Reassessing the Use of Membranes in Peri-Implantitis Surgery: A Systematic Review and Meta-Analysis of In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Protocol and Registration
2.3. PICO
2.3.1. Population
2.3.2. Intervention
2.3.3. Comparison
2.3.4. Outcomes
2.4. Search Strategy
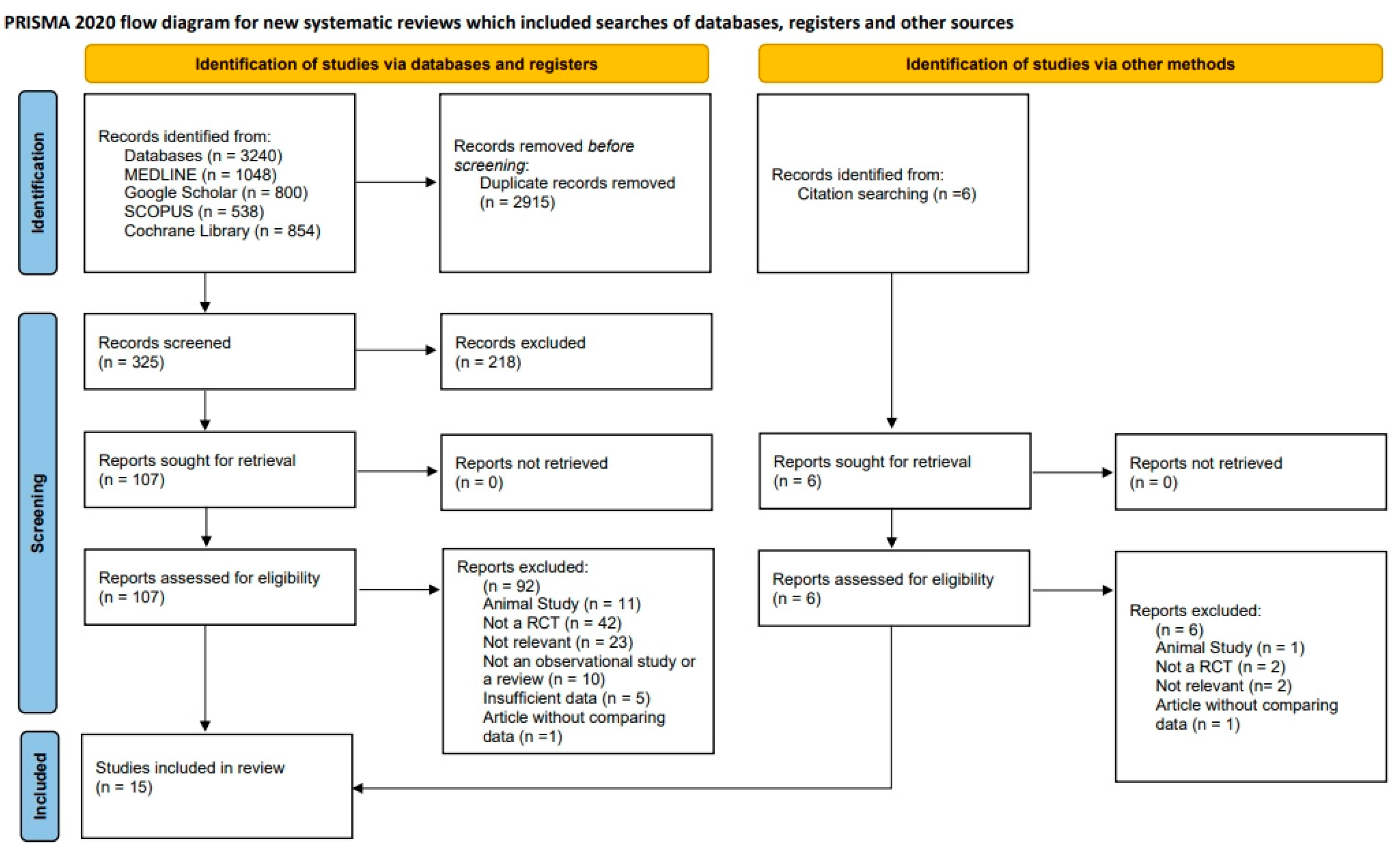
2.5. Study Selection, Assessment, and Agreement
2.6. Radiographic MLB Gain Assessment
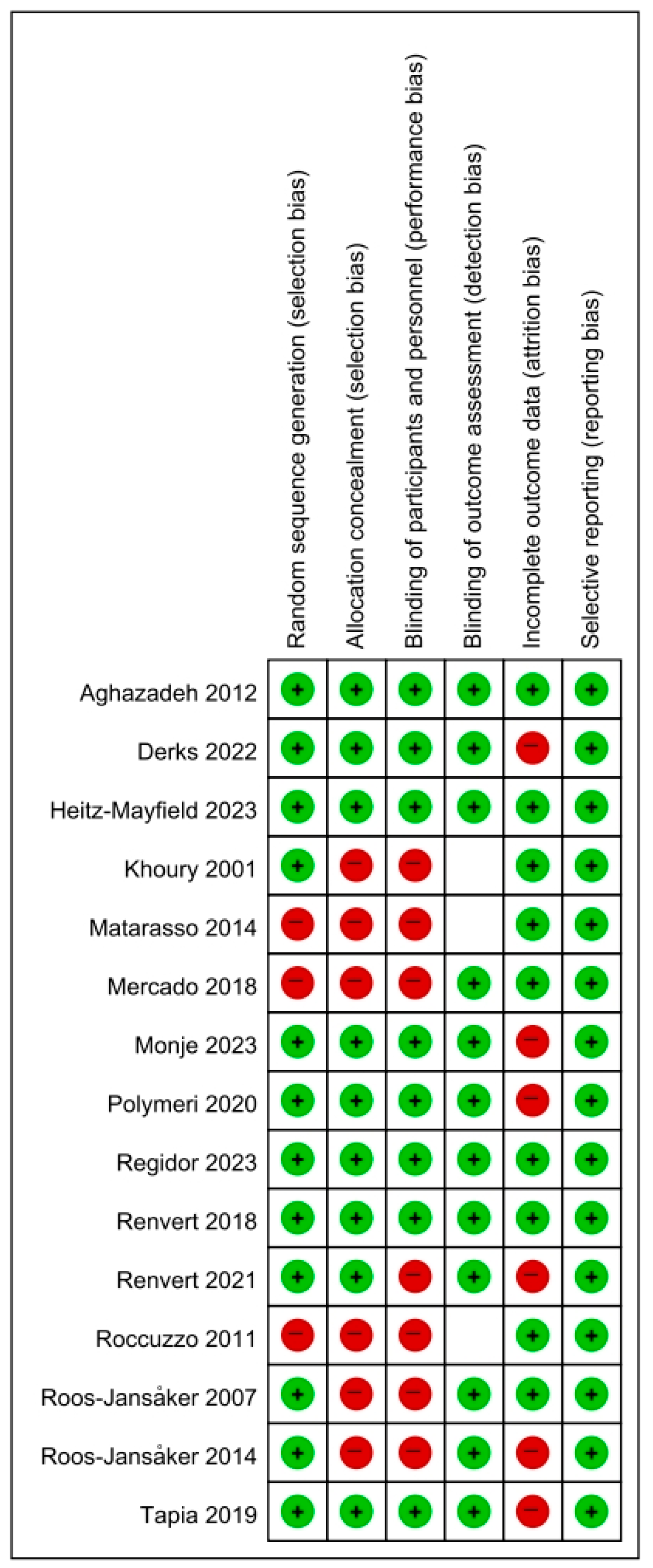
2.7. Risk of Bias Assessment
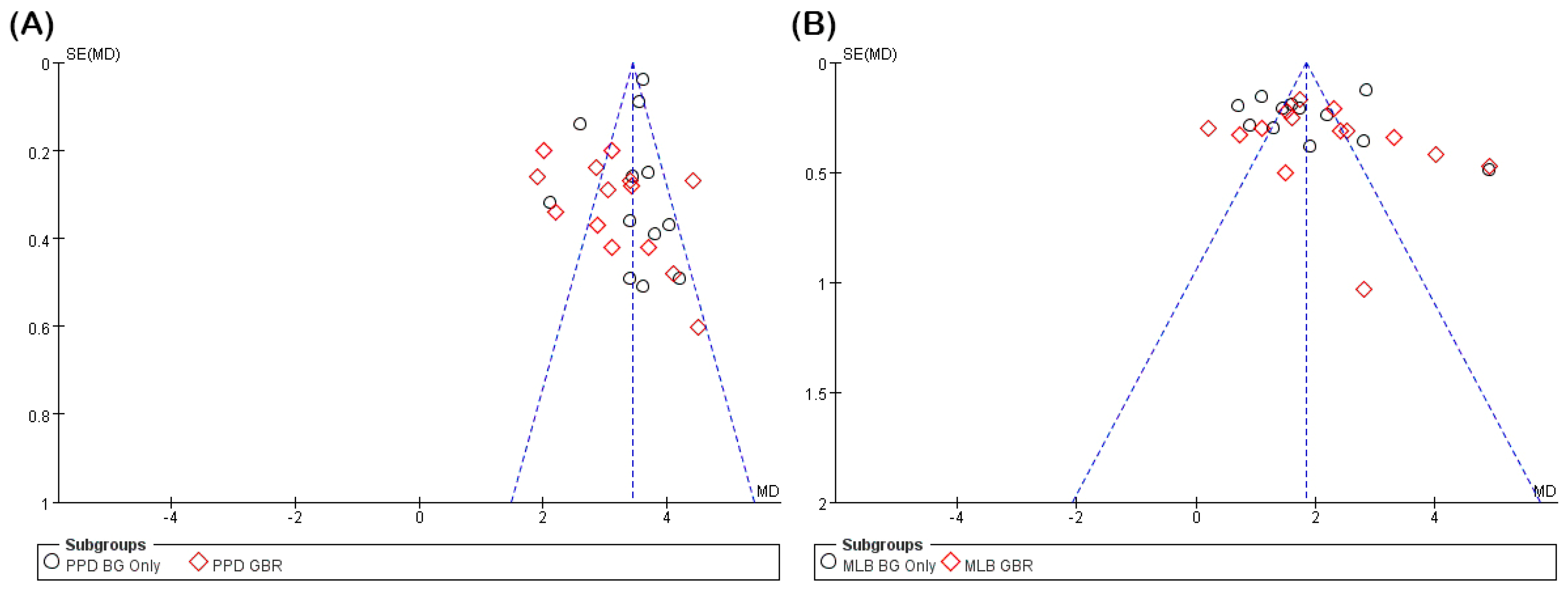
2.8. Data Analysis
3. Results
3.1. Overview of Included Studies (Table 1)
| Author | Year | Graft Material | Membrane | Other Factors | Defect Type | Radiograph Type and Evaluation Method | Mean ± SD | Sample Size | SE | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPD Reduction (mm) | MLB Gain (mm) | PPD Reduction | MLB Gain | ||||||||
| Khoury [12] | 2001 | Autogenous | No | Intrabony | Periapical | 2.6 ± 0.5 | 4.9 ± 1.7 | 12 | 0.14 | 0.49 | |
| e-PTFE (Gore-Tex) | Linear Measurement (Manual) | 3.4 ± 1.2 | 4 ± 1.9 | 20 | 0.27 | 0.42 | |||||
| Bio-Gide | 4.4 ± 0.8 | 4.9 ± 1.4 | 9 | 0.27 | 0.47 | ||||||
| Roos-Jansåker [9] | 2007 | Algipore | No | N/A | Periapical | 3.44 ± 1.58 | 1.44 ± 1.27 | 36 | 0.26 | 0.21 | |
| Osseoquest | N/A | 2.86 ± 2 | 1.52 ± 1.16 | 29 | 0.37 | 0.22 | |||||
| Roccuzzo [13] | 2011 | Bio-Oss collagen | No | TPS | Crater-Like | Periapical | 2.1 ± 1.2 | 1.6 ± 0.7 | 14 | 0.32 | 0.19 |
| SLA | N/A | 3.4 ± 1.7 | 1.9 ± 1.3 | 12 | 0.49 | 0.38 | |||||
| Aghazadeh [14] | 2012 | Autogenous | OsseoGuard | ≥ 3 mm Angular Peri-Implant | Periapical | 2 ± 0.2 | 0.2 ± 1.4 | 22 | 0.04 | 0.3 | |
| Bio-Oss | Software-Assisted | 3.1 ± 0.2 | 1.1 ± 1.43 | 23 | 0.04 | 0.3 | |||||
| Matarasso [15] | 2014 | Bio-Oss | Bio-Gide | Intrabony and Suprabony | Periapical | 4.1 ± 1.6 | 2.8 ± 3.4 | 11 | 0.48 | 1.03 | |
| Software-Assisted | |||||||||||
| Roos-Jansåker [16] | 2014 | Algipore | No | 1-Wall (14.6%) 2-Wall (29.2%) 3-Wall (43.8%) Circumferential (10.4%) Not Classified (2.1%) | Periapical | 3.4 ± 1.7 | 1.3 ± 1.4 | 22 | 0.36 | 0.30 | |
| Osseoquest | N/A | 3.1 ± 2 | 1.6 ± 1.2 | 23 | 0.42 | 0.25 | |||||
| Mercado [17] | 2018 | Bio-Oss collagen | No | EMD, Doxy | Crater-Like or Circumferential | Periapical | 3.55 ± 0.5 | 2.85 ± 0.73 | 30 | 0.09 | 0.13 |
