Stannous Fluoride in Toothpastes: A Review of Its Clinical Effects and Likely Mechanisms of Action
Abstract
1. Introduction
2. Background
3. Toothpaste
4. Dental Caries and Fluoride
- (i)
- (ii)
- (iii)
5. Stannous Fluoride in Toothpaste
6. Stannous Fluoride Toothpastes and Tooth Mineral
7. Stannous Fluoride Toothpastes and Dental Plaque
8. Stannous Fluoride Toothpastes and Hypersensitivity
9. Aqueous Solutions of Stannous Fluoride
10. Stabilisation and Speciation of Stannous Fluoride Toothpastes
11. Mechanism of Action of Stannous Fluoride
12. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fejerskov, O.; Cury, J.A.; Tenuta, L.M.; Marinho, V.C. Fluorides in Caries Control. In Dental Caries: The Disease and Its Clinical Management, 3rd ed; Fejerskov, O., Nyrad, B., Kidd, E.A.M., Eds.; Blackwell and Munksgaard: Oxford, UK, 2015; Chapter 14; pp. 245–286. [Google Scholar]
- Griffin, S.O.; Regnier, E.; Griffin, P.M.; Huntley, V. Effectiveness of fluoride in prventing caries in adults. J. Dent. Res. 2007, 86, 410–415. [Google Scholar] [CrossRef]
- Urbansky, E.T. Fate of fluorosilicate drinking water additives. Chem. Rev. 2002, 102, 2837–2854. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.M.; Ellwood, R.P.; Davies, G.M. The rational use of fluoride toothpaste. Int. Dent. J. Hyg. 2003, 1, 3–8. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.T.; Logan, S.; Sheiham, A. Systematic review of controlled trials on the effectiveness of fluoride gels for the prevention of dental caries in children. J. Dent. Educ. 2003, 67, 448–458. [Google Scholar] [CrossRef]
- White, D.J. A “return” to stannous fluoride dentifrices. J. Clin. Dent. 1995, 6, 29–36. [Google Scholar] [PubMed]
- Gaffar, A.; Asflitto, J.; Jabi, N. Chemical agents for the control of plaque and plaque microflora: An overview. Eur. J. Oral Sci. 1997, 14, 502–507. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Laino, L.; Cicciu, M. Stannous fluoride effects on enamel: A systematic review. Biomimetics 2020, 5, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Addy, M.; Greenman, J.; Renton-Harper, P.; Newcombe, R.; Doherty, F. Studies on stannous fluoride toothpaste and gel (2). Effects on salivary bacterial counts and plaque regrowth in vivo. J. Clin. Periodontol. 1997, 24, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kou, H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv. Dent. Res. 2008, 20, 17–21. [Google Scholar] [CrossRef]
- Abreu-Placerese, N.; Martinez-Mier, E.A. Stabilized stannous fluoride(SnF2) toothpastes may be effective in the management of hypersensitivity, while more research is needed for its effectiveness in dental caries and erosion prevention. J. Evid.-Based Dent. Pract. 2021, 21, 101651. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, A.; Emilson, C.-G.; Johannsen, G.; Konradson, K.; Lingstron, P.; Ramberg, P. Effect of stabilized stannous fluoride dentifrice on dental calculus, dental plaque, gingivitis, halitosis and stain: A systematic review. Heliyon 2019, 5, e02850. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Chen, K.J.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Arresting early childhood caries using silver and fluoride products—A randomised trial. J. Dent. 2020, 103, 103522. [Google Scholar] [CrossRef]
