Evaluation of Physicochemical and Mechanical Properties of a Modified Adhesive System by Resveratrol Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Design
2.3. Preparation of Adhesive System
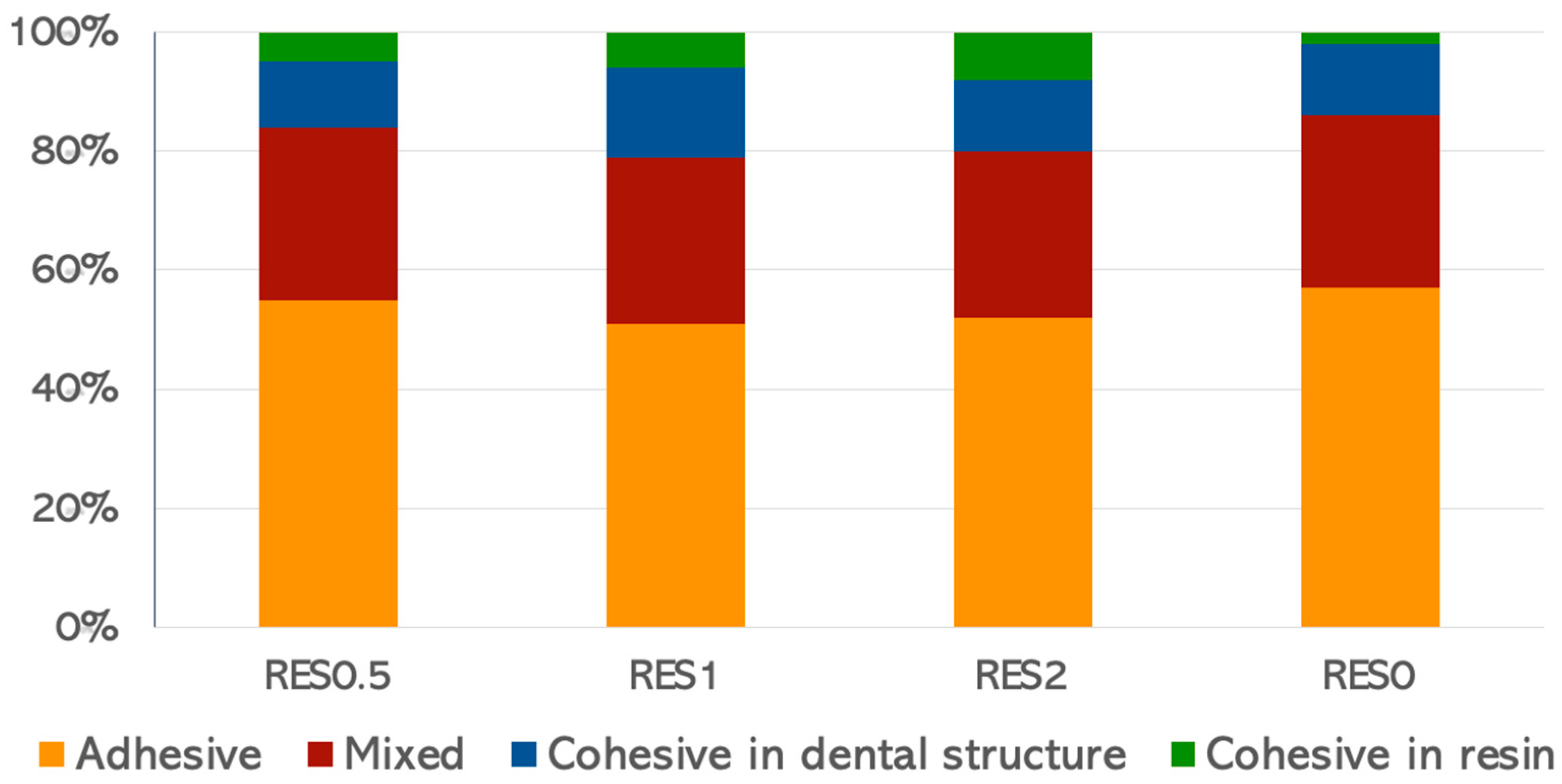
2.4. Microtensile Bond Strength (µTBS)
2.5. Degree of Conversion (DC)
2.6. Mini-Flexural Strength (MFS)
2.7. Antibacterial Activity
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Cenci, M.S.; Montagner, A.F.; de Lima, V.P.; Correa, M.B.; Moraes, R.R.; Opdam, N.J.M. Longevity of Composite Restorations Is Definitely Not Only about Materials. Dent. Mater. 2023, 39, 1–12. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is Secondary Caries with Composites a Material-Based Problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef] [PubMed]
- Hickel, R.; Mesinger, S.; Opdam, N.; Loomans, B.; Frankenberger, R.; Cadenaro, M.; Burgess, J.; Peschke, A.; Heintze, S.D.; Kühnisch, J. Revised FDI Criteria for Evaluating Direct and Indirect Dental Restorations—Recommendations for Its Clinical Use, Interpretation, and Reporting. Clin. Oral Investig. 2023, 27, 2573–2592. [Google Scholar] [CrossRef]
- Pucci, C.R.; Gu, L.S.; Zeng, C.; Gou, Y.P.; Tay, F.R.; Niu, L.N. Susceptibility of Contemporary Single-Bottle Self-Etch Dentine Adhesives to Intrinsic Water Permeation. J. Dent. 2017, 66, 52–61. [Google Scholar] [CrossRef]
- Gou, Y.-P.; Meghil, M.M.; Pucci, C.R.; Breschi, L.; Pashley, D.H.; Cutler, C.W.; Niu, L.-N.; Li, J.-Y.; Tay, F.R. Optimizing Resin-Dentin Bond Stability Using a Bioactive Adhesive with Concomitant Antibacterial Properties and Anti-Proteolytic Activities. Acta Biomater. 2018, 75, 171–182. [Google Scholar] [CrossRef]
- Porto, I.C.C.d.M.; Lôbo, T.d.L.G.F.; Rodrigues, R.F.; Lins, R.B.E.; da Silva, M.A.B. Insight into the Development of Versatile Dentin Bonding Agents to Increase the Durability of the Bonding Interface. Front. Dent. Med. 2023, 4, 1127368. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nagano, F.; Endo, K.; Ohno, H. A Review: Biodegradation of Resin-Dentin Bonds. Jpn. Dent. Sci. Rev. 2011, 47, 5–12. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, Fatigue, and Failure of Resin Dental Composite Materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef]
