Designing Superhydrophilic 3D Porous Surfaces on Polyetherketoneketone Surfaces to Promote Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
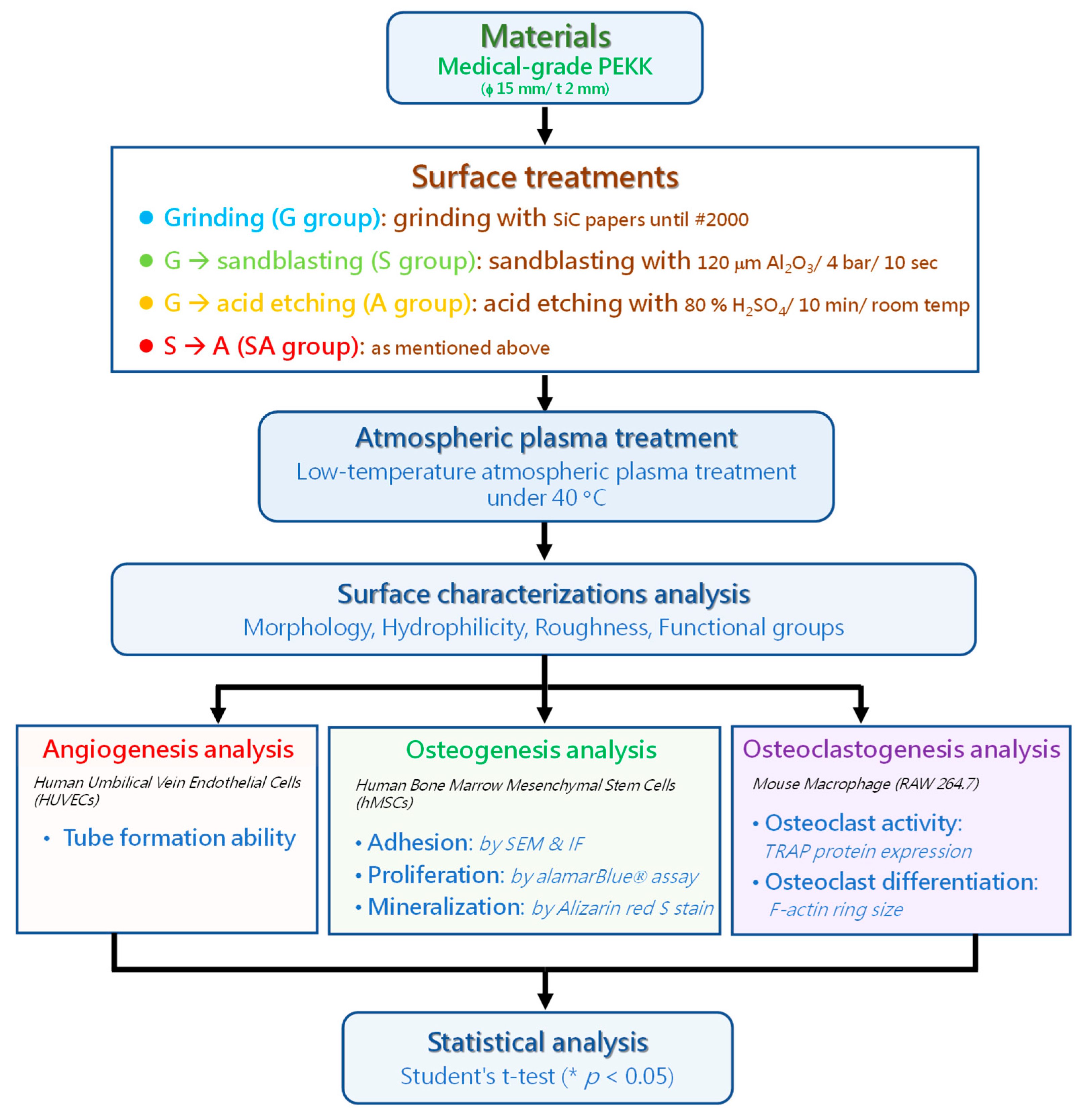
2.1. Materials’ Preparations and Surface Treatments
2.2. Surface Characterization Analysis
2.3. Angiogenesis Analysis
Tube Formation Ability
2.4. Bone Remodeling Analysis
2.4.1. Osteoblastic Response
2.4.2. Osteoclastic Activity
2.5. Statistical Methods
3. Results
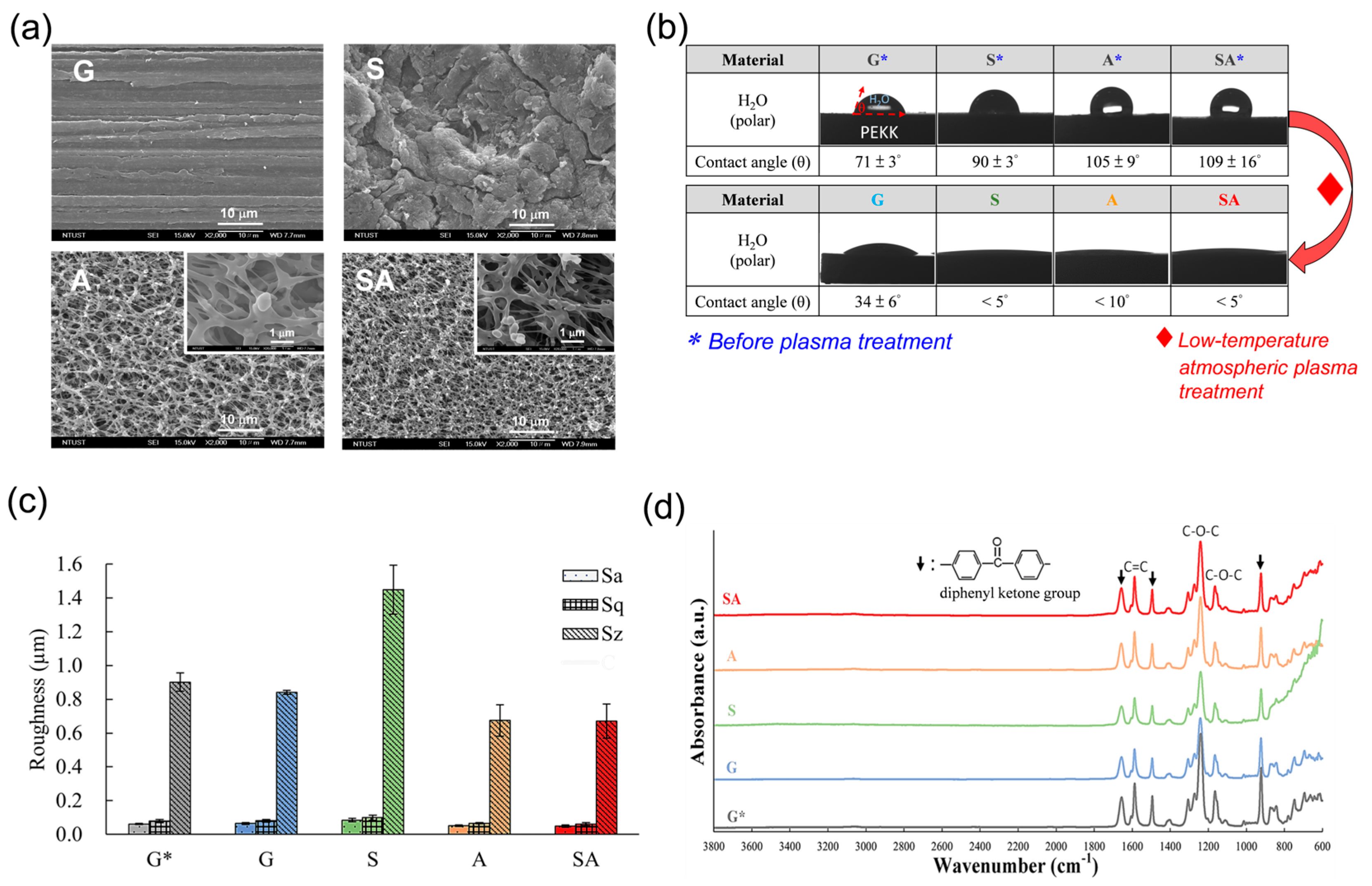
3.1. Surface Characterization
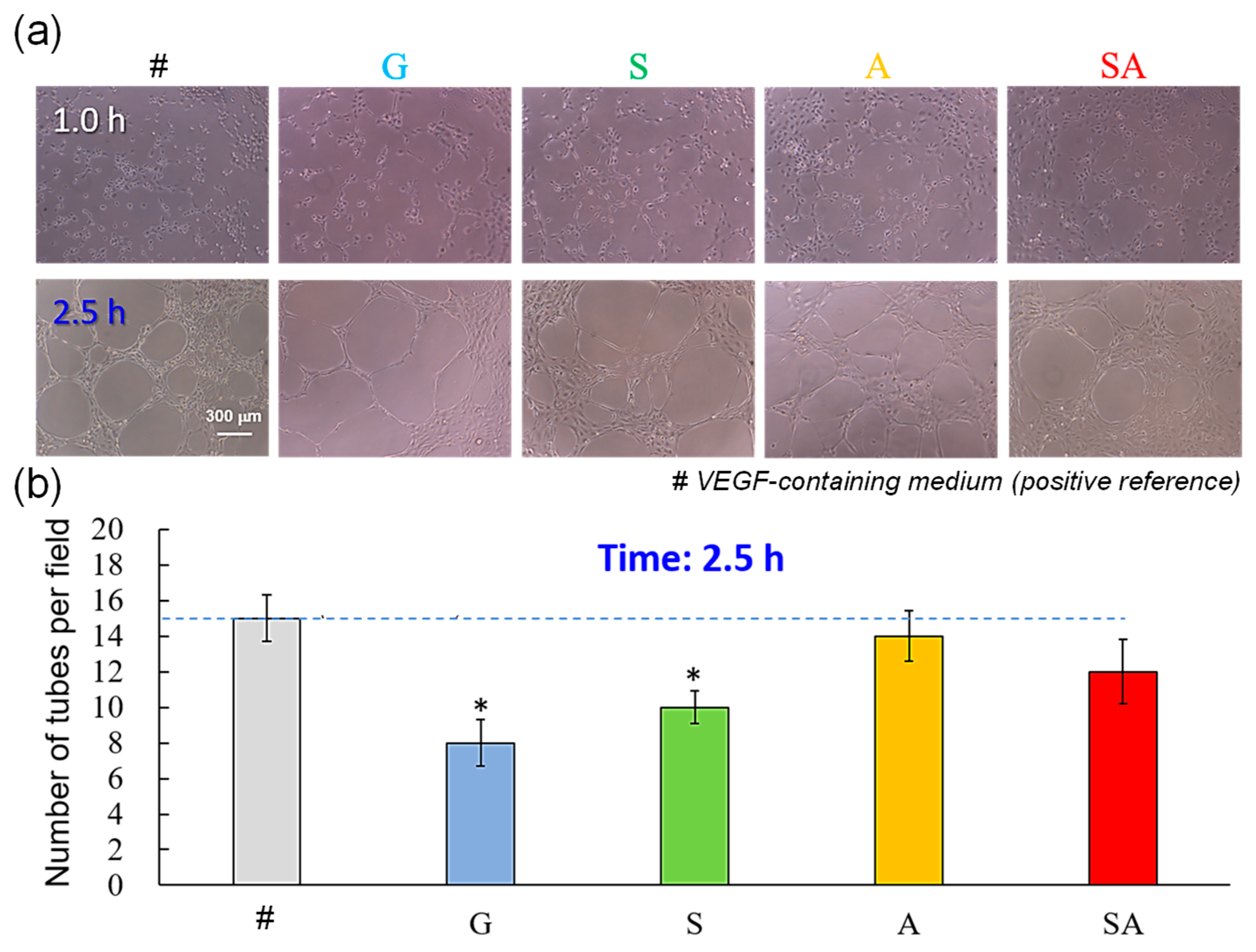
3.2. Angiogenesis
3.3. Osteogenesis
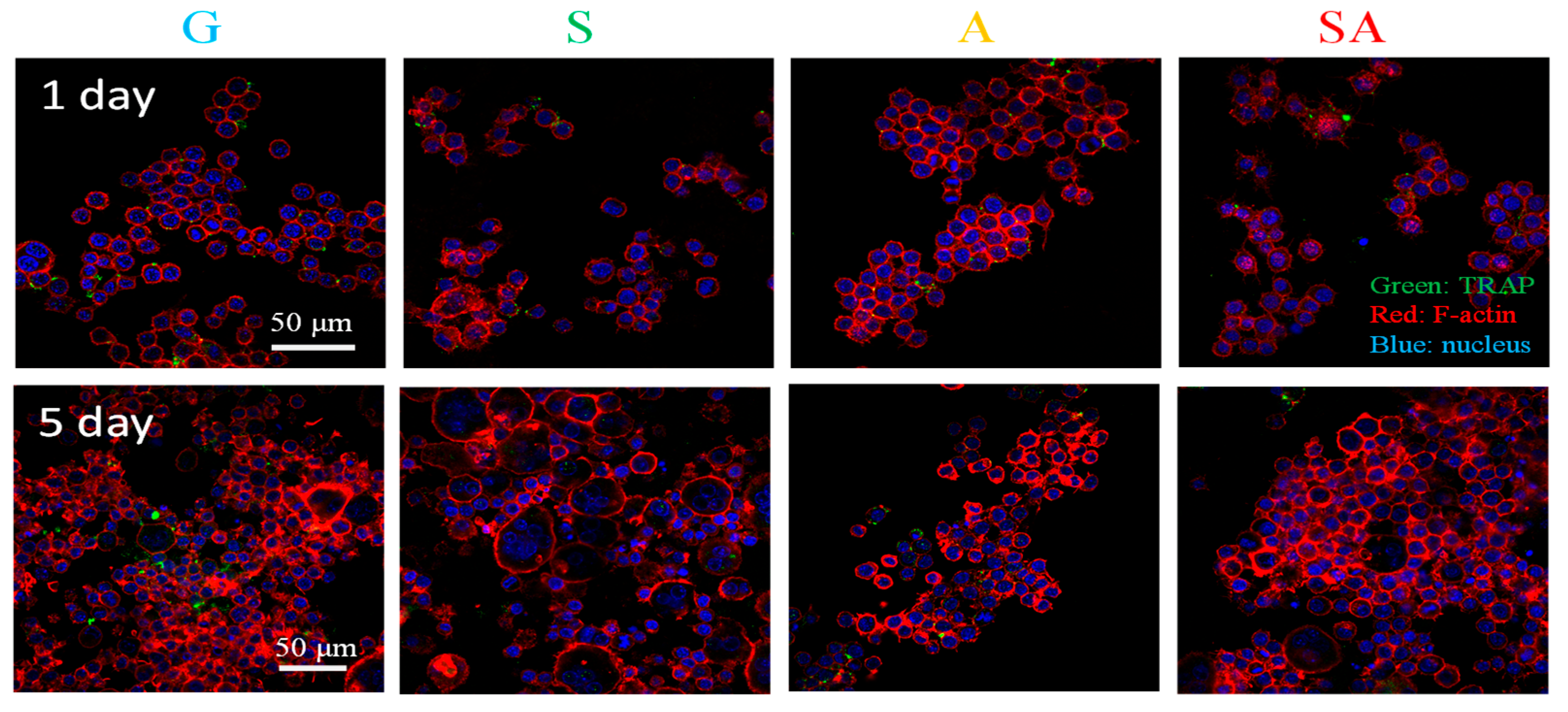
3.4. Osteoclast Genesis
4. Discussion
4.1. Surface Characterization
4.1.1. Roughness
4.1.2. Porosity
4.1.3. Hydrophilicity
4.2. Angiogenesis
4.3. Osteogenesis
4.4. Osteoclastogenesis
4.5. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Chen, Y.; Ding, J.; Yu, L. Blending strategy to modify PEEK-based orthopedic implants. Compos. B Eng. 2023, 250, 110427. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Zheng, W.; Huang, D.; Zhang, Y.; He, H.; Xiong, S.; Li, C.; Wang, C.; Lin, H.; et al. Immunological mechanism of Sr/Cu ion synergistically promote implant osseointegration. Mater. Today Adv. 2023, 20, 100435. [Google Scholar] [CrossRef]
- Peng, T.Y.; Lin, D.J.; Mine, Y.; Tsai, C.Y.; Li, P.J.; Shih, Y.H.; Chiu, K.-C.; Wang, T.-H.; Hsia, S.-M.; Shieh, T.-M. Biofilm formation on the surface of (poly)ether-ether-ketone and in vitro antimicrobial efficacy of photodynamic therapy on peri-implant mucositis. Polymers 2021, 13, 940. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Mei, S.; Wang, F.; Qian, J.; Xie, D.; Zhao, J.; Yang, L.; Wu, Z.; Wei, J. Implantable PEKK/tantalum microparticles composite with improved surface performances for regulating cell behaviors, promoting bone formation and osseointegration. Bioact. Mater. 2021, 6, 928–940. [Google Scholar] [CrossRef]
- Wei, W.; Yu, Q.; Yang, R.; Zhang, X.; Li, W.; Zhao, J. Germanium/niobium/polyetherketoneketone ternary biocomposite with crisscross micro-nano patterns created by femtosecond laser for enhancing bone-bonding and resisting infection. Mater. Des. 2023, 234, 112337. [Google Scholar] [CrossRef]
- Suphangul, S.; Rokaya, D.; Kanchanasobhana, C.; Rungsiyakull, P.; Chaijareenont, P. PEEK biomaterial in long-term provisional implant restorations: A review. J. Funct. Biomater. 2022, 13, 33. [Google Scholar] [CrossRef]
- Shu, T.; Wang, X.; Li, M.; Ma, S.; Cao, J.; Sun, G.; Lai, T.; Liu, S.; Li, A.; Qu, Z.; et al. Nanoscaled titanium oxide layer provokes quick osseointegration on 3D-printed dental implants: A domino effect induced by hydrophilic surface. ACS Nano 2024, 18, 783–797. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Perrin, D.; Ahossi, V.; Magnin, G.; Bernard, J.P. Biological properties of acid etched titanium implants: Effect of sandblasting on bone anchorage. J. Biomed. Mater. Res. B 2004, 68, 149–159. [Google Scholar] [CrossRef]
- Komatsu, K.; Matsuura, T.; Cheng, J.; Kido, D.; Park, W.; Ogawa, T. Nanofeatured surfaces in dental implants: Contemporary insights and impending challenges. Int. J. Implant Dent. 2024, 10, 34. [Google Scholar] [CrossRef]
