Efficacy of an All-Natural Polyherbal Mouthwash in Patients With Periodontitis: A Single-Blind Randomized Controlled Trial. Front. Physiol. 2019, 10, 632. [Google Scholar] [CrossRef]
- Giammarinaro, E.; Marconcini, S.; Genovesi, A.; Poli, G.; Lorenzi, C.; Covani, U. Propolis as an Adjuvant to Non-Surgical Periodontal Treatment: A Clinical Study with Salivary Anti-Oxidant Capacity Assessment. Minerva Stomatol. 2018, 67, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Tanasiewicz, M.; Skucha-Nowak, M.; Dawiec, M.; Król, W.; Skaba, D.; Twardawa, H. Influence of Hygienic Preparations with a 3% Content of Ethanol Extract of Brazilian Propolis on the State of the Oral Cavity. Adv. Clin. Exp. Med. 2012, 21, 81–92. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Cochrane Library About PICO. Available online: https://www.cochranelibrary.com/about-pico (accessed on 1 February 2025).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.; Nagarajappa, R.; Ramesh, G.; Bapat, K. Effect of Propolis Mouth Rinse on Oral Microorganisms—a Randomized Controlled Trial. Clin. Oral Investig. 2021, 25, 6139–6146. [Google Scholar] [CrossRef]
- Dehghani, M.; Abtahi, M.; Hasanzadeh, N.; Farahzad, Z.; Noori, M.; Noori, M. Effect of Propolis Mouthwash on Plaque and Gingival Indices over Fixed Orthodontic Patients. J. Clin. Exp. Dent. 2019, 11, e244–e249. [Google Scholar] [CrossRef] [PubMed]
- Ercan, N.; Erdemir, E.O.; Ozkan, S.Y.; Hendek, M.K. The Comparative Effect of Propolis in Two Different Vehicles; Mouthwash and Chewing-Gum on Plaque Accumulation and Gingival Inflammation. Eur. J. Dent. 2015, 09, 272–276. [Google Scholar] [CrossRef]
- Gunjal, S.; Pateel, D.G.S. Comparative Effectiveness of Propolis with Chlorhexidine Mouthwash on Gingivitis—a Randomized Controlled Clinical Study. BMC Complement. Med. Ther. 2024, 24, 154. [Google Scholar] [CrossRef]
- Khabazian, A.; Mirhashemi, F.S.; Sadeghi, F. Investigating the Effect of Propolis-Containing Chewing Gum in Comparison with Propolis-Containing Mouthwash on Reducing Gingival Inflammation in Patients with Gingivitis. BMC Oral Health 2025, 25, 231. [Google Scholar] [CrossRef]
- Kiani, S.; Birang, R.; Jamshidian, N. Effect of Propolis Mouthwash on Clinical Periodontal Parameters in Patients with Gingivitis: A Double-Blinded Randomized Clinical Trial. Int. J. Dent. Hyg. 2022, 20, 434–440. [Google Scholar] [CrossRef]
- Koo, H.; Cury, J.A.; Rosalen, P.L.; Ambrosano, G.M.B.; Ikegaki, M.; Park, Y.K. Effect of a Mouthrinse Containing Selected Propolis on 3-Day Dental Plaque Accumulation and Polysaccharide Formation. Caries Res. 2002, 36, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Kripal, K.; Sm, M.; Sm, S.; Kumar Sm, D.; Sm, S.S.; Bhavanam, S.R.; Chandrasekaran, K.; Dileep, A.; Sm, S. Health from the Hive: 5% Propolis Mouth Wash as an Adjunct in the Treatment of Chronic Generalized Gingivitis-A Randomized Controlled Clinical Trial. Dentistry 2019, 9, 533. [Google Scholar] [CrossRef]
- Mallikarjun, S.A.; Sathyanarayana, S.; Nanaiah, P.; Devi, P.R. Evaluation of Clinical and Antimicrobial Efficacy of Propolis Mouthwash in Treatment of Gingivitis. A Randomized Controlled Clinical Trial. Int. J. Innov. Res. Growth 2022, 5, 254–262. [Google Scholar]
- Murray, M.C.; Worthington, H.V.; Blinkhorn, A.S. A Study to Investigate the Effect of a Propolis-Containing Mouthrinse on the Inhibition of de Novo Plaque Formation. J. Clin. Periodontol. 1997, 24, 796–798. [Google Scholar] [CrossRef]
- Pereira, E.M.R.; da Silva, J.L.D.C.; Silva, F.F.; De Luca, M.P.; Ferreira, E.F.e; Lorentz, T.C.M.; Santos, V.R. Clinical Evidence of the Efficacy of a Mouthwash Containing Propolis for the Control of Plaque and Gingivitis: A Phase II Study. Evid.-Based Complement. Altern. Med. 2011, 2011, 750249. [Google Scholar] [CrossRef]
- Porwal, S.; Mathur, A.; Shetty, N.; Manohar, B.; Makhijani, B.; Mundra, R. Comparative Evaluation of the Effect of Chlorhexidine Gluconate, Raw Propolis and Hydrogen Peroxide on Dental Plaque and Gingival Inflammation. J. Nepal. Soc. Perio. Oral Implantol. 2018, 2, 14–19. [Google Scholar] [CrossRef][Green Version]