- Cadenaro, M.; Josic, U.; Maravić, T.; Mazzitelli, C.; Marchesi, G.; Mancuso, E.; Breschi, L.; Mazzoni, A. Progress in Dental Adhesive Materials. J. Dent. Res. 2023, 102, 254–262. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 2nd ed.; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Porto, I.C.C.d.M.; Rocha, A.B.d.B.; Ferreira, I.I.S.; de Barros, B.M.; Ávila, E.C.; da Silva, M.C.; de Oliveira, M.P.S.; Lôbo, T.d.L.G.F.; Oliveira, J.M.d.S.; do Nascimento, T.G.; et al. Polyphenols and Brazilian Red Propolis Incorporated into a Total-Etching Adhesive System Help in Maintaining Bonding Durability. Heliyon 2021, 7, e06237. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, M.C.G.; Osorio, R.; Viseras, C.; Toledano, M. Adjunctive Use of an Anti-Oxidant Agent to Improve Resistance of Hybrid Layers to Degradation. J. Dent. 2011, 39, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Castellan, C.S.; Pereira, P.N.; Grande, R.H.M.; Bedran-Russo, A.K. Mechanical Characterization of Proanthocyanidin-Dentin Matrix Interaction. Dent. Mater. 2010, 26, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Castellan, C.S.; Bedran-Russo, A.K.; Karol, S.; Pereira, P.N.R. Long-Term Stability of Dentin Matrix Following Treatment with Various Natural Collagen Cross-Linkers. J. Mech. Behav. Biomed. Mater. 2011, 4, 1343–1350. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.B.; Pereira, P.N.R.; Duarte, W.R.; Drummond, J.L.; Yamaychi, M. Application of Crosslinkers to Dentin Collagen Enhances the Ultimate Tensile Strength. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 268–272. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.B.; Pashley, D.H.; Agee, K.; Drummond, J.L.; Miescke, K.J. Changes in Stiffness of Demineralized Dentin Following Application of Collagen Crosslinkers. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 330–334. [Google Scholar] [CrossRef]
- Al-Ammar, A.; Drummond, J.L.; Bedran-Russo, A.K. The Use of Collagen Cross-Linking Agents to Enhance Dentin Bond Strength. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 419–424. [Google Scholar] [CrossRef]
