Multiple Recessions Coverage Using the Modified Tunnel Technique and Connective Tissue Graft with or Without Cross-Linked Hyaluronic Acid: 2-Year Outcomes of RCT
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Exclusion and Inclusion Criteria
2.3. Surgical Procedure and Postoperative Care
2.4. Clinical Assessment
2.5. Examiner Calibration
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S204–S213. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Neiva, R. Periodontal soft tissue non-root coverage procedures: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. 2), S56–S72. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Gumber, B.; Makker, K.; Gupta, V.; Tewari, N.; Khanduja, P.; Yadav, R. Global prevalence of gingival recession: A systematic review and meta-analysis. Oral Dis. 2023, 29, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Kassab, M.M.; Cohen, R.E. The etiology and prevalence of gingival recession. J. Am. Dent. Assoc. 2003, 134, 220–225. [Google Scholar] [CrossRef]
- Gennai, S.; Guiza, Z.B.; Orsolini, C.; Gosset, M. The influence of non-carious lesions in the surgical treatment of gingival recession: A systematic review & meta-analysis. J. Dent. 2022, 117, 103922. [Google Scholar] [CrossRef]
- Cairo, F.; Nieri, M.; Cincinelli, S.; Mervelt, J.; Pagliaro, U. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: An explorative and reliability study. J. Clin. Periodontol. 2011, 38, 661–666. [Google Scholar] [CrossRef]
- Chambrone, L.; Salinas Ortega, M.A.; Sukekava, F.; Rotundo, R.; Kalemaj, Z.; Buti, J.; Pini Prato, G.P. Root coverage procedures for treating localised and multiple recession-type defects. Cochrane. Database. Syst. Rev. 2018, 10, CD007161. [Google Scholar] [CrossRef]