- Shapira, L.; Schatzker, Y.; Gedalia, I.; Borinski, R.; Bouchard, D.; Gaffar, A. Effect of amine and stannous fluoride on human neutrofil functions in vitro. J. Dent. Res. 1997, 73, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Truory, T.; Heu, R.; Stranik, M.; Bouchard, D.; Gaffar, A. Recent advances in stannous fluoride technology: Antibacterial efficacy and mechanism of action towards hypersensitivity. Int. Dent. J. 1994, 44 (Suppl. S1), 83–98. [Google Scholar]
- Rolla, G.; Ellingsen, J.E. Clinical effects and possible mechanisms of action of stannous fluoride. Int. Dent. J. 1994, 44 (Suppl. S1), 99–105. [Google Scholar] [PubMed]
- Donaldson, J.D.; Grimes, S.M. The Inorganic Chemistry of Tin. In Chemistry of Tin, 2nd ed; Smith, P.J., Ed.; Springer-Nature: London, UK, 1998; pp. 62–94. [Google Scholar]
- Meutterties, E.L. Chemistry of the difluorides of germanium and tin. Inorg. Chem. 1962, 1, 342–345. [Google Scholar] [CrossRef]
- Birchall, T.; Denes, G. A 19F, 119Sn nuclear magnetic resonance and 119Sn Mossbauer study of the SnF2-MF-H2O system. Can. J. Chem. 1984, 62, 591–595. [Google Scholar] [CrossRef]
- Moharamzadeh, K. Biocompatibility of Oral Care Produects. In Biocompatibility of Dental Biomaterials; Shelton, R., Ed.; Woodhead Publishing Series in Biomaterials: Duxford, UK, 2017; Chapter 8; pp. 113–129. [Google Scholar]
- Featherstone, J.B.D. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeronic, A. Fluoride toothpaste of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 4, CD-007868. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.B.; Keels, M.A.; Slayton, R.L. Fluoride use in caries prevention in the primary care setting. Pediatrics 2020, 146, e2020034637. [Google Scholar] [CrossRef]
- Zero, D.T.; Fontana, M.; Martinez-Miller, E.A.; Ferreira-Zandona, E.A.; Ando, A.; Gonzales-Cabezas, C.; Bayne, S. The biology, prevention, diagnosis and treatment of dental caries: Scientific advances in the United States. J. Am. Dent. Assoc. 2009, 140, 25S–34S. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Lavanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Hojo, S.; Takahashi, N.; Yamada, T. Acid profile in caries dentin. J. Dent. Res. 1991, 70, 182–186. [Google Scholar] [CrossRef]
- Featherstone, J.B.D. The continuum of dental caries—Evidence for a dynamic disease process. J. Dent. Res. 2004, 83, C39–C42. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphates in Oral Biology and Medicine; Karger: Basel, Belgium, 1991. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Teaford, M.F.; Smith, M.M.; Ferguson, M.W.J. Development, Function and Evolution of Teeth; Cambridge University Press: Cambridge, UK, 2000; p. 314. [Google Scholar]
- Epple, M.; Enax, J.; Meyer, F. Prevention of caries and dental erosion by fluorides–A critical discussion based on physico-chemical data and principles. Dent. J. 2022, 10, 6–19. [Google Scholar] [CrossRef]
- Eder, M.; Amini, S.; Fratzl, P. Biological composites-complex structures for functional diversity. Science 2018, 362, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Gradl, R.; Zanette, I.; Ruiz-Yaniz, M.; Dierolf, M.; Rack, A.; Zaslansky, P.; Pfeiffer, F. Mass density measurement of mineralized tissue with grating-based X-ray phase tomography. PLoS ONE 2016, 11, e0167797. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Yu, H.J.; Chen, C.Z. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O.; Larsen, M.J. Demineralization and remineralization: The key to understanding clinical manifestations of dental caries. In Dental Caries: The Disease and Its Clinical Management, 3rd ed; Fejerskov, O., Nyrad, B., Kidd, E.A.M., Eds.; Blackwell and Munksgaard: Oxford, UK, 2015; Chapter 9; pp. 155–172. [Google Scholar]
