Spatial Platform for Periodontal Ligament Angulation and Regeneration: In Vivo Pilot Study
Abstract
1. Introduction
2. Materials and Methods
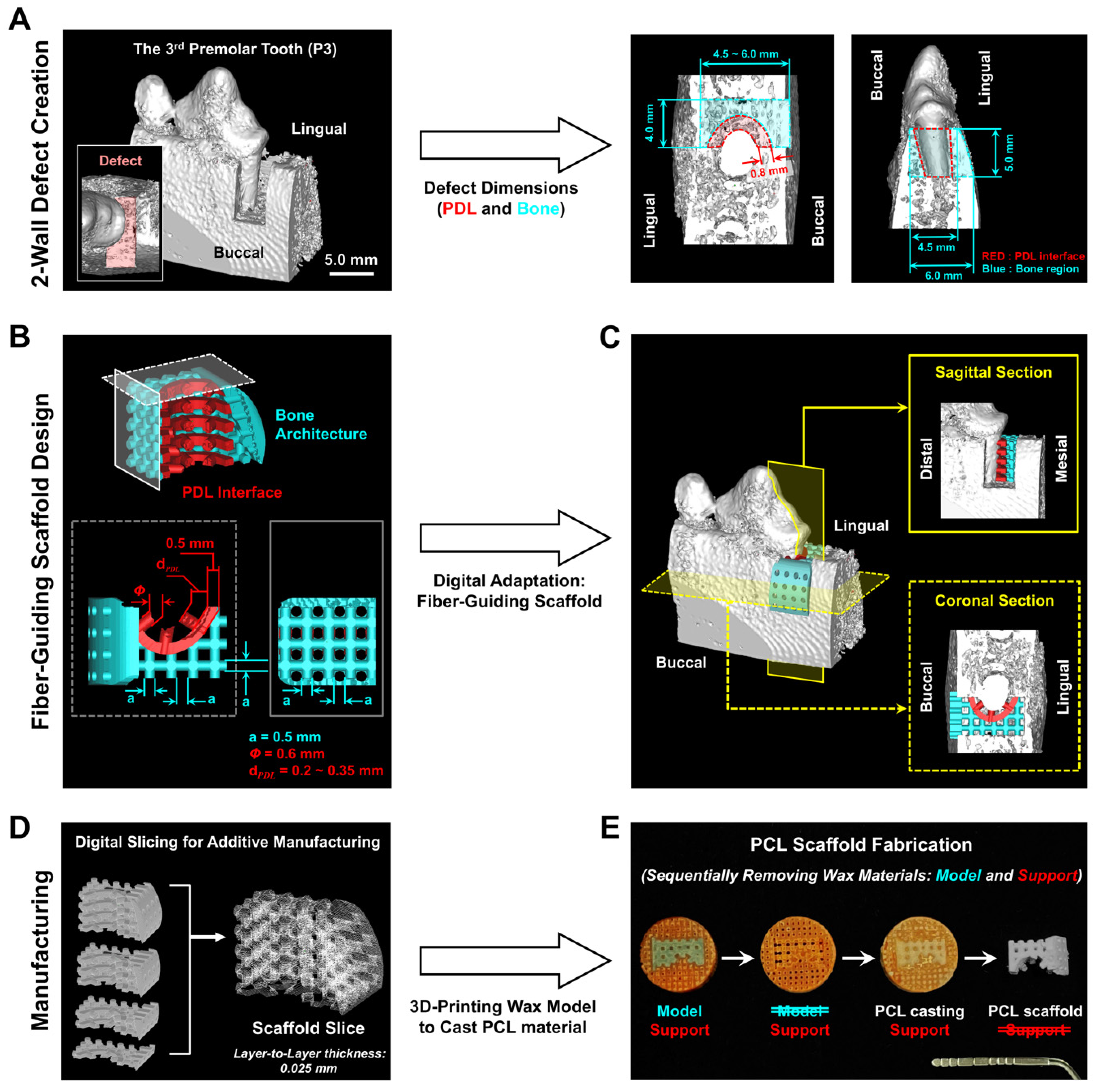
2.1. Customized Perio-Complex Scaffolds Using a Two-Wall Periodontal Defect Model
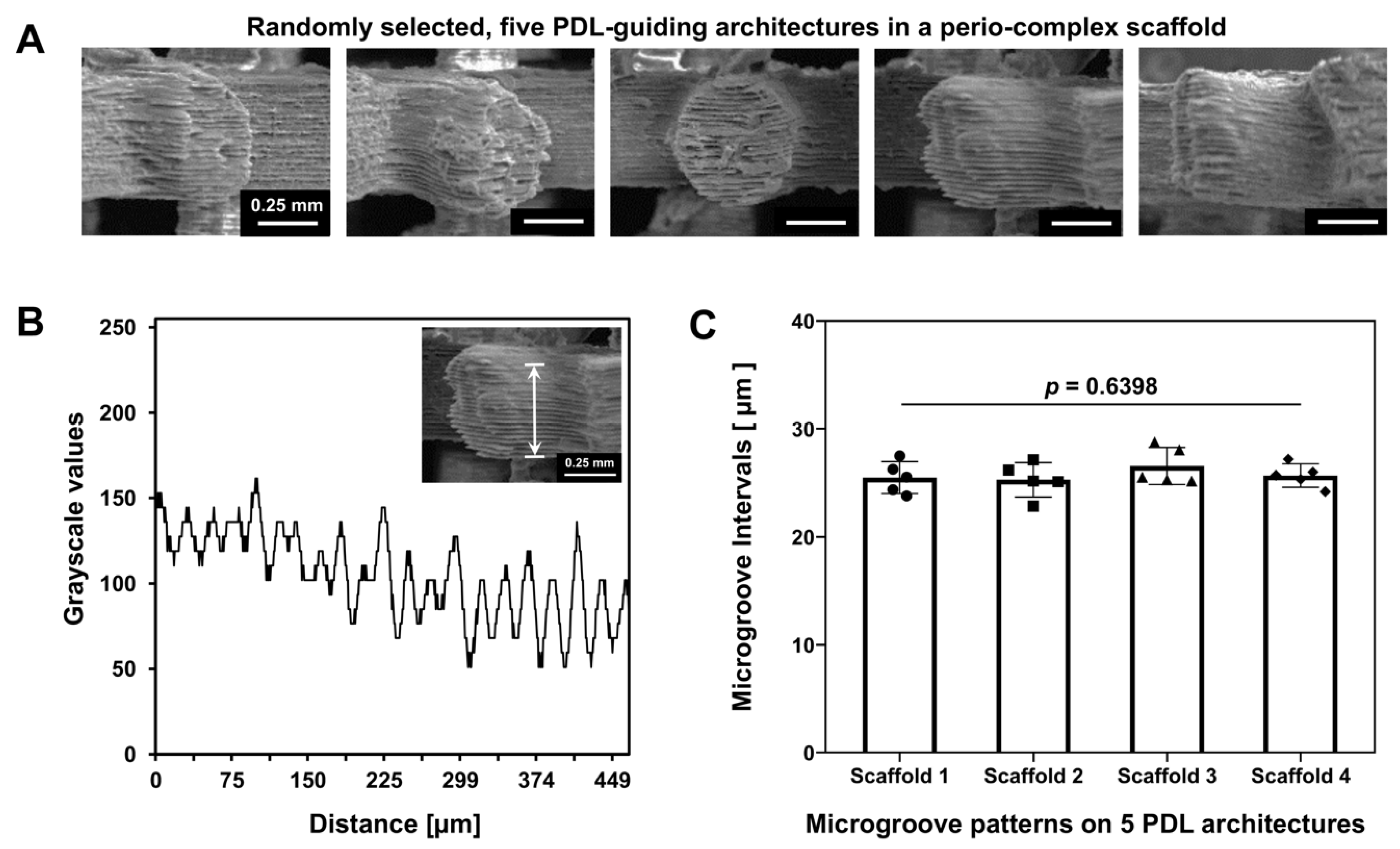
2.2. Surface Characterization of PDL-Guiding Architectures in a Perio-Complex Scaffold
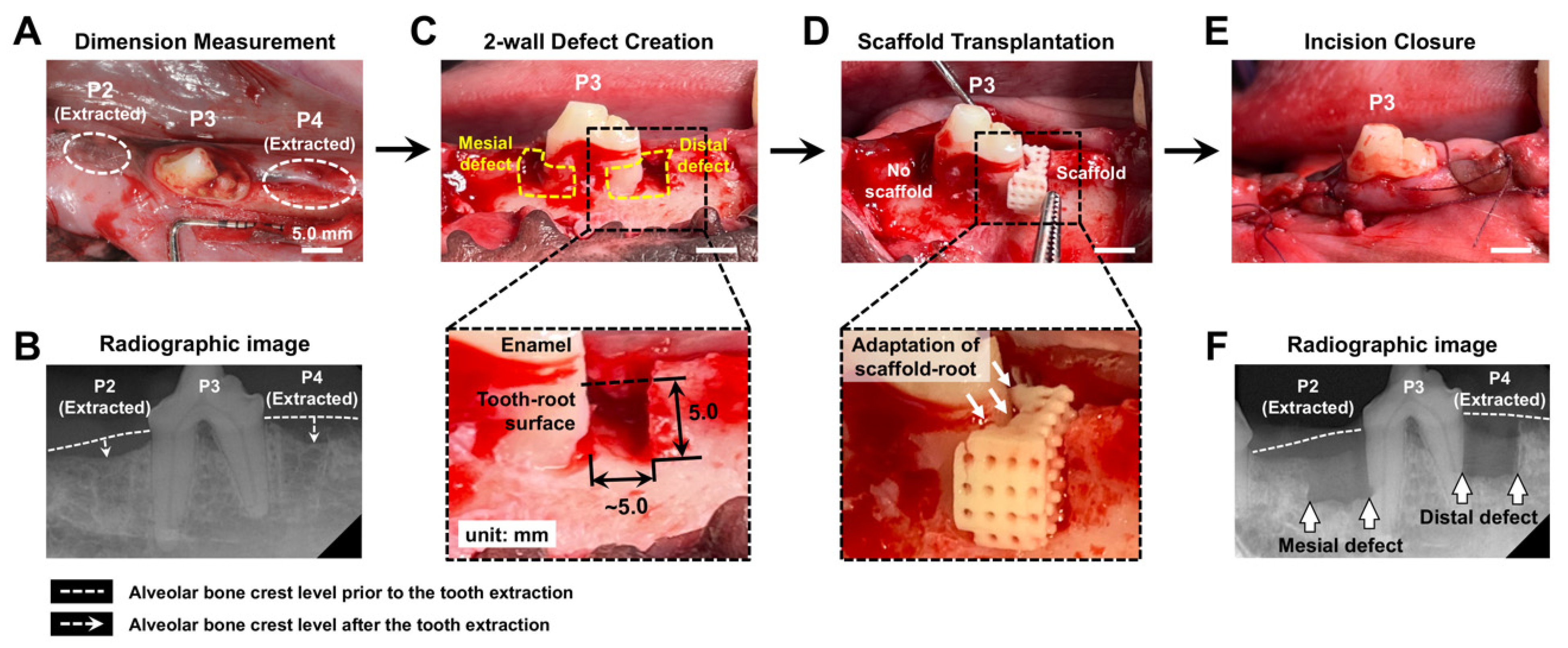
2.3. Surgical Creation of 2-Wall Periodontal Defects and Scaffold Transplantation
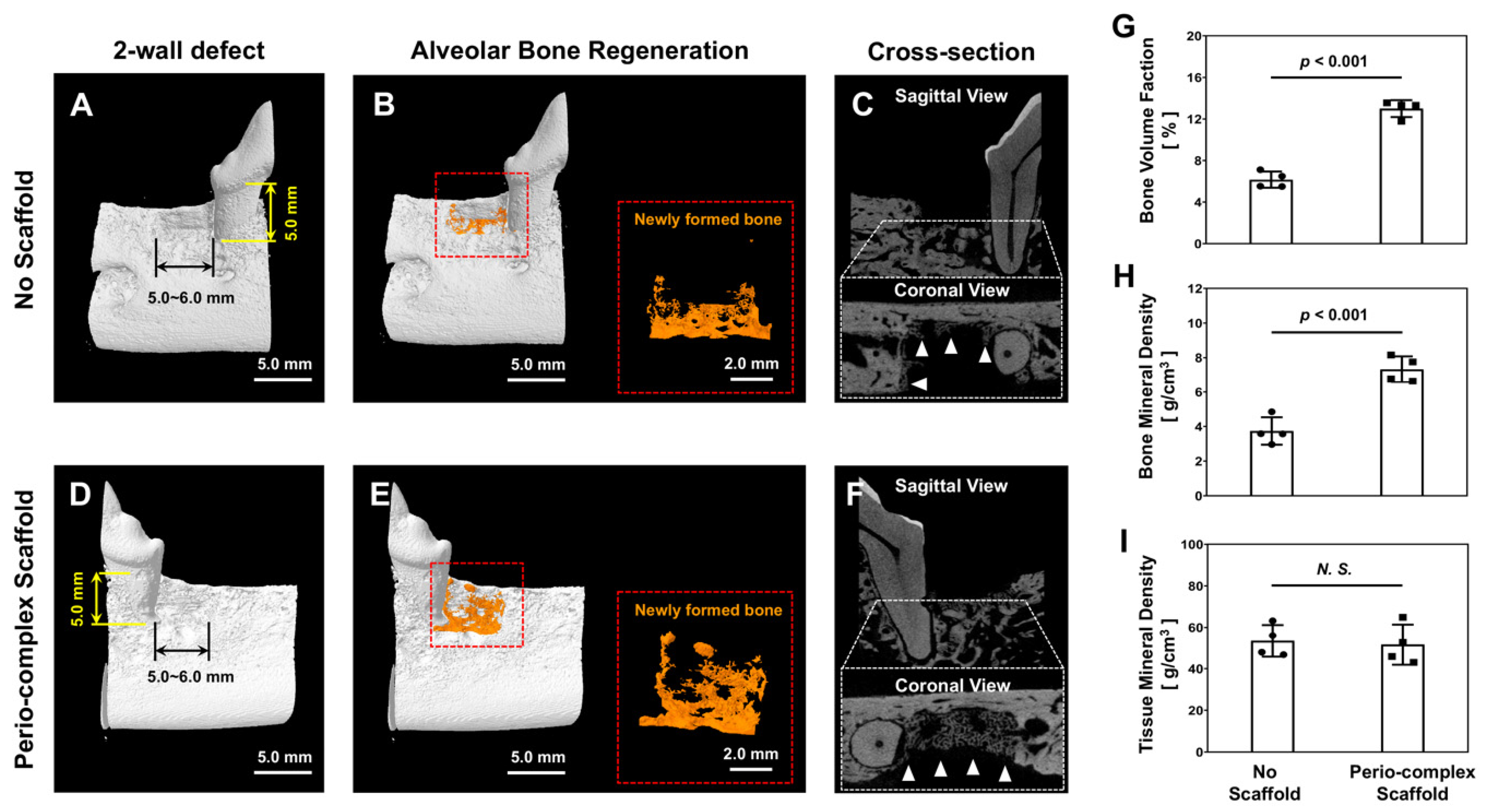
2.4. Micro-Computed Tomgoraphy (Micro-CT) Assessments
2.5. Histological and Immunohistochemical Analyses for Oriented Ligament Formations
2.6. Statistical Analysis
3. Results
3.1. Customized Perio-Complex Scaffold Design Based on Two-Wall Periodontal Defects
3.2. Volumetric Analyses of Bone Formation in Micro-CT
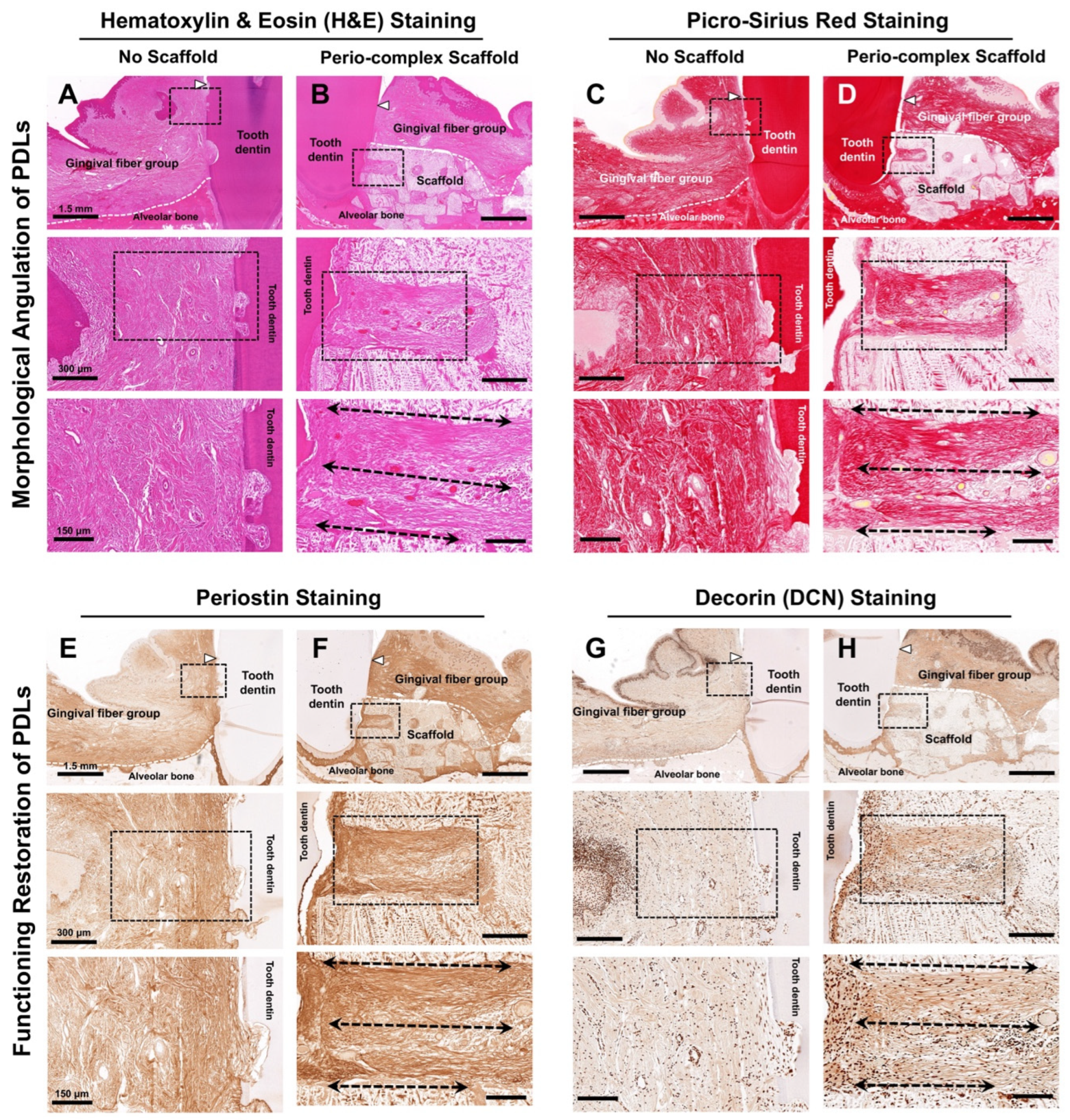
3.3. Histological and Immunohistochemical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDL | Periodontal ligament |
| 3D | Three-dimensional |
| AM | Additive manufacturing |
| P2 | The 2nd premolar tooth |
| P3 | The 3rd premolar tooth |
| P4 | The 4th premolar tooth |
| PCL | Poly-ε-caprolactone |
| FE-SEM | Field emission scanning electron microscope |
| IM | Intramuscularly |
| IV | Intravenous |
| KCl | Potassium chloride |
| Micro-CT | Micro-computed tomography |
| HU | Hounsfield Unit |
| VOI | Volume of interest |
| H&E | Hematoxylin and eosin |
| DCN | Decorin |