- Aroca, S.; Molnár, B.; Windisch, P.; Gera, I.; Salvi, G.E.; Nikolidakis, D.; Sculean, A. Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: A randomized, controlled clinical trial. J. Clin. Periodontol. 2013, 40, 713–720. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Nguyen, T.V.N.; Tattan, M.; Ravidà, A.; Wang, H.L. Efficacy of tunnel technique in the treatment of localized and multiple gingival recessions: A systematic review and meta-analysis. J. Periodontol. 2018, 89, 1075–1090. [Google Scholar] [CrossRef]
- Mayta-Tovalino, F.; Barboza, J.J.; Pasupuleti, V.; Hernandez, A.V. Efficacy of Tunnel Technique (TUN) versus Coronally Advanced Flap (CAF) in the Management of Multiple Gingival Recession Defects: A Meta-Analysis. Int. J. Dent. 2023, 6, 8671484. [Google Scholar] [CrossRef]
- Yadav, A.P.; Kulloli, A.; Shetty, S.; Ligade, S.S.; Martande, S.S.; Gholkar, M.J. Sub-epithelial connective tissue graft for the management of Miller’s class I and class II isolated gingival recession defect: A systematic review of the factors influencing the outcome. J. Investig. Clin. Dent. 2018, 9, e12325. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.L.; Roberts, J.L.; Moseley, R.; Griffiths, P.C.; Thomas, D.W. Evaluation of the physical and biological properties of hyaluronan and hyaluronan fragments. Int. J. Pharm. 2011, 420, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Srinivas, M.; Pai, J.; Suragimath, G.; Prasad, K.; Polepalle, T. Efficacy of hyaluronic acid (hyaluronan) in root coverage procedures as an adjunct to coronally advanced flap in Millers Class I recession: A clinical study. J. Indian Soc. Periodontol. 2014, 18, 746–750. [Google Scholar] [CrossRef]
- Tian, X.; Wen, Y.; Zhang, Z.; Zhu, J.; Song, X.; Phan, T.T.; Li, J. Recent advances in smart hydrogels derived from polysaccharides and their applications for wound dressing and healing. Biomaterials 2025, 318, 123134. [Google Scholar] [CrossRef]
- Rajan, P.; Rao, N.; Nera, M.; Rahaman, S. Hyaluronon As an adjunct to coronally advanced flap for the treatment of gingival recession defects—A clinical study. Natl. J. Integr. Res. Med. 2015, 6, 94–100. [Google Scholar]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2019, 23, 1133–1141. [Google Scholar] [CrossRef]