- Brown, W.E.; Gregory, T.M.; Chow, L.C. Effects of fluoride on enamel solubility and cariostasis. Caries Res. 1977, 11, 118–141. [Google Scholar] [CrossRef]
- ten Cate, J.N.; Featherstone, J.D. Mechanistic aspects of the interaction between fluoride and dental enamel. Crit. Rev. Oral Biol. Med. 1991, 2, 283–286. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, N.H. Resisting the onset of hydroxyapatite dissolution through incorpration of fluoride. J. Phys. Chem. B 2004, 108, 1809–1811. [Google Scholar] [CrossRef]
- Robinson, C.A.; Shore, R.C.; Brooks, S.J.; Stafford, S.; Wood, S.R.; Kirkham, J. The chemistry of enamel caries. Crit. Rev. Oral Biol. Med. 2000, 11, 481–495. [Google Scholar] [CrossRef]
- Yehia, A.; Ezzat, K. Fluoride uptake by synthetic apatites. Adsorp. Sci. Technol. 2009, 27, 337–347. [Google Scholar] [CrossRef]
- Faidt, T.; Friedrichs, A.; Grandthyll, S.; Spengler, C.; Jacobs, K.; Müller, F. Effect of fluoride treatment on the acid resistance of hydroxyapatite. Langmuir 2018, 34, 15253–15258. [Google Scholar] [CrossRef]
- Larsen, M.J.; Fejerskov, O. Chemical and structural challenges in remineralization of dental enamel lesions. Scand. J. Dent. Res. 1989, 97, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cury, J.A.; Tenuta, L.M.A. Enamel remineralization: Controlling the caries disease or treating early caries lesions? Braz. Oral Res. 2009, 23 (Suppl. S1), 23–30. [Google Scholar] [CrossRef] [PubMed]
- Veneri, F.; Vinceti, S.R.; Filippini, T. Fluoride and caries prevention: A scoping review of public health policies. Ann. Ig. 2024, 36, 270–280. [Google Scholar] [PubMed]
- Larsen, M.J.; von der Fehr, F.R.; Birkeland, J.M. Effect of fluoride on the saturation of an acetate buffer with respect to hydroxyapatite. Arch. Oral. Biol. 1976, 21, 723–728. [Google Scholar] [CrossRef]
- Marchetti, E.; Casalena, F.; Capestro, A.; Tecco, S.; Mattei, A.; Marzo, G. Efficacy of two mouthwashes on 3-day supragingival plaque regrowth: A randomized crossover clinical trial. Int. J. Dent. Hyg. 2017, 15, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Mozaffari, E.; Jonas, L. Scanning electron microscopy of growing dental plaque: A quantitative study with different mouth rinses. Ultrastruct. Pathol. 2013, 37, 233–240. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; Addy, M.; Newcombe, R.; Macdonald, E.; Chapman, A.; Davies, M.; Moran, J.; Claydon, N. A randomised crossover trial to compare the potential of stannous fluoride and essential oil mouth rinses to induce tooth and tongue staining. Clin. Oral Investig. 2012, 16, 821–826. [Google Scholar] [CrossRef]