- Atalayin, C.; Tezel, H.; Ergucu, Z.; Unlu, N.; Armagan, G.; Dagci, T.; Kose, T. The Improvement of Biocompatibility of Adhesives: The Effects of Resveratrol on Biocompatibility and Dentin Micro-Tensile Bond Strengths of Self-Etch Adhesives. Clin. Oral Investig. 2019, 23, 3213–3218. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Peng, W.; Zhu, Y. Effects of Resveratrol on Cariogenic Virulence Properties of Streptococcus Mutans. BMC Microbiol. 2020, 20, 99. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lin, C. Effect of Resveratrol and Pterostilbene on Aging and Longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Hsieh, Y.; Yang, S.; Chen, C.; Tang, C.; Chou, M.; Chuang, Y.; Lin, C.; Chen, M. Resveratrol Suppresses TPA-induced Matrix Metalloproteinase-9 Expression through the Inhibition of MAPK Pathways in Oral Cancer Cells. J. Oral Pathol. Med. 2015, 44, 699–706. [Google Scholar] [CrossRef]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K.H. Bioactive Polyphenol Antioxidants Protect Oral Fibroblasts from ROS-Inducing Agents. Arch. Oral Biol. 2012, 57, 1657–1667. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin Adhesion and MMPs: A Comprehensive Review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, D.C.; Fonseca, B.M.; Pucci, C.R.; Cavalcanti, B.d.N.; Persici, E.D.S.; Gonçalves, S.E.d.P. Zn-Doped Etch-and-Rinse Model Dentin Adhesives: Dentin Bond Integrity, Biocompatibility, and Properties. Dent. Mater. 2016, 32, 940–950. [Google Scholar] [CrossRef]
- Lopes, S.R.; Matuda, A.G.N.; Campos, R.P.; Mafetano, A.P.V.P.; Barnabe, A.H.M.; Chagas, G.S.; Barcellos, D.C.; Niu, L.N.; Tay, F.R.; Pucci, C.R. Development of an Antibacterial Dentin Adhesive. Polymers 2022, 14, 2502. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.R.; De Oliveira, R.S.; Caneppele, T.M.F.; Torres, C.R.G.; Borges, A.B.; Tay, F.R. Effects of Surface Treatment, Hydration and Application Method on the Bond Strength of a Silorane Adhesive and Resin System to Dentine. J. Dent. 2013, 41, 278–286. [Google Scholar] [CrossRef]
- ISO/TS 11405:2015; Dental Materials—Testing of Adhesion to Tooth Structure. International Organization for Standardization: Geneva, Switzerland, 2015.
