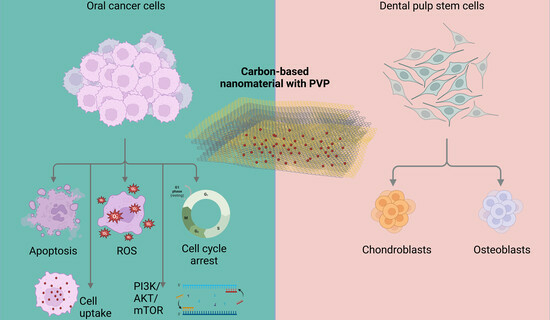
A Carbon-Based Nanomaterial with Dichotomous Effects: Antineoplastic on Oral Cancer Cells and Osteoinductive/Chondroinductive on Dental Pulp Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and PVP Functionalization of the Carbon-Based Nanomaterial
2.2. Characterization of Pristine Graphite and CBN/PVP
2.3. OSCC and DPSC Cell Cultures
2.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.5. Cell-Cycle Analysis
2.6. Transwell Invasion Assay and Wound Healing Assay
2.7. ROS Detection by Flow Cytometry
2.8. Nanoparticle Uptake Assay
2.9. CBN/PVP Effects on DPSCs’ Differentiation Induction
2.10. Isolation of RNA and Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
2.11. Quantitative Polymerase Chain Reaction (qPCR)
2.12. Statistical Analysis
3. Results
3.1. Material’s Characteristics
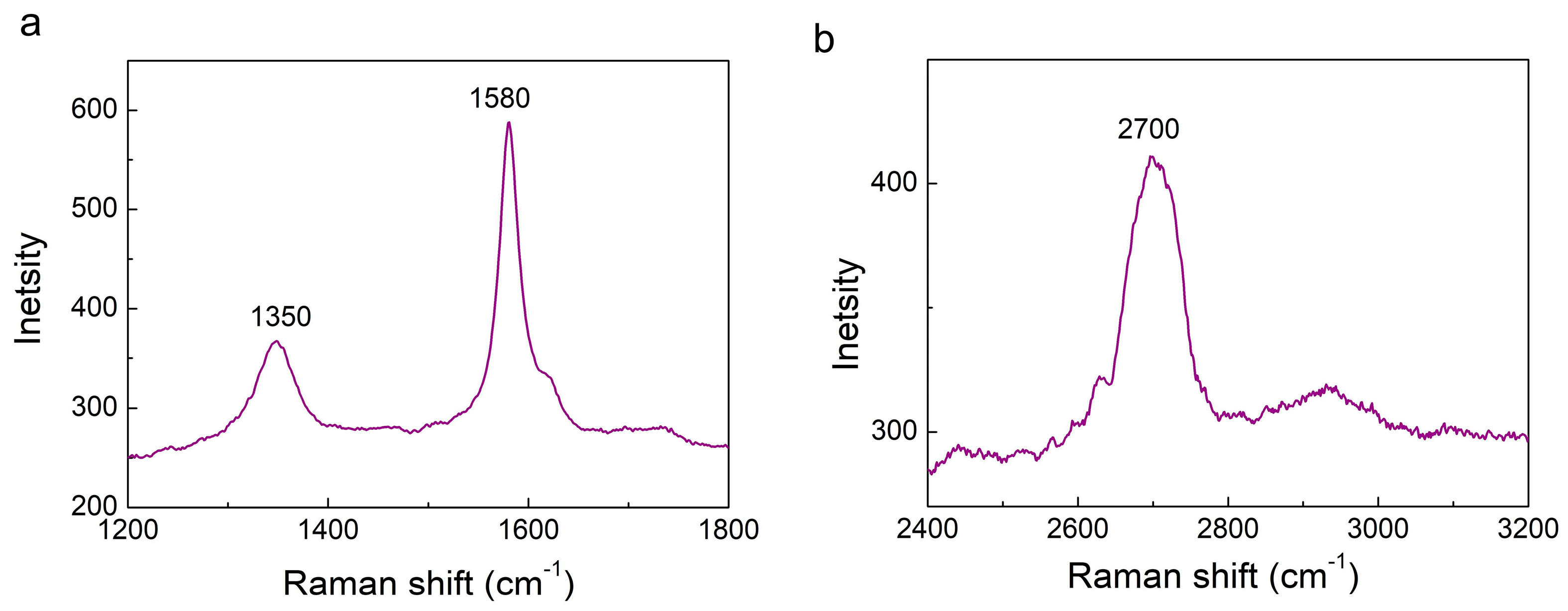
3.1.1. Raman Spectroscopy
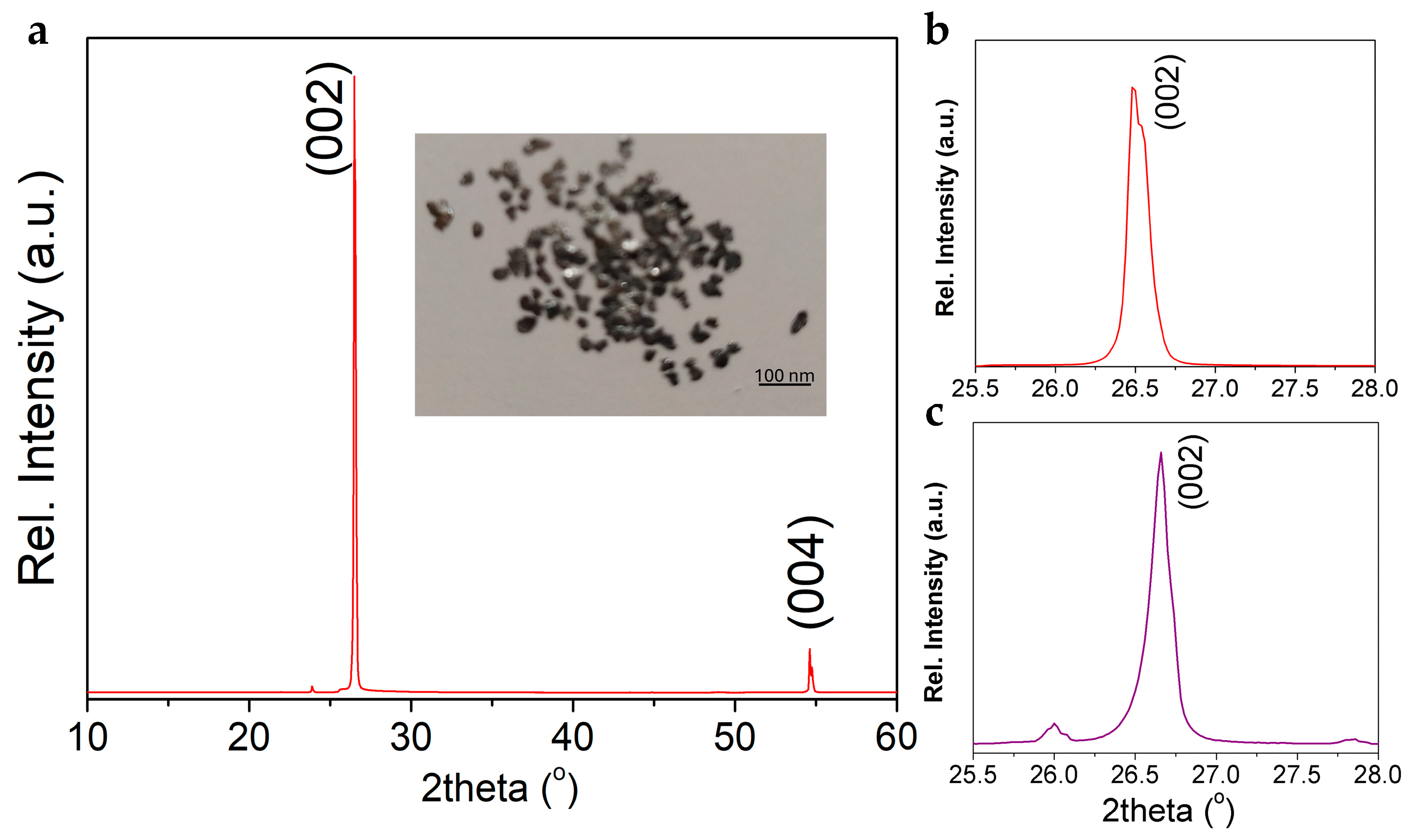
3.1.2. XRD Analysis
3.1.3. ATR-FTIR Spectroscopy
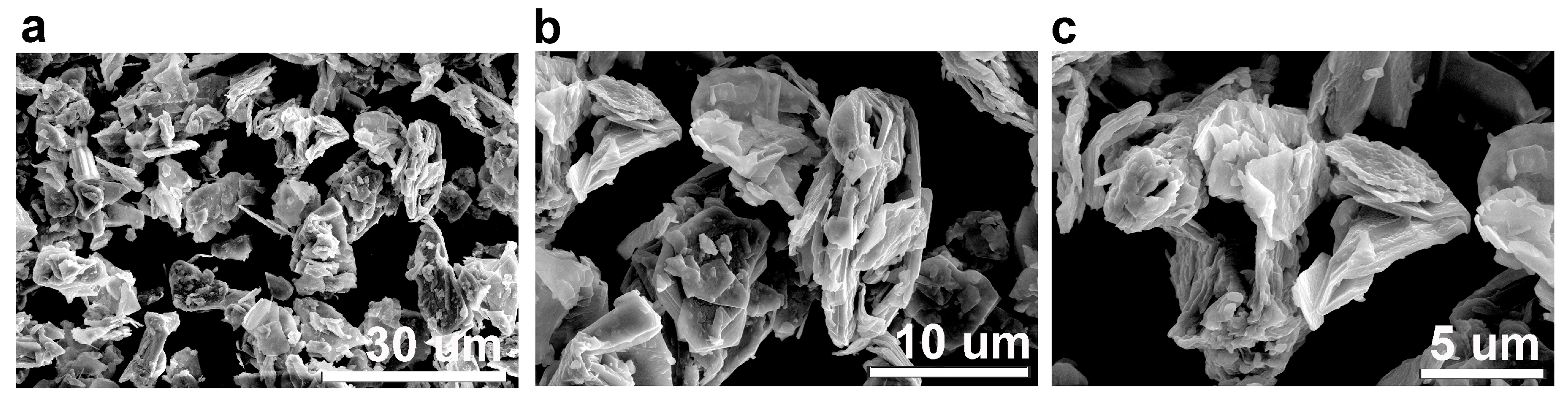
3.1.4. CBN/PVP Morphology
3.1.5. SEM Analysis
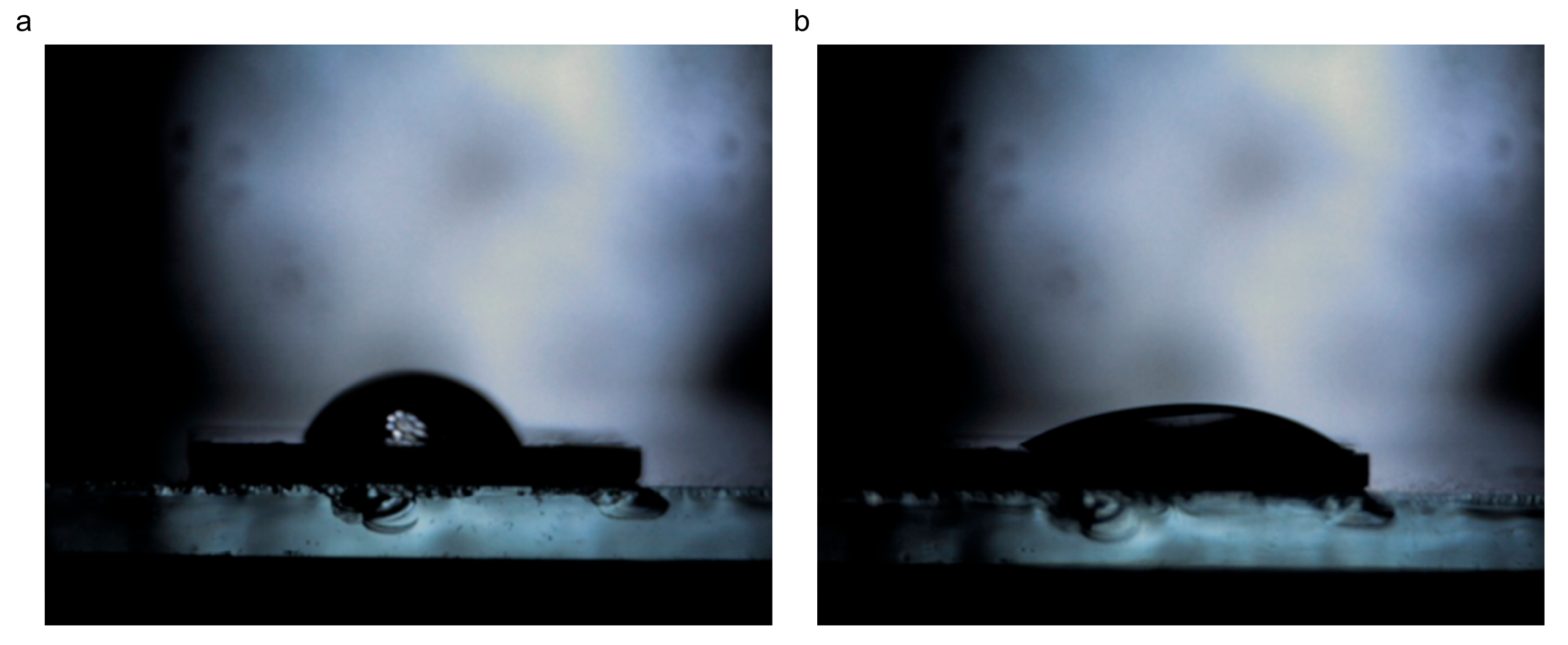
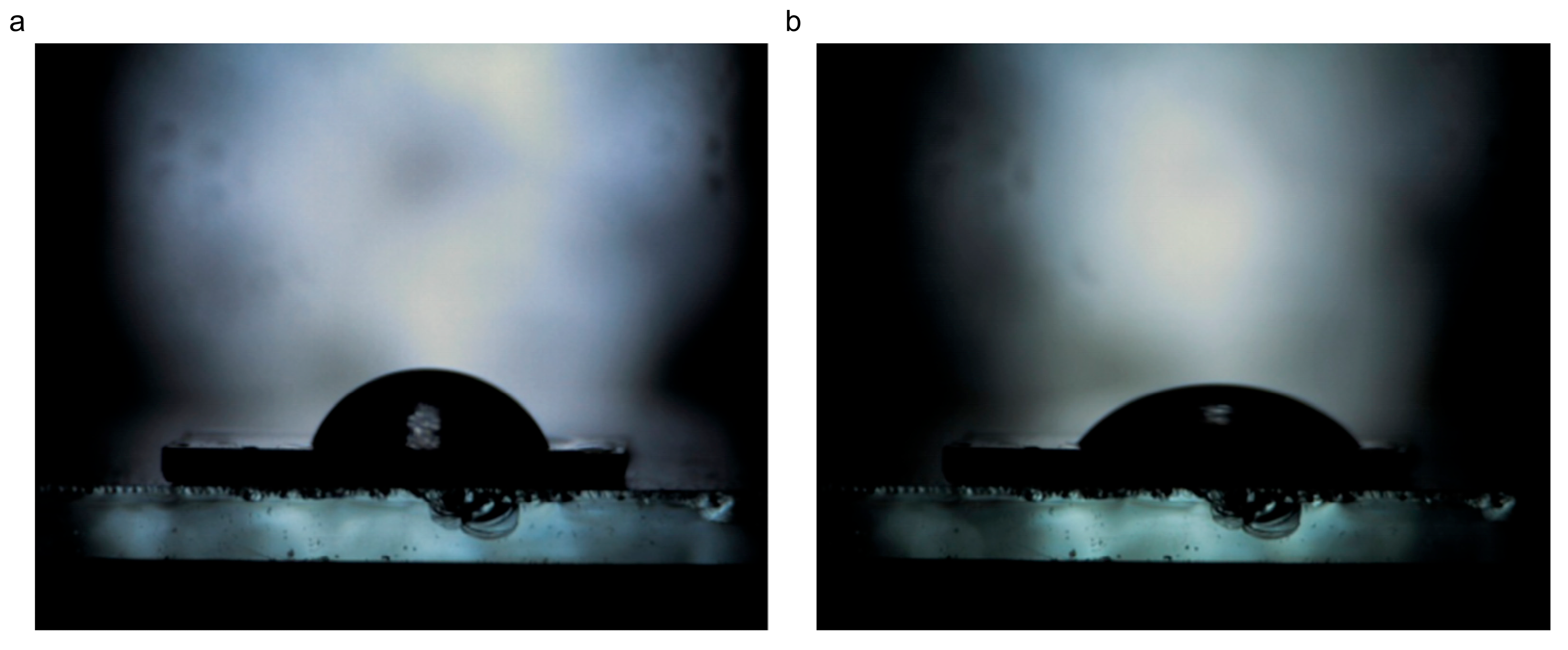
3.1.6. Wettability
3.2. Cytotoxic Effects of CBN/PVP Nanoparticles on Cancer Cells
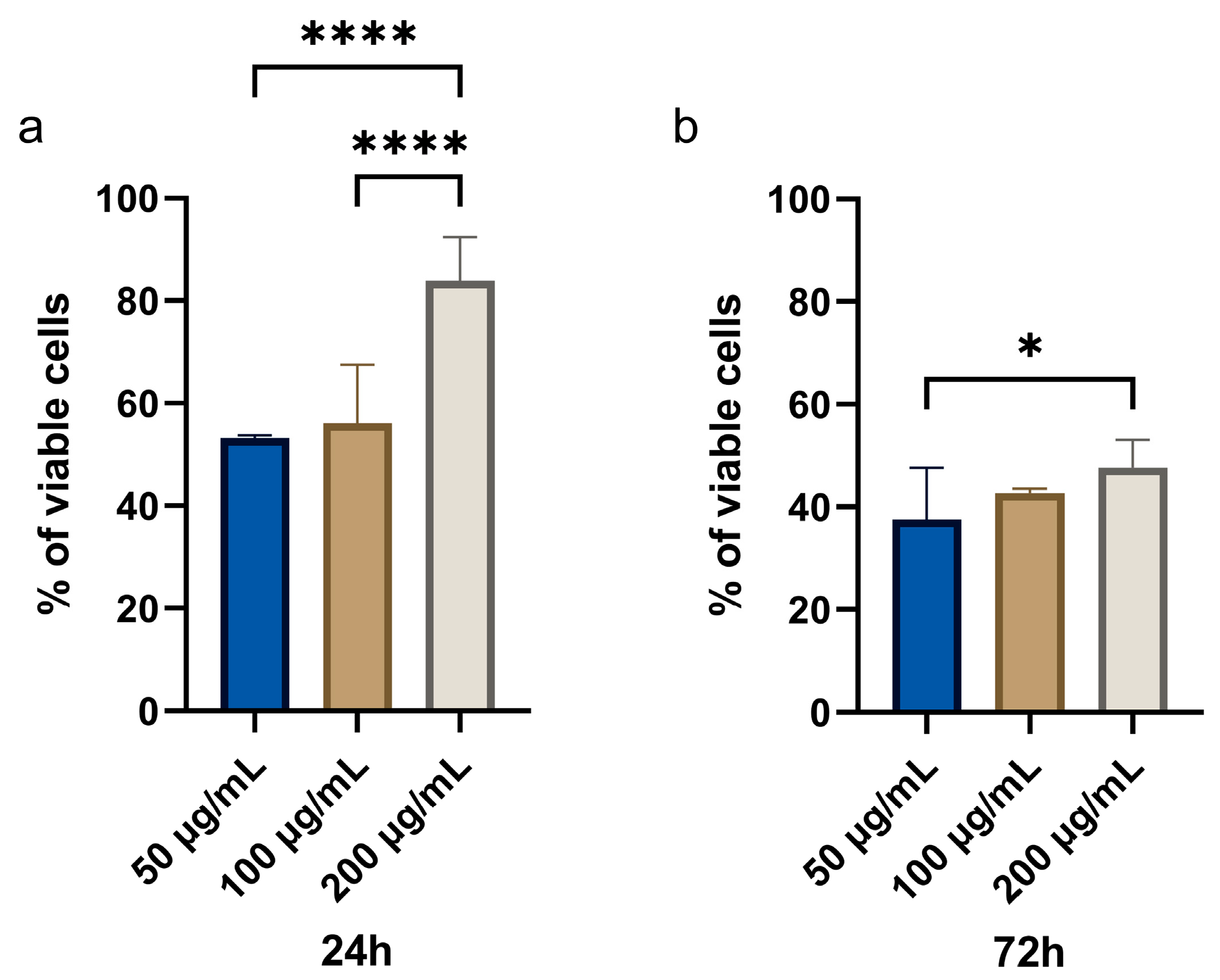
3.2.1. MTT Assay
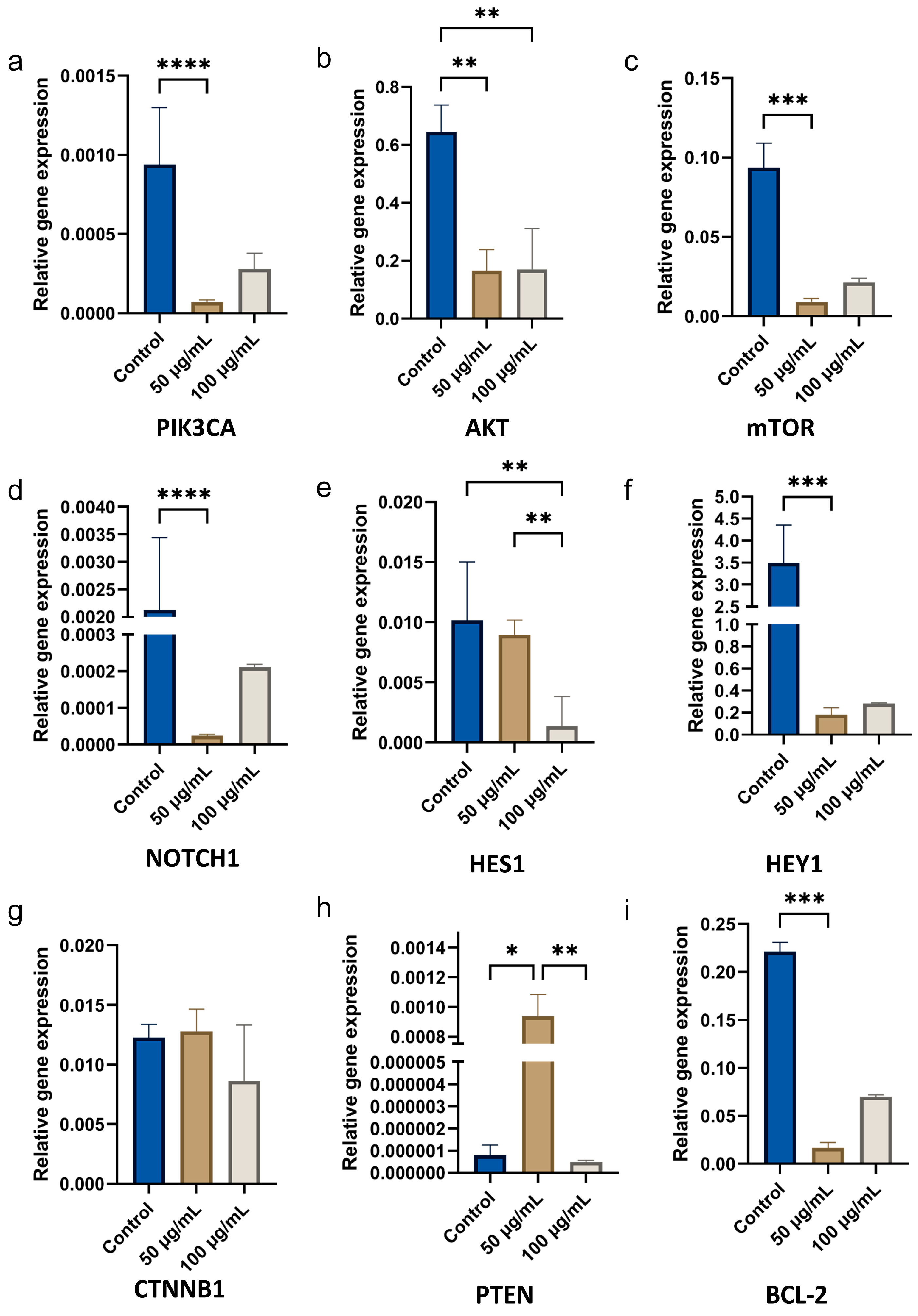
3.2.2. Gene Expression in OSCC Cells
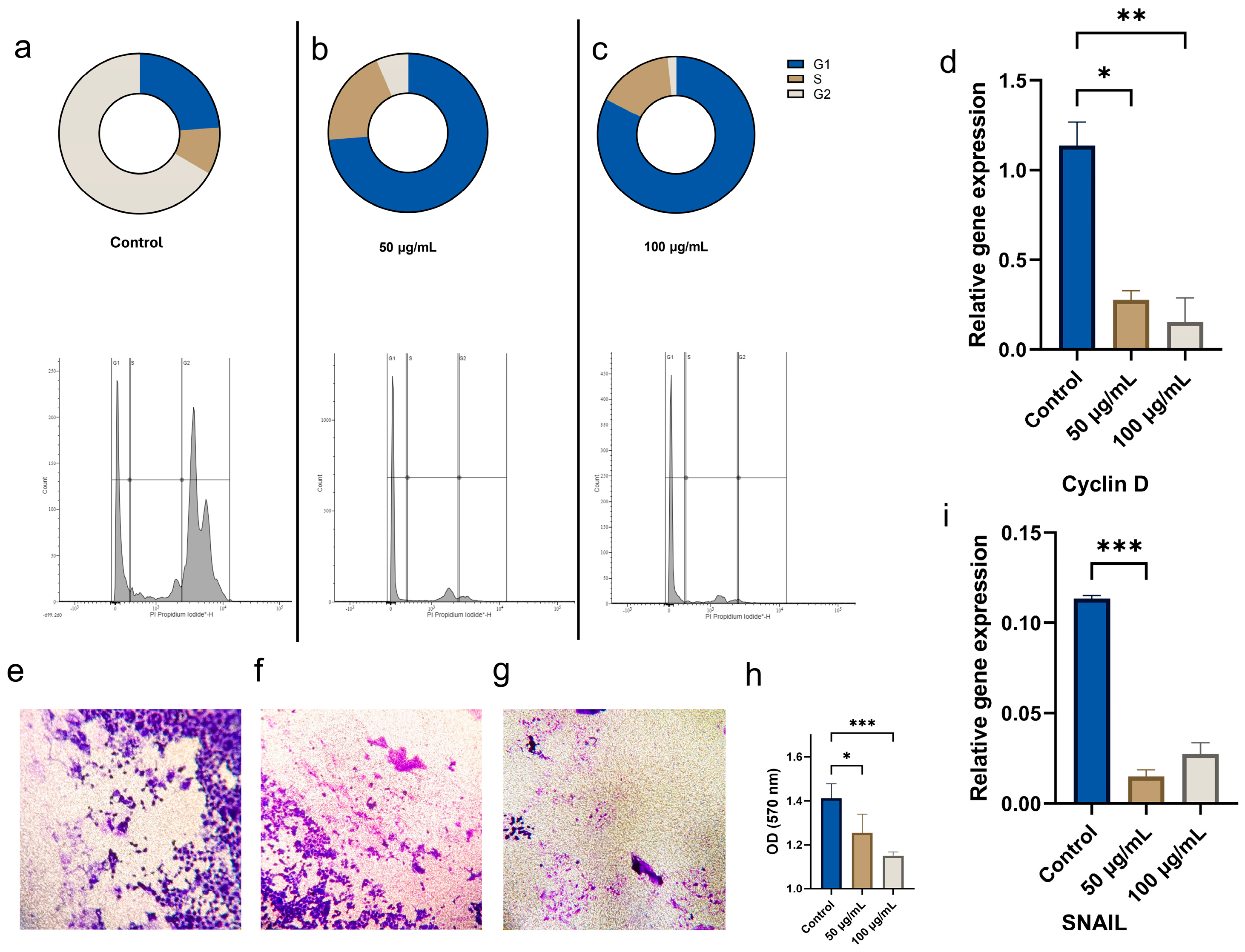
3.2.3. Effects of CBN/PVP Nanoparticles on Cell Cycle and Invasion of Oral Cancer Cells
3.2.4. Effects of CBN/PVP Nanoparticles on Reactive Oxygen Species (ROS) Production in OSCC Cells
3.2.5. Nanoparticle Uptake
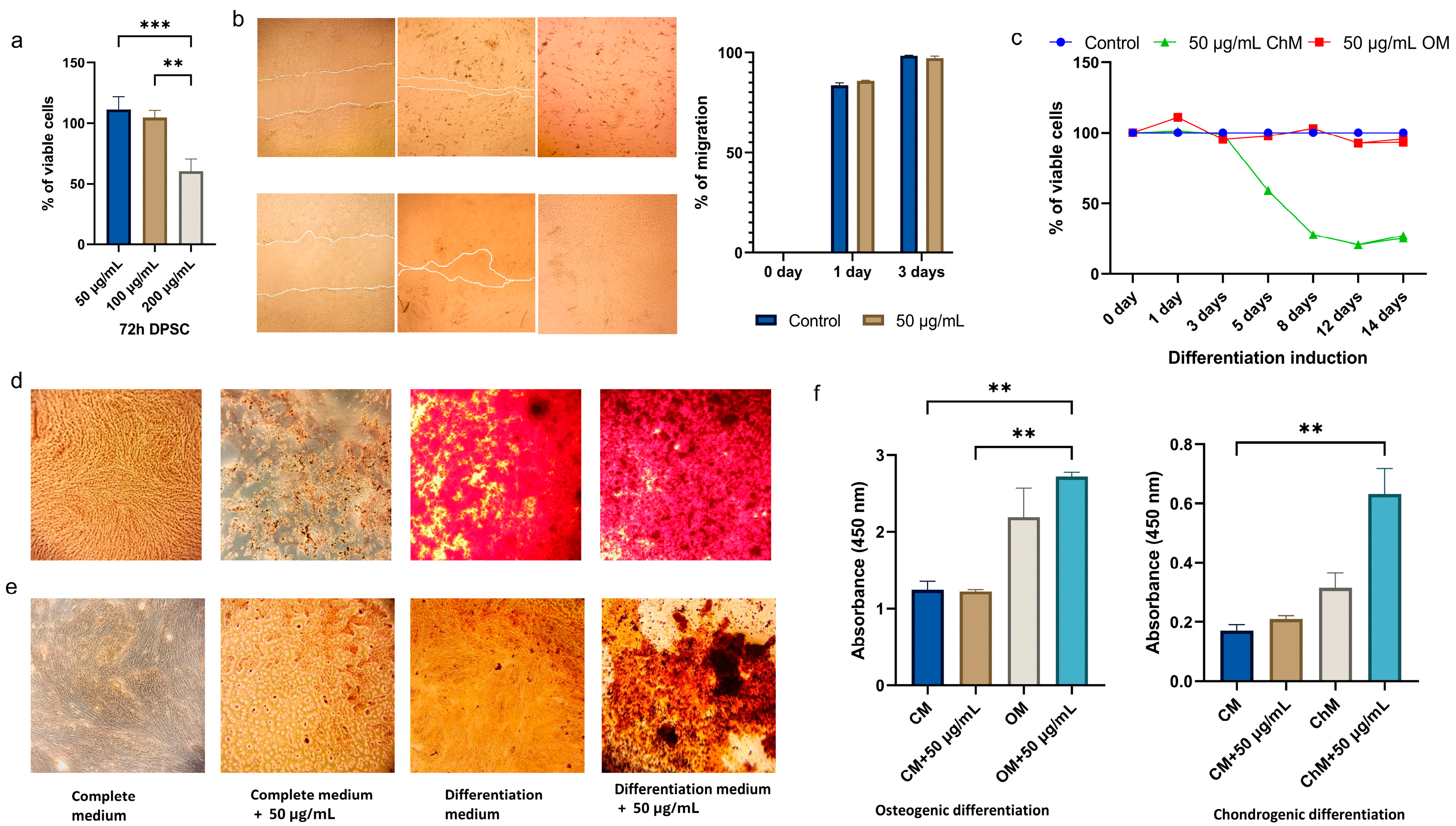
