Enhanced Osteoconductivity of Zirconia Implants with One-Step Femtosecond Laser Treatment Through Morphological and Chemical Modifications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Zirconia Specimens
2.2. Femtosecond Laser Treatment
2.3. Surface Morphology Characterisation
2.4. Surface Chemical Composition and Wettability Characterisation
2.5. Biocompatibility Assessment In Vitro
2.5.1. Cell Culture
2.5.2. Cell Morphology and Adhesion
2.5.3. Cell Proliferation
2.6. In Vitro Osteogenic Potential Assessment
3. Results
3.1. Surface Morphology of the 3Y-TZP Specimens
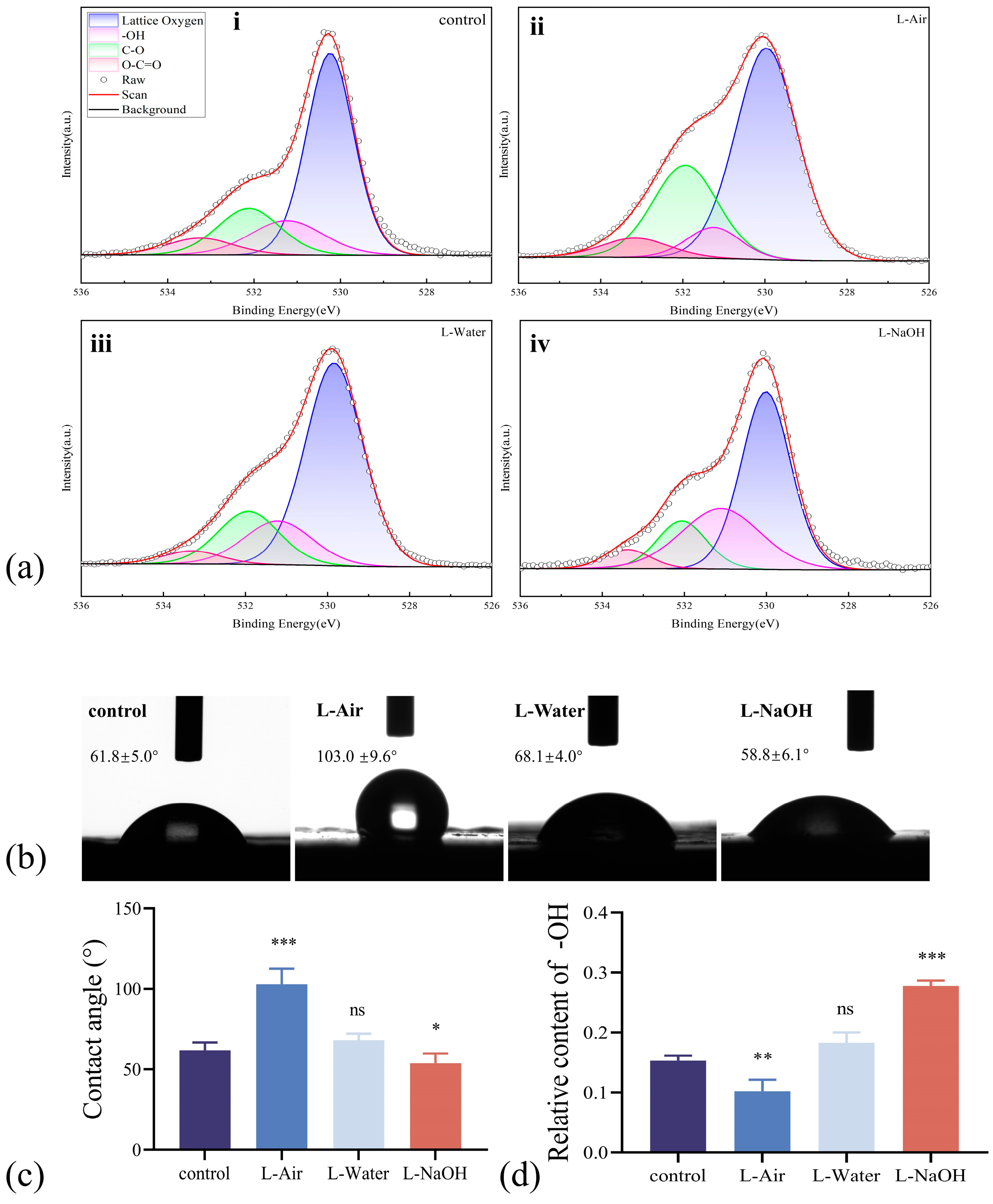
3.2. Surface Chemical Composition and Wettability Modified by Laser Ablation
3.3. Biocompatibility Assessment In Vitro
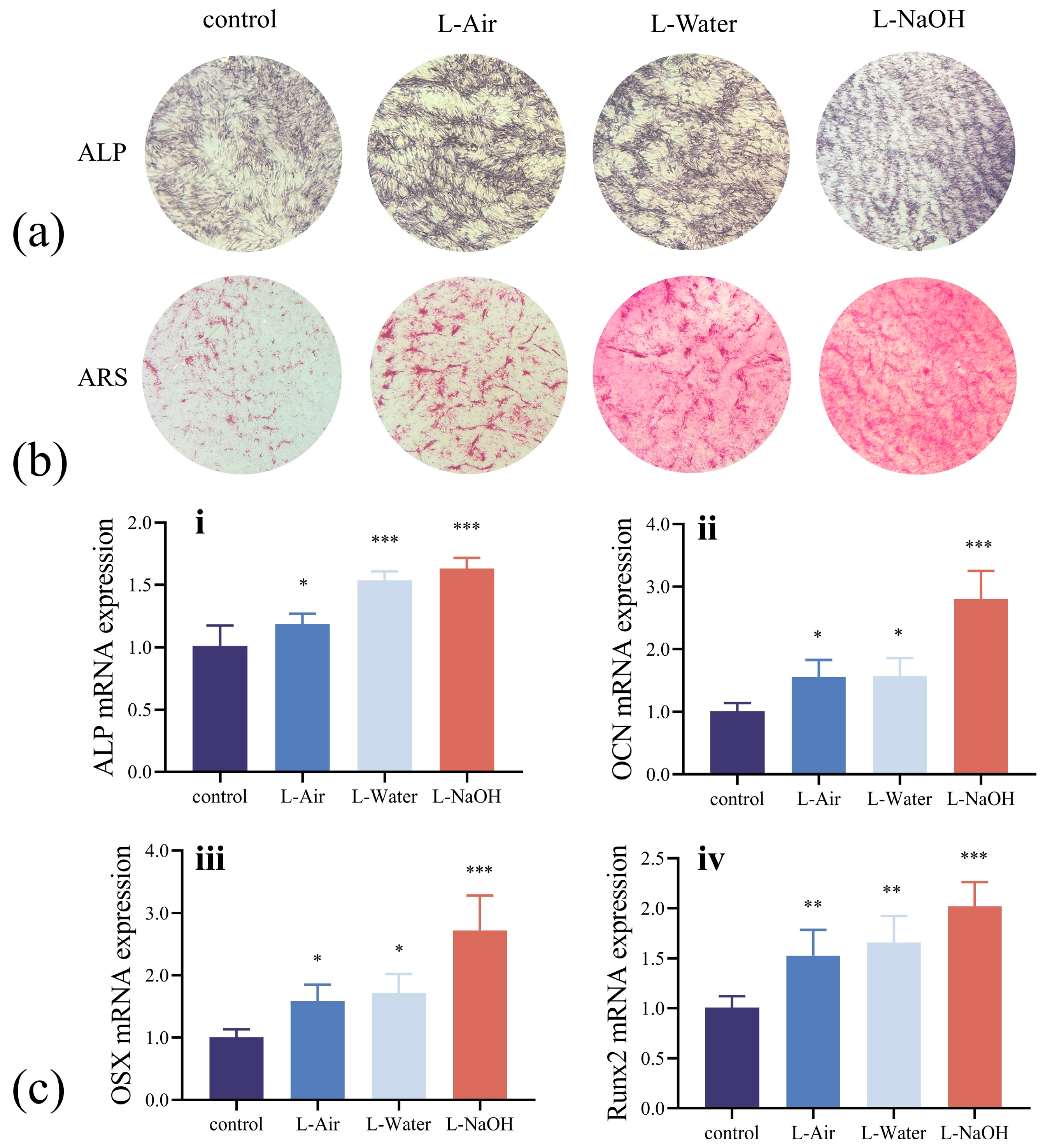
3.4. In Vitro Osteogenic Potential Assessment
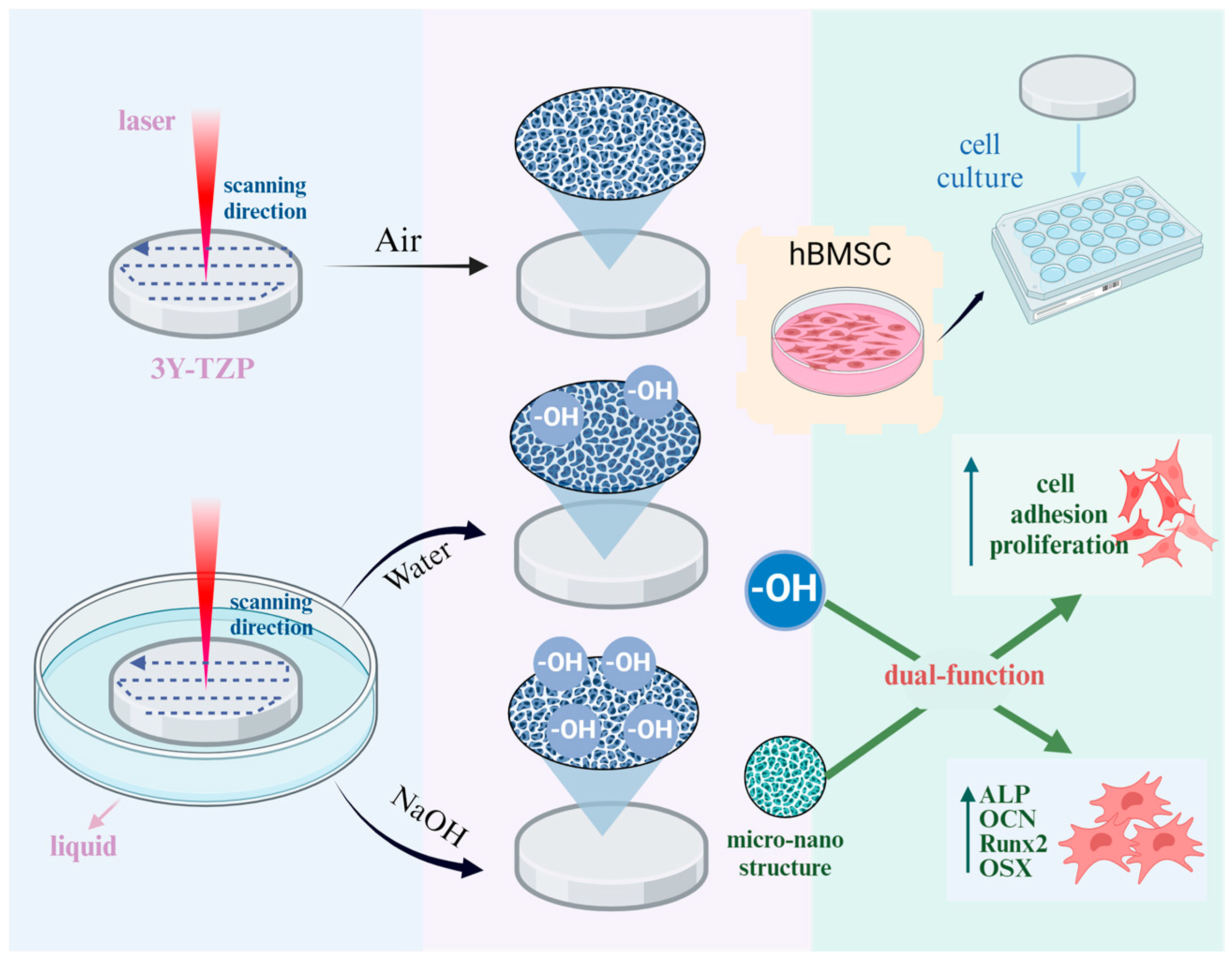
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ARS | Alizarin red S |
| DI | Deionized |
| FSL | Femtosecond lase |
| hBMSCs | Human bone marrow mesenchymal stem cells |
| NaOH | Sodium hydroxide |
| hBMSCs | Human bone marrow mesenchymal stem cells |
| OCN | Osteocalcin |
| OD | Optical density |
| OSX | Osterix |
| PBS | Phosphate-buffered saline |
| Runx2 | Runt-related transcription factor 2 |
| UV | Ultraviolet |
| 3Y-TZP | 3 mol% yttrium oxide-stabilised zirconia |
References
- Cattani-Lorente, M.; Scherrer, S.S.; Ammann, P.; Jobin, M.; Wiskott, H.W.A. Low Temperature Degradation of a Y-TZP Dental Ceramic. Acta Biomater. 2011, 7, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Haro Adanez, M.; Att, W. Current Status of Zirconia Implants in Dentistry: Preclinical Tests. J. Prosthodont. Res. 2019, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Matei, M.; Voinea, E.-A.; Rîcă, R.; Manolea, H.; Mogoantă, L.; Salan, A.; Rîcă, A.; Dinescu, V.C.; Cioateră, N. New Zirconia-Based Materials for Dental Applications. Structural, Morphological and Histological Evaluation. Ceram. Int. 2019, 45, 14859–14866. [Google Scholar] [CrossRef]