- Hong, S.O.; Pyo, J.Y.; On, S.W.; Seo, J.Y.; Choi, J.Y. The Biocompatibility and the effect of titanium and PEKK on the osseointegration of customized facial implants. Material 2024, 17, 4435. [Google Scholar] [CrossRef]
- Ourahmoune, R.; Salvia, M.; Mathia, T.G.; Mesrati, N. Surface morphology and wettability of sandblasted PEEK and its composites. Scanning Microsc. 2014, 36, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Wen, Z.; Bao, L.; Wang, Y.; Ouyang, P.; Lu, T.; Li, J.; Li, J.; Jiang, M.; Li, N.; et al. New synthetic PEKK/bioceramic hybrids and their surface sulfonation counterparts have increased cellular osteogenic capacity and promoted osseointegration. Mater. Des. 2022, 224, 111283. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, Y.; Lin, H.; Song, Y.; Yang, X.; Tang, H.; Xie, E.; Hsu, T.; Yang, X.; Zhu, X.; et al. Processing and properties of bioactive surface-porous PEKK. ACS Biomater. Sci. Eng. 2016, 2, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Ma, Z.; Li, M.; Liu, R.; Ren, L.; Zhang, Y.; Pan, H.; Zhao, Y.; Yang, K. In vitro study on an antibacterial Ti–5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016, 27, 91. [Google Scholar] [CrossRef]
- Han, X.; Sharma, N.; Xu, Z.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F.; Thieringer, F.M.; Spintzyk, S. An in vitro study of osteoblast response on fused-filament fabrication 3D printed PEEK for dental and cranio-maxillofacial implants. J. Clin. Med. 2019, 8, 771. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Wei, J.; Tang, Z.; Wei, S. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite composite. Int. J. Nanomed. 2015, 10, 1425–1447. [Google Scholar]