| HRP | Horseradish peroxidase |
| DAB | Diaminobenzidine |
| IHC | Immunohistochemistry |
| ANOVA | Analysis of variance |
| BVF | Bone volume fraction |
| BMD | Bone mineral density |
| BV | Bone volume |
| BC | Bone content |
| TV | Total volume |
| ROI | Region of interest |
| TMD | Tissue mineral density |
References
- Kim, J.I.; Kim, J.Y.; Bhattarai, G.; So, H.S.; Kook, S.H.; Lee, J.C. Periodontal Ligament-Mimetic Fibrous Scaffolds Regulate YAP-Associated Fibroblast Behaviors and Promote Regeneration of Periodontal Defect in Relation to the Scaffold Topography. ACS Appl. Mater. Interfaces 2023, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, B.; Yang, F.; Jiang, D.; Izadikhah, I.; Chen, Y.; Li, N.; Yan, B. Understanding the hierarchical structure of collagen fibers of the human periodontal ligament: Implications for biomechanical characteristics. Acta Biomater. 2024, 188, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Niver, E.L.; Leong, N.; Greene, J.; Curtis, D.; Ryder, M.I.; Ho, S.P. Reduced functional loads alter the physical characteristics of the bone-periodontal ligament-cementum complex. J. Periodontal Res. 2011, 46, 730–741. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Svanberg, S.; Hirth, E.; Mitsiadis, T.A.; Dittrich, P.S. “Periodontal ligament-on-chip” as a Novel Tool for Studies on the Physiology and Pathology of Periodontal Tissues. Adv. Healthc. Mater. 2024, 13, e2303942. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Cortellini, P.; Stalpers, G.; Mollo, A.; Tonetti, M.S. Periodontal regeneration versus extraction and dental implant or prosthetic replacement of teeth severely compromised by attachment loss to the apex: A randomized controlled clinical trial reporting 10-year outcomes, survival analysis and mean cumulative cost of recurrence. J. Clin. Periodontol. 2020, 47, 768–776. [Google Scholar]
- Orishko, A.; Imber, J.C.; Roccuzzo, A.; Stahli, A.; Salvi, G.E. Tooth- and implant-related prognostic factors in treatment planning. Periodontol. 2000 2024, 95, 102–128. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D Printed and Micropatterned Polycaprolactone Scaffolds for Guidance of Oriented Collagenous Tissue Formation In Vivo. Adv. Healthc. Mater. 2016, 5, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Silva, J.C.; Carvalho, M.S. Hierarchical Biomaterial Scaffolds for Periodontal Tissue Engineering: Recent Progress and Current Challenges. Int. J. Mol. Sci. 2024, 25, 8562. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Z.; Bi, F.; Tang, H.; Chen, J.; Huo, F.; Chen, J.; Lan, T.; Qiao, X.; Sima, X.; et al. Personalized 3D-Printed Scaffolds with Multiple Bioactivities for Bioroot Regeneration. Adv. Healthc. Mater. 2023, 12, e2300625. [Google Scholar] [CrossRef] [PubMed]
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Samuel Ipe, D.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Luo, D.; Qiao, J.; Guo, J.; He, D.; Jin, S.; Tang, L.; Wang, Y.; Shi, X.; Mao, J.; et al. A hierarchical bilayer architecture for complex tissue regeneration. Bioact. Mater. 2022, 10, 93–106. [Google Scholar] [CrossRef]