| Linear Measurement (Manual) | |||||||||||
| Renvert [18] | 2018 | Endobon | No | Crater-Like | Periapical | 3.6 ± 0.2 | 0.7 ± 0.9 | 21 | 0.04 | 0.34 | |
| Software-Assisted | |||||||||||
| Tapia [19] | 2019 | BoneCeramic | Cytoplast (Resorbable) | 3% H2O2 | Intrabony | Periapical | 2.19 ± 1.31 | 0.73 ± 1.26 | 15 | 0.34 | 0.33 |
| Ti-Brush | Software-Assisted | 2.84 ± 0.93 | 2.51 ± 1.21 | 15 | 0.24 | 0.31 | |||||
| Polymeri [20] | 2020 | Bio-Oss | No | Intrabony | Periapical | 3.6 ± 1.7 | 2.2 ± 0.8 | 11 | 0.51 | 0.24 | |
| Endobon | Software-Assisted | 3.8 ± 1.4 | 2.8 ± 1.3 | 13 | 0.39 | 0.36 | |||||
| Renvert [21] | 2021 | Bio-Oss | Bio-Gide | Intrabony | Periapical | 1.9 ± 1.5 | 2.3 ± 1.2 | 34 | 0.26 | 0.21 | |
| Software-Assisted | |||||||||||
| Derks [22] | 2022 | Bio-Oss collagen | No | ≥ 3 mm Circumferential and No Minimum Number of Walls | Periapical | 3.7 ± 2.1 | 1.1 ± 1.4 | 72 | 0.25 | 0.16 | |
| Software-Assisted | |||||||||||
| Monje [10] | 2023 | Bio-Oss | No | Intrabony and Suprabony | Periapical | 4.03 ± 1.47 | 1.73 ± 0.83 | 16 | 0.37 | 0.21 | |
| Allograft | RTM | Linear Measurement (Manual) | 3.41 ± 1.15 | 1.72 ± 0.72 | 17 | 0.28 | 0.17 | ||||
| Heitz-Mayfield [23] | 2023 | Bio-Oss | Bio-Gide | Intrabony | Periapical and CBCT | 3.7 ± 1.9 | 2.4 ± 1.4 | 20 | 0.42 | 0.31 | |
| Software-Assisted | |||||||||||
| Regidor [24] | 2023 | Bio-Oss collagen | No | Intrabony (Minimum 2 Walls) | Periapical | 4.2 ± 2.2 | 0.9 ± 1.3 | 20 | 0.49 | 0.29 | |
| Bio-Gide | Software-Assisted | 4.5 ± 2.6 | 1.5 ± 2.2 | 19 | 0.60 | 0.50 | |||||
3.2. Quality Assessment and Reporting Bias
3.3. Clinical Outcomes of Studies
3.3.1. PPD Reduction
3.3.2. Radiographic MLB Gain
3.3.3. CAL Gain
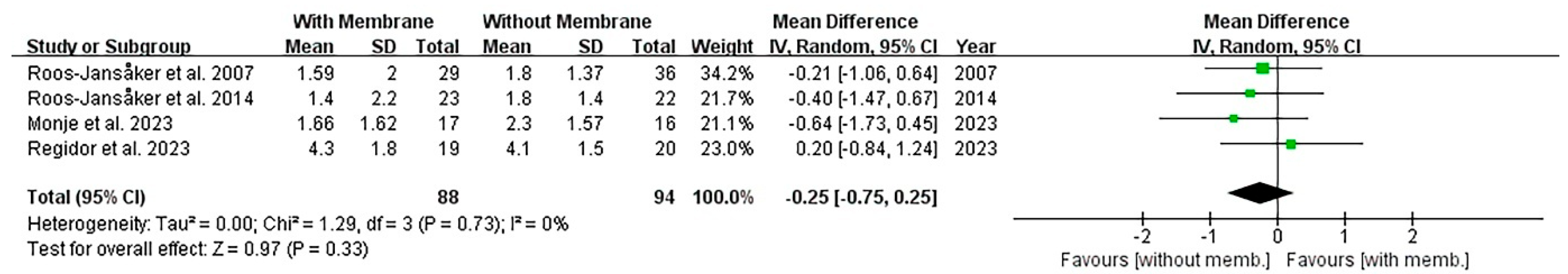
3.3.4. Comparison of Membrane Type
4. Discussion
4.1. Summary of Findings and Meta-Analytical Interpretation
4.2. Comparison with Previous Literature
4.3. Clinical Implications and Decision Making
4.4. Limitations of the Evidence and Methodological Considerations