- Ban, S. Classification and Properties of Dental Zirconia as Implant Fixtures and Duperstructures. Materials 2021, 14, 4879. [Google Scholar] [CrossRef]
- Belli, R.; Hurle, K.; Schürrlein, J.; Petschelt, A.; Werbach, K.; Peterlik, H.; Rabe, T.; Mieller, B.; Lohbauer, U. Relationships between Fracture Toughness, Y2O3 Fraction and Phases Content in Modern Dental Yttria-Doped Zirconias. J. Eur. Ceram. Soc. 2021, 41, 7771–7782. [Google Scholar] [CrossRef]
- Saito, M.M.; Onuma, K.; Yamakoshi, Y. Nanoscale Osseointegration of Zirconia Evaluated from the Interfacial Structure between Ceria-Stabilized Tetragonal Zirconia and Cell-Induced Hydroxyapatite. J. Oral Biosci. 2024, 66, 281–287. [Google Scholar] [CrossRef]
- Zhang, Y. Making Yttria-Stabilized Tetragonal Zirconia Translucent. Dent. Mater. 2014, 30, 1195–1203. [Google Scholar] [CrossRef]
- Jin, H.W.; Noumbissi, S.; Wiedemann, T.G. Comparison of Zirconia Implant Surface Modifications for Optimal Osseointegration. J. Funct. Biomater. 2024, 15, 91. [Google Scholar] [CrossRef]
- Bienz, S.P.; Hilbe, M.; Hüsler, J.; Thoma, D.S.; Hämmerle, C.H.F.; Jung, R.E. Clinical and Histological Comparison of the Soft Tissue Morphology between Zirconia and Titanium Dental Implants under Healthy and Experimental Mucositis Conditions—A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2021, 48, 721–733. [Google Scholar] [CrossRef]
- Jaeggi, M.; Gyr, S.; Astasov-Frauenhoffer, M.; Zitzmann, N.U.; Fischer, J.; Rohr, N. Influence of Different Zirconia Surface Treatments on Biofilm Formation in Vitro and in Situ. Clin. Oral Implant. Res. 2022, 33, 424–432. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia Surface Modifications for Implant Dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An Overview of Zirconia Ceramics: Basic Properties and Clinical Applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A Review of Biomimetic Surface Functionalization for Bone-Integrating Orthopedic Implants: Mechanisms, Current Approaches, and Future Directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Ogawa, T.; Saita, M.; Ikeda, T.; Yamada, M.; Kimoto, K.; Lee, M. UV Photofunctionalization Promotes Nano-Biomimetic Apatite Deposition on Titanium. Int. J. Nanomed. 2016, 223–234. [Google Scholar] [CrossRef]
