The Integration of Micro-CT Imaging and Finite Element Simulations for Modelling Tooth-Inlay Systems for Mechanical Stress Analysis: A Preliminary Study
Abstract
1. Introduction
- –
- The analysis of stress and strain distribution;
- –
- The evaluation of implant design and performance;
- –
- The assessment of new dental materials;
- –
- The optimisation of prosthetic restorations;
- –
2. Materials and Methods
2.1. Selection of Tooth
2.2. Preparation/Fabrication/Creation of Typodont Models and Obturating Constructions
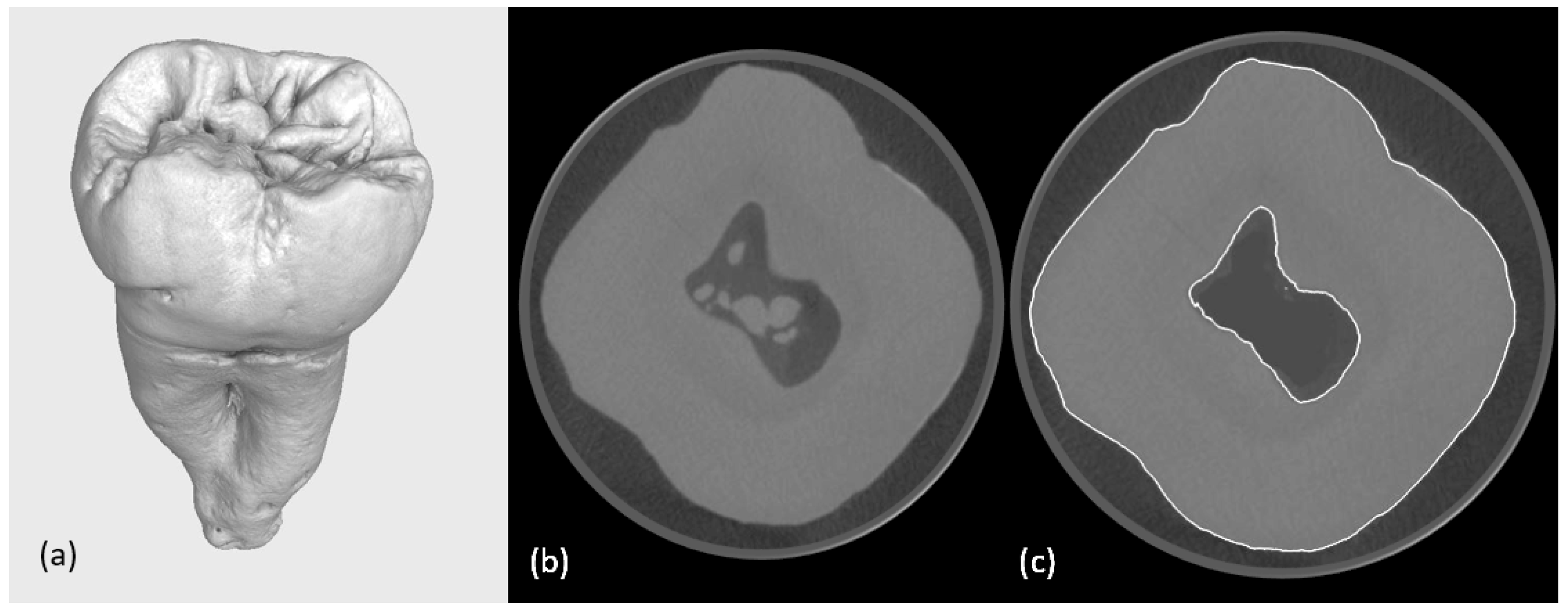
2.2.1. First Micro-CT Scanning
2.2.2. Printing the Typodonts
2.2.3. Preparation of Cavities
2.2.4. Second Micro-CT Scanning (Post-Cavity Preparation)
2.2.5. Virtual Design of Indirect Restorations
2.3. Preparation of Models for FEA
- A cement layer was created using the “Boolean” function based on the predefined gap between the tooth structure and the obturating construction.
- A base element simulating the supporting bone was modelled to provide appropriate boundary conditions during simulation.
2.4. Finite Element Analysis
- Geometrical Model Discretisation: The 3D geometry of the tooth inlay from the STEP file was imported into the ANSYS pre-processor and discretised using tetrahedral second-order finite elements. Mesh refinement was applied in critical regions, particularly near interfaces between materials, to capture potential stress gradients and ensure numerical stability.
