Impact of Vitamin D3 Functionalization on the Osteogenic Capacity of Bioinspired 3D Scaffolds Based on Ce-Doped Bioactive Glass and Spongia Agaricina
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffold Preparation
2.3. Scaffold Functionalization
2.4. Characterization of Functionalized/Non-Functionalized Scaffolds
2.5. Evaluation of Biological Properties
2.5.1. In Vitro Cytocompatibility Testing
2.5.2. Genotoxicity Assessment
2.5.3. In Vitro Osteogenic Capacity Testing
2.6. Statistical Analysis
3. Results and Discussion
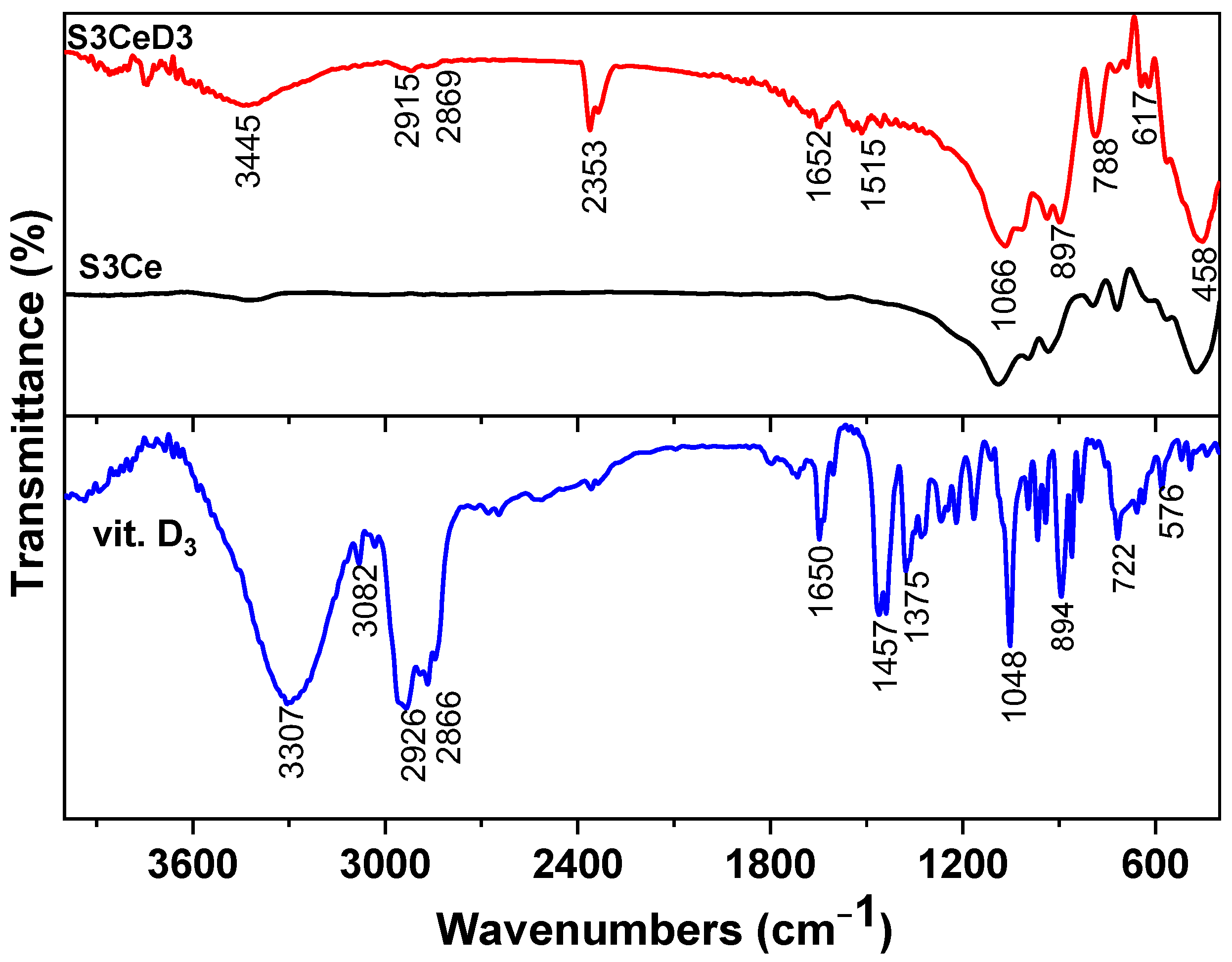
3.1. Surface Characterization of Functionalized/Non-Functionalized Ce-Containing MBG-Based Scaffolds
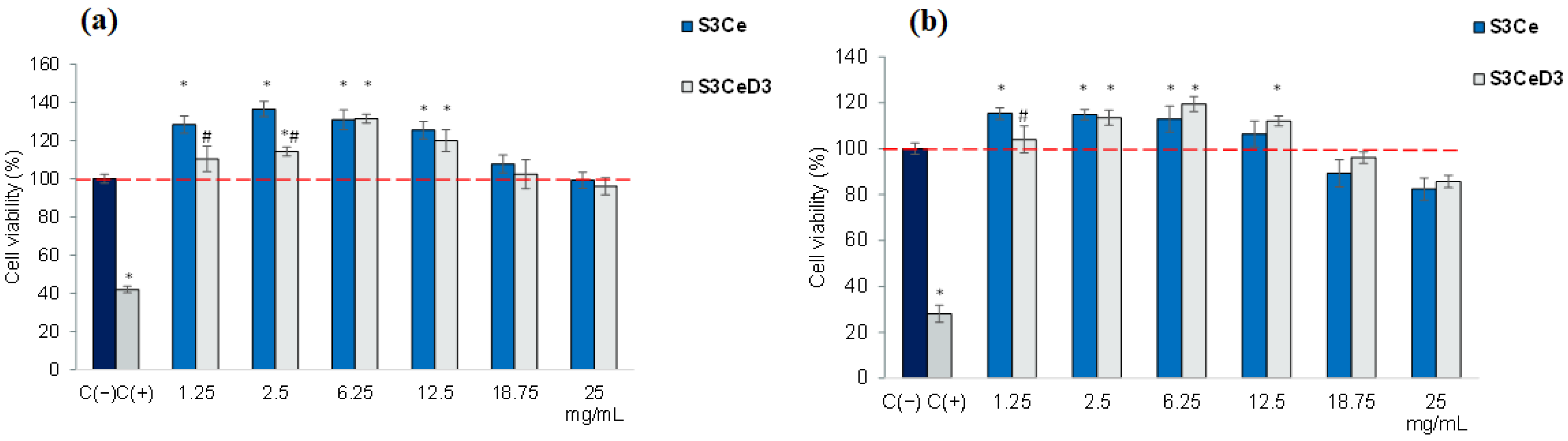
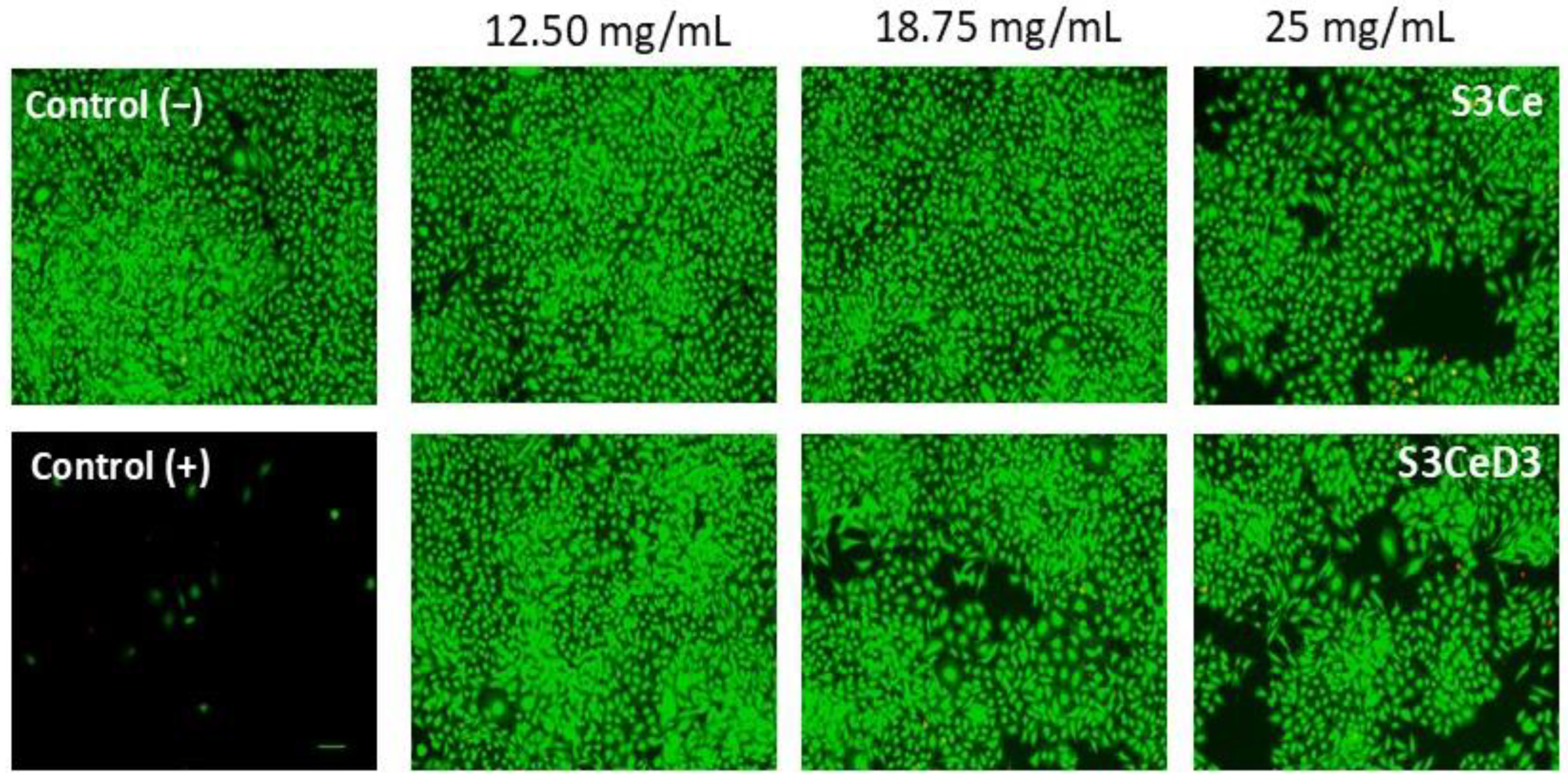
3.2. Cytocompatibility Testing
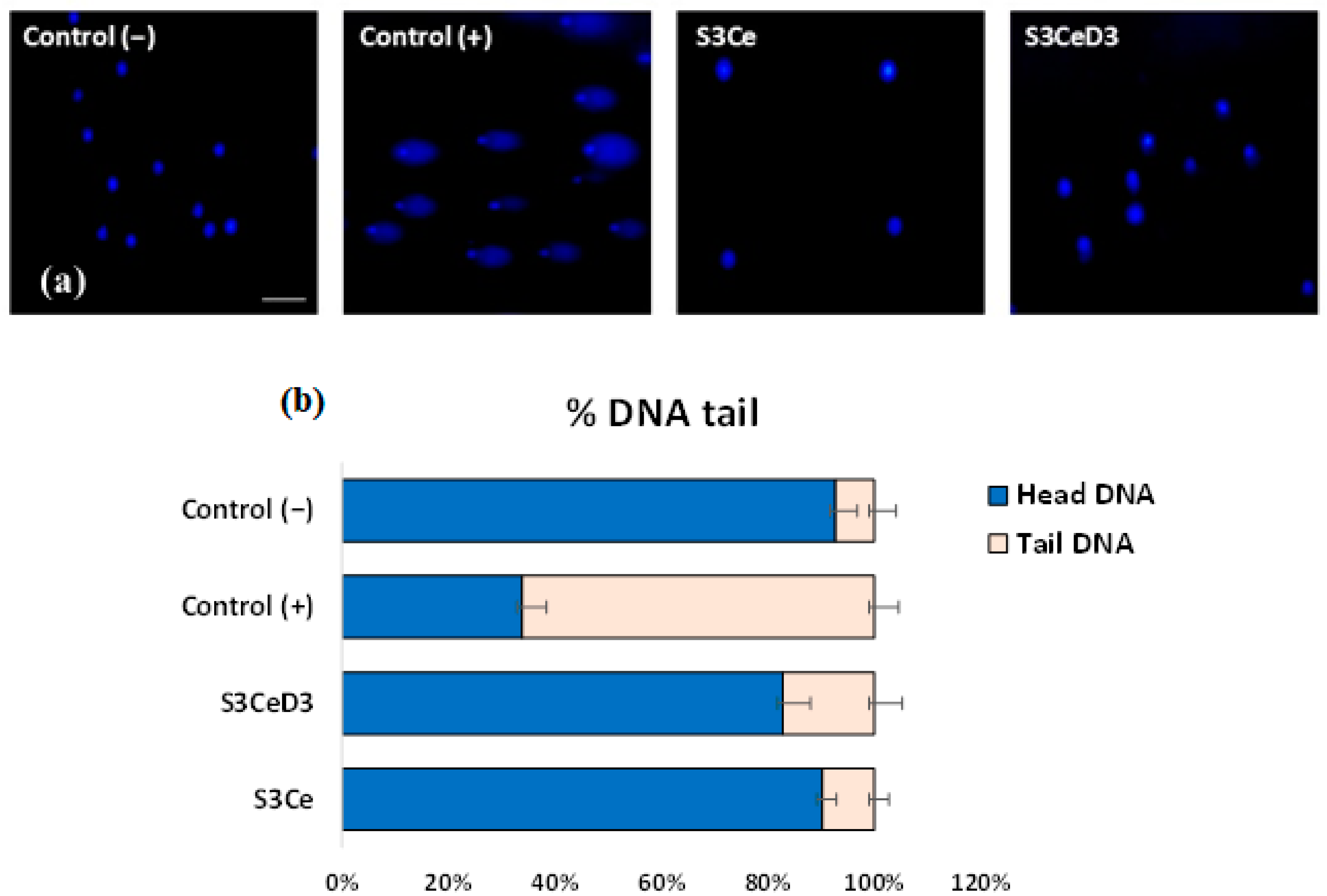
3.3. Genotoxicity Testing
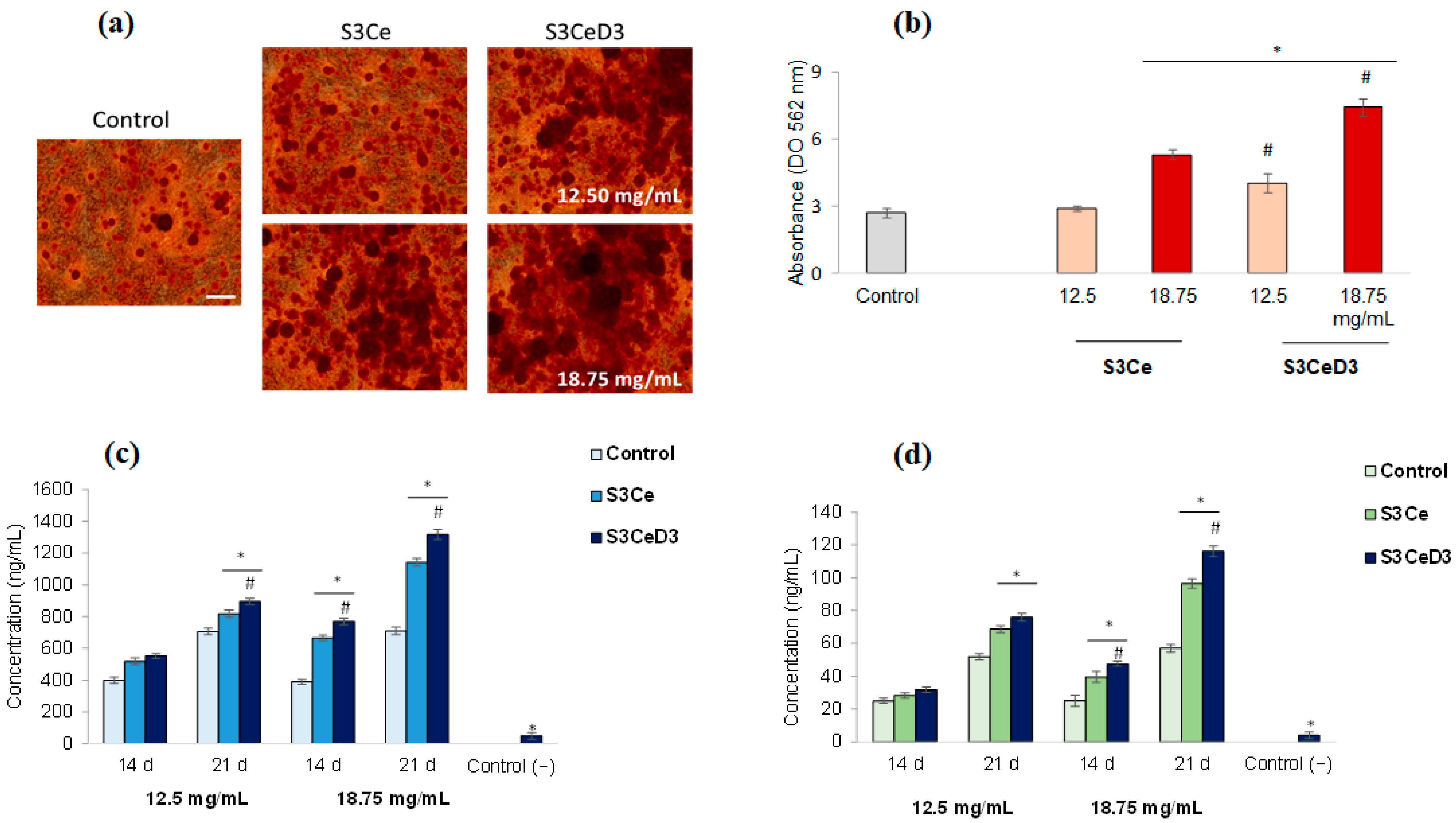
3.4. Osteogenic Capacity of Functionalized Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Res. 2006, 17, 967–973. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Biano, F. Bioactive glasses-When glass science and technology meet regenerative medicine. Ceram. Int. 2018, 44, 14953–14966. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Piatti, E.; Miola, M.; Verné, E. Tailoring of bioactive glass and glass-ceramics properties for in vitro and in vivo response optimization: A review. Biomater. Sci. 2024, 12, 4546–4589. [Google Scholar] [CrossRef]