- Wigger-Alberti, W.; Gysen, K.; Axmann, E.M.; Wilhelm, K.P. Efficacy of a new mouthrinse formulation on the reduction of oral malodour in vivo. A randomized, double-blind, placebo-controlled, 3 week clinical study. J. Breath Res. 2010, 4, 017102. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Lussi, A.; Grunau, O.; Klimek, J.; Schlueter, N. Conventional and anti-erosion fluoride toothpastes: Effect on enamel erosion and erosion-abrasion. Caries Res. 2011, 45, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Zero, D.T.; Lippert, F.; Hara, A.T.; Creeth, J.E.; Newby, E.E.; Butler, A.; Constantin, P.; Bosma, M.L. In situ anticaries efficacy of dentifrices with different formulations-A pooled analysis of results from three randomized clinical trials. J. Dent. 2018, 77, 93–105. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; Seong, J.; Hellin, N.; Macdonald, E.L.; Jones, S.B.; Creeth, J.E. Assessment of tubule occlusion properties of an experimental stannous fluoride toothpaste: A randomised clinical in situ study. J. Dent. 2018, 76, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hove, L.H.; Stenhagen, K.R.; Holme, B.; Tveit, A.B. The protective effect of SnF2 containing toothpastes and solution on enamel surfaces subjected to erosion and abrasion in situ. Eur. Arch. Paediatr. Dent. 2014, 15, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.C.; Oliveira, P.H.C.; Oliveira, L.H.C.; Horliana, A.; César, P.F.; Moura, S.K.; Bussadori, S.K. Microhardness of bovine enamel after different fluoride application protocols. Dent. Mater. J. 2019, 38, 61–67. [Google Scholar] [CrossRef]
- Stenhagen, K.R.; Hove, L.H.; Holme, B.; Tveit, A.B. The effect of daily fluoride mouth rinsing on enamel erosive/abrasive wear in situ. Caries Res. 2013, 47, 2–8. [Google Scholar] [CrossRef]
- Huysmans, M.C.; Jager, D.H.; Ruben, J.L.; Unk, D.E.; Klijn, C.P.; Vieira, A.M. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011, 45, 518–523. [Google Scholar] [CrossRef]
- West, N.X.; He, T.; Hellin, N.; Claydon, N.; Seong, J.; Macdonald, E.; Farrell, S.; Eusebio, R.; Wilberg, A. Randomized in situ clinical trial evaluating erosion protection efficacy of a 0.454% stannous fluoride dentifrice. Int. J. Dent. Hyg. 2019, 17, 261–267. [Google Scholar] [CrossRef]
- Seriwatanachai, D.; Triratana, T.; Kraivaphan, P.; Amaornchat, C.; Mateo, L.R.; Sabharwal, A.; Delgado, E.; Szewczyk, G.; Ryan, M.; Zhang, Y.P. Effect of stannous fluoride and zinc phosphate dentifrice on dental plaque and gingivitis: A randomized clinical trial with 6-month follow-up. J. Am. Dent. Assoc. 2019, 150, S25–S31. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, X.; Liu, H.; Mateo, L.R.; Sabharwal, A.; Xu, G.; Szewczyk, G.; Ryan, M.; Zhang, Y.P. Evaluation of a stabilized stannous fluoride dentifrice on dental plaque and gingivitis in a randomized controlled trial with 6-month follow-up. J. Am. Dent. Assoc. 2019, 150, S32–S37. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Raylae, C.C.; Sreenivasan, P.K. Antimicrobial effects of a stannous fluoride toothpaste in distinct oral microenvironments. J. Am. Dent. Assoc. 2019, 150, S14–S24. [Google Scholar] [CrossRef] [PubMed]
- Hagenfeld, D.; Prior, K.; Harks, I.; Jockel-Schneider, Y.; May, T.W.; Harmsen, D.; Schlagenhauf, U.; Ehmke, B. No differences in microbiome changes between anti-adhesive and antibacterial ingredients in toothpastes during periodontal therapy. J. Periodontal Res. 2019, 54, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, P.G.; Harris, R.; Date, R.F.; Mussett, A.J.; Manley, A.; Barker, M.L.; Hellin, N.; West, N.X. In situ clinical evaluation of a stabilised, stannous fluoride dentifrice. Int. Dent. J. 2014, 64 (Suppl. S1), 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Sreenivasan, P.K.; McKiernan, M.; Tischio-Bereski, D.; Furgang, D. Whole mouth antimicrobial effects after oral hygiene: Comparison of three dentifrice formulations. J. Clin. Periodontol. 