Three-Dimensional-Printed Biomimetic Scaffolds for Investigating Osteoblast-Like Cell Interactions in Simulated Microgravity: An In Vitro Platform for Bone Tissue Engineering Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomimetic Scaffold Design
2.2. Three-Dimensional Printing Set-Up and Scaffold Evaluation
2.3. Cell Culture
2.4. RCCS Bioreactor
2.5. Biological Assays
2.5.1. Cell Proliferation and Metabolic Activity Analysis
2.5.2. Quantification of TNF-α
2.5.3. Scanning Electron Microscopy (SEM) Analysis
2.6. Statistical Analysis
3. Results
3.1. Scaffold Design Assessment
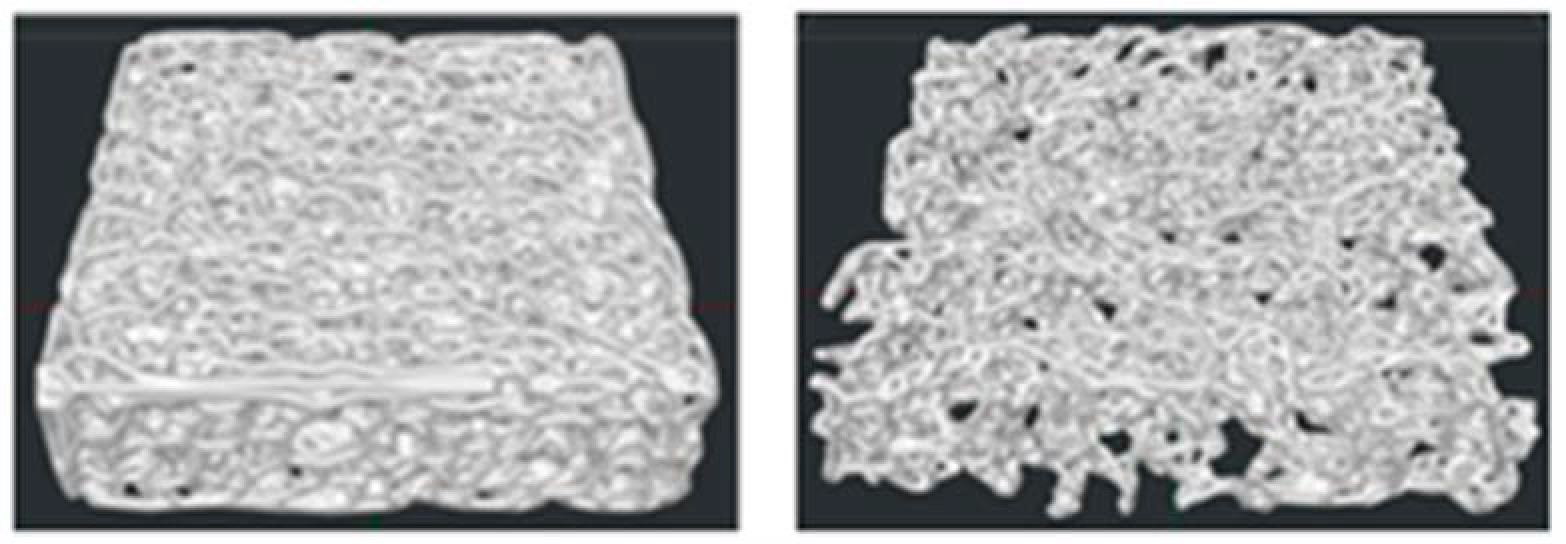
3.2. Three-Dimensional-Printed Scaffolds and Morphometric Analysis
3.3. Cell Growth and Metabolic Activity Study in RCCS Conditions
3.4. Cell Adhesion Study in RCCS Conditions
3.5. TNF-α Secretion Analysis
4. Discussion
4.1. Bioreactor System and Scaffold Design
4.2. Cellular Responses and Biocompatibility
4.3. Implications for Bone Tissue Engineering Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Li, C. Advances in 3D printing technology for preparing bone tissue engineering scaffolds from biodegradable materials. Front. Bioeng. Biotechnol. 2024, 12, 1483547. [Google Scholar] [CrossRef] [PubMed]
- Winarso, R.; Anggoro, P.W.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Application of fused deposition modeling (FDM) on bone scaffold manufacturing process: A review. Heliyon 2022, 8, e11701. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Su, G.N.; Chen, J.K.; Mukaisho, K.; Hattori, T.; Yamamoto, G. Transplantation of engineered bone tissue using a rotary three-dimensional culture system. In Vitro Cell. Dev. Biol. Anim. 2007, 43, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A. Mineralization, Structure and Function of Bone; Academic Press: San Diego, CA, USA, 2006; pp. 201–212. [Google Scholar]
- Weiner, S.; Wagner, H.D. The material bone: Structure mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E. The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol. Metab. Clin. N. Am. 2003, 32, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Hammond, J.M. Optimized suspension culture: The rotating-wall vessel. Am. J. Physiol. Renal. Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, Y.; Xiao, Z.; Zhao, Y.; Li, J.; Han, S.; Chen, L.; Dai, B.; Wang, L.; Chen, B.; et al. The combination of three-dimensional and rotary cell culture system promotes the proliferation and maintains the differentiation potential of rat BMSCs. Sci. Rep. 2017, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, A.H.; Valeri, C.; Jiang, D.; Ning, F.; Razavi, M.; Razavi, M.J. Understanding compressive viscoelastic properties of additively manufactured PLA for bone-mimetic scaffold design. Med. Eng. Phys. 2023, 114, 103972. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Bu, W.; Mao, Y.; Wang, E.; Yang, Y.; Liu, C.; Guo, F.; Mai, H.; You, H.; Long, Y. Magnesium Hydroxide as a Versatile Nanofiller for 3D-Printed PLA Bone Scaffolds. Polymers 2024, 16, 198. [Google Scholar] [CrossRef] [PubMed]