- Guo, S.; Liu, N.; Liu, K.; Li, Y.; Zhang, W.; Zhu, B.; Gu, B.; Wen, N. Effects of Carbon and Nitrogen Plasma Immersion Ion Implantation on Bioactivity of Zirconia. RSC Adv. 2020, 10, 35917–35929. [Google Scholar] [CrossRef]
- Hong, G.; Liao, M.; Wu, T.; Zhou, Q.; Xie, H.; Chen, C. Improving Osteogenic Activity of Y-TZP (Yttria-Stabilized Tetragonal Zirconia Polycrystal) Surfaces by Grafting of Silanes with Different end Groups. Appl. Surf. Sci. 2021, 570, 151144. [Google Scholar] [CrossRef]
- Han, A.; Ding, H.; Tsoi, J.K.H.; Imazato, S.; Matinlinna, J.P.; Chen, Z. Prolonged UV-C Irradiation is a Double-Edged Sword on the Zirconia Surface. ACS Omega 2020, 5, 5126–5133. [Google Scholar] [CrossRef]
- Han, A.; Tsoi, J.K.H.; Matinlinna, J.P.; Zhang, Y.; Chen, Z. Effects of Different Sterilization Methods on Surface Characteristics and Biofilm Formation on Zirconia in Vitro. Dent. Mater. 2018, 34, 272–281. [Google Scholar] [CrossRef]
- Carvalho, A.; Grenho, L.; Fernandes, M.H.; Daskalova, A.; Trifonov, A.; Buchvarov, I.; Monteiro, F.J. Femtosecond Laser Microstructuring of Alumina Toughened Zirconia for Surface Functionalization of Dental Implants. Ceram. Int. 2020, 46, 1383–1389. [Google Scholar] [CrossRef]
- Lu, J.; Huang, T.; Liu, Z.; Zhang, X.; Xiao, R. Long-Term Wettability of Titanium Surfaces by Combined Femtosecond Laser Micro/Nano Structuring and Chemical Treatments. Appl. Surf. Sci. 2018, 459, 257–262. [Google Scholar] [CrossRef]
- Her, T.-H.; Finlay, R.J.; Wu, C.; Deliwala, S.; Mazur, E. Microstructuring of Silicon with Femtosecond Laser Pulses. Appl. Phys. Lett. 1998, 73, 1673–1675. [Google Scholar] [CrossRef]
- Sakka, T.; Iwanaga, S.; Ogata, Y.H.; Matsunawa, A.; Takemoto, T. Laser Ablation at Solid–Liquid Interfaces: An Approach from Optical Emission Spectra. J. Chem. Phys. 2000, 112, 8645–8653. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Liang, C.; Wang, H.; Zhu, X.; Zhang, N. Surface Microstructuring of Ti Plates by Femtosecond Lasers in Liquid Ambiences: A New Approach to Improving Biocompatibility. Opt. Express 2009, 17, 21124–21133. [Google Scholar] [CrossRef]