- Ranjbar, F.E.; Farzad-Mohajeri, S.; Samani, S.; Saremi, J.; Khademi, R.; Dehghan, M.M.; Azami, M. Kaempferol-loaded bioactive glass-based scaffold for bone tissue engineering: In vitro and in vivo evaluation. Sci. Rep. 2023, 13, 12375. [Google Scholar] [CrossRef]
- Krishnan, L.; Chakrabarty, P.; Govarthanan, K.; Rao, S.; Santra, T.S. Bioglass and nano bioglass: A next-generation biomaterial for therapeutic and regenerative medicine applications. Int. J. Biol. Macromol. 2024, 277, 133073. [Google Scholar] [CrossRef]
- Kowalska, K.J.; Czechowska, J.P.; Yousef, E.S.; Zima, A. Novel phosphate bioglasses and bioglass-ceramics for bone regeneration. Ceram. Int. 2024, 50 Pt A, 45976–45985. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, P.; Jiang, Y.; You, J.; Xu, Z.; Yu, K.; Boccaccini, A.R.; Ma, J.; Zheng, K. Extracellular and intracellular effects of bioactive glass nanoparticles on osteogenic differentiation of bone marrow mesenchymal stem cells and bone regeneration in zebrafish osteoporosis model. Acta Biomater. 2024, 174, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, E.; Subramanian, B. Synergistic effects of silica-enriched bioactive glass and tri-calcium phosphate nanocomposites on BMP2 gene expression for bone repair and regeneration applications. Int. J. Pharm. 2025, 669, 125026. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ran, X.; Wei, X.; Zhu, L.; Chen, S.; Liao, Z.; Xu, K.; Xia, W. Bioactive glass 1393 promotes angiogenesis and accelerates wound healing through ROS/P53/MMP9 signaling pathway. Regen. Ther. 2024, 26, 132–144. [Google Scholar] [CrossRef]