3.3. Effects of CBN/PVP Nanoparticles on DPSC Viability and Osteogenic/Chondrogenic Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Chen, X.; Luo, H.; Meng, C.; Zhu, D. Cancer Stem Cells of Head and Neck Squamous Cell Carcinoma; Distance towards Clinical Application: A Systematic Review of Literature. Am. J. Cancer Res. 2023, 13, 4315–4345. [Google Scholar] [PubMed]
- Liu, Y.; Yang, M.; Luo, J.; Zhou, H. Radiotherapy Targeting Cancer Stem Cells “Awakens” Them to Induce Tumour Relapse and Metastasis in Oral Cancer. Int. J. Oral. Sci. 2020, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Imbesi Bellantoni, M.; Picciolo, G.; Pirrotta, I.; Irrera, N.; Vaccaro, M.; Vaccaro, F.; Squadrito, F.; Pallio, G. Oral Cavity Squamous Cell Carcinoma: An Update of the Pharmacological Treatment. Biomedicines 2023, 11, 1112. [Google Scholar] [CrossRef]
- Murray Walker, D.; Boey, G.; McDonald, L.A. The Pathology of Oral Cancer. Pathology 2003, 35, 376–383. [Google Scholar] [CrossRef]
- Ou, L.; Sun, T.; Liu, M.; Zhang, Y.; Zhou, Z.; Zhan, X.; Lu, L.; Zhao, Q.; Lai, R.; Shao, L. Efficient miRNA Inhibitor Delivery with Graphene Oxide-Polyethylenimine to Inhibit Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2020, 15, 1569–1583. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Zhi, X.; Fang, H.; Bao, C.; Shen, G.; Zhang, J.; Wang, K.; Guo, S.; Wan, T.; Cui, D. The Immunotoxicity of Graphene Oxides and the Effect of PVP-Coating. Biomaterials 2013, 34, 5254–5261. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, H.-Y.; Wang, J.-Q.; Wu, G.-D.; Wang, L. Graphene Oxide and Reduced Graphene Oxide Exhibit Cardiotoxicity Through the Regulation of Lipid Peroxidation, Oxidative Stress, and Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2021, 9, 616888. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Z.; Jiang, F.; Zeng, B.; Huang, M.; Yu, D. Cellular Behaviours of Bone Marrow-Derived Mesenchymal Stem Cells towards Pristine Graphene Oxide Nanosheets. Cell Prolif. 2017, 50, e12367. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene Oxide Loaded with Tumor-Targeted Peptide and Anti-Cancer Drugs for Cancer Target Therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Sekar, R.; Dhayasankar, P.S.; Ali, E.M.; Abdelsalam, S.A.; Balaraman, S.; Chellappan, B.V.; Metwally, A.M.; Abdallah, B.M. PI3K/AKT Signaling Pathway Mediated Autophagy in Oral Carcinoma—A Comprehensive Review. Int. J. Med. Sci. 2024, 21, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dong, X.; Huang, D.C.S.; Xu, P.; Zhao, Q.; Chen, B. Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers 2023, 15, 4957. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Huang, C.; Li, Q.; Kazi, S.A.; Byers, L.A.; Wang, J.; Johnson, F.M.; Frederick, M.J. NOTCH1 Signaling in Head and Neck Squamous Cell Carcinoma. Cells 2020, 9, 2677. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Garcia-Godoy, F. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. J. Funct. Biomater. 2024, 15, 241. [Google Scholar] [CrossRef]
- Vukovic, M.; Lazarevic, M.; Mitic, D.; Jaksic Karisik, M.; Ilic, B.; Andric, M.; Jevtic, B.; Roganovic, J.; Milasin, J. Acetylsalicylic-Acid (ASA) Regulation of Osteo/Odontogenic Differentiation and Proliferation of Human Dental Pulp Stem Cells (DPSCs) in Vitro. Arch. Oral Biol. 2022, 144, 105564. [Google Scholar] [CrossRef]
- Herendija, E.; Jakšić Karišik, M.; Milašin, J.; Lazarević, M.; Ignjatović, N. Anti-Cancer Activities of Nano Amorphous Calcium Phosphates toward Premalignant and Oral Cancer Cells. Biomedicines 2024, 12, 1499. [Google Scholar] [CrossRef]