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 2000, 49, 155–166. [Google Scholar] [CrossRef]
- Adhitya, K.; Mustika, T.; Manawan, M.; Ulfah, I.M.; Hanafi, R.; Setyadi, I.; Suryadi, A.; Hidayat, A.; Wibisono, M.; Sah, J.; et al. Optimizing surface properties in pure titanium for dental implants: A crystallographic analysis of sandblasting and acid-etching techniques. Powder Diffr. 2024, 39, 206–216. [Google Scholar] [CrossRef]
- Yang, W.E.; Huang, H.H. TiO2 nanonetwork on rough Ti enhanced osteogenesis in vitro and in vivo. J. Dent. Res. 2021, 100, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Torstrick, F.B.; Lin, A.S.P.; Safranski, D.L.; Potter, D.; Sulchek, T.; Lee, C.S.D.; Gall, K.; Guldberg, R.E. Effects of surface topography and chemistry on polyether-ether-ketone (PEEK) and titanium osseointegration. Spine 2020, 45, e417–e424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.W.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterial 2013, 34, 9264–9277. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterial 2018, 170, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef] [PubMed]
- Baek, I.; Lim, C.M.; Park, K.Y.; Ryu, B.K. Enhanced metal coating adhesion by surface modification of 3D printed PEKKs. Coatings 2022, 12, 854. [Google Scholar] [CrossRef]