- Moll, M.; Scheurle, A.; Nawaz, Q.; Walker, T.; Kunisch, E.; Renkawitz, T.; Boccaccini, A.R.; Westhauser, F. Osteogenic and angiogenic potential of molybdenum-containing mesoporous bioactive glass nanoparticles: An ionic approach to bone tissue engineering. J. Trace Elem. Med. Biol. 2024, 86, 127518. [Google Scholar] [CrossRef]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio 2020, 5, 100041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, X.; Mo, M.; Hu, X.; Liu, J.; Chen, L. Systematic review of the osteogenic effect of rare earth nanomaterials and the underlying mechanisms. J. Nanobiotechnol. 2024, 22, 185. [Google Scholar] [CrossRef]
- Mostajeran, H.; Baheiraei, N.; Bagheri, H. Effects of cerium-doped bioactive glass incorporation on an alginate/gelatin scaffold for bone tissue engineering: In vitro characterizations. Int. J. Biol. Macromol. 2024, 255, 128094. [Google Scholar] [CrossRef]
- Beserra dos Santos, M.V.; Bastos Nogueira Rocha, L.; Gomes Vieira, E.; Oliveira, A.L.; Oliveira Lobo, A.; Martins de Carvalho, M.A.; Osajima, J.A.; Silva-Filho, E.C. Development of Composite Scaffolds Based on Cerium Doped-Hydroxyapatite and Natural Gums—Biological and Mechanical Properties. Materials 2019, 12, 2389. [Google Scholar] [CrossRef]