- Kalimeri, E.; Roccuzzo, A.; Stähli, A.; Oikonomou, I.; Berchtold, A.; Sculean, A.; Kloukos, D. Adjunctive use of hyaluronic acid in the treatment of gingival recessions: A systematic review and meta-analysis. Clin. Oral Investig. 2024, 28, 329. [Google Scholar] [CrossRef]
- Talebi Ardakani, M.; Moscowchi, A.; Talebi, A.; Talebi, M.H. Hyaluronic acid efficacy in root coverage procedures: A systematic review and meta-analysis. BMC Oral Health 2025, 25, 119. [Google Scholar] [CrossRef]
- Górski, B.; Skierska, I.; Szerszeń, M.; Mańka-Malara, K. Tunnel technique with cross-linked hyaluronic acid in addition to subepithelial connective tissue graft, compared with connective tissue graft alone, for the treatment of multiple gingival recessions: 6-month outcomes of a randomized clinical trial. Clin. Oral. Investig. 2023, 27, 2395–2406. [Google Scholar] [CrossRef]
- Skierska, I.; Górski, B.; Fus, Ł. Tunnel technique and subepithelial connective tissue graft, with or without cross-linked hyaluronic acid, in the treatment of multiple gingival recessions: 12-month outcomes of a randomized clinical trial. J. Periodontol. 2024, 95, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Cosgarea, R.; Stähli, A.; Katsaros, C.; Arweiler, N.B.; Miron, R.J.; Deppe, H. Treatment of multiple adjacent maxillary Miller Class I, II, and III gingival recessions with the modified coronally advanced tunnel, enamel matrix derivative, and subepithelial connective tissue graft: A report of 12 cases. Quintessence Int. 2016, 47, 653–659. [Google Scholar] [CrossRef]
- Zucchelli, G.; Mele, M.; Stefanini, M.; Mazzotti, C.; Marzadori, M.; Montebugnoli, L.; de Sanctis, M. Patient morbidity and root coverage outcome after subepithelial connective tissue and de-epithelialized grafts: A comparative randomized-controlled clinical trial. J. Clin. Periodontol. 