- Material Properties and Constitutive Laws: Each domain in the model (enamel, dentin, inlay material, and adhesive interface) was assigned material properties obtained from published experimental data. A linear elastic, isotropic constitutive model was used for all materials under the assumption of small strains and negligible time-dependent effects. Material parameters such as Young’s modulus and Poisson’s ratio were defined individually for each component.
- Boundary and Initial Conditions: To replicate physiological conditions, the base of the tooth was constrained in all directions to simulate its anchorage within the alveolar socket. A vertical compressive load was applied to a selected location on the occlusal surface to mimic masticatory loading. The load magnitude was selected based on the upper range of functional bite forces observed in fully dentate individuals. The load application point was chosen based on anatomical relevance and the location of expected peak stress. A static linear analysis was performed, assuming quasi-static loading conditions and neglecting dynamic effects [13,14,15].
- Contact Modelling: The interfaces between dissimilar materials—such as between the dentin and inlay or enamel and adhesive—were modelled assuming perfect (full) contact, i.e., without interfacial separation or sliding. This assumption implies ideal bonding and stress continuity across interfaces, allowing for a simplified representation of load transfer between materials. While this approach may not capture all interfacial failure mechanisms, it provides an initial approximation for evaluating internal stress distributions. This modelling strategy enabled a detailed assessment of stress distributions within the tooth–restoration assembly and the identification of zones with elevated mechanical risk under functional loading.
3. Results
4. Discussion
- Adjacent structures are omitted.
- The current model excludes the periodontal ligament (PDL), neighbouring teeth, and soft tissues. These structures may significantly influence stress distribution and displacement behaviour under clinical loading.
- Polygon reduction effects are present.
- Although polygon count reduction was necessary to improve computational efficiency, it may have resulted in the loss of fine anatomical details in localised regions.
- Micro-CT applicability was limited.
- While micro-CT provides exceptional image resolution, it is limited to ex vivo use. For clinical translation, the workflow must be adapted for lower-resolution CBCT, which may reduce model accuracy.
Clinical Applicability and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asaad, Y.M.; Alhudaithi, M.K.; Alazraqi, M.S.; Almugren, S.S.; Alhumizi, N.A.; Albesher, F.A.; Alhammad, Y.M.; Alzahrani, A.M.; Aljutaili, A.A.; Albaijan, A.A.; et al. The Impact of Occlusal Forces on the Longevity of Restorations. Int. J. Community Med. Public Health 2023, 10, 3899–3903. [Google Scholar] [CrossRef]
- Alakkad, T.M.A.; Alshammari, N.M.; Alhertani, S.M.R.; Rizq, I.M.; Musawi, K.A.; Alalwan, A.A.; Alammari, K.A.; Alhumaidan, S.A.; Asiri, Y.N.; Alkurbi, A.A.; et al. Long-Term Success of Inlay and Onlay Procedures in Dental Repairs. Int. J. Community Med. Public Health 2024, 11, 4089–4092. [Google Scholar] [CrossRef]
- Galiatsatos, A.; Galiatsatos, P.; Bergou, D. Clinical Longevity of Indirect Composite Resin Inlays and Onlays: An Up to 9-Year Prospective Study. Eur. J. Dent. 2022, 16, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.V.; Nunes, M.F.; Swift, E.J., Jr. Longevity of ceramic inlays/onlays, Part I. J. Esthet. Restor. Dent. 2002, 14, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Guijarro, M.; Pérez-Pevida, E.; Chávarri-Prado, D.; Estrada-Martínez, A.; Diéguez-Pereira, M.; Sánchez-Lasheras, F.; Brizuela-Velasco, A. Biomechanical Effects of Ti-Base Abutment Height on the Dental Implant System: A Finite Element Analysis. J. Funct. Biomater. 2024, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, D.B.; Todoh, M.; Huang, S.J. Advancing 3D Dental Implant Finite Element Analysis: Incorporating Biomimetic Trabecular Bone with Varied Pore Sizes in Voronoi Lattices. J. Funct. Biomater. 2024, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Ghavami-Lahiji, M.; Davalloo, R.T.; Tajziehchi, G.; Shams, P. Micro-computed tomography in preventive and restorative dental research: A review. Imaging Sci. Dent. 2021, 51, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Conway, R.C.; Ben-Nissan, B. Finite-element modeling and analysis in nanomedicine and dentistry. Nanomedicine 2014, 9, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Marcián, P.; Borák, L.; Zikmund, T.; Horáčková, L.; Kaiser, J.; Joukal, M.; Wolff, J. On the limits of finite element models created from (micro) CT datasets and used in studies of bone-implant-related biomechanical problems. J. Mech. Behav. Biomed. Mater. 2021, 117, 104393. [Google Scholar] [CrossRef] [PubMed]