- Sorce, S.; Krause, K.-H. NOX Enzymes in the Central Nervous System: From Signaling to Disease. Antioxid. Redox. Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiao, X.; Qiu, Y.; Zhang, L.; Zhang, X. Comparison of the Characteristic Properties of Reduced Graphene Oxides Synthesized from Natural Graphites with Different Graphitization Degrees. RSC Adv. 2017, 7, 52337–52344. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Kozbial, A.; Shenoy, G.; Zhou, F.; McGinley, R.; Ireland, P.; Morganstein, B.; Kunkel, A.; Surwade, S.P.; et al. Effect of Airborne Contaminants on the Wettability of Supported Graphene and Graphite. Nat. Mater. 2013, 12, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Flower-Like Morphology of Naphthalene Diimides Containing tetra-L- and D-Alanine—Shaikh—2020—ChemistrySelect—Wiley Online Library. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.202003108 (accessed on 3 March 2025).
- Kozbial, A.; Trouba, C.; Liu, H.; Li, L. Characterization of the Intrinsic Water Wettability of Graphite Using Contact Angle Measurements: Effect of Defects on Static and Dynamic Contact Angles. Langmuir 2017, 33, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Pierfelice, T.V.; Lazarevic, M.; Mitic, D.; Nikolic, N.; Radunovic, M.; Iezzi, G.; Piattelli, A.; Milasin, J. Red Light and 5% Aminolaevulinic Acid (5%) Inhibit Proliferation and Migration of Dysplastic Oral Keratinocytes via ROS Production: An In Vitro Study. Gels 2023, 9, 604. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghahramani, Y.; Azhdari, R.; Yousefi, K.; Gholami, A.; Fallahi Nezhad, F.; Vijayakameswara Rao, N.; Omidifar, N.; Chiang, W.-H. Antiproliferative and Apoptotic Effects of Graphene Oxide @AlFu MOF Based Saponin Natural Product on OSCC Line. Pharmaceuticals 2022, 15, 1137. [Google Scholar] [CrossRef]