4.5. Evolution of Membrane Use and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S246–S266. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Galindo-Fernández, P.; Nart, J. Supportive Therapy Following Peri-Implantitis Treatment. Clin. Oral Implants Res. 2024, 35, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Mombelli, A.; Loup, P.-J.; Heitz, F.; Kruger, E.; Lang, N.P. Supportive Peri-Implant Therapy Following Anti-Infective Surgical Peri-Implantitis Treatment: 5-Year Survival and Success. Clin. Oral Implants Res. 2018, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.; Claffey, N. Surgical Therapy for the Control of Peri-Implantitis. Clin. Oral Implants Res. 2012, 23 (Suppl. S6), 84–94. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The Recent Advances in Scaffolds for Integrated Periodontal Regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Chapple, I.L.; on behalf of Working Group 4 of the VIII European Workshop on Periodontology. Clinical Research on Peri-implant Diseases: Consensus Report of W Orking G Roup 4. J. Clin. Periodontol. 2012, 39, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.; Mombelli, A. The Therapy of Peri-Implantitis: A Systematic Review. Int. J. Oral Maxillofac. Implants 2014, 29, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.-M.; Renvert, H.; Lindahl, C.; Renvert, S. Surgical Treatment of Peri-Implantitis Using a Bone Substitute with or without a Resorbable Membrane: A Prospective Cohort Study. J. Clin. Periodontol. 2007, 34, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Vilarrasa, J.; Nart, J.; Wang, H. Significance of Barrier Membrane on the Reconstructive Therapy of Peri-implantitis: A Randomized Controlled Trial. J. Periodontol. 2023, 94, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Buchmann, R. Surgical Therapy of Peri-Implant Disease: A 3-Year Follow-Up Study of Cases Treated with 3 Different Techniques of Bone Regeneration. J. Periodontol. 2001, 72, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Bonino, F.; Bonino, L.; Dalmasso, P. Surgical Therapy of Peri-Implantitis Lesions by Means of a Bovine-Derived Xenograft: Comparative Results of a Prospective Study on Two Different Implant Surfaces. J. Clin. Periodontol. 2011, 38, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, A.; Rutger Persson, G.; Renvert, S. A Single-centre Randomized Controlled Clinical Trial on the Adjunct Treatment of Intra-bony Defects with Autogenous Bone or a Xenograft: Results after 12 Months. J. Clin. Periodontol. 