- Wan, T.; Jiao, Z.; Guo, M.; Wang, Z.; Wan, Y.; Lin, K.; Zhang, P. Gaseous sulfur trioxide induced controllable sulfonation promoting biomineralization and osseointegration of polyetheretherketone implants. Bioact. Mater. 2020, 5, 1004–1017. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Wang, Y.; Deng, J.; Wang, X.; Wang, Q.; Liu, Y.; Ding, J.; Yu, L. Polyetheretherketone implants with hierarchical porous structure for boosted osseointegration. Biomater. Res. 2023, 27, 61. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, J.; Liu, X.; Sun, S.; Wu, J. Effects of combined modification of sulfonation, oxygen plasma and silane on the bond strength of PEEK to resin. Dent. Mater. 2024, 40, e1–e11. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, J.; Pei, X.; Sun, S.; Cai, J.; Liu, Y.; Wang, Y.; Zheng, L.; Zhou, H. Ultrasound-driven electrical stimulation based on 3D hierarchical porous piezoelectric nanofiber-aerogel scaffold promotes bone defect repair. Chem. Eng. J. 2023, 470, 144305. [Google Scholar] [CrossRef]
- Guo, C.; Lu, R.; Wang, X.; Chen, S. Antibacterial activity, bio-compatibility and osteogenic differentiation of graphene oxide coating on 3D-network poly-ether-ether-ketone for orthopaedic implants. J. Mater. Sci. Mater. Med. 2021, 32, 135. [Google Scholar] [CrossRef]
- Ma, R.; Wang, J.; Li, C.; Ma, K.; Wei, J.; Yang, P.; Guo, D.; Wang, K.; Wang, W. Effects of different sulfonation times and post-treatment methods on the characterization and cytocompatibility of sulfonated PEEK. J. Biomater. Appl. 2020, 35, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Omrani, M.; Kumar, H.; Mohamed, M.G.; Golovin, K.S.; Milani, A.; Hadjizadeh, A.; Kim, K. Polyether ether ketone surface modification with plasma and gelatin for enhancing cell attachment. J. Biomed. Mater. Res. 2021, 109, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, K.; Zhang, Y. A review on research progress in plasma-controlled superwetting surface structure and properties. Polymers 2022, 14, 3759. [Google Scholar] [CrossRef]