2012, 39, 1056–1064. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, J.; Li, J.; Zhou, X.; Wang, L.; Liu, J.; Xu, X. Comparative effect of a stannous fluoride toothpaste and a sodium fluoride toothpaste on a multispecies biofilm. Arch. Oral Biol. 2017, 74, 5–11. [Google Scholar] [CrossRef]
- Tobler, D.; Braissant, O.; Waltimo, T.; Bornstein, M.M.; Astasov-Fraunhoffer, M. Stannous source in toothpastes leads to differences in their antimicrobial efficacy. Oral Health Prev. Dent. 2023, 21, 3019–3324. [Google Scholar]
- Chen, D.; Chew, D.; Xiang, Q.; Lam, T.; Dai, Y.; Wang, L.; He, T.; Strand, R.; Zhang, X.; Lim, L.; et al. Interactions and effects of stannous-containing sodium fluoride dentifrice on oral pathogens and the oral microbiome. Front. Microbiol. 2024, 15, 1327913. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Periodontol. 2018, 89 (Suppl. S1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, C.R.; Milleman, K.R.; Milleman, J.L. Gingivitis efficacy of a 0.454% w/w stannous fluoride dentifrice: A 24-week randomized controlled trial. BMC Oral Health 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Acherkouk, A.; Patel, N.; Butler, A.; Amini, P. A randomised clinical study investigating efficacy of a stannous fluoride toothpaste in improving gingival health after 3 weeks’ use. BMC Oral Health 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.; Barbour, A.; Kaura, K.; Kerns, K.A.; Chen, D.; Trivedi, H.M.; Gomez, J.; Sabharwal, A.; McLean, J.S.; Darveau, R.; et al. Effects of a stabilized stannous fluoride dentifrice on clinical, immunomodulatory, and microbial outcomes in a human experimental gingivitis model. J. Periodontol. 2024, 95, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.; Shiyou, X.; Stranik, M.; Lavender, S.; Pilch, S.; Zhang, Y.-P.; Sullian, R.; Montesani, L.; Montesani, L.; Mateo, L.R.; et al. Effect of stannous fluoride toothpaste on dentinal hypersensitivity. In vitro and clinical evaluation. J. Am. Dent. Assoc. 2019, 150, S47–S59. [Google Scholar] [CrossRef]
- Tao, D.; Ling, M.R.; Feng, X.-P.; Gallob, J.; Souverain, A.; Yamg, W.; Alavi, A. Efficacy of a stannous fluoride dentifrice for relief of dentine hypersensitivity: A randomized clinical study. J. Clin. Periodontol. 2020, 47, 962–969. [Google Scholar] [CrossRef]
- Creeth, J.E.; Goyal, C.; Qaqish, J.; Maclure, R.; Holt, J.S. Efficacy of an occluding toothpaste on dental hypersensitivity over 14 days. BDJ Open 2021, 7, 26. [Google Scholar] [CrossRef]
- Li, R.; Yang, W.; Grimaldi, R.; Zeng, P.; Smith, G.; Chen, X. Efficacy of a stannous fluoride dentifrice for relieving dental hypersensitivity in Chinese population: An 8-week randomized clinical trial. Clin. Oral Invest. 2024, 28, 230. [Google Scholar] [CrossRef] [PubMed]
- Schaap, W.B.; Davies, J.A.; Nebergall, W.H. Polarographic study of the complex ions of tin in fluoride solutions. J. Am. Chem. Soc. 1954, 76, 5226–5229. [Google Scholar] [CrossRef]
- Kriegsmann, H.H.; Kessler, G. Investigation on tin compounds, IV. Vibrational spectra and structure of some inorganic tin-fluorine compounds. Z. Anorg. Allg. Chem. 1962, 318, 277–286. [Google Scholar] [CrossRef]