2012, 39, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.; Iorio Siciliano, V.; Aglietta, M.; Andreuccetti, G.; Salvi, G.E. Clinical and Radiographic Outcomes of a Combined Resective and Regenerative Approach in the Treatment of Peri-implantitis: A Prospective Case Series. Clin. Oral Implants Res. 2014, 25, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.-M.; Persson, G.R.; Lindahl, C.; Renvert, S. Surgical Treatment of Peri-Implantitis Using a Bone Substitute with or without a Resorbable Membrane: A 5-Year Follow-Up. J. Clin. Periodontol. 2014, 41, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.; Hamlet, S.; Ivanovski, S. Regenerative Surgical Therapy for Peri-implantitis Using Deproteinized Bovine Bone Mineral with 10% Collagen, Enamel Matrix Derivative and Doxycycline—A Prospective 3-year Cohort Study. Clin. Oral Implants Res. 2018, 29, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Roos-Jansåker, A.-M.; Persson, G.R. Surgical Treatment of Peri-Implantitis Lesions with or without the Use of a Bone Substitute-a Randomized Clinical Trial. J. Clin. Periodontol. 2018, 45, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- De Tapia, B.; Valles, C.; Ribeiro-Amaral, T.; Mor, C.; Herrera, D.; Sanz, M.; Nart, J. The Adjunctive Effect of a Titanium Brush in Implant Surface Decontamination at Peri-implantitis Surgical Regenerative Interventions: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2019, 46, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Polymeri, A.; Anssari-Moin, D.; Van Der Horst, J.; Wismeijer, D.; Laine, M.L.; Loos, B.G. Surgical Treatment of Peri-implantitis Defects with Two Different Xenograft Granules: A Randomized Clinical Pilot Study. Clin. Oral Implants Res. 2020, 31, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Giovannoli, J.-L.; Roos-Jansåker, A.-M.; Rinke, S. Surgical Treatment of Peri-Implantitis with or without a Deproteinized Bovine Bone Mineral and a Native Bilayer Collagen Membrane: A Randomized Clinical Trial. J. Clin. Periodontol. 2021, 48, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Ortiz-Vigón, A.; Guerrero, A.; Donati, M.; Bressan, E.; Ghensi, P.; Schaller, D.; Tomasi, C.; Karlsson, K.; Abrahamsson, I.; et al. Reconstructive Surgical Therapy of Peri-Implantitis: A Multicenter Randomized Controlled Clinical Trial. Clin. Oral Implants Res. 2022, 33, 921–944. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Heitz, F.; Koong, B.; Huang, T.; Chivers, P. Surgical Peri-Implantitis Treatment with and without Guided Bone Regeneration. A Randomized Controlled Trial. Clin. Oral Implants Res. 2023, 34, 892–910. [Google Scholar] [CrossRef] [PubMed]