- Della Bona, A.; Borba, M.; Benetti, P.; Duan, Y.; Griggs, J.A. Three-dimensional finite element modelling of all-ceramic restorations based on micro-CT. J. Dent. 2013, 41, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, W.; Ding, Z. 3D finite element mesh generation of complicated tooth model based on CT slices. Comput. Methods Programs Biomed. 2006, 82, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ordinola-Zapata, R.; Lin, F.; Nagarkar, S.; Perdigão, J. A critical analysis of research methods and experimental models to study the load capacity and clinical behaviour of the root filled teeth. Int. Endod. J. 2022, 55, 471–494. [Google Scholar] [CrossRef] [PubMed]
- Hongbin, L.; Xinwei, L.; Yuejiao, Z.; Qimei, G.; Zhongchun, T. Effect of different endodontic access preparations on the biomechanical behavior of lithium disilicate and resin nanoceramic onlay restorations: An in vitro and 3D finite element analysis study. J. Prosthet. Dent. 2024, 131, 64–74. [Google Scholar] [CrossRef]
- Bansal, P.; Seth, T.; Kumar, M.; Bhatt, M.; Arora, P.; Gupta, I.; Chaudhary, S.; Akkanapally, S.; Arora, A.; Singh, S. Comparative Evaluation of Stress Distribution and Deformation in Class II Cavities Restored with Two Different Biomimetic Restorative Materials: A Three-Dimensional Finite Element Analysis. Cureus 2024, 16, e69179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harikrishnan, P.; Magesh, V. Stress Trajectory Variations During Occlusal Loading in Human Skull with a Maxillofacial Defect: A Finite Element Analysis. J. Maxillofac. Oral Surg. 2023, 22, 1022–1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Huang, T. Investigation on the smoothing algorithms used in the coarse aggregate texture characterization. Constr. Build. Mater. 2022, 346, 128348. [Google Scholar] [CrossRef]
- Kilpattu Ramaniharan, A.; Parpura, V.; Zhang, M.W.; Martin, R.; Ver Hoef, L. Development of an objective method to quantify hippocampal dentation. Hum. Brain Mapp. 2023, 44, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Elraggal, A.; Abdelraheem, I.M.; Watts, D.C.; Roy, S.; Dommeti, V.K.; Alshabib, A.; Althaqafi, K.A.; Afifi, R.R. Biomechanical reinforcement by CAD-CAM materials affects stress distributions of posterior composite bridges: 3D finite element analysis. Dent. Mater. 2024, 40, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Kaleli, N.; Sarac, D.; Külünk, S.; Öztürk, Ö. Effect of different restorative crown and customized abutment materials on stress distribution in single implants and peripheral bone: A three-dimensional finite element analysis study. J. Prosthet. Dent. 2018, 119, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Demircan, B.; Demir, P. 3D finite element analysis of stress distribution as a result of oblique and horizontal forces after regenerative endodontic treatment part II: Comparison of material thickness. BMC Oral Health 2023, 23, 869. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.K.R.; Pilecco, R.O.; da Rosa, L.S.; Machry, R.V.; Baldi, A.; Scotti, N.; Valandro, L.F.; Tribst, J.P.M.; Kleverlaan, C.J. Does the Restoration Design and Material Affect Indirect Restorations’ Marginal and Internal Gap, Interfacial Volume, and Fatigue Behavior? Eur. J. Dent. 2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lai, C.J.; Whitman, G.J.; Geiser, W.R.; Shen, Y.; Yi, Y.; Shaw, C.C. Effects of exposure equalization on image signal-to-noise ratios in digital mammography: A simulation study with an anthropomorphic breast phantom. Med. Phys. 2011, 38, 6489–6501. [Google Scholar] [CrossRef] [PubMed]
- Raykovska, M.; Georgiev, I. A new surface treatment method for simplifying and enhancing the segmentation of open space pore object. 12th Conference on Industrial Computed Tomography (iCT) 2023, 27 February–2 March 2023 in Fürth, Germany. e-J. Nondestruct. Test. 2023, 28. [Google Scholar] [CrossRef]
- Raykovska, M.; Koytchev, E.; Georgiev, I.; Datcheva, M. The Application of Region Growing as a Segmentation Method in Micro–CT Studies in Endodontics. In Advanced Computing in Industrial Mathematics; Springer: Cham, Switzerland, 2025; pp. 155–160. Available online: https://link.springer.com/chapter/10.1007/978-3-031-76786-9_13 (accessed on 31 May 2025).