2010, 37, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Lanzrein, C.; Guldener, K.; Imber, J.C.; Katsaros, C.; Stähli, A.; Sculean, A. Treatment of multiple adjacent recessions with the modified coronally advanced tunnel or laterally closed tunnel in conjunction with cross-linked hyaluronic acid and subepithelial connective tissue graft: A report of 15 cases. Quintessence Int. 2020, 51, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Górski, B.; Górska, R.; Szerszeń, M.; Kaczyński, T. Modified coronally advanced tunnel technique with enamel matrix derivative in addition to subepithelial connective tissue graft compared with connective tissue graft alone for the treatment of multiple gingival recessions: Prognostic parameters for clinical treatment outcomes. Clin. Oral Investig. 2022, 26, 673–688. [Google Scholar] [CrossRef]
- Tatakis, D.N.; Chambrone, L.; Allen, E.P.; Langer, B.; McGuire, M.K.; Richardson, C.R.; Zabalegui, I.; Zadeh, H.H. Periodontal soft tissue root coverage procedures: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (2 Suppl), S52–S55. [Google Scholar] [CrossRef]
- Cairo, F.; Rotundo, R.; Miller, P.D.; Pini Prato, G.P. Root coverage esthetic score: A system to evaluate the esthetic outcome of the treatment of gingival recession through evaluation of clinical cases. J. Periodontol. 2009, 80, 705–710. [Google Scholar] [CrossRef]
- Nandanwar, J.; Bhongade, M.L.; Puri, S.; Dhadse, P.; Datir, M.; Kasatwar, A. Comparison of effectiveness of hyaluronic acid in combination with polylactic acid/polyglycolic acid membrane and subepi-thelial connective tissue graft for the treatment of multiple gingival recession defects in human: A clinical study. J. Datta Meghe Inst. Med. Sci. Univ. 2018, 13, 48–53. [Google Scholar] [CrossRef]