- Li, R.; Gao, R.; Zhao, Y.; Zhang, F.; Wang, X.; Li, B.; Wang, L.; Ma, L.; Du, J. pH-Responsive Graphene Oxide Loaded with Targeted Peptide and Anticancer Drug for OSCC Therapy. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Shen, J.; Dong, J.; Shao, F.; Zhao, J.; Gong, L.; Wang, H.; Chen, W.; Zhang, Y.; Cai, Y. Graphene Oxide Induces Autophagy and Apoptosis via the ROS-Dependent AMPK/mTOR/ULK-1 Pathway in Colorectal Cancer Cells. Nanomedicine 2022, 17, 591–605. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Qiao, S.; Zhang, W. Carbon-Based Nanomaterials for Bone and Cartilage Regeneration: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4718–4735. [Google Scholar] [CrossRef]
- Moharil, R.B.; Khandekar, S.; Dive, A.; Bodhade, A. Cyclin D1 in Oral Premalignant Lesions and Oral Squamous Cell Carcinoma: An Immunohistochemical Study. J. Oral Maxillofac. Pathol. 2020, 24, 397. [Google Scholar] [CrossRef] [PubMed]
- Masamha, C.P.; Benbrook, D.M. Cyclin D1 Degradation Is Sufficient to Induce G1 Cell Cycle Arrest despite Constitutive Expression of Cyclin E2 in Ovarian Cancer Cells. Cancer Res. 2009, 69, 6565–6572. [Google Scholar] [CrossRef]
- Schwock, J.; Bradley, G.; Ho, J.C.; Perez-Ordonez, B.; Hedley, D.W.; Irish, J.C.; Geddie, W.R. SNAI1 Expression and the Mesenchymal Phenotype: An Immunohistochemical Study Performed on 46 Cases of Oral Squamous Cell Carcinoma. BMC Clin. Pathol. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, M.; Milosevic, M.; Jelovac, D.; Milenkovic, S.; Tepavcevic, Z.; Baldan, F.; Suboticki, T.; Toljic, B.; Trisic, D.; Dragovic, M.; et al. Marked Epithelial to Mesenchymal Transition in Surgical Margins of Oral Cancer-an in Vitro Study. Oncol. Lett. 2020, 19, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Ghosh, S.; Ali, A.; Pal, M.; Chakrabarti, P. Inhibition of Microtubule Assembly and Cytotoxic Effect of Graphene Oxide on Human Colorectal Carcinoma Cell HCT116. Arch. Biochem. Biophys. 2021, 708, 108940. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Salaheldin, T.A.; Ramadan, M.A.; Farroh, K.Y.; Abdallah, Z.F.; Youssef, T. Synthesis, Characterization and Cytotoxic Evaluation of Graphene Oxide Nanosheets: In Vitro Liver Cancer Model. Asian Pac. J. Cancer Prev. 2017, 18, 955–961. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; He, Y.; Liu, X.; Wang, S.; Ma, C.; Tian, X.; Wang, J.; Wu, X. Graphene Oxide Inhibits Cell Migration and Invasion by Destroying Actin Cytoskeleton in Cervical Cancer Cells. Aging 2020, 12, 17625–17633. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, J.; Wu, J.; Yin, Q.; Liang, H.; Chen, A.; Shao, L. Graphene Oxide and Reduced Graphene Oxide Induced Neural Pheochromocytoma-Derived PC12 Cell Lines Apoptosis and Cell Cycle Alterations via the ERK Signaling Pathways. Int. J. Nanomed. 2017, 12, 5501–5510. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.-J.; Gao, X.; Nie, G. Deciphering the Underlying Mechanisms of Oxidation-State Dependent Cytotoxicity of Graphene Oxide on Mammalian Cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive Oxygen Species (ROS) Control the Expression of Bcl-2 Family Proteins by Regulating Their Phosphorylation and Ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein Corona-Mediated Mitigation of Cytotoxicity of Graphene Oxide. ACS Nano. 2011, 5, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Rani, A.; Greenlaw, R.; Smith, R.A.; Galustian, C. HES1 in Immunity and Cancer. Cytokine Growth Factor Rev. 2016, 30, 113–117. [Google Scholar] [CrossRef]