- Shen, H.; Jiang, J.; Feng, D.; Xing, C.; Zhao, X.; Xiao, P. Environmental Effect on the Crack Behavior of Yttria-Stabilized Zirconia during Laser Drilling. J. Manuf. Sci. Eng. 2019, 141, 054501. [Google Scholar] [CrossRef]
- Bashir, S.; Rafique, M.S.; Husinsky, W. Liquid Assisted Ablation of Zirconium for the Growth of LIPSS at Varying Pulse Durations and Pulse Energies by Femtosecond Laser Irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 349, 230–238. [Google Scholar] [CrossRef]
- Sugioka, K. Hybrid Femtosecond Laser Three-Dimensional Micro-and Nanoprocessing: A Review. Int. J. Extrem. Manuf. 2019, 1, 012003. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Wang, H.J.; Zhou, L.C.; Zhang, Z.H.; Zhou, Z.R. Temperature-Responsive, Femtosecond Laser-Ablated Ceramic Surfaces with Switchable Wettability for On-Demand Droplet Transfer. ACS Appl. Mater. Interfaces 2023, 15, 13740–13752. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.-M.; Kokubo, T.; Nawa, M.; Asano, T.; Tanaka, K.; Nakamura, T. Apatite-Forming Ability of a Zirconia/Alumina Nano-Composite Induced by Chemical Treatment. J. Biomed. Mater. Res. 2002, 60, 277–282. [Google Scholar] [CrossRef]
- Baxter, S.; Cassie, A.B.D. The Water Repellency of Fabrics and a New Water Repellency Test. J. Text. Inst. Trans. 1945, 36, T67–T90. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, M.; Lin, J.; Cheng, K.; Weng, W.; Wang, H. Surface Hydroxyl Groups Direct Cellular Response on Amorphous and Anatase TiO2 Nanodots. Colloids Surf. B Biointerfaces 2014, 123, 68–74. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Franco, G. Defective Bone Mineralization and Osteopenia in Young Adult FGFR3-/- Mice. Hum. Mol. Genet. 