- Amano, S.; Matsumoto, M.; Morimoto, M.; Kawamoto, H.; Takeshita, F.; Yasui, T.; Sakagami, H. Efficacy of Toothpaste Containing Brazilian Green Propolis Extracts with an Optimal Kaempferide/Betuletol Ratio for Improving Oral Microbiota: A Randomized, Controlled, Paired Crossover Study. J. Ethnopharmacol. 2025, 337, 118762. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Bapat, S.; Asawa, K.; Tak, M.; Chaturvedi, P.; Gupta, V.V.; George, P.P. The Antiplaque Efficacy of Propolis-Based Herbal Toothpaste: A Crossover Clinical Study. J. Nat. Sci. Biol. Med. 2015, 6, 364–368. [Google Scholar] [CrossRef]
- Biria, M.; Rezvani, Y.; Haeri, A.; Parhiz, Z.; Amirabadi, N.E.; Eftekhar, L. Evaluation of Antiplaque Efficacy of a Propolis-Based Herbal Toothpaste: A Single-Blind Parallel Clinical Trail. J. Islam. Dent. Assoc. Iran. 2019, 31, 126–131. [Google Scholar] [CrossRef]
- Fereidooni, M.; Khosravi Samani, M.; Amiri, A.; Seyed, M.; Haji Ahmadi, M. Comparison of the Effect of Propolis and Traditional Toothpaste on Bacterial Plaque. J. Babol. Univ. Med. Sci. 2014, 16, 17–22. [Google Scholar]
- Penmetsa, G.S.; Meghana, G.; Kumar, P.M.; Sruthima, N.; Ksv, R.; Kondapally, M. Comparison of Propolis Containing Dentifrice Versus Commercially Available Dentifrice in Gingivitis Treatment: A Randomized Double-Blinded Clinical Trial. Adv. Pharmacol. Pharm. 2023, 11, 329–334. [Google Scholar] [CrossRef]
- Ranjan, P.; Anshuman, P. A Hospital-Based a Single-Blind Parallel Clinical Trial Assessing the Antiplaque Efficacy of Propolis-Based Herbal Toothpaste. Int. J. Curr. Pharm. Rev. Res. 2023, 15, 41–45. [Google Scholar]
- Suriamah, N.; Lessang, R.; Kemal, Y. Effectiveness of Toothpaste Containing Propolis, Tea Tree Oil, and Sodium Monofluorophosphate Against Plaque and Gingivitis. Int. J. App. Pharm. 2019, 11, 14–116. [Google Scholar] [CrossRef]
- Dumitriu, A.S.; Păunică, S.; Nicolae, X.A.; Bodnar, D.C.; Albu, Ș.D.; Suciu, I.; Ciongaru, D.N.; Giurgiu, M.C. The Effectiveness of the Association of Chlorhexidine with Mechanical Treatment of Peri-Implant Mucositis. Healthcare 2023, 11, 1918. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine Mouthrinse as an Adjunctive Treatment for Gingival Health. Cochrane Database Syst. Rev. 2017, 2021, CD008676. [Google Scholar] [CrossRef]
- Sycinska-Dziarnowska, M.; Bielawska-Victorini, H.; Budzyńska, A.; Woźniak, K. The Implications of the COVID-19 Pandemic on the Interest in Orthodontic Treatment and Perspectives for the Future. Real-Time Surveillance Using Google Trends. Int. J. Environ. Res. Public Health 2021, 18, 5647. [Google Scholar] [CrossRef]
- Sycińska-Dziarnowska, M.; Szyszka-Sommerfeld, L.; Ziąbka, M.; Woźniak, K.; Spagnuolo, G. Propolis in Dental Implantology: A Systematic Review of Its Effects and Benefits. J. Funct. Biomater. 2024, 15, 339. [Google Scholar] [CrossRef]
| N | Author, Year | Study Design | Inclusion Criteria | Study Groups | Type of Measurements | Timing of Measurements | Results |
|---|---|---|---|---|---|---|---|
| Mouthwash | |||||||
| 1 | Bapat et al., 2021 [28] | Patients were randomized into four groups: hot ethanolic propolis extract, cold ethanolic propolis extract, chlorhexidine (CHX) and distilled water. Patients rinsed twice a day for three months | Age group: 18–22 years old with overall good health | 120 participants divided into four equal groups: hot ethanolic propolis extract, cold ethanolic propolis extract, CHX, and distilled water | GI, PI micro-biological analysis | At the beginning of the study and after 15 days, 1 month, and 3 months | Similar plaque reduction was observed in the groups using CHX (0.45), cold ethanolic propolis (0.46) and hot ethanolic propolis (0.47). Propolis was found to be as effective as CHX in reducing GI and PI. |
| 2 | Dehghani et al., 2019 [29] | Patients were randomly assigned to two groups: propolis or CHX mouthwash. Patients were asked to rinse their mouths with 15 mL of the liquid for 1 min twice a day after brushing their teeth | Age group: 15–35 years old, with good general health; group: with fixed orthodontic appliances, mild to moderate gingivitis, and completion of a patient satisfaction questionnaire | 18 patients in the propolis group and 19 in the CHX group | GI, PI, Community Periodontal Index (CPI) | At the beginning of the study and 22 days apart | The difference between PI (p < 0.001), GI (p = 0.006), and CPI (p = 0.005) before and after propolis administration was statistically significant. However, it was not statistically significant between the two groups of mouthwashes. |