- Figarol, A.; Pourchez, J.; Boudard, D.; Forest, V.; Akono, C.; Tulliani, J.-M.; Lecompte, J.-P.; Cottier, M.; Bernache-Assollant, D.; Grosseau, P. In Vitro Toxicity of Carbon Nanotubes, Nano-Graphite and Carbon Black, Similar Impacts of Acid Functionalization. Toxicol. In Vitro 2015, 30, 476–485. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; She, L.; Sun, L.; Ma, Z.; Chen, M.; Hu, P.; Wang, D.; Yang, F. Immunotoxicity Assessment of Ordered Mesoporous Carbon Nanoparticles Modified with PVP/PEG. Colloids Surf. B 2018, 171, 485–493. [Google Scholar] [CrossRef]
- Rahimi, S.; Chen, Y.; Zareian, M.; Pandit, S.; Mijakovic, I. Cellular and Subcellular Interactions of Graphene-Based Materials with Cancerous and Non-Cancerous Cells. Adv. Drug Deliv. Rev. 2022, 189, 114467. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, B.-J.; Jeon, R.-H.; Jang, S.-J.; Son, Y.-B.; Lee, S.-L.; Rho, G.-J.; Kim, S.-J.; Lee, W.-J. Alteration of Apoptosis during Differentiation in Human Dental Pulp-Derived Mesenchymal Stem Cell. J. Anim. Reprod. Biotechnol. 2019, 34, 2–9. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced Graphene Oxide Coating Enhances Osteogenic Differentiation of Human Mesenchymal Stem Cells on Ti Surfaces. Biomater. Res. 2021, 25, 4. [Google Scholar] [CrossRef]
- Milosevic, M.; Lazarevic, M.; Toljic, B.; Petrovic, M.; Vukadinovic, M.; Jezdic, Z.; Anicic, B.; Jelovac, D.; Jovanovic, S.; Milasin, J. Basal Cell Carcinoma Stem Cells Exhibit Osteogenic and Chondrogenic Differentiation Potential. Biocell 2021, 45, 1543–1550. [Google Scholar] [CrossRef]