2003, 13, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T.; Hinoi, E.; Nakazato, R.; Ochi, H.; Xu, C.; Tsuchikane, A.; Takeda, S.; Karsenty, G.; Abe, T.; Kiyonari, H.; et al. An Analysis of Skeletal Development in Osteoblast-Specific and Chondrocyte-Specific Runt-Related Transcription Factor-2 (Runx2) Knockout Mice. J. Bone Miner. Res. 2013, 28, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Padilla, R.J.; Ambrose, W.; De Kok, I.J.; Cooper, L.F. The Effect of Hydrofluoric Acid Treatment of TiO2 Grit Blasted Titanium Implants on Adherent Osteoblast Gene Expression in Vitro and in Vivo. Biomaterials 2007, 28, 5418–5425. [Google Scholar] [CrossRef]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone Tissue Responses to Surface-modified Zirconia Implants: A Histomorphometric and Removal Torque Study in the Rabbit. Clin. Implant. Dent. Rel. Res. 2005, 7, s13–s20. [Google Scholar] [CrossRef]
- Zinger, O.; Zhao, G.; Schwartz, Z.; Simpson, J.; Wieland, M.; Landolt, D.; Boyan, B. Differential Regulation of Osteoblasts by Substrate Microstructural Features. Biomaterials 2005, 26, 1837–1847. [Google Scholar] [CrossRef]
- Li, D.; Ferguson, S.J.; Beutler, T.; Cochran, D.L.; Sittig, C.; Hirt, H.P.; Buser, D. Biomechanical Comparison of the Sandblasted and Acid-Etched and the Machined and Acid-Etched Titanium Surface for Dental Implants. J. Biomed. Mater. Res. 2002, 60, 325–332. [Google Scholar] [CrossRef]
- Wennerberg, A.; Hallgren, C.; Johansson, C.; Danelli, S. A Histomorphometric Evaluation of Screw-Shaped Implants Each Prepared with Two Surface Roughnesses. Clin. Oral Implant. Res. 1998, 9, 11–19. [Google Scholar] [CrossRef]
- Shao, K.; Zhou, Q.; Chen, Q.; Liu, Y.; Wang, C.; Li, X. Research Progress of Water–Laser Compound Machining Technology. Coatings 2022, 12, 1887. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel Bioactive Materials with Different Mechanical Properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Z.; Liang, C. Diverse Nanomaterials Synthesized by Laser Ablation of Pure Metals in Liquids. Sci. China Phys. Mech. Astron. 2022, 65, 274203. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J.; Low, D.K.Y.; Lim, G.C.; Zheng, H.Y. Correlation of the Hydroxyl Bond and Wettability Characteristics of a Magnesia Partially Stabilised Zirconia Following CO2 Laser Irradiation. Thin. Solid Film. 2004, 468, 12–16. [Google Scholar] [CrossRef]