- Daghrery, A.; Ferreira, J.A.; Xu, J.; Golafshan, N.; Kaigler, D.; Bhaduri, S.B.; Malda, J.; Castilho, M.; Bottino, M.C. Tissue-specific melt electrowritten polymeric scaffolds for coordinated regeneration of soft and hard periodontal tissues. Bioact. Mater. 2023, 19, 268–281. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Z.; Han, J.; Jiang, X.; Lei, L.; Yang, X.; Sun, W.; Gou, Z.; Chen, L. Modularized bioceramic scaffold/hydrogel membrane hierarchical architecture beneficial for periodontal tissue regeneration in dogs. Biomater. Res. 2022, 26, 68. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Z.; Guo, W. The 3-dimensional printing for dental tissue regeneration: The state of the art and future challenges. Front. Bioeng. Biotechnol. 2024, 12, 1356580. [Google Scholar] [CrossRef]
- Kim, M.G.; Park, C.H. The Topographical Optimization of 3D Microgroove Pattern Intervals for Ligamentous Cell Orientations: In Vitro. Int. J. Mol. Sci. 2020, 21, 9358. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. 3D Printed, Microgroove Pattern-Driven Generation of Oriented Ligamentous Architectures. Int. J. Mol. Sci. 2017, 18, 1927. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Park, C.H. Spatial Controls of Ligamentous Tissue Orientations Using the Additively Manufactured Platforms in an In Vivo Model: A Pilot Study. Appl. Sci. 2021, 11, 7847. [Google Scholar] [CrossRef]