- Atkinson, I.; Seciu-Grama, A.M.; Serafim, A.; Petrescu, S.; Voicescu, M.; Anghel, E.M.; Marinescu, C.; Mitran, R.A.; Mocioiu, O.C.; Cusu, J.P.; et al. Bioinspired 3D scaffolds with antimicrobial, drug delivery, and osteogenic functions for bone regeneration. Drug Deliv. Transl. Res. 2024, 14, 1028–1047. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Vitamin D-Effects on Skeletal and Extraskeletal Health and the Need for Supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Van de Peppel, J.; Van Leeuwen, J.P.T.M. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, S.; Goula, T.; Ververidis, A.; Drosos, G. Vitamin D and Bone Disease. BioMed Res. Int. 2012, 27, 396541. [Google Scholar] [CrossRef]
- Sundar, R.; Rai, A.B.; Naveen Kumar, J.; Devang Divakar, D. The role of Vitamin D as an adjunct for bone regeneration: A systematic review of literature. Saudi Dent. J. 2023, 35, 220–232. [Google Scholar] [CrossRef]
- Abad-Javier, M.E.; Cajero-Juárez, M.; Nuñez-Anita, R.E.; Contreras-García, M.E. Effect of collagen type I and vitamin D3 functionalization of biomimetic bioglass scaffolds on hydroxyapatite condensation. J. Eur. Ceram. Soc. 2019, 39, 3505–3512. [Google Scholar] [CrossRef]
- Negut, I.; Gradisteanu-Pircalabioru, G.; Dinu, M.; Bita, B.; Constantina Parau, A.; Grumezescu, V.; Ristoscu, C.; Chifiriuc, C.M. Bioglass and Vitamin D3 Coatings for Titanium Implants: Osseointegration and Corrosion Protection. Biomedicines 2023, 11, 2772. [Google Scholar] [CrossRef]