- Guo, X.; Peng, Z.; Spencer, P.; Wang, Y. Effect of Initiator on Photopolymerization of Acidic, Aqueous Dental Model Adhesives. J. Biomed. Mater. Res. A 2009, 90A, 1120–1127. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y. Effect of Proanthocyanidins and Photo-Initiators on Photo-Polymerization of a Dental Adhesive. J. Dent. 2013, 41, 71–79. [Google Scholar] [CrossRef]
- Sano, H.; Shono, T.; Sonoda, H.; Takatsu, T.; Ciucchi, B.; Carvalho, R.; Pashley, D.H. Relationship between Surface Area for Adhesion and Tensile Bond Strength—Evaluation of a Micro-Tensile Bond Test. Dent. Mater. 1994, 10, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.; Kumar, A.; Telang, A.; Kumari, P.; Verma, G.; Bharti, K. Evaluation of the Effect of Different Contaminants on the Shear Bond Strength of Different Adhesive Systems—An In Vitro Study. J. Pharm. Bioallied Sci. 2024, 16, S3598–S3600. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, T.M.A.; Basting, R.T.; Turssi, C.P.; França, F.M.G.; Amaral, F.L.B. Influence of Natural and Synthetic Metalloproteinase Inhibitors on Bonding Durability of an Etch-and-Rinse Adhesive to Dentin. Int. J. Adhes. Adhes. 2013, 47, 83–88. [Google Scholar] [CrossRef]
- Hass, V.; Dobrovolski, M.; Zander-Grande, C.; Martins, G.C.; Gordillo, L.A.A.; Rodrigues Accorinte, M.d.L.; Gomes, O.M.M.; Loguercio, A.D.; Reis, A. Correlation between Degree of Conversion, Resin–Dentin Bond Strength and Nanoleakage of Simplified Etch-and-Rinse Adhesives. Dent. Mater. 2013, 29, 921–928. [Google Scholar] [CrossRef]
- Perote, L.C.; Kamozaki, M.B.; Gutierrez, N.C.; Tay, F.R.; Pucci, C.R. Effect of Matrix Metalloproteinase-Inhibiting Solutions and Aging Methods on Dentin Bond Strength. J. Adhes. Dent. 2015, 17, 347–352. [Google Scholar] [PubMed]
- da Silva, E.M.; de Sá Rodrigues, C.U.F.; de Oliveira Matos, M.P.; de Carvalho, T.R.; dos Santos, G.B.; Amaral, C.M. Experimental Etch-and-Rinse Adhesive Systems Containing MMP-Inhibitors: Physicochemical Characterization and Resin-Dentin Bonding Stability. J. Dent. 2015, 43, 1491–1497. [Google Scholar] [CrossRef]
- Guo, R.; Peng, W.; Yang, H.; Yao, C.; Yu, J.; Huang, C. Evaluation of Resveratrol-Doped Adhesive with Advanced Dentin Bond Durability. J. Dent. 2021, 114, 103817. [Google Scholar] [CrossRef]
- Poptani, B.; Gohil, K.S.; Ganjiwale, J.; Shukla, M. Microtensile Dentin Bond Strength of Fifth with Five Seventh-Generation Dentin Bonding Agents after Thermocycling: An In Vitro Study. Contemp. Clin. Dent. 2012, 3, S167–S171. [Google Scholar] [CrossRef]
- Gotti, V.B.; Feitosa, V.P.; Sauro, S.; Correr-Sobrinho, L.; Leal, F.B.; Stansbury, J.W.; Correr, A.B. Effect of Antioxidants on the Dentin Interface Bond Stability of Adhesives Exposed to Hydrolytic Degradation. J. Adhes. Dent. 2015, 17, 35–44. [Google Scholar] [CrossRef]
- Miletic, V.; Santini, A.; Trkulja, I. Quantification of Monomer Elution and Carbon–Carbon Double Bonds in Dental Adhesive Systems Using HPLC and Micro-Raman Spectroscopy. J. Dent. 2009, 37, 177–184. [Google Scholar] [CrossRef]