- Lin, J.D.; Jang, A.T.; Kurylo, M.P.; Hurng, J.; Yang, F.; Yang, L.; Pal, A.; Chen, L.; Ho, S.P. Periodontal ligament entheses and their adaptive role in the context of dentoalveolar joint function. Dent. Mater. 2017, 33, 650–666. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Rios, H.F.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J. Dent. Res. 2014, 93, 1304–1312. [Google Scholar] [CrossRef]
- Daghrery, A.; Bottino, M.C. Advanced biomaterials for periodontal tissue regeneration. Genesis 2022, 60, e23501. [Google Scholar] [CrossRef]
- He, Z.; Lv, J.C.; Zheng, Z.L.; Gao, C.T.; Xing, J.W.; Li, B.L.; Liu, H.H.; Liu, Y.; Xu, J.Z.; Li, Z.M.; et al. Hierarchically structured nanofibrous scaffolds spatiotemporally mediate the osteoimmune micro-environment and promote osteogenesis for periodontitis-related alveolar bone regeneration. Acta Biomater. 2024, 189, 323–336. [Google Scholar] [CrossRef]
- Swanson, W.B.; Yao, Y.; Mishina, Y. Novel approaches for periodontal tissue engineering. Genesis 2022, 60, e23499. [Google Scholar] [CrossRef]
- Hashmi, A.W.; Mali, H.S.; Meena, A. A comprehensive review on surface quality improvement methods for additively manufactured parts. Rapid Prototyp. J. 2023, 29, 504–557. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, J.; Wang, K.; Weijian, Q.; Shi, L.; Lei, L.; He, W.; Wu, S. State-of-art review on the process-structure-properties-performance linkage in wire arc additive manufacturing. Virtual Phys. Prototyp. 2024, 19, e2390495. [Google Scholar] [CrossRef]