- Regidor, E.; Ortiz-Vigón, A.; Romandini, M.; Dionigi, C.; Derks, J.; Sanz, M. The Adjunctive Effect of a Resorbable Membrane to a Xenogeneic Bone Replacement Graft in the Reconstructive Surgical Therapy of Peri-implantitis: A Randomized Clinical Trial. J. Clin. Periodontol. 2023, 50, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Solderer, A.; Schmidlin, P.R. Regenerative Surgical Therapy of Peri-Implantitis: An Umbrella Review of Answered/Unanswered Questions and Future Perspectives. Front. Dent. Med. 2020, 1, 614240. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Obreja, K.; Sader, R.; Khoury, F.; Romanos, G.; Koo, K.T.; Keeve, P.L.; Sculean, A.; Schwarz, F. Surgical Treatment of Periimplantitis With Augmentative Techniques. Implant Dent. 2019, 28, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, S.; Sharma, L.A.; Alavi, S.E.; Sharma, A. Peri-Implantitis Therapy Using Surgical Methods: A Systematic Review. Appl. Sci. 2023, 13, 3166. [Google Scholar] [CrossRef]
- Merli, M.; Merli, I.; Raffaelli, E.; Pagliaro, U.; Nastri, L.; Nieri, M. Bone Augmentation at Implant Dehiscences and Fenestrations. A Systematic Review of Randomised Controlled Trials. Eur. J. Oral Implant. 2016, 9, 111–127. [Google Scholar] [PubMed]
- Toledano, M.; Osorio, M.T.; Vallecillo-Rivas, M.; Toledano-Osorio, M.; Rodríguez-Archilla, A.; Toledano, R.; Osorio, R. Efficacy of Local Antibiotic Therapy in the Treatment of Peri-Implantitis: A Systematic Review and Meta-Analysis. J. Dent. 2021, 113, 103790. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Derks, J.; Charalampakis, G.; Abrahamsson, I.; Wennström, J.; Berglundh, T. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Peri-Implantitis: A Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Vallecillo, C.; Toledano, R.; Aguilera, F.S.; Osorio, M.T.; Muñoz-Soto, E.; García-Godoy, F.; Vallecillo-Rivas, M. A Systematic Review and Meta-Analysis of Systemic Antibiotic Therapy in the Treatment of Peri-Implantitis. Int. J. Environ. Res. Public. Health 2022, 19, 6502. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Fretwurst, T.; Schwarz, F. Efficacy of Alternative or Adjunctive Measures to Conventional Non-Surgical and Surgical Treatment of Peri-Implant Mucositis and Peri-Implantitis: A Systematic Review and Meta-Analysis. Int. J. Implant Dent. 2021, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Riben Grundström, C.; Lund, B.; Kämpe, J.; Belibasakis, G.N.; Hultin, M. Systemic Antibiotics in the Surgical Treatment of Peri-implantitis: A Randomized Placebo-controlled Trial. J. Clin. Periodontol. 2024, 51, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Ghisolfi, M.; Murgolo, N.; Chiapasco, M.; Lops, D.; Vogel, G. Therapy of Peri-Implantitis with Resective Surgery. A 3-Year Clinical Trial on Rough Screw-Shaped Oral Implants. Part I: Clinical Outcome. Clin. Oral Implants Res. 2005, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of Peri-Implantitis with Resective Surgery. A 3-Year Clinical Trial on Rough Screw-Shaped Oral Implants. Part II: Radiographic Outcome. Clin. Oral Implants Res. 2007, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Bieling, K.; Becker, J. Surgical Regenerative Treatment of Peri-Implantitis Lesions Using a Nanocrystalline Hydroxyapatite or a Natural Bone Mineral in Combination with a Collagen Membrane: A Four-Year Clinical Follow-up Report. J. Clin. Periodontol. 2009, 36, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Englezos, E.; Cosyn, J.; Koole, S.; Jacquet, W.; De Bruyn, H. Resective Treatment of Peri-Implantitis: Clinical and Radiographic Outcomes After 2 Years. Int. J. Periodontics Restor. Dent. 2018, 38, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-W.; Ashnagar, S.; Gianfilippo, R.D.; Arnett, M.; Kinney, J.; Wang, H.-L. Laser-Assisted Regenerative Surgical Therapy for Peri-Implantitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2021, 92, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, R.; Nikparto, N.; Gharibpour, F.; Pourhajibagher, M.; Bahador, A. Antimicrobial Photodynamic Therapy for Managing the Peri-Implant Mucositis and Peri-Implantitis: A Systematic Review of Randomized Clinical Trials. Photodiagnosis Photodyn. Ther. 2024, 45, 103990. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.; Amato, A.; Sammartino, P.; Iandolo, A.; Martina, S.; Caggiano, M. Laser Therapy in the Treatment of Peri-Implantitis: State-of-the-Art, Literature Review and Meta-Analysis. Appl. Sci. 2021, 11, 5290. [Google Scholar] [CrossRef]