- Gupta, A.A.; Kheur, S.; Badhe, R.V.; Raj, A.T.; Bhonde, R.; Jaisinghani, A.; Vyas, N.; Patil, V.R.; Alhazmi, Y.A.; Parveen, S.; et al. Assessing the potential use of chitosan scaffolds for the sustained localized delivery of vitamin D. Saudi J. Biol. Sci. 2021, 4, 2210–2215. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Oancea, A.; Cotarlet, M.; Vasile, A.M.; Bahrim, G.E.; Shaposhnikov, S.; Craciunescu, O.; Oprita, E.I. Angiotensin-converting enzyme inhibition, antioxidant activity and cytotoxicity of bioactive peptides from fermented bovine colostrum. Int. J. Dairy Technol. 2020, 73, 108–116. [Google Scholar] [CrossRef]
- Craciunescu, O.; Seciu, A.M.; Zarnescu, O. In vitro and in vivo evaluation of a biomimetic scaffold embedding silver nanoparticles for improved treatment of oral lesions. Mater. Sci. Eng. C 2021, 123, 112015. [Google Scholar] [CrossRef]
- Seciu, A.M.; Craciunescu, O.; Zarnescu, O. Advanced regenerative techniques based on dental pulp stem cells for the treatment of periodontal disease. In Periodontology and Dental Implantology; Manakil, J., Ed.; InTechOpen Ltd.: London, UK, 2020; pp. 129–148. [Google Scholar] [CrossRef]
- Jan, Y.; Al-Keridis, L.A.; Malik, M.; Haq, A.; Ahmad, S.; Kaur, J.; Adnan, M.; Alshammari, N.; Ashraf, S.A.; Panda, B.P. Preparation, modelling, characterization and release profile of vitamin D3 nanoemulsion. LWT 2022, 169, 113980. [Google Scholar] [CrossRef]