- Gupta, A.; Woods, M.D.; Illingworth, K.D.; Niemeier, R.; Schafer, I.; Cady, C.; Filip, P.; El-Amin, S.F., III. Single Walled Carbon Nanotube Composites for Bone Tissue Engineering. J. Orthop. Res. 2013, 31, 1374–1381. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaksic Karisik, M.; Jović Orsini, N.; Carkic, J.; Lazarevic, M.; Mitić, D.; Jokanovic, B.; Jokanović, V.; Milasin, J. A Carbon-Based Nanomaterial with Dichotomous Effects: Antineoplastic on Oral Cancer Cells and Osteoinductive/Chondroinductive on Dental Pulp Stem Cells. J. Funct. Biomater. 2025, 16, 109. https://doi.org/10.3390/jfb16030109
Jaksic Karisik M, Jović Orsini N, Carkic J, Lazarevic M, Mitić D, Jokanovic B, Jokanović V, Milasin J. A Carbon-Based Nanomaterial with Dichotomous Effects: Antineoplastic on Oral Cancer Cells and Osteoinductive/Chondroinductive on Dental Pulp Stem Cells. Journal of Functional Biomaterials. 2025; 16(3):109. https://doi.org/10.3390/jfb16030109
Chicago/Turabian StyleJaksic Karisik, Milica, Nataša Jović Orsini, Jelena Carkic, Milos Lazarevic, Dijana Mitić, Bojan Jokanovic, Vukoman Jokanović, and Jelena Milasin. 2025. "A Carbon-Based Nanomaterial with Dichotomous Effects: Antineoplastic on Oral Cancer Cells and Osteoinductive/Chondroinductive on Dental Pulp Stem Cells" Journal of Functional Biomaterials 16, no. 3: 109. https://doi.org/10.3390/jfb16030109
APA StyleJaksic Karisik, M., Jović Orsini, N., Carkic, J., Lazarevic, M., Mitić, D., Jokanovic, B., Jokanović, V., & Milasin, J. (2025). A Carbon-Based Nanomaterial with Dichotomous Effects: Antineoplastic on Oral Cancer Cells and Osteoinductive/Chondroinductive on Dental Pulp Stem Cells. Journal of Functional Biomaterials, 16(3), 109. https://doi.org/10.3390/jfb16030109