- Donaldson, J.D.; O’Donoghue, J.D. Complex tin(II) fluorides. J. Chem. Soc. 1964, 271–275. [Google Scholar] [CrossRef]
- Hall, F.M.; Slater, S.J. The determination of the stability constants of the fluoride complexes of tin (II) using the fluoride electrode. Aust. J. Chem. 1968, 21, 2663–2667. [Google Scholar] [CrossRef]
- Bond, A.M.; Taylor, R.J. Polarographic studies of the fluoride complexes of tin (II) in neutral and acidic media. J. Electroanal. Chem. Interfac. Electrochem. 1970, 28, 207–215. [Google Scholar] [CrossRef]
- Abrahams, I.; Clark, S.J.; Donaldson, J.D.; Khan, Z.I.; Southern, J.T. Hydrolysis of tin (II) fluoride and crystal structure of Sn4OF6. J. Chem. Soc. Dalton Trans. 1994, 23–28. [Google Scholar] [CrossRef]
- Denes, G.; Lazarus, G. Oxidation of SnF2 stannous fluoride in aqueous solutions. Hyperfine Int. 1994, 90, 435–439. [Google Scholar] [CrossRef]
- Turner, D.; Czarnecka, B.; Nicholson, J.W. Interaction of stannous fluoride solutions with synthetic hydroxyapatite. Ceramics Silikaty 2013, 57, 1–6. [Google Scholar]
- McDonald, R.R.; Larson, A.C.; Cromar, D.T. The crystal structure of sodium pentafluorodistannate (II). Acta Cryst. 1964, 17, 1104–1108. [Google Scholar] [CrossRef]
- Donaldson, J.D.; O’Donohue, J.D.; Oteng, R. Formation of complex tin(II) species in molten tin(II) fluoride. J. Chem. Soc. 1965, 3876–3879. [Google Scholar]
- Alsina, M.A.; Gaillard, J.-F. Structural characterization of metal complexes in aqueous solutions: An XAS study of stannous fluoride. Phys. Chem. Chem. Phys. 2018, 20, 12727–12735. [Google Scholar] [CrossRef] [PubMed]
- Brauniger, T.; Ghedia, S.; Jansen, M. Covalent bonds in α-SnF2 monitored by J-couplings in solid-state NMR spectra. J. Inorg. Gen. Chem. 2010, 636, 2399–2404. [Google Scholar] [CrossRef]
- Donaldson, J.D.; Senior, B.J. The Mossbauer effect in tin(II) compounds. Part II. The spectra of complex tin(II) fluorides. J. Chem. Soc. A 1996, 1798–1800. [Google Scholar] [CrossRef]
- Tricker, M.J.; Donaldson, J.D. Comments on the structure, bonding and 119Sn Mossbauer parameters of tin(II) derivatives of the type MSnX3. Inorg. Chim. Acta 1978, 31, L445–L446. [Google Scholar] [CrossRef]
- Cigala, R.M.; Crea, F.; De Stefano, C.; Lando, G.; Milea, D.; Sammartano, S. The inorganic speciation of tin(II) in aqueous solution. Geochem. Cosmochem. Acta 2012, 87, 1–20. [Google Scholar] [CrossRef]
- Pettine, M.; Macchi, G. Hydrolysis of tin(II) in aqueous solutions. Anal. Chem. 1981, 53, 1039–1043. [Google Scholar] [CrossRef]
- Desmau, M.; Alsina, M.A.; Gaillard, J.-F. XAS study of Sn speciation in toothpaste. J. Anal. At. Spectrom. 2020, 36, 407–415. [Google Scholar] [CrossRef]
- Myers, C.P.; Pappas, I.; Makwana, E.; Begum-Gafur, R.; Utgikar, N.; Alsina, M.A.; Fitzgerald, M.; Trivedi, H.M.; Gaillard, J.-F.; Masters, J.G.; et al. Solving the problem with stannous fluoride. J. Am. Dent. Assoc. 2019, 150, S5–S13. [Google Scholar] [CrossRef]
- Faller, R.V.; Noble, W.H. Protection from dental erosion: All fluorides are not equal. Compend. Contin. Educ. Dent. 2018, 39, e13–e17. [Google Scholar] [PubMed]
- Pearson, R.G. G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Bond Enthalpy Values. Available online: https://labs.chem.ucsb.edu/zakarian/armen/11---bonddissociationenergy.pdf (accessed on 5 December 2024).