| 3 | Dodwad et al., 2011 [20] | Patients randomly assigned to three groups: mouthwash containing propolis, CHX 0.2% or control (saline) | Age group: 18–50 years old with chronic gingivitis, systemically healthy patient | 10 patients in the propolis group, 10 patients in the CHX group, and 10 patients in the saline group | GI, PI | At the beginning of the study and at an interval of five days | CHX mouthwash was found to provide better results than propolis and saline in inhibiting plaque formation. Propolis was found to be slightly better than CHX in improving the GI status. |
| 4 | Ercan et al., 2015 [30] | Two study groups: a group using propolis mouthwash or a group using chewing gum. Chewing gum was used after meals three times a day for 20 min. The mouthwash group was asked to rinse their mouths with propolis two times a day for 1 min. | Age group: 18–22-year-old students, free of systemic diseases with good oral hygiene | 5 patients in chewing gum group, 5 patients in mouthwash group | GI, PI | At an interval of five days | GI and PI in the propolis mouthwash group were significantly lower than in the propolis chewing gum group (p = 0.005). |
| 5 | Gunjal et al., 2024 [31] | The study group used a mouthwash containing propolis extract, while the control group used an identical mouthwash without the propolis extract and Chlorhexidine mouthwash. | Age group: 8–30 years old patients with Gingival Index > 1, pocket depth ≤ 3 mm, no clinical attachment loss Exclusion criteria: Severe periodontitis | 45 adults: three groups of 15 patients: propolis mouthwash, Chlorhexidine mouthwash, and placebo mouthwash—each used by all participants in different phases | GI, PI | Baseline and after 21 day use of each mouthwash, 15 day washout between phases | After 21 days: significant reduction in GI and PI across all groups (p < 0.001). Propolis showed greater reduction than Chlorhexidine (p < 0.001) and placebo in both GI and PI. |
| 6 | Khabazian et al., 2025 [32] | The study group used a chewing gum containing propolis extract, while the comparison group used a mouthwash containing propolis extract. | Age group: patients aged 18–65 with gingivitis, ≥20 teeth, no systemic disease, non-smokers, not pregnant/breastfeeding | 20 patients. Two arms study: Propolis chewing gum vs. Propolis mouthwash, assigned randomly (10 patients per group) | GI, PI, Papillary Bleeding Index (PBI) | Baseline and after one week of product use | Both groups had significant reductions in PI (p = 0.0001) and GI (p = 0.006 for mouthwash). |
| 7 | Kiani et al., 2022 [33] | The study group used a mouthwash containing propolis extract. The control group rinsed their mouths with the same mouthwash without propolis extract. | Age group: patients over 18 years old with gingivitis, absence of dental calculus, with a minimum of 20 teeth regardless of wisdom teeth | 32 patients: 16 patients in the study group, 16 patients in the control group | PI, PBI, tooth discoloration | At the beginning of the study and after 15 and 30 days | No significant difference between groups in PI (p = 0.91). The decrease in papillary bleeding was significantly greater in the propolis group compared to the placebo group (p < 0.001). |
| 8 | Koo et al., 2002 [34] | Two groups: propolis mouthwash and placebo. During study, the patients withheld from all oral hygiene and rinsed with 20% sucrose solution five times a day to increase dental plaque formation, and with mouthrinse placebo or experimental two times a day. | Age group: patients aged 20–38, good health, at least 24 teeth, no carious lesions, no gingivitis or periodontitis, no crowns or removable orthodontic appliances, no antibiotic therapy in the six months preceding the examination | 6 patients, cross-over design | PI | On day four | The PI for the propolis group was significantly lower than in the placebo group. |