- Gu, X.; Sun, X.; Sun, Y.; Wang, J.; Liu, Y.; Yu, K.; Wang, Y.; Zhou, Y. Bioinspired modifications of PEEK implants for bone tissue Engineering. Front. Bioeng. Biotechnol. 2021, 8, 631616. [Google Scholar] [CrossRef]
- Heinl, P.; Müller, L.; Korner, C.; Singer, R.F.; Müller, F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Q.; Li, C.; Wang, Y.; Chen, X. VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget 2017, 8, 77020–77027. [Google Scholar] [CrossRef]
- Libby, J.R.; Royce, H.; Walker, S.R.; Li, L. The role of extracellular matrix in angiogenesis: Beyond adhesion and structure. Biomater. Biosyst. 2024, 15, 100097. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Yovos, J.G. Osteoclastogenesis—Current knowledge and future perspectives. J. Musculoskelet. Neuronal Interact. 2008, 8, 204–216. [Google Scholar]
- Matsubara, T.; Kinbara, M.; Maeda, T.; Yoshizawa, M.; Kokabu, S.; Takano Yamamoto, T. Regulation of osteoclast differentiation and actin ring formation by the cytolinker protein plectin. Biochem. Biophys. Res. Commun. 2017, 489, 472–476. [Google Scholar] [CrossRef]
- Sidorov, I.D.; Oleynikova, E.I.; Pudovkin, M.S.; Nizamutdinov, A.S.; Semashko, V.V.; Gafurov, M.R.; Pavlov, I.; Zobkova, Y.; Petrakova, N.; Sirotinkin, V.; et al. Spectral and kinetic characteristics of precipitated and heat-treated hydroxyapatite and tricalcium phosphate doped with europium. Opt. Mater. 2025, 159, 116609. [Google Scholar] [CrossRef]