- Torrisi, A.; Cutroneo, M.; Torrisi, L.; Lavalle, S.; Forzina, A.; Pegreffi, F. Unveiling the Potential of Vitamin D3 Orodispersible Films: A Comprehensive FTIR and UV–Vis Spectroscopic Study. Molecules 2024, 29, 3762. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, I.; Seciu-Grama, A.M.; Petrescu, S.; Culita, D.; Mocioiu, O.C.; Voicescu, M.; Mitran, R.A.; Lincu, D.; Prelipcean, A.M.; Craciunescu, O. Cerium-Containing Mesoporous Bioactive Glasses (MBGs)-Derived Scaffolds with Drug Delivery Capability for Potential Tissue Engineering Applications. Pharmaceutics 2022, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, L.; Roszkowska, A.M.; Silipigni, L.; Cutroneo, M.; Torrisi, A. Effects of 365nm UV lamp irradiation of polymethyl methacrylate (PMMA). Radiat. Eff. Defects Solids 2024, 179, 264–274. [Google Scholar] [CrossRef]
- Alkentar, R.; Kladovasilakis, N.; Tzetzis, D.; Mankovits, T. Effects of pore size parameters of titanium additively manufactured lattice structures on the osseointegration process in orthopedic applications: A comprehensive review. Crystals 2022, 13, 113. [Google Scholar] [CrossRef]
- Bose, S.; Chaudhari, V.C.; Kushram, P. 3D printed scaffolds with quercetin and vitamin D3 nanocarriers: In vitro cellular evaluation. J. Biomed. Mater. Res. A 2024, 112, 2110–2123. [Google Scholar] [CrossRef]
- Owens, D.; Tang, J.C.Y.; Bradley, W.J.; Sparks, A.S.; Fraser, W.D.; Morton, J.P.; Close, G.L. Efficacy of high-dose vitamin D supplements for elite athletes. Med. Sci. Sports Exerc. 2017, 49, 349–356. [Google Scholar] [CrossRef]