- Broomfield, C.; Meis, N.; Johnson, J.; Regan, D.; McGilvray, K.; Puttlitz, C. Optimization of ovine bone decalcification for increased cellular detail: A parametric study. J. Histotechnol. 2022, 45, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Hong, S.W.; Yoon, S.O. Proposal of an appropriate decalcification method of bone marrow biopsy specimens in the era of expanding genetic molecular study. J. Pathol. Transl. Med. 2015, 49, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Balli, U.; Keles, Z.P.; Avci, B.; Guler, S.; Cetinkaya, B.O.; Keles, G.C. Assessment of periostin levels in serum and gingival crevicular fluid of patients with periodontal disease. J. Periodontal Res. 2015, 50, 707–713. [Google Scholar] [CrossRef]
- Yamada, S.; Tauchi, T.; Awata, T.; Maeda, K.; Kajikawa, T.; Yanagita, M.; Murakami, S. Characterization of a novel periodontal ligament-specific periostin isoform. J. Dent. Res. 2014, 93, 891–897. [Google Scholar] [CrossRef]
- Häkkinen, L.; Strassburger, S.; Kähäri, V.M.; Scott, P.G.; Eichstetter, I.; Lozzo, R.V.; Larjava, H. A role for decorin in the structural organization of periodontal ligament. Lab. Investig. 2000, 80, 1869–1880. [Google Scholar] [CrossRef]
- Rios, H.F.; Ma, D.; Xie, Y.; Giannobile, W.V.; Bonewald, L.F.; Conway, S.J.; Feng, J.Q. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J. Periodontol. 2008, 79, 1480–1490. [Google Scholar] [CrossRef]