- Escobar, M.; Souza, J.C.M.; Barra, G.M.O.; Fredel, M.C.; Ozcan, M.; Henriques, B. On the synergistic effect of sulfonic functionalization and acidic adhesive conditioning to enhance the adhesion of PEEK to resin-matrix composites. Dent. Mater. 2021, 37, 741–754. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-C.; Chen, C.-S.; Lin, K.-Y.; Huang, Y.-L.; Hsu, H.-H.; Kuo, Y.-L.; Chen, W.-C.; Huang, H.-H. Designing Superhydrophilic 3D Porous Surfaces on Polyetherketoneketone Surfaces to Promote Biocompatibility. J. Funct. Biomater. 2025, 16, 106. https://doi.org/10.3390/jfb16030106
Lin H-C, Chen C-S, Lin K-Y, Huang Y-L, Hsu H-H, Kuo Y-L, Chen W-C, Huang H-H. Designing Superhydrophilic 3D Porous Surfaces on Polyetherketoneketone Surfaces to Promote Biocompatibility. Journal of Functional Biomaterials. 2025; 16(3):106. https://doi.org/10.3390/jfb16030106
Chicago/Turabian StyleLin, Hui-Ching, Chiang-Sang Chen, Kai-Yi Lin, Ya-Lin Huang, Hao-Hsiang Hsu, Yu-Lin Kuo, Wei-Cheng Chen, and Her-Hsiung Huang. 2025. "Designing Superhydrophilic 3D Porous Surfaces on Polyetherketoneketone Surfaces to Promote Biocompatibility" Journal of Functional Biomaterials 16, no. 3: 106. https://doi.org/10.3390/jfb16030106
APA StyleLin, H.-C., Chen, C.-S., Lin, K.-Y., Huang, Y.-L., Hsu, H.-H., Kuo, Y.-L., Chen, W.-C., & Huang, H.-H. (2025). Designing Superhydrophilic 3D Porous Surfaces on Polyetherketoneketone Surfaces to Promote Biocompatibility. Journal of Functional Biomaterials, 16(3), 106. https://doi.org/10.3390/jfb16030106