- Almohandes, A.; Carcuac, O.; Abrahamsson, I.; Lund, H.; Berglundh, T. Re-Osseointegration Following Reconstructive Surgical Therapy of Experimental Peri-Implantitis. A Pre-Clinical in Vivo Study. Clin. Oral Implants Res. 2019, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Bougas, K.; Alibegovic, L.; Hosseini, S.; Carcuac, O.; Berglundh, T.; Derks, J. Long-Term Outcomes and Prognostic Factors of Surgical Treatment of Peri-Implantitis—A Retrospective Study. Clin. Oral Implants Res. 2024, 35, 321–329. [Google Scholar] [CrossRef] [PubMed]
- La Monaca, G.; Pranno, N.; Annibali, S.; Polimeni, A.; Cristalli, M.P. A 10-Year Follow-Up of Reconstructive Treatment of Peri-Implantitis Using Mineralized Dehydrated Allograft and Resorbable Membrane: A Retrospective Case Series. Clin. Oral Implants Res. 2025, 36, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Mordini, L.; Sun, N.; Chang, N.; De Guzman, J.-P.; Generali, L.; Consolo, U. Peri-Implantitis Regenerative Therapy: A Review. Biology 2021, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lessem, J.; Dahlén, G.; Renvert, H.; Lindahl, C. Mechanical and Repeated Antimicrobial Therapy Using a Local Drug Delivery System in the Treatment of Peri-Implantitis: A Randomized Clinical Trial. J. Periodontol. 2008, 79, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Brägger, U.; Hämmerle, C.H.F.; Sutter, F. Immediate Transmucosal Implants Using the Principle of Guided Tissue Regeneration. I. Rationale, Clinical Procedures and 30-month Results. Clin. Oral Implants Res. 1994, 5, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.; Nevins, M. Bone Formation Utilizing Titanium Reinforced Barrier Membranes. Implant Dent. 1995, 4, 293. [Google Scholar] [CrossRef]
- Annen, B.M.; Ramel, C.F.; Hämmerle, C.H.F.; Jung, R.E. Use of a New Cross-Linked Collagen Membrane for the Treatment of Peri-Implant Dehiscence Defects: A Randomised Controlled Double-Blinded Clinical Trial. Eur. J. Oral Implant. 2011, 4, 123–134. [Google Scholar] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Khouly, I.; Pardiñas-López, S.; Ruff, R.R.; Strauss, F.