- Navarra, C.O.; Breschi, L.; Turco, G.; Diolosà, M.; Fontanive, L.; Manzoli, L.; Di Lenarda, R.; Cadenaro, M. Degree of Conversion of Two-Step Etch-and-Rinse Adhesives: In Situ Micro-Raman Analysis. J. Dent. 2012, 40, 711–717. [Google Scholar] [CrossRef]
- Eick, J.D.; Gwinnett, A.J.; Pashley, D.H.; Robinson, S.J. Current Concepts On Adhesion To Dentin. Crit. Rev. Oral Biol. Med. 1997, 8, 306–335. [Google Scholar] [CrossRef]
- Faria-e-Silva, A.L.; Lima, A.F.; Moraes, R.R.; Piva, E.; Martins, L.R. Degree of Conversion of Etch-and-Rinse and Self-Etch Adhesives Light-Cured Using QTH or LED. Oper. Dent. 2010, 35, 649–654. [Google Scholar] [CrossRef]
- Bertolo, M.V.L.; Guarda, M.B.; Fronza, B.M.; Abuna, G.F.; Vitti, R.P.; Geraldeli, S.; Sinhoreti, M.A.C. Electric Current Effects on Bond Strength, Nanoleakage, Degree of Conversion and Dentinal Infiltration of Adhesive Systems. J. Mech. Behav. Biomed. Mater. 2021, 119, 104529. [Google Scholar] [CrossRef]
- Ferracane, J.L. Correlation between Hardness and Degree of Conversion during the Setting Reaction of Unfilled Dental Restorative Resins. Dent. Mater. 1985, 1, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Cocco, A.R.; Lima, G.S.; Leal, F.B.; Munchow, E.A.; Ogliari, F.A.; Piva, E. Addition of Nanoparticles for Development of Radiopaque Dental Adhesives. Int. J. Adhes. Adhes. 2018, 80, 122–127. [Google Scholar] [CrossRef]
- ISO 4049; Dentistry—Resin-Based Filling Materials. International Organization for Standardization: Geneva, Switzerland, 1998.
- Arrais, C.A.G.; Pontes, F.M.; dos Santos, L.P.S.; Leite, E.R.; Giannini, M. Degree of Conversion of Adhesive Systems Light-Cured by LED and Halogen Light. Braz. Dent. J. 2007, 18, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Luiz Souto Borges, A.; Bühler Borges, A.; Câmara Barcellos, D.; De Siqueira Ferreira Anzaloni Saavedra, G.; De José Arruda Paes Junior, T.; Mello Rode, S. Avaliação da Resistência Flexural e Módulo de Elasticidade de Diferentes Resinas Compostas Indiretas. RPG Rev. Pós-Graduação 2012, 19, 50–56. [Google Scholar]
- Bahrami, R.; Gharibpour, F.; Pourhajibagher, M.; Bahador, A. The Flexural Strength of Orthodontic Acrylic Resin Containing Resveratrol Nanoparticles as Antimicrobial Agent: An In Vitro Study. Int. Orthod. 2024, 22, 100846. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Z.; Guo, R.; Peng, W.; Yang, H.; Huang, C. Epigallocatechin-3-Gallate/Nanohydroxyapatite Platform Delivery Approach to Adhesive-Dentin Interface Stability. Mater. Sci. Eng. C 2021, 122, 111918. [Google Scholar] [CrossRef]
- Abedini, E.; Khodadadi, E.; Zeinalzadeh, E.; Moaddab, S.R.; Asgharzadeh, M.; Mehramouz, B.; Dao, S.; Samadi Kafil, H. A Comprehensive Study on the Antimicrobial Properties of Resveratrol as an Alternative Therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 8866311. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Yi, L.; Wang, Z.; Yang, H.; Huang, C. Effects of Resveratrol/Ethanol Pretreatment on Dentin Bonding Durability. Mater. Sci. Eng. C 2020, 114, 111000. [Google Scholar] [CrossRef] [PubMed]