- Zenobi, E.; Merco, M.; Mochi, F.; Ruspi, J.; Pecci, R.; Marchese, R.; Convertino, A.; Lisi, A.; Del Gaudio, C.; Ledda, M. Tailoring the Microarchitectures of 3D Printed Bone-like Scaffolds for Tissue Engineering Applications. Bioengineering 2023, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Molino, G.; Dalpozzi, A.; Ciapetti, G.; Lorusso, M.; Novara, C.; Cavallo, M.; Baldini, N.; Giorgis, F.; Fiorilli, S.; Vitale-Brovaron, C. Osteoporosis-related variations of trabecular bone properties of proximal human humeral heads at different scale lengths. J. Mech. Behav. Biomed. 2019, 100, 103373. [Google Scholar] [CrossRef] [PubMed]
- Ciarelli, T.E.; Fyhrie, D.P.; Schaffler, M.B.; Goldstein, S.A. Variations in three-dimensional cancellous bone architecture of the proximal femur in female hip fractures and in controls. J. Bone Miner. Res. 2000, 15, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sandino, C.; McErlain, D.D.; Schipilow, J.; Boyd, S.K. Mechanical stimuli of trabecular bone in osteoporosis: A numerical simulation by finite element analysis of microarchitecture. J. Mech. Behav. Biomed. Mater. 2017, 66, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Porrelli, D.; Abrami, M.; Pelizzo, P.; Formentin, C.; Ratti, C.; Turco, G.; Grassi, M.; Canton, G.; Grassi, G.; Murena, L. Trabecular bone porosity and pore size distribution in osteoporotic patients-A low field nuclear magnetic resonance and microcomputed tomography investigation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104933. [Google Scholar] [CrossRef] [PubMed]
- Viveen, J.; Perilli, E.; Zahrooni, S.; Jaarsma, R.L.; Doornberg, J.N.; Bain, G.I. Three-dimensional cortical and trabecular bone microstructure of the proximal ulna. Arch. Orthop. Trauma. Surg. 2023, 143, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.C.; Pereira, M.F.C.; Maurício, A.; Amaral, P.; Rosa, L.G.; Lopes, A.; Rodrigues, A.; Caetano-Lopes, J.; Vidal, B.; Monteiro, J.; et al. Micro-computed tomography and compressive characterization of trabecular bone. Colloid. Surf. A 2013, 438, 199–205. [Google Scholar] [CrossRef]
- Nikodem, A. Correlations between structural and mechanical properties of human trabecular femur bone. Acta Bioeng. Biomech. 2012, 14, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ozan, F.; Pekedis, M.; Koyuncu, S.; Altay, T.; Yildiz, H.; Kayali, C. Micro-computed tomography and mechanical evaluation of trabecular bone structure in osteopenic and osteoporotic fractures. J. Orthop. Surg. 2017, 25, 2309499017692718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, G.; Junka, R.; Chang, N.; Anwar, A.; Wang, H.; Yu, X. Fabrication of polylactic acid (PLA)-based porous scaffold through the combination of traditional bio-fabrication and 3D printing technology for bone regeneration. Colloids Surf. B Biointerfaces 2021, 197, 111420. [Google Scholar] [CrossRef] [PubMed]
- Gremare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’Heureux, N.; Fricain, J.C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M.; Wijenayaka, A.R.; Kumarasinghe, D.D.; Ormsby, R.T.; Evdokiou, A.; Findlay, D.M.; Atkins, G.J. SaOS2 Osteosarcoma Cells as an In Vitro Model for Studying the Transition of Human Osteoblasts to Osteocytes. Calcif. Tissue Int. 2014, 95, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In Search of an Osteoblast Cell Model for Research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mornet, E.; Stura, E.; Lia-Baldini, A.S.; Stigbrand, T.; Ménez, A.; Le Du, M.H. Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J. Biol. Chem. 2001, 276, 31171–31178. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Shimada, T. Effects of phosphates on the expression of tissue-nonspecific alkaline phosphatase gene and phosphate-regulating genes in short-term cultures of human osteosarcoma cell lines. Mol. Cell. Biochem. 2006, 282, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, P.; Agrawal, G.; Sally, J.; Ravi, M. Dynamic three-dimensional cell-culture systems for enhanced applications. Curr. Sci. 2022, 122, 149–160. [Google Scholar] [CrossRef]
- Zerath, E. Effects of microgravity on bone and calcium homeostasis. Adv. Space Res. 1998, 21, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Evans, H.; Spector, E.; Ploutz-Snyder, R.; et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporosis Int. 2013, 24, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.; Techy, G.B.; Saroufeem, R.; Yazan, O.; Narayan, K.S.; Goodwin, T.J.; Spaulding, G.F. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell. Dev.-An. 1997, 33, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Zhang, Y.; Xuan, K.; He, D.; Deng, T.; Tang, L.; Lu, W.; Duan, Y. Establishment of three-dimensional tissue-engineered bone constructs under microgravity-simulated conditions. Artif. Organs 2010, 34, 118–125. [Google Scholar] [CrossRef] [PubMed]







| Sample | PD (µm) | PS (µm) | TT (µm) | TS (µm) |
|---|---|---|---|---|
| P | 700 | 700 | 200 | 600 |
| O | 800 | 500 | 150 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenobi, E.; Gramigna, G.; Scatena, E.; Panizza, L.; Achille, C.; Pecci, R.; Convertino, A.; Del Gaudio, C.; Lisi, A.; Ledda, M. Three-Dimensional-Printed Biomimetic Scaffolds for Investigating Osteoblast-Like Cell Interactions in Simulated Microgravity: An In Vitro Platform for Bone Tissue Engineering Research. J. Funct. Biomater. 2025, 16, 271. https://doi.org/10.3390/jfb16080271
Zenobi E, Gramigna G, Scatena E, Panizza L, Achille C, Pecci R, Convertino A, Del Gaudio C, Lisi A, Ledda M. Three-Dimensional-Printed Biomimetic Scaffolds for Investigating Osteoblast-Like Cell Interactions in Simulated Microgravity: An In Vitro Platform for Bone Tissue Engineering Research. Journal of Functional Biomaterials. 2025; 16(8):271. https://doi.org/10.3390/jfb16080271
Chicago/Turabian StyleZenobi, Eleonora, Giulia Gramigna, Elisa Scatena, Luca Panizza, Carlotta Achille, Raffaella Pecci, Annalisa Convertino, Costantino Del Gaudio, Antonella Lisi, and Mario Ledda. 2025. "Three-Dimensional-Printed Biomimetic Scaffolds for Investigating Osteoblast-Like Cell Interactions in Simulated Microgravity: An In Vitro Platform for Bone Tissue Engineering Research" Journal of Functional Biomaterials 16, no. 8: 271. https://doi.org/10.3390/jfb16080271
APA StyleZenobi, E., Gramigna, G., Scatena, E., Panizza, L., Achille, C., Pecci, R., Convertino, A., Del Gaudio, C., Lisi, A., & Ledda, M. (2025). Three-Dimensional-Printed Biomimetic Scaffolds for Investigating Osteoblast-Like Cell Interactions in Simulated Microgravity: An In Vitro Platform for Bone Tissue Engineering Research. Journal of Functional Biomaterials, 16(8), 271. https://doi.org/10.3390/jfb16080271