- Barioni, E.D.; Lopes, S.L.P.d.C.; Silvestre, P.R.; Yasuda, C.L.; Costa, A.L.F. Texture Analysis in Volumetric Imaging for Dentomaxillofacial Radiology: Transforming Diagnostic Approaches and Future Directions. J. Imaging 2024, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Magne, P. Efficient 3D finite element analysis of dental restorative procedures using micro-CT data. Dent. Mater. 2007, 23, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.P.; Li, J.; Silikas, N.; Ballester, R.Y.; Watts, D.C. Sequential software processing of micro-XCT dental-images for 3D-FE analysis. Dent. Mater. 2009, 25, e47–e55. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Adıgüzel, Ö.; Cangül, S. An evaluation using micro-CT data of the stress formed in the crown and periodontal tissues from the use of PEEK post and PEEK crown: A 3D finite element analysis study. Int. Dent. Res. 2018, 8, 144–150. [Google Scholar] [CrossRef]
- Wicaksono, S.; Prasetia, W.; Muryani, A.; Dirgantara, T.; Mahyuddin, A.I. Finite element stress analysis of dental cement application on endocrown and onlay restoration. Aust. Endod. J. 2023, 49, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, C.L.; Hou, C.H. Investigating inlay designs of class II cavity with deep margin elevation using finite element method. BMC Oral Health 2021, 21, 264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Q.; Gao, Y.; Zhou, M.; Li, H.; Lin, J.; Zhang, W.; Chen, X. Semi or fully automatic tooth segmentation in CBCT images: A review. PeerJ Comput. Sci. 2024, 10, e1994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camargos, G.D.V.; Lazari-Carvalho, P.C.; de Carvalho, M.A.; Castro, M.B.; Neris, N.W.; Del Bel Cury, A.A. 3D Finite Element Model Based on CT Images of Tooth: A Simplified Method of Modeling. Braz. J. Oral Sci. 2020, 19, e208910. [Google Scholar] [CrossRef]
- Özcan, C.; Lestriez, P.; Özcan, M.; Josset, Y. Finite element analysis of dental structures: The role of mandibular kinematics and model complexity. Front. Dent. Med. 2024, 5, 1461909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasegawa, A.; Shinya, A.; Nakasone, Y.; Lassila, L.V.J.; Vallittu, P.K.; Shinya, A. Development of 3D CAD/FEM analysis system for natural teeth and jaw bone constructed from X-ray CT images. Int. J. Biomater. 2010, 2010, 659802. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, P.; Faghihian, H.; Richert, R.; Jacobs, R.; EzEldeen, M. Finite element models: A road to in-silico modeling in the age of personalized dentistry. J. Dent. 2024, 150, 105348. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, N.; Raykovska, M.; Petkov, N.; Tsvetkov, M.; Georgiev, I.; Koytchev, E.; Iankov, R.; Dimova-Gabrovska, M.; Gusiyska, A. The Integration of Micro-CT Imaging and Finite Element Simulations for Modelling Tooth-Inlay Systems for Mechanical Stress Analysis: A Preliminary Study. J. Funct. Biomater. 2025, 16, 267. https://doi.org/10.3390/jfb16070267
Nikolova N, Raykovska M, Petkov N, Tsvetkov M, Georgiev I, Koytchev E, Iankov R, Dimova-Gabrovska M, Gusiyska A. The Integration of Micro-CT Imaging and Finite Element Simulations for Modelling Tooth-Inlay Systems for Mechanical Stress Analysis: A Preliminary Study. Journal of Functional Biomaterials. 2025; 16(7):267. https://doi.org/10.3390/jfb16070267
Chicago/Turabian StyleNikolova, Nikoleta, Miryana Raykovska, Nikolay Petkov, Martin Tsvetkov, Ivan Georgiev, Eugeni Koytchev, Roumen Iankov, Mariana Dimova-Gabrovska, and Angela Gusiyska. 2025. "The Integration of Micro-CT Imaging and Finite Element Simulations for Modelling Tooth-Inlay Systems for Mechanical Stress Analysis: A Preliminary Study" Journal of Functional Biomaterials 16, no. 7: 267. https://doi.org/10.3390/jfb16070267
APA StyleNikolova, N., Raykovska, M., Petkov, N., Tsvetkov, M., Georgiev, I., Koytchev, E., Iankov, R., Dimova-Gabrovska, M., & Gusiyska, A. (2025). The Integration of Micro-CT Imaging and Finite Element Simulations for Modelling Tooth-Inlay Systems for Mechanical Stress Analysis: A Preliminary Study. Journal of Functional Biomaterials, 16(7), 267. https://doi.org/10.3390/jfb16070267