- Du, J.; Li, M. Functions of Periostin in Dental Tissues and Its Role in Periodontal Tissue Regeneration. Adv. Exp. Med. Biol. 2019, 1132, 63–72. [Google Scholar]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef]
- Jang, A.T.; Merkle, A.P.; Fahey, K.P.; Gansky, S.A.; Ho, S.P. Multiscale biomechanical responses of adapted bone-periodontal ligament-tooth fibrous joints. Bone 2015, 81, 196–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.G.; Kim, D.-Y.; Ko, H.-G.; Byun, J.-S.; Kim, J.-H.; Park, C.H. Spatial Platform for Periodontal Ligament Angulation and Regeneration: In Vivo Pilot Study. J. Funct. Biomater. 2025, 16, 99. https://doi.org/10.3390/jfb16030099
Kim MG, Kim D-Y, Ko H-G, Byun J-S, Kim J-H, Park CH. Spatial Platform for Periodontal Ligament Angulation and Regeneration: In Vivo Pilot Study. Journal of Functional Biomaterials. 2025; 16(3):99. https://doi.org/10.3390/jfb16030099
Chicago/Turabian StyleKim, Min Guk, Do-Yeon Kim, Hyoung-Gon Ko, Jin-Seok Byun, Joong-Hyun Kim, and Chan Ho Park. 2025. "Spatial Platform for Periodontal Ligament Angulation and Regeneration: In Vivo Pilot Study" Journal of Functional Biomaterials 16, no. 3: 99. https://doi.org/10.3390/jfb16030099
APA StyleKim, M. G., Kim, D.-Y., Ko, H.-G., Byun, J.-S., Kim, J.-H., & Park, C. H. (2025). Spatial Platform for Periodontal Ligament Angulation and Regeneration: In Vivo Pilot Study. Journal of Functional Biomaterials, 16(3), 99. https://doi.org/10.3390/jfb16030099