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heinemann: Oxford, UK, 1984. [Google Scholar]
- Marcus, Y. Volumes of aqueous hydrogen and hydroxide ions at 0 to 200 °C. J. Chem. Phys. 2012, 137, 154501. [Google Scholar] [CrossRef] [PubMed]
- Radike, A.W.; Gish, C.W.; Peterson, J.K.; King, J.D.; Segreto, V.A. Clinical evaluation of stannous fluoride as anticaries mouthrinse. J. Am. Dent. Assoc. 1973, 86, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response. A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; D’Amato, A.; Launa, G.; Saturnino, C.; Andreu, I.; Longo, P.; Sinicropi, M.S. Treatment of dental caries. Antibiotics 2023, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyl transferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Hardt, M.; Lussi, A.; Klimek, J.; Ganss, C. Tin-containing fluoride solutions as anti-erosive agents in enamel: An in vitro tin-uptake, tissue loss, and scanning electron micrograph study. Eur. J. Oral Sci. 2009, 117, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Hardt, M.; Cocks, A.K.; Klimek, J.; Schlueter, N. Mechanism of action of tin-containing fluoride solutions as anti-erosive agents in dentine—An in vitro tin-uptake, tissue loss, and scanning electron microscopy study. Eur. J. Oral Sci. 2010, 119, 376–384. [Google Scholar] [CrossRef]
- Berndt, A.F. Reaction of stannous fluoride with hydroxyapatite: The crystal structure of Sn3PO4F3. J. Dent. Res. 1972, 51, 53–57. [Google Scholar] [CrossRef]
- Babcock, F.D.; King, J.C.; Jordan, T.H. The reaction of stannous fluoride and hydroxyapatite. J. Dent. Res. 1978, 57, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Thamaraiselvi, T.V.; Prabakaran, K.; Rajeswari, S. Synthesis of hydroxyapatite that mimic bone minerology. Trends Biomater. Artif. Organs 2006, 19, 81–88. [Google Scholar]
- Koumoulidid, G.C.; Katsoulidis, A.R.; Ladavos, A.K.; Pomonis, P.J.; Trapalis, C.G.; Sdoukos, A.T.; Vaimakis, T.C. Preparation of hydroxyapatite via microemulsion route. J. Colloid Interfac. Sci. 2003, 259, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Celotti, G.; Logroscino, G.; Tamperi, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thain, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomater. 2006, 1, 317–332. [Google Scholar]

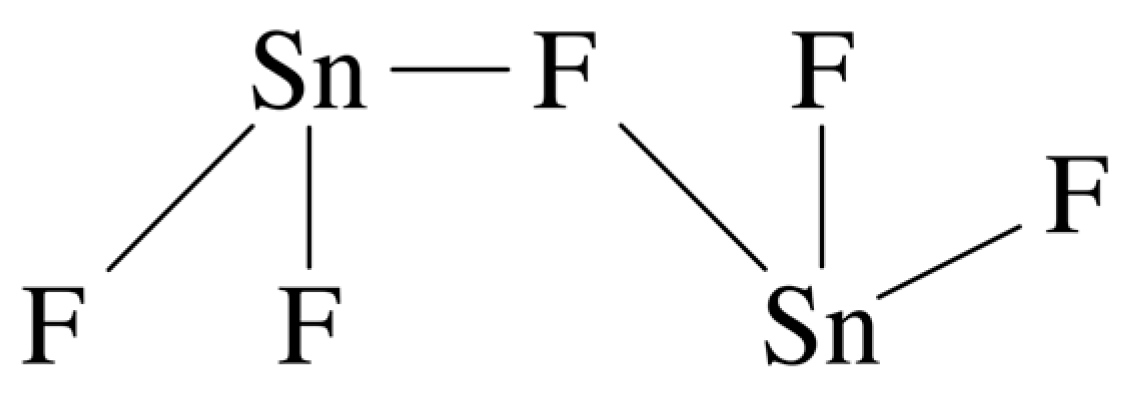
| Toothpaste Groups | Sample | Time/Days | Results | Reference |
|---|---|---|---|---|
| Four including SnF2 | 168 enamel surfaces | 14 | SnF2 gave greater remineralisation | [51] |