| 9 | Kripal et al., 2019 [35] | The first group used a 5% propolis mouthwash. The second group used CHX mouthwash, and the control group used saline. | Age group: 18–70 years old patients with chronic gingivitis | 45 patients: 15 patients in propolis group, 15 patients in chlorhexidine group, 15 patients in placebo group | GI, PI | At baseline and six weeks after | Significant improvement in clinical parameters (p < 0.05). |
| 10 | Mallikarjun et al., 2022 [36] | Three groups: propolis mouthwash 20%, CHX 0.2%, and control—saline. The subjects were asked to rinse their mouth for 1 min with 10 mL of the liquid, twice a day for two weeks. | Age group: 18–65 years old, suffering from chronic gingivitis, good general health | 20 patients in the propolis group, 20 patients in the CHX group, and 20 patients in the saline group | GI, PI micro-biological examination | At the beginning of the study and on day 15 | In all three groups for GI there was no statistically significant difference between groups at the follow-up visits (p = 0.204). For PI it was statistically significant (p = 0.002). |
| 11 | Murray et al., 1997 [37] | Patients were assigned to three groups: a mouthwash containing propolis, a second Periogard® mouthwash with CHX, and placebo mouthwash (without propolis). All patients were instructed to rinse their mouth twice a day with 150 mL of mouthwash for 1 min. | General population, good general health | 42 patients: 14 patients in propolis group, 14 patients in CHX group, 14 patients in placebo group | PI | At baseline and five days later | There were significant differences between the mean plaque scores for the active and placebo groups compared to the CHX group (p < 0.001). A 14% reduction in plaque was found comparing the test mouthwash with placebo and it was not significant (p = 0.19). |
| 12 | Pereira et al., 2011 [38] | Patients were instructed to rinse their mouths with 10 mL of alcohol-free mouthwash containing 5.0% Brazilian green propolis for 1 min, immediately after brushing twice a day. | Age group: 18–60 years old, overall good health, at least 20 natural teeth, average GI of at least 1.0, and average PI of at least 1.5 | 22 patients | GI, PI | During the first visit, after 45 and 90 days | The results showed a significant reduction in GI (p < 0.05) and PI (p < 0.05) compared to the baseline of the study. |
| 13 | Porwal et al., 2018 [39] | Group one received 0.2% CHX, group two received propolis diluted with distilled water (1:1), and group three 3% hydrogen peroxide (1:1). Patients were instructed to rinse their mouths with 10 mL of the liquid twice a day for 15 days. | Age group: 20–40 years old, generally healthy, PI of 4, no clinical attachment loss | 10 patients in each group | PI, Modified Gingival Index (MGI) | At the beginning of the study, after 7 and 28 days | All three mouthwashes were effective in reducing GI and PI. CHX 0.2% was most effective in reducing plaque. Propolis was most effective in reducing GI. |
| Toothpaste | |||||||
| 14 | Amano et al., 2025 [40] | Patients brushed for one minute with the assigned toothpaste. After a washout period, the procedure was repeated with the alternate toothpaste in a paired crossover design. Participants were randomly assigned to start with either the herbal toothpaste containing Brazilian green propolis (BGP) or the toothpaste without propolis. | Age group: healthy students aged 18–40 years | 48 participants (24 males, 24 females), used both toothpastes (with and without BGP) in two separate phases | GI, PI and analysis of oral microbiota | Baseline, after one week, and after two weeks for each toothpaste | BGP toothpaste significantly reduced PI (p < 0.05) but not GI. |
| 15 | Bhat et al., 2015 [41] | Patients were asked to brush their teeth for 1 min. Baseline plaque levels were recorded. The subjects then withheld oral hygiene for 24 h, and the plaque formation measurements were repeated. After a two-week washout period, the procedure was repeated according to a cross-over design. | Age group: dental students aged 18–22 with at least 24 natural teeth who volunteered and agreed to stop using oral hygiene products for 24 h after their first visit | 30 participants | Modified Gingival and Plaque Index (MGMPI) | Baseline and two weeks | Toothpaste with propolis resulted in significantly (p < 0.05) lower MGMPI scores than Colgate Total and Miswak toothpastes. |