-J. Efficacy of Growth Factors for the Treatment of Peri-Implant Diseases: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2020, 24, 2141–2161. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Sculean, A.; Nart, J.; Wang, H.-L. Defect Angle as Prognostic Indicator in the Reconstructive Therapy of Peri-Implantitis. Clin. Implant Dent. Relat. Res. 2023, 25, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; John, G.; Becker, J. The Influence of Implantoplasty on the Diameter, Chemical Surface Composition, and Biocompatibility of Titanium Implants. Clin. Oral Investig. 2017, 21, 2355–2361. [Google Scholar] [CrossRef] [PubMed]


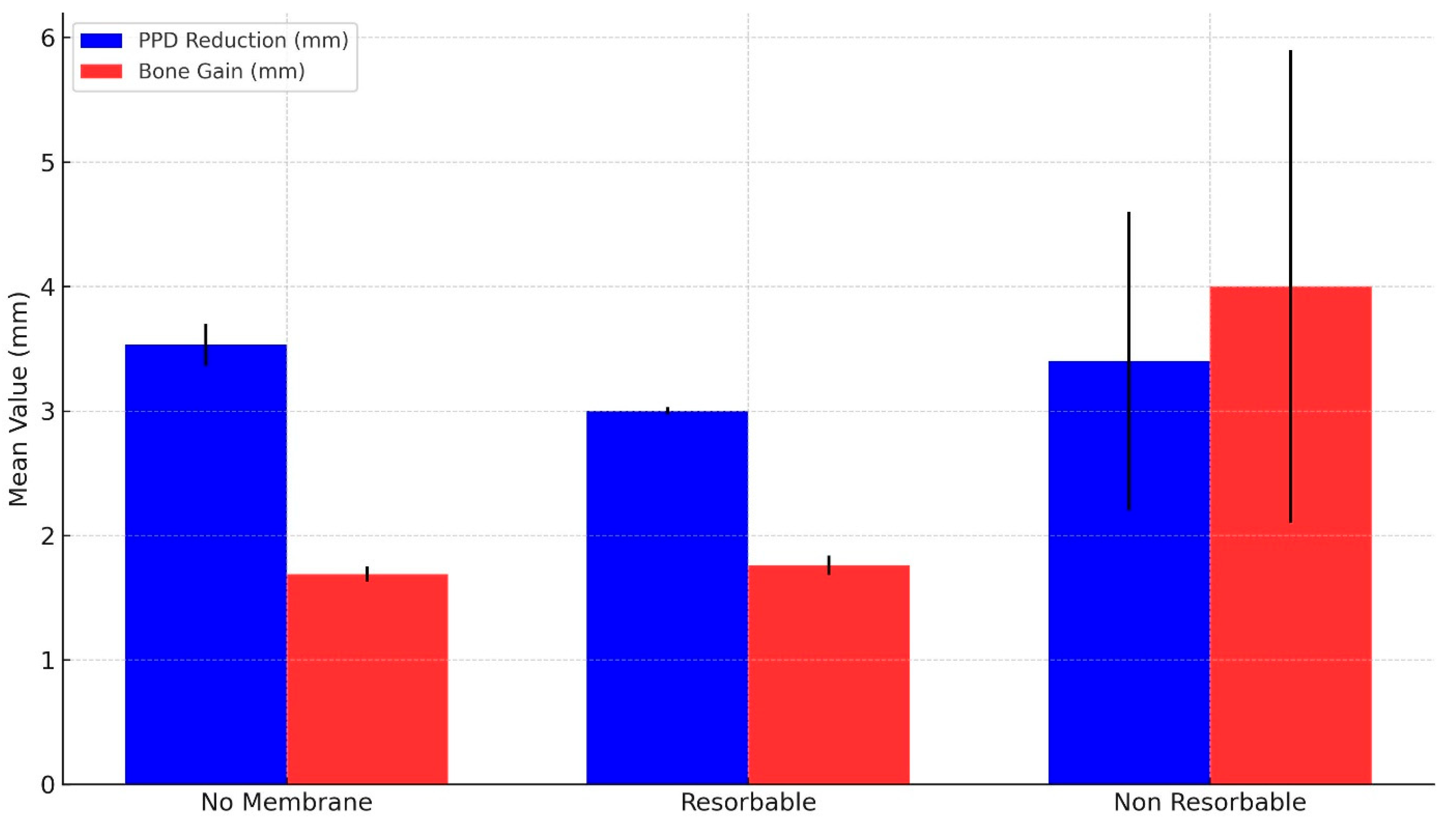
| PPD Reduction (mm) | MLB Gain (mm) | Number of Studies | Total Sample Size | |||
|---|---|---|---|---|---|---|
| Weighted Mean | 95% CI | Weighted Mean | 95% CI | |||
| No Membrane | 3.53 ± 0.17 | [3.21, 3.86] | 1.69 ± 0.06 | [1.56, 1.81] | 10 | 279 |
| Resorbable | 3 ± 0.03 | [2.95, 3.06] | 1.76 ± 0.08 | [1.61, 1.92] | 10 | 237 |
| Non-resorbable | 3.4 ± 1.2 | [1.05, 5.75] * | 4 ± 1.9 | [0.28, 7.72] * | 1 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.J.; Jeong, Y.T.; Woo, H.N.; Cho, H.W.; Kang, M.G.; Hwang, S.-M.; Lee, J.-M. Reassessing the Use of Membranes in Peri-Implantitis Surgery: A Systematic Review and Meta-Analysis of In Vivo Studies. J. Funct. Biomater. 2025, 16, 262. https://doi.org/10.3390/jfb16070262
Cho YJ, Jeong YT, Woo HN, Cho HW, Kang MG, Hwang S-M, Lee J-M. Reassessing the Use of Membranes in Peri-Implantitis Surgery: A Systematic Review and Meta-Analysis of In Vivo Studies. Journal of Functional Biomaterials. 2025; 16(7):262. https://doi.org/10.3390/jfb16070262
Chicago/Turabian StyleCho, Young Joon, Yong Tak Jeong, Hyun Nyun Woo, Hyun Woo Cho, Min Gu Kang, Sung-Min Hwang, and Jae-Mok Lee. 2025. "Reassessing the Use of Membranes in Peri-Implantitis Surgery: A Systematic Review and Meta-Analysis of In Vivo Studies" Journal of Functional Biomaterials 16, no. 7: 262. https://doi.org/10.3390/jfb16070262
APA StyleCho, Y. J., Jeong, Y. T., Woo, H. N., Cho, H. W., Kang, M. G., Hwang, S.-M., & Lee, J.-M. (2025). Reassessing the Use of Membranes in Peri-Implantitis Surgery: A Systematic Review and Meta-Analysis of In Vivo Studies. Journal of Functional Biomaterials, 16(7), 262. https://doi.org/10.3390/jfb16070262