| Two, one SnF2 + NaF | 33 enamel surfaces | 15 | SnF2 reduced enamel loss | [52] |
| Two SnF2 (0.4 and 0.454%) | 64 human teeth | 9 | Both reduced enamel wear | [53] |
| One SnF2 | 27 enamel surfaces | 17 | SnF2 reduced plaque | [54] |
| Four including SnF2 | 16 human molars | 9 | SnF2 no different | [55] |
| Three, two with SnF2 | 20 enamel surfaces | 5 | SnF2 better than NaF | [56] |
| Toothpaste Groups | Time | Results | Reference |
|---|---|---|---|
| SnF2, SnF2 + zinc lactate, F | 6 months | SnF2 reduced plaque | [58] |
| SnF2 and NaF | 6 months | SnF2 reduced plaque | [59] |
| SnF2 and NaMFP | 8 weeks | SnF2 reduced plaque | [60] |
| Zn-HAP and amine fluoride + SnF2 | 12 weeks | SnF2 reduced plaque | [61] |
| SnF2 and NaF | 17 days | SnF2 same as NaF | [62] |
| Species Identified | Additional Species Proposed | Technique | Reference | Year Published |
|---|---|---|---|---|
| SnF3− | SnF62− | Polarography | [75] | 1954 |
| SnF3− | - | IR and Raman | [76] | 1962 |
| SnF3− | - | IR and 119Sn nmr | [77] | 1965 |
| SnF+, SnF2, SnF3− | F− | Fluoride electrode | [78] | 1968 |
| SnF+, SnF2, SnF3− | - | Polarography | [79] | 1970 |
| SnF2, SnF3− | - | 119Sn nmr, 19F nmr and 119Sn Mossbauer | [19] | 1984 |
| Sn4OF6 | - | Hydrolysis of SnF2 | [80] | 1994 |
| SnF2, SnF3− | - | 119Sn Mossbauer | [81] | 1994 |
| F−, SnF2 | SnF+ | Fluoride electrode | [82] | 2013 |
| Toothpaste Number | Unopened | Opened |
|---|---|---|
| 1 | 85 ± 1 | 62 ± 1 |
| 2 | 78 ± 1 | 60 ± 2 |
| 3 | 87 ± 1 | 61 ± 1 |
| 4 | 67 ± 1 | 67 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholson, J.W. Stannous Fluoride in Toothpastes: A Review of Its Clinical Effects and Likely Mechanisms of Action. J. Funct. Biomater. 2025, 16, 73. https://doi.org/10.3390/jfb16030073
Nicholson JW. Stannous Fluoride in Toothpastes: A Review of Its Clinical Effects and Likely Mechanisms of Action. Journal of Functional Biomaterials. 2025; 16(3):73. https://doi.org/10.3390/jfb16030073
Chicago/Turabian StyleNicholson, John W. 2025. "Stannous Fluoride in Toothpastes: A Review of Its Clinical Effects and Likely Mechanisms of Action" Journal of Functional Biomaterials 16, no. 3: 73. https://doi.org/10.3390/jfb16030073
APA StyleNicholson, J. W. (2025). Stannous Fluoride in Toothpastes: A Review of Its Clinical Effects and Likely Mechanisms of Action. Journal of Functional Biomaterials, 16(3), 73. https://doi.org/10.3390/jfb16030073