| 16 | Biria et al., 2019 [42] | Patients were randomly divided into two groups: herbal toothpaste with propolis and herbal toothpaste without propolis. | Age group: students aged 24–30 who volunteered to participate in the study and agreed to use the prescribed toothpaste | 60 patients: 30 in both groups | PI | At the beginning of the study and after four weeks | There was a significant difference in PI after four weeks (p ˂ 0.001). |
| 17 | Fereidooni et al., 2014 [43] | Patients were divided into two groups: with propolis toothpaste (Colgate) and regular toothpaste (Colgate). | Age group: students with a mean age of 22 ± 1.2 years | 40 participants divided into two groups | PI | At the beginning of the study, at the end of two weeks and after two weeks a third time | The results of this study showed that toothpastes reduce plaque index, and this reduction is more in propolis toothpaste than normal toothpaste. |
| 18 | Penmetsa et al., 2023 [44] | Patients were divided into two groups using propolis toothpaste and Colgate toothpaste. | Age group: patients aged 20–35 years, mild to moderate gingivitis, without recent periodontal treatment | 40 patients: 20 patients in propolis group, 20 patients in Colgate group | GI, PI | At the baseline and after 30 days | No statistically significant differences were observed between the two groups. Statistically significant differences (p ≤ 0.05) occurred in the comparison between baseline and 30 day. |
| 19 | Ranjan et al., 2023 [45] | Fifty participants were included in the study. Each participant tested three different toothpastes. Propolis-based toothpaste (Forever Bright), Dabur toothpaste, and Pepsodent. | Age group: healthy dental students aged 24–30, no orthodontic appliances, cavities, probing depth ≤ 3 mm | 50 participants (25 male, 25 female); tested three toothpastes: Propolis (Forever Bright), Dabur, and Pepsodent | Modified Gingival Marginal Plaque Index (MGMPI) | At the baseline, after 24 h without brushing, with two-week washout between pastes | After 24 h, Propolis toothpaste showed the lowest plaque accumulation (36.74 ± 2.40). The increase in plaque from baseline to 24h was smallest with Propolis (21.09 ± 1.12), indicating it had the best antiplaque effect. |
| 20 | Suriamah et al., 2019 [46] | Two groups: in the test group, patients were instructed to use toothpaste containing propolis, tea tree oil, and sodium monofluorophosphate, while the control group used toothpaste without any natural ingredients. | Age group: students, age group 17–25, good general health, diagnosed gingivitis | 40 patients: 20 patients in the study group, 20 in the control group | PI, Papillary Bleeding Index (PBI) | During the first visit and within seven days | Significant decrease (p < 0.05) in PI and PBI scores in the test group compared to the control group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sycińska-Dziarnowska, M.; Szyszka-Sommerfeld, L.; Bugajska, M.; Ziąbka, M.; Szućko-Kociuba, I.; Spagnuolo, G.; Woźniak, K.; Park, H.-S. Propolis as a Natural Remedy in Reducing Dental Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis. J. Funct. Biomater. 2025, 16, 336. https://doi.org/10.3390/jfb16090336
Sycińska-Dziarnowska M, Szyszka-Sommerfeld L, Bugajska M, Ziąbka M, Szućko-Kociuba I, Spagnuolo G, Woźniak K, Park H-S. Propolis as a Natural Remedy in Reducing Dental Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis. Journal of Functional Biomaterials. 2025; 16(9):336. https://doi.org/10.3390/jfb16090336
Chicago/Turabian StyleSycińska-Dziarnowska, Magdalena, Liliana Szyszka-Sommerfeld, Monika Bugajska, Magdalena Ziąbka, Izabela Szućko-Kociuba, Gianrico Spagnuolo, Krzysztof Woźniak, and Hyo-Sang Park. 2025. "Propolis as a Natural Remedy in Reducing Dental Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis" Journal of Functional Biomaterials 16, no. 9: 336. https://doi.org/10.3390/jfb16090336
APA StyleSycińska-Dziarnowska, M., Szyszka-Sommerfeld, L., Bugajska, M., Ziąbka, M., Szućko-Kociuba, I., Spagnuolo, G., Woźniak, K., & Park, H.-S. (2025). Propolis as a Natural Remedy in Reducing Dental Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis. Journal of Functional Biomaterials, 16(9), 336. https://doi.org/10.3390/jfb16090336