- Osamudiamen, P.M.; Oluremi, B.B.; Oderinlo, O.O.; Aiyelaagbe, O.O. Trans-Resveratrol, Piceatannol and Gallic Acid: Potent Polyphenols Isolated from Mezoneuron Benthamianum Effective as Anticaries, Antioxidant and Cytotoxic Agents. Sci. Afr. 2020, 7, e00244. [Google Scholar] [CrossRef]
- Pournasir, M.; Alavi, F.N.; Davalloo, R.T.; Ebrahim-Saraie, H.S.; Ghaffari, M.E. Antibacterial Effect of Resveratrol Extract Compared to Chlorhexidine Mouthwash against Primary Cariogenic Pathogen, Streptococcus Mutans. J. Clin. Exp. Dent. 2024, 16, e802–e807. [Google Scholar] [CrossRef]
- Rêgo, H.M.C.; Alves, T.S.; Bresciani, E.; Niu, L.N.; Tay, F.R.; Pucci, C.R. Can Long-Term Dentine Bonding Created in Real Life Be Forecasted by Parameters Established in the Laboratory? Sci. Rep. 2016, 6, 37799. [Google Scholar] [CrossRef] [PubMed]

| Product | Manufacturer | Composition | Batch Number |
|---|---|---|---|
| Adper Single Bond 2 | 3M ESPE, St. Paul, MN, USA | Bisphenol-A-glycidyl-dimethacrylate, 2-hydroxylethyl methacrylate, dimethacrylate, water, ethanol, photoinitiator, silica nanoparticles, methacrylate functional copolymer of polyacrylic and polyalkenoic acids | 2129900256 |
| Resveratrol | Sigma-Aldrich, St. Louis, MO, USA | 3,4′,5-Tri-hidroxi-trans-estilbeno 5-[(1E)-2-(4-hidroxifenil) etenil]-1,3-benzenodiol | SLBV8562 |
| Groups | µTBS (±SD) * | % DC (±SD) | MFS (±SD) * | CFUs (±SD) |
|---|---|---|---|---|
| RES0 | 41.01 (±2.64) A | 77.75 (±3.22) A | 29.72 (±11.95) A | 0.75 × 107 (±0.03) A |
| RES0.5 | 42.93 (±15.49) A | 81.02 (±1.95) A | 33.14 (±13.83) A | 0.67 × 107 (±0.37) B |
| RES1 | 42.61 (±13.97) A | 76.02 (±9.00) A | 31.1 (±12.21) A | 0.68 × 107 (±0.34) B |
| RES2 | 39.43 (±9.14) A | 58.86 (±15.94) A | 19.72 (±5.43) B | 0.60 × 107 (±0.02) C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuda, A.G.N.; Yui, K.C.K.; Gomes, N.M.; Chagas, G.d.S.; Rocha, M.B.; Senefonte, F.L.; Mailart, M.C.; Pucci, C.R. Evaluation of Physicochemical and Mechanical Properties of a Modified Adhesive System by Resveratrol Incorporation. J. Funct. Biomater. 2025, 16, 178. https://doi.org/10.3390/jfb16050178
Matuda AGN, Yui KCK, Gomes NM, Chagas GdS, Rocha MB, Senefonte FL, Mailart MC, Pucci CR. Evaluation of Physicochemical and Mechanical Properties of a Modified Adhesive System by Resveratrol Incorporation. Journal of Functional Biomaterials. 2025; 16(5):178. https://doi.org/10.3390/jfb16050178
Chicago/Turabian StyleMatuda, Amanda Guedes Nogueira, Karen Cristina Kazue Yui, Nathália Moreira Gomes, Gabriela da Silva Chagas, Marcella Batista Rocha, Fernanda Labiapari Senefonte, Mariane Cintra Mailart, and Cesar Rogério Pucci. 2025. "Evaluation of Physicochemical and Mechanical Properties of a Modified Adhesive System by Resveratrol Incorporation" Journal of Functional Biomaterials 16, no. 5: 178. https://doi.org/10.3390/jfb16050178
APA StyleMatuda, A. G. N., Yui, K. C. K., Gomes, N. M., Chagas, G. d. S., Rocha, M. B., Senefonte, F. L., Mailart, M. C., & Pucci, C. R. (2025). Evaluation of Physicochemical and Mechanical Properties of a Modified Adhesive System by Resveratrol Incorporation. Journal of Functional Biomaterials, 16(5), 178. https://doi.org/10.3390/jfb16050178