- Guldener, K.; Lanzrein, C.; Eliezer, M.; Katsaros, C.; Stähli, A.; Sculean, A. Treatment of single mandibular recessions with the modified coronally advanced tunnel or laterally closed tunnel, hyaluronic acid, and subepithelial connective tissue graft: A report of 12 cases. Quintessence Int. 2020, 51, 456–463. [Google Scholar] [CrossRef]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhao, Y.; Xu, K.; He, Y.; Qin, M. Hyaluronic Acid Receptor-Mediated Nanomedicines and Targeted Therapy. Small Methods 2024, 8, e2400513. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal. Res. 2019, 54, 33–45. [Google Scholar] [CrossRef]
- McGuire, M.K.; Cochran, D.L. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 2: Histological evaluation. J. Periodontol. 2003, 74, 1126–1135. [Google Scholar] [CrossRef]
- Stähli, A.; Duong, H.Y.; Imber, J.C.; Roccuzzo, A.; Salvi, G.E.; Katsaros, C.; Ramseier, C.A.; Sculean, A. Recession coverage using the modified coronally advanced tunnel and connective tissue graft with or without enamel matrix derivative: 5-year results of a randomised clinical trial. Clin. Oral Investig. 2023, 27, 105–113. [Google Scholar] [CrossRef]
- Meza Mauricio, J.; Furquim, C.P.; Bustillos-Torrez, W.; Soto-Peñaloza, D.; Peñarrocha-Oltra, D.; Retamal-Valdes, B.; Faveri, M. Does enamel matrix derivative application provide additional clinical benefits in the treatment of maxillary Miller class I and II gingival recession? A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 1613–1626. [Google Scholar] [CrossRef]
- Mancini, L.; Tarallo, F.; Quinzi, V.; Fratini, A.; Mummolo, S.; Marchetti, E. Platelet-Rich Fibrin in Single and Multiple Coronally Advanced Flap for Type 1 Recession: An Updated Systematic Review and Meta-Analysis. Medicina 2021, 57, 144. [Google Scholar] [CrossRef]
- Lu, K.H.; Lu, P.W.; Lin, C.W.; Lu, E.W.; Yang, S.F. Different molecular weights of hyaluronan research in knee osteoarthritis: A state-of-the-art review. Matrix. Biol. 2023, 117, 46–71. [Google Scholar] [CrossRef]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- Liu, H.; Yin, Y.; Yao, K.; Ma, D.; Cui, L.; Cao, Y. Influence of the concentrations of hyaluronic acid on the properties and biocompatibility of Cs-Gel-HA membranes. Biomaterials 2004, 25, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Pini-Prato, G.P.; Cairo, F.; Nieri, M.; Franceschi, D.; Rotundo, R.; Cortellini, P. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: A split-mouth study with a 5-year follow-up. J. Clin. Periodontol. 2010, 37, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Nieri, M.; Pagliaro, U. Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. J. Clin. Periodontol. 2014, 41 (Suppl. 15), S44–S62. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.C.; Joly, J.C.; Botelho, J.; Machado, V.; Avila-Ortiz, G.; Cairo, F.; Chambrone, L. Long-term stability of gingival margin and periodontal soft-tissue phenotype achieved after mucogingival therapy: A systematic review. J. Clin. Periodontol. 2024, 51, 177–195. [Google Scholar] [CrossRef]
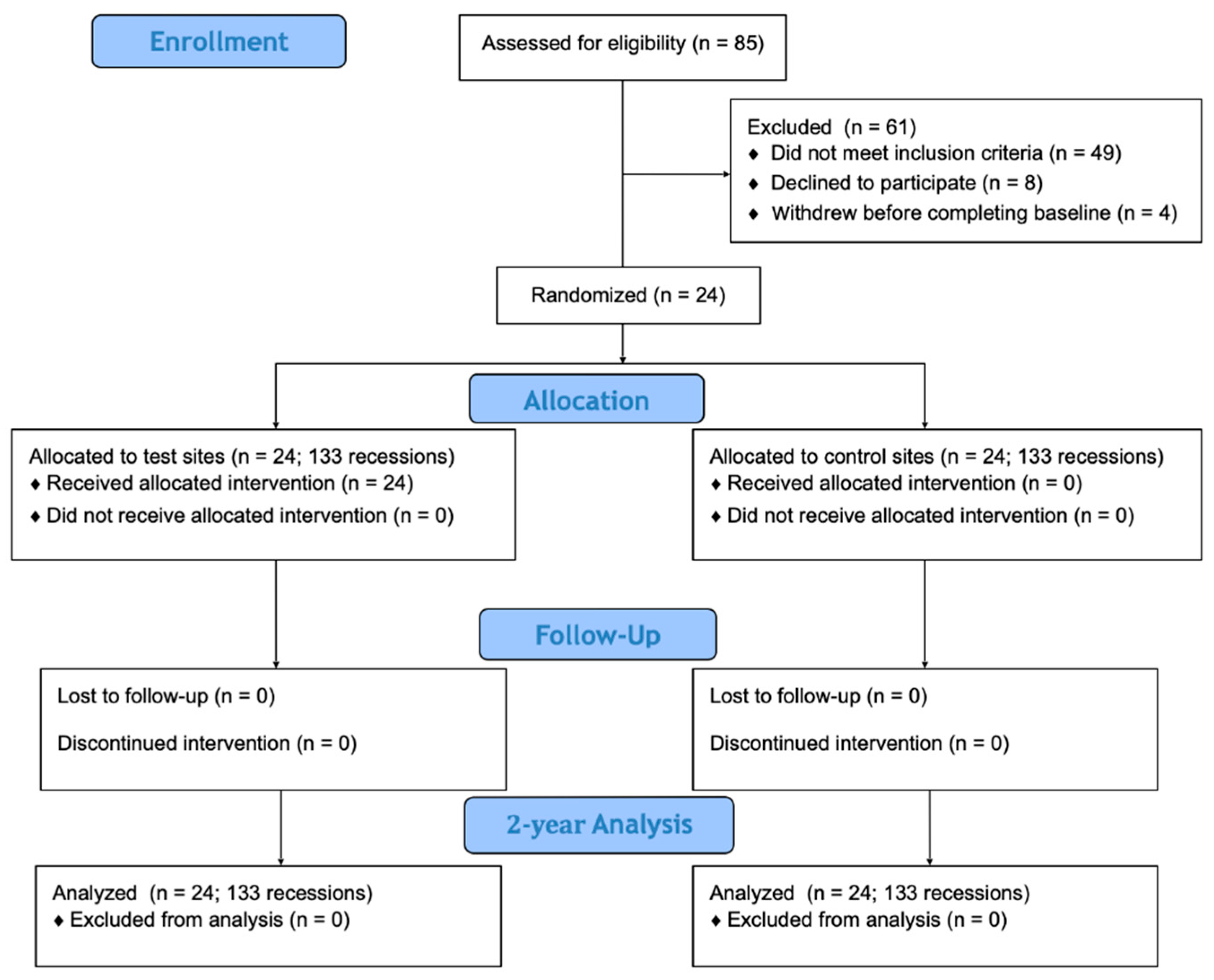
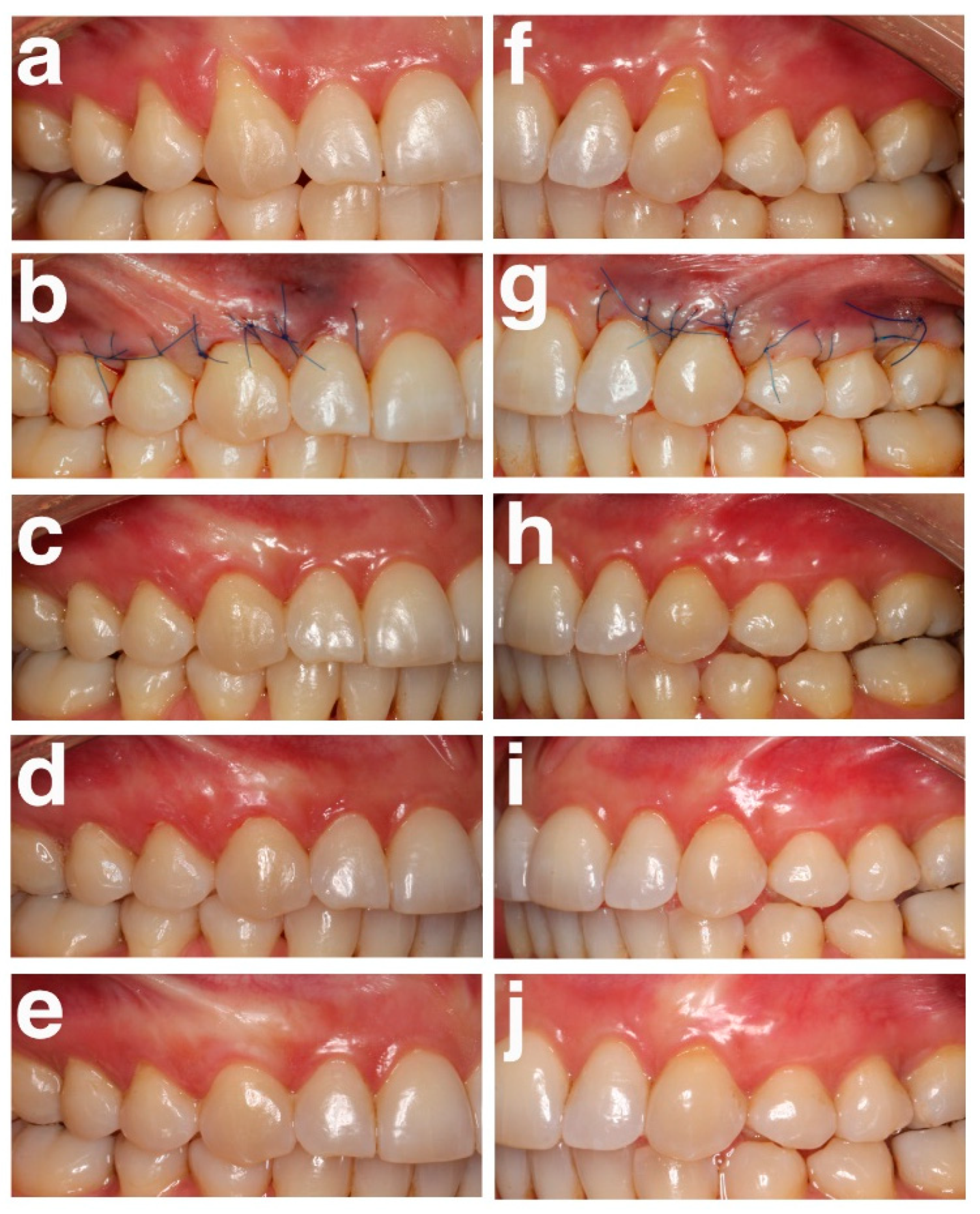



| Variables | Test Group (n = 24, 133 Recessions) | Control Group (n = 24, 133 Recessions) |
|---|---|---|
| Sex (n) | ||
| Women | 19 | 19 |
| Men | 5 | 5 |
| Tooth type (n) | ||
| Incisors | 42 | 42 |
| Canines | 23 | 23 |
| Premolars | 45 | 45 |
| Molars | 23 | 23 |
| Tooth position (n) | ||
| Maxillary teeth | 108 | 105 |
| Mandibular teeth | 25 | 28 |
| Type of recession (n,%) | ||
| RT1 | 59 (44%) | 54 (41%) |
| RT2 | 74 (56%) | 79 (59%) |
| Test Group (n = 24, 133 Recessions) | Control Group (n = 24, 133 Recessions) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
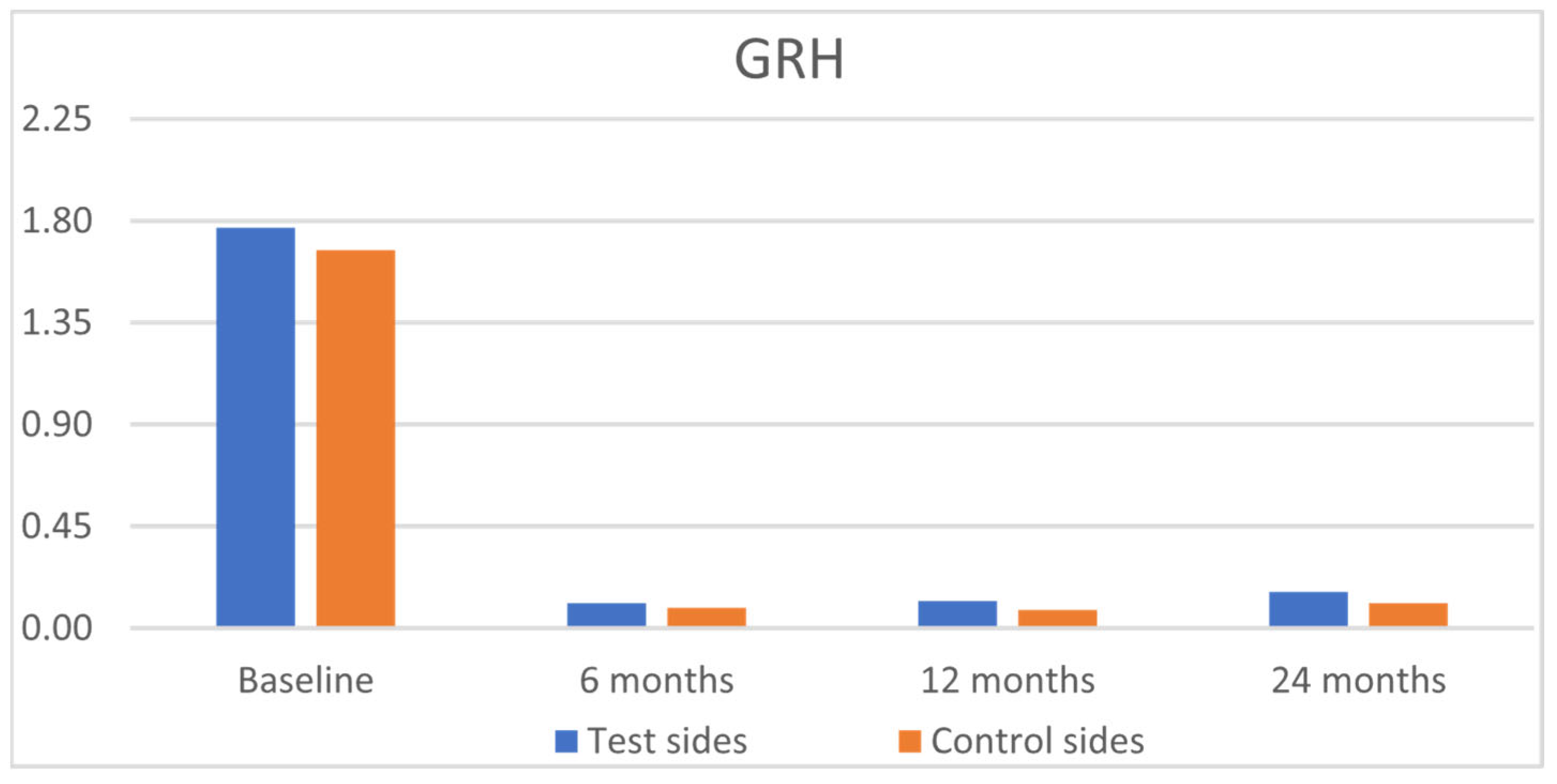
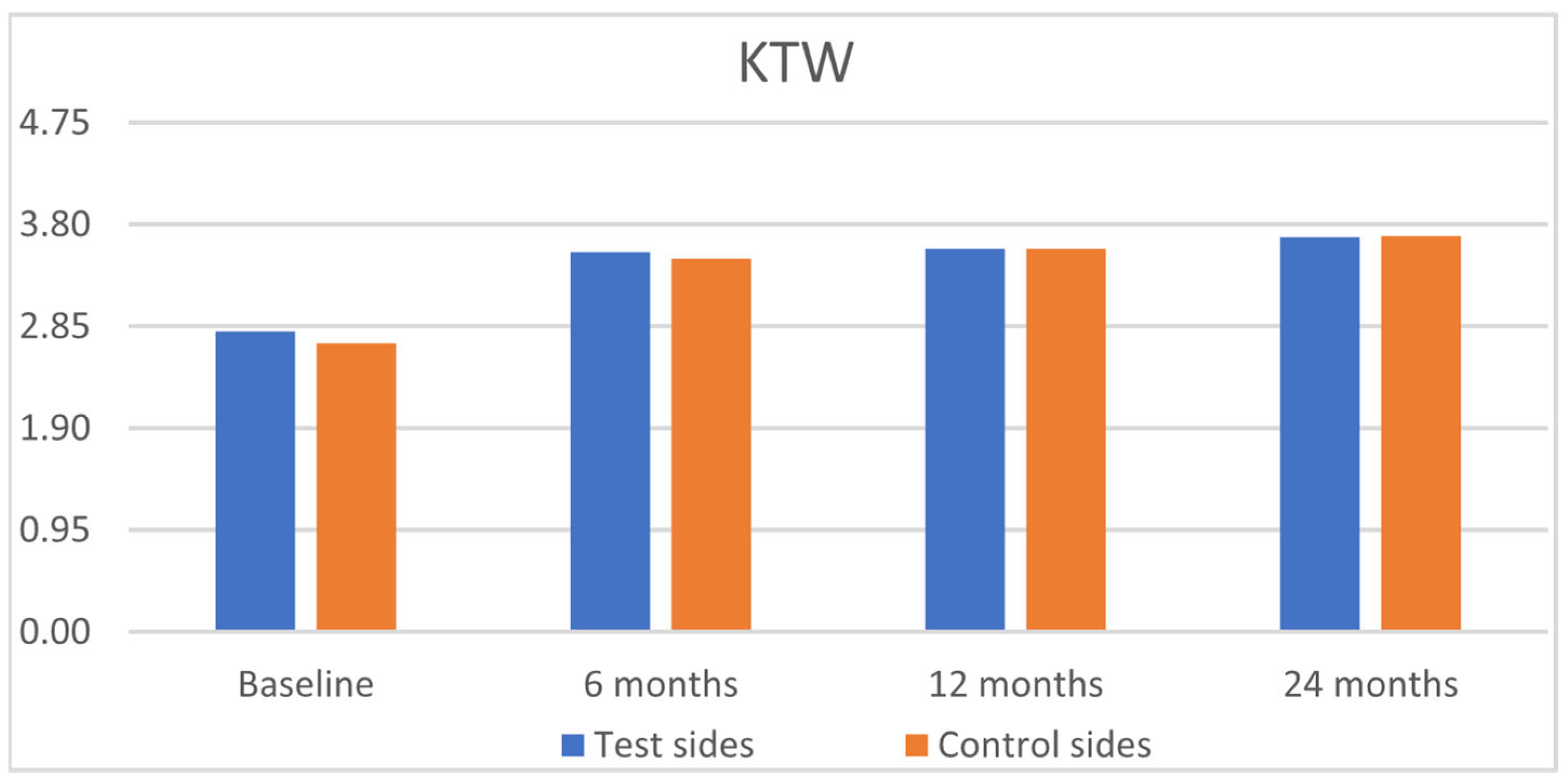
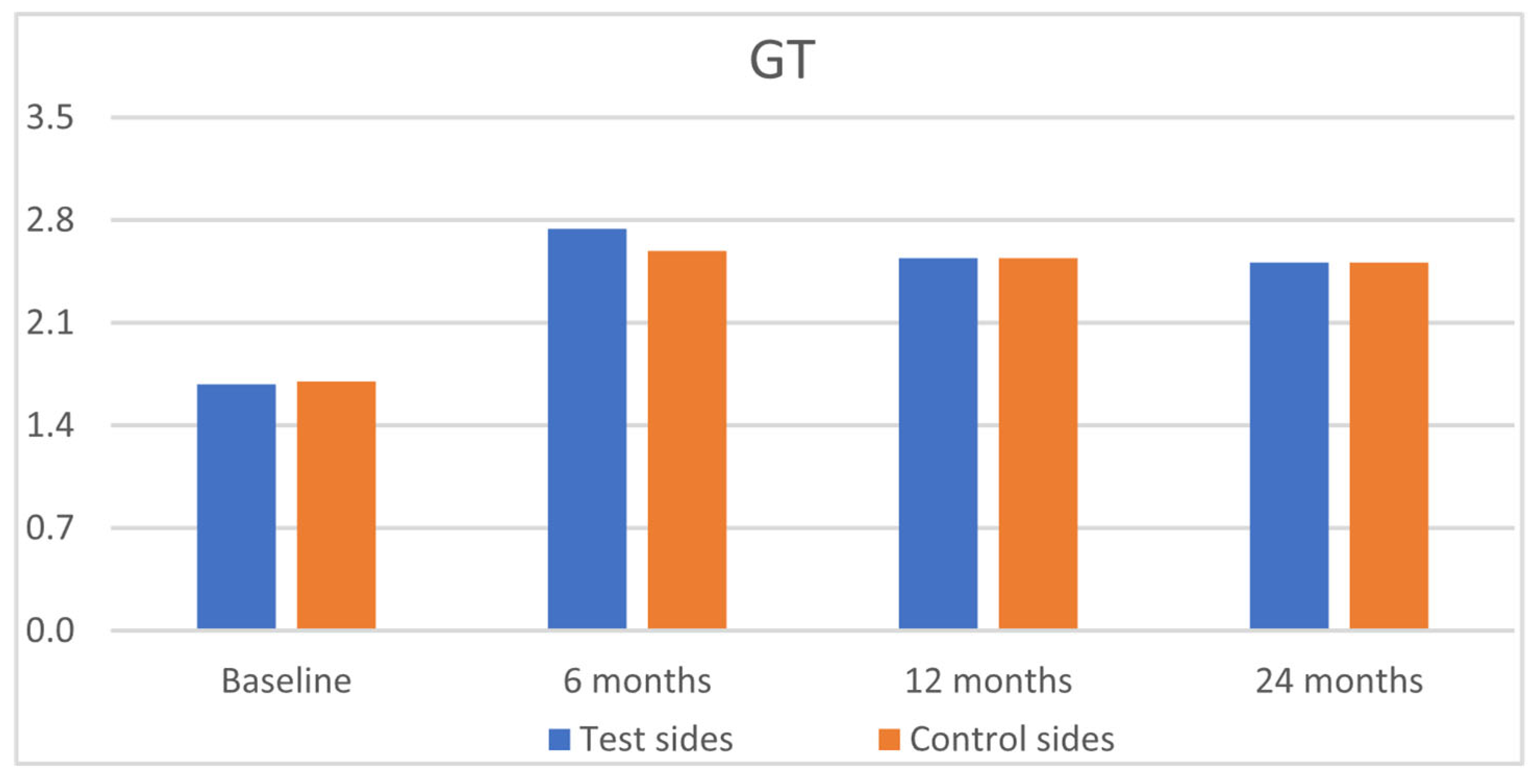
| GRH [mm] | RW [mm] | CAL [mm] | PPD [mm] | KTW [mm] | GT [mm] | GRH [mm] | RW [mm] | CAL [mm] | PPD [mm] | KTW [mm] | GT [mm] | |
| Baseline | ||||||||||||
| Mean | 1.77 a | 3.24 a | 3.08 a | 1.42 a | 2.80 a | 1.68 a | 1.67 a | 3.32 a | 3.08 a | 1.49 a | 2.69 a | 1.70 a |
| SD | 1.13 | 1.88 | 1.28 | 0.54 | 1.38 | 0.72 | 1.12 | 1.82 | 1.22 | 0.57 | 1.28 | 0.75 |
| 6 months | ||||||||||||