- Irving, A.A.; Plum, L.A.; Blaser, W.J.; Ford, M.R.; Weng, C.; Clipson, L.; DeLuca, H.F.; Dove, W.F. Cholecalciferol or 25-Hydroxycholecalciferol neither prevents nor treats adenomas in a rat model of familial colon cancer. J. Nutr. 2015, 145, 291–298. [Google Scholar] [CrossRef]
- Koren, R.; Hadari-Naor, I.; Zuck, E.; Rotem, C.; Liberman, U.A.; Ravid, A. Vitamin D is a prooxidant in breast cancer cells. Cancer Res. 2001, 61, 1439–1444. [Google Scholar]
- Liu, Y.; Ma, Y.; Zhang, J.; Xie, Q.; Wang, Z.; Yu, S.; Yuan, Y.; Liu, C. MBG-Modified β-TCP Scaffold Promotes Mesenchymal Stem Cells Adhesion and Osteogenic Differentiation via a FAK/MAPK Signaling Pathway. ACS Appl. Mater. Interfaces 2017, 9, 30283–30296. [Google Scholar] [CrossRef]
- Sattary, M.; Rafienia, M.; Kazemi, M.; Salehi, H.; Mahmoudzade, M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 19, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, J.; Wiesnerova1, L.; Chocholata, P.; Kulda, V.; Landsmann, L.; Cedikova, M.; Kripnerova, M.; Eberlova, L.; Babuska, V. Human cells with osteogenic potential in bone tissue research. BioMed. Eng. OnLine 2023, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K.W.K.; Chu, P.K. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv. Sci 2017, 5, 1700678. [Google Scholar] [CrossRef]