- Srivas, P.K.; Kapat, K.; Das, B.; Pal, P.; Ray, P.G.; Dhara, S. Hierarchical Surface Morphology on Ti6Al4V via Patterning and Hydrothermal Treatment towards Improving Cellular Response. Appl. Surf. Sci. 2019, 478, 806–817. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, J.M.; Park, J.-G.; Chung, B.G. A Self-Assembled Monolayer-Based Micropatterned Array for Controlling Cell Adhesion and Protein Adsorption. Biotechnol. Bioeng. 2011, 108, 1194–1202. [Google Scholar] [CrossRef]



| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | GGAAGCTTGTCATCAATGGAAATC | TGATGACCCTTTTGGCTCCC |
| ALP | CTCCTCGGAAGACACTCTGACC | CTGCGCCTGGTAGTTGTTGTG |
| OCN | CCTCACACTCCTCGCCCTATT | CCGATGTGGTCAGCCAACTC |
| OSX | TTTACCCGAAGCGACCACC | GAGTGATTGGCAAGCAGTGGTC |
| Runx2 | CTACTATGGCACTTCGTCAGGAT | ATCAGCGTCAACACCATCATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fu, Y.; Li, N.; Liu, G.; Li, J.; Wen, J.; Han, J. Enhanced Osteoconductivity of Zirconia Implants with One-Step Femtosecond Laser Treatment Through Morphological and Chemical Modifications. J. Funct. Biomater. 2025, 16, 142. https://doi.org/10.3390/jfb16040142
Li Y, Fu Y, Li N, Liu G, Li J, Wen J, Han J. Enhanced Osteoconductivity of Zirconia Implants with One-Step Femtosecond Laser Treatment Through Morphological and Chemical Modifications. Journal of Functional Biomaterials. 2025; 16(4):142. https://doi.org/10.3390/jfb16040142
Chicago/Turabian StyleLi, Yuqi, Yanzhe Fu, Nan Li, Guanqi Liu, Jiebo Li, Jiao Wen, and Jianmin Han. 2025. "Enhanced Osteoconductivity of Zirconia Implants with One-Step Femtosecond Laser Treatment Through Morphological and Chemical Modifications" Journal of Functional Biomaterials 16, no. 4: 142. https://doi.org/10.3390/jfb16040142
APA StyleLi, Y., Fu, Y., Li, N., Liu, G., Li, J., Wen, J., & Han, J. (2025). Enhanced Osteoconductivity of Zirconia Implants with One-Step Femtosecond Laser Treatment Through Morphological and Chemical Modifications. Journal of Functional Biomaterials, 16(4), 142. https://doi.org/10.3390/jfb16040142