| Mean | 0.11 b | 0.31 b | 0.87 b | 1.40 a | 3.54 a | 2.74 b | 0.09 b | 0.29 b | 0.90 b | 1.51 a | 3.48 a | 2.59 b |
| SD | 0.41 | 1.11 | 0.80 | 0.54 | 1.46 | 0.80 | 0.38 | 1.11 | 0.82 | 0.57 | 1.32 | 0.68 |
| 12 months | ||||||||||||
| Mean | 0.12 b | 0.35 b | 0.50 b | 1.42 a | 3.57 a | 2.54 b | 0.08 b | 0.25 b | 0.57 b | 1.49 a | 3.57 a | 2.54 b |
| SD | 0.48 | 1.29 | 0.85 | 0.53 | 1.49 | 0.74 | 0.39 | 1.09 | 0.80 | 0.54 | 1.26 | 0.67 |
| 24 months | ||||||||||||
| Mean | 0.16 b | 0.49 b | 0.53 b | 1.35 a | 3.68 a | 2.51 b | 0.11 b | 0.33 b | 0.62 b | 1.36 a | 3.69 a | 2.51 b |
| SD | 0.50 | 1.41 | 0.88 | 0.49 | 1.52 | 0.72 | 0.41 | 1.15 | 0.82 | 0.53 | 1.22 | 0.64 |
| Test Group (n = 24, 133 Recessions) | Control Group (n = 24, 133 Recessions) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRC [%] | CRC [%] | GRred [mm] | KTWgain [mm] | GTgain [mm] | MRC (%) | CRC (%) | GRred [mm] | KTWgain [mm] | GTgain [mm] | |
| Baseline—6 months | ||||||||||
| Mean | 85.23 a | 91.32 a | 1.66 a | 0.67 a | 1.00 a | 83.37 a | 93.39 a | 1.59 a | 0.65 a | 0.80 a |
| SD | 34.21 | 29.11 | 1.06 | 1.33 | 0.99 | 35.54 | 26.34 | 1.15 | 1.35 | 0.98 |
| Baseline—12 months | ||||||||||
| Mean | 84.32 a | 92.12 a | 1.65 a | 0.68 a | 0.81 a | 85.71 a | 94.61 a | 1.59 a | 0.76 a | 0.77 a |
| SD | 34.46 | 28.14 | 1.09 | 1.40 | 0.79 | 36.43 | 24.71 | 1.14 | 1.36 | 0.74 |
| Baseline—24 months | ||||||||||
| Mean | 81.37 a | 87.02 a | 1.61 a | 0.80 a | 0.78 a | 84.63 a | 91.90 a | 1.57 a | 0.88 a | 0.75 a |
| SD | 37.17 | 34.73 | 1.10 | 1.50 | 0.93 | 35.33 | 29.85 | 1.15 | 1.40 | 0.92 |
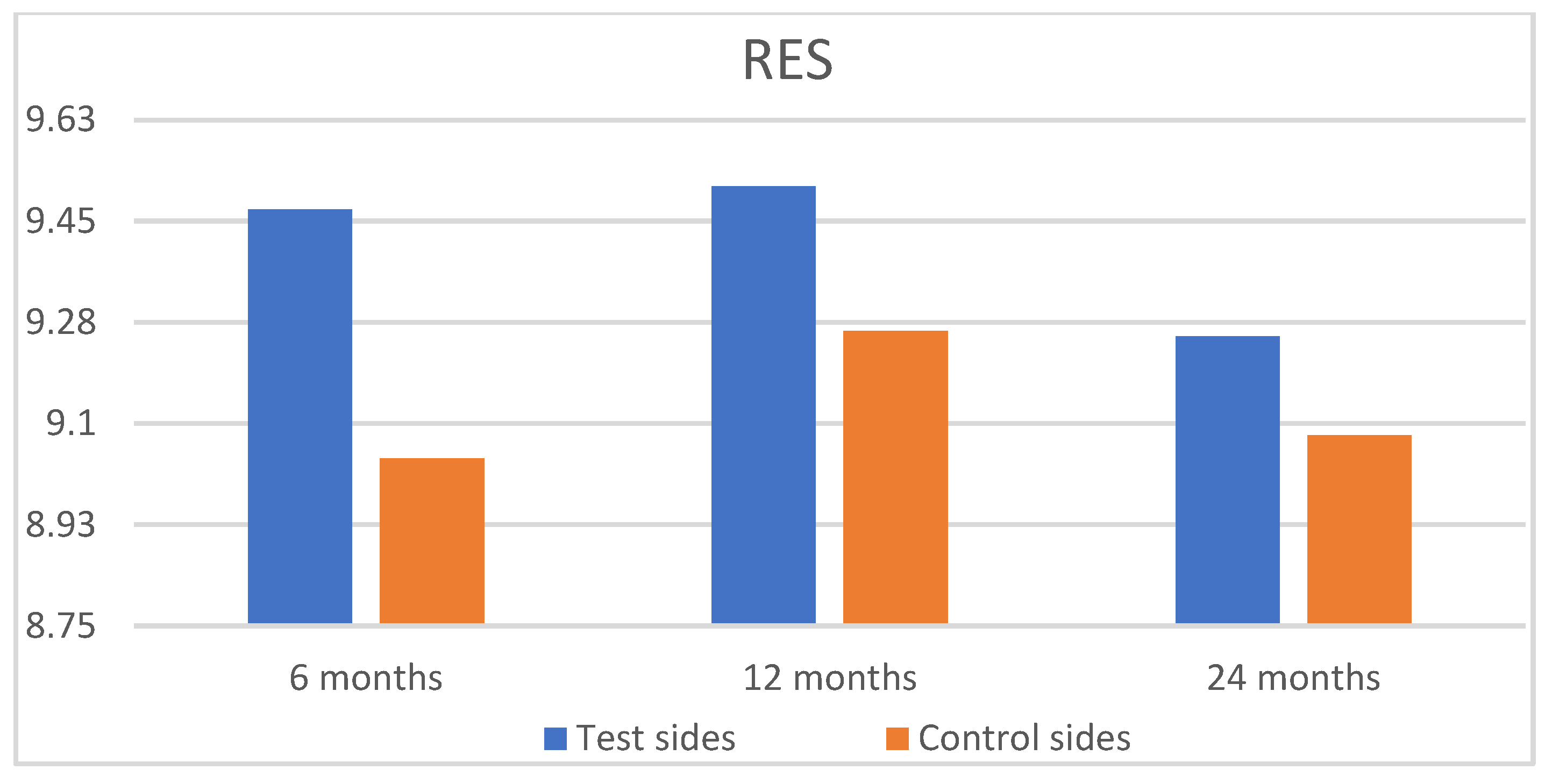
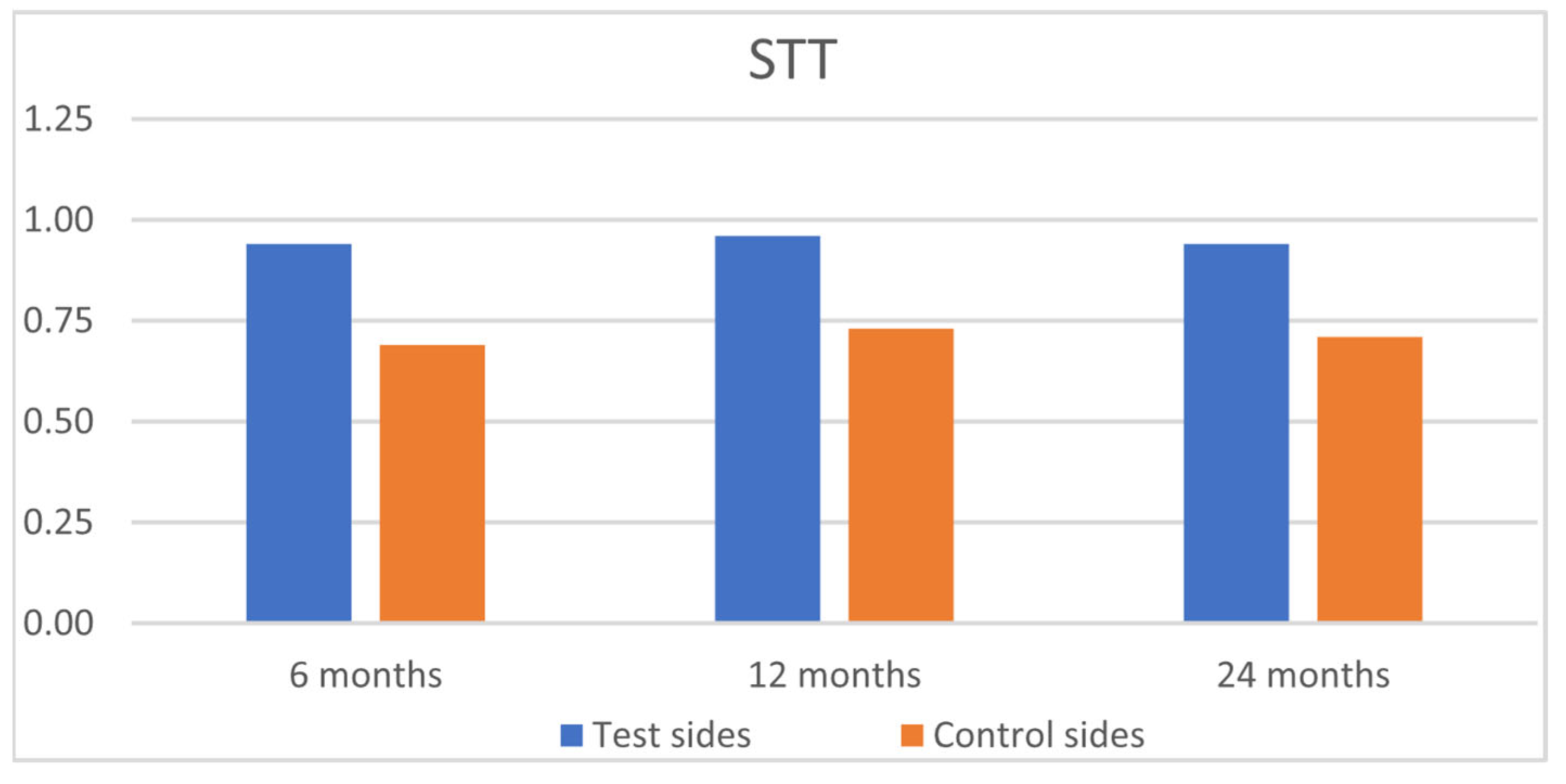
| Test Group (n = 24, 133 Recessions) | Control Group (n = 24, 133 Recessions) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | MTC | STT | MGJ | GC | RES | GM | MTC | STT | MGJ | GC | RES | |
| 6 months | ||||||||||||
| Mean | 5.73 Aa | 0.88 Aa | 0.94 Aa | 0.92 Aa | 0.98 Aa | 9.47 Aa | 5.70 Aa | 0.83 Aa | 0.69 Ba | 0.86 Aa | 0.95 Aa | 9.04 Aa |