- Lou, Y.R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D3 induces osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 2017, 7, 42816. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seciu-Grama, A.-M.; Lazăr, S.E.; Petrescu, S.; Mocioiu, O.C.; Crăciunescu, O.; Atkinson, I. Impact of Vitamin D3 Functionalization on the Osteogenic Capacity of Bioinspired 3D Scaffolds Based on Ce-Doped Bioactive Glass and Spongia Agaricina. J. Funct. Biomater. 2025, 16, 141. https://doi.org/10.3390/jfb16040141
Seciu-Grama A-M, Lazăr SE, Petrescu S, Mocioiu OC, Crăciunescu O, Atkinson I. Impact of Vitamin D3 Functionalization on the Osteogenic Capacity of Bioinspired 3D Scaffolds Based on Ce-Doped Bioactive Glass and Spongia Agaricina. Journal of Functional Biomaterials. 2025; 16(4):141. https://doi.org/10.3390/jfb16040141
Chicago/Turabian StyleSeciu-Grama, Ana-Maria, Sorana Elena Lazăr, Simona Petrescu, Oana Cătălina Mocioiu, Oana Crăciunescu, and Irina Atkinson. 2025. "Impact of Vitamin D3 Functionalization on the Osteogenic Capacity of Bioinspired 3D Scaffolds Based on Ce-Doped Bioactive Glass and Spongia Agaricina" Journal of Functional Biomaterials 16, no. 4: 141. https://doi.org/10.3390/jfb16040141
APA StyleSeciu-Grama, A.-M., Lazăr, S. E., Petrescu, S., Mocioiu, O. C., Crăciunescu, O., & Atkinson, I. (2025). Impact of Vitamin D3 Functionalization on the Osteogenic Capacity of Bioinspired 3D Scaffolds Based on Ce-Doped Bioactive Glass and Spongia Agaricina. Journal of Functional Biomaterials, 16(4), 141. https://doi.org/10.3390/jfb16040141