| SD | 0.87 | 0.32 | 0.23 | 0.28 | 0.16 | 1.00 | 1.12 | 0.38 | 0.46 | 0.35 | 0.22 | 1.00 |
| 12 months | ||||||||||||
| Mean | 5.75 Aa | 0.90 Aa | 0.96 Aa | 0.92 Aa | 0.98 Aa | 9.51 Aa | 5.78 Aa | 0.87 Aa | 0.73 Ba | 0.89 Aa | 0.98 Aa | 9.26 Aa |
| SD | 0.83 | 0.30 | 0.20 | 0.28 | 0.14 | 1.01 | 0.87 | 0.34 | 0.22 | 0.31 | 0.13 | 1.10 |
| 24 months | ||||||||||||
| Mean | 5.58 Aa | 0.87 Aa | 0.94 Aa | 0.87 Aa | 0.98 Aa | 9.25 Aa | 5.69 Aa | 0.84 Aa | 0.71 Ba | 0.86 Aa | 0.98 Aa | 9.08 Aa |
| SD | 1.04 | 0.34 | 0.23 | 0.34 | 0.13 | 1.24 | 1.00 | 0.37 | 0.46 | 0.35 | 0.13 | 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górski, B.; Skierska, I.M.; Gelemanović, A.; Roguljić, M.; Bozic, D. Multiple Recessions Coverage Using the Modified Tunnel Technique and Connective Tissue Graft with or Without Cross-Linked Hyaluronic Acid: 2-Year Outcomes of RCT. J. Funct. Biomater. 2025, 16, 87. https://doi.org/10.3390/jfb16030087
Górski B, Skierska IM, Gelemanović A, Roguljić M, Bozic D. Multiple Recessions Coverage Using the Modified Tunnel Technique and Connective Tissue Graft with or Without Cross-Linked Hyaluronic Acid: 2-Year Outcomes of RCT. Journal of Functional Biomaterials. 2025; 16(3):87. https://doi.org/10.3390/jfb16030087
Chicago/Turabian StyleGórski, Bartłomiej, Izabela Maria Skierska, Andrea Gelemanović, Marija Roguljić, and Darko Bozic. 2025. "Multiple Recessions Coverage Using the Modified Tunnel Technique and Connective Tissue Graft with or Without Cross-Linked Hyaluronic Acid: 2-Year Outcomes of RCT" Journal of Functional Biomaterials 16, no. 3: 87. https://doi.org/10.3390/jfb16030087
APA StyleGórski, B., Skierska, I. M., Gelemanović, A., Roguljić, M., & Bozic, D. (2025). Multiple Recessions Coverage Using the Modified Tunnel Technique and Connective Tissue Graft with or Without Cross-Linked Hyaluronic Acid: 2-Year Outcomes of RCT. Journal of Functional Biomaterials, 16(3), 87. https://doi.org/10.3390/jfb16030087








