Abstract
This comprehensive review of dental resin adhesives explores their historical development, key components, recent innovations, and potential future directions, highlighting a dynamic and continually advancing field. From Buonocore’s breakthrough acid-etching technique and Bowen’s pioneering dental resin invention, successive generations of clinicians and scientists have pushed forward the technological and materials development for secure bonding, while preserving dental tissues. The review discusses the substantial advances in improving adhesive reliability, enabling more conservative treatment approaches. It also delves into enhancing fundamental adhesive components and their synergistic combinations. Recent innovations, including biostable and functional resins, nanotechnology, and bioactive components, address persistent challenges such as durability, antimicrobial efficacy, and therapeutic functionality. Emerging technologies, such as digital dentistry, artificial intelligence, and bioinspired adhesives, portend an exciting and promising future for dental adhesives. This review underscores the critical role of ongoing research in developing biocompatible, multifunctional, and durable adhesives. It aims to support dental professionals and researchers by providing a comprehensive understanding of the dynamic progression of dental adhesives, inspiring continued innovation and excellence in restorative dentistry.
1. Introduction
Methacrylate-based resin composites were pioneered in the early 1960s by Dr. Rafael Bowen of the American Dental Association (ADA). The acronyms used throughout this proposal are listed in Appendix A. A decade later, these composites began to be widely used by clinicians to treat teeth affected by dental caries. Six decades on, methacrylate-based restoratives remain dominant in dental adhesives and restorative materials, owing to their natural tooth-like appearance, strong bonding capability with teeth, and versatility for small and large restorations. These unique properties have positioned them ahead of alternatives such as amalgam and glass ionomer cement [1,2,3].
Modern dental resin composite restoratives, along with their adhesives for dentin and enamel, typically consist of three essential components: (1) a resin network, (2) reinforcing filler particles, and (3) functional additives. Clinicians and researchers blend these components—like artists mixing colors—to address a wide range of complex dental cases. In addition, new materials and advanced technologies are consistently emerging, owing to more than fifty years of clinical experience and ongoing development [4,5,6,7].
Dental resin adhesives have emerged as foundational tools in restorative dentistry. Their development marked a pivotal shift from traditional mechanical retention methods to advanced adhesive techniques that enabled micromechanical and chemical bonding to dentin and enamel [8,9]. This transition has enabled more conservative treatment approaches, as dental adhesives minimized the removal of natural tooth structure and preserved more healthy tissue [1]. Additionally, by strengthening the bond between restorative materials and tooth surfaces, adhesives have enhanced the longevity and success of restorations, ultimately benefiting patients through increased comfort and reduced likelihood of restoration failure. This literature review provides an in-depth exploration of evolution, significant advancements, and the current state of dental adhesives. By highlighting their crucial role within dental science and projecting their future potential, the review underscores the transformative impact of adhesives on clinical practice and patient outcomes.
To gain a thorough insight into these developments, we conducted a systematic search that was conducted across multiple databases, including PubMed, Sco-pus, Web of Science, ScienceDirect, and Google Scholar, focusing on peer-reviewed articles published up to 2024 without a specific starting date, ensuring the inclusion of foundational studies to provide historical context. The search employed Boolean combinations of relevant keywords and MeSH terms, including “dental resin adhesives”, “adhesive dentistry”, “bioactive adhesives”, “self-etch adhesives”, “universal adhesives”, “bond strength of adhesives”, “composite restoration failure”, “remineralizing dental adhesives”, “antibacterial dental adhesives”, “nanotechnology in adhesive dentistry”, “polymerization shrinkage”, “hydrolytic degradation of adhesives”, “AI and machine learning in adhesive dentistry”, “digital dentistry adhesive bonding” and “clinical performance of adhesive restorations.” Articles were selected based on inclusion criteria, prioritizing clinical trials, systematic reviews, meta-analyses, and experimental studies evaluating adhesive performance, biomaterial innovations, antibacterial and remineralizing properties, digital dentistry applications, and AI-driven material advancements. Exclusion criteria were applied to eliminate non-peer-reviewed sources, conference abstracts, duplicated studies, non-English articles without translations, and publications lacking quantitative data or scientific validation.
Through our analysis, several key themes emerged over the development of adhesives from rudimentary to highly specialized formulae; continuous compositional improvements in search of better bond strength and biocompatibility; the addition of functional agents to tackle some clinical challenges; how to overcome deficiencies of existing materials; and the introduction of new concepts that could change the paradigm for dental adhesive applications soon [10,11,12,13].
This review first outlines the historical trajectory of dental adhesives, highlighting pivotal advancements in dental material science that have met the evolving demands of clinical practice. It then delves into the primary components of contemporary adhesives, such as resins and fillers, discussing their respective advantages and the practical challenges they address. Following this, we evaluate emerging alternative materials and technologies that show significant promise in addressing current limitations and advancing the science of dental materials. Finally, we offer a forward-looking perspective on anticipated trends in dentistry, focusing on research advancements and practical applications in clinical settings. Through this comprehensive review, we aim to assist dental professionals and researchers in understanding the dynamic progression of dental adhesives, thereby inspiring continued innovation and excellence in the field.
2. Historical Development of Dental Resin Adhesives
The trajectory of dental resin adhesives, integral to modern restorative dentistry, is rich with incremental advancements and innovations. Tracing back to its roots, the historical development of these materials reflects the progress in dental material sciences and the evolving needs of clinical practice.
2.1. Early Beginnings
The early beginnings of dental adhesives are to improve the retention of restorations within the oral cavity. Before the mid-20th century, dental restorative materials focused predominantly on mechanical retention. The principle was to design restorations physically locked into tooth structures, often necessitating extensive tooth preparation. This approach, while adequate to an extent, had several drawbacks, including removing healthy tooth tissue and potentially weakening the tooth structure.
Various materials, including zinc phosphate and silicate cement, were designed and tested in the early stages to enhance adhesion to tooth structures. However, these materials lacked bond strength and durability for long-term restoration success [14]. The search for improved adhesives led to the exploration of synthetic resins in the 1940s. Initially, these resins, such as polymethyl methacrylate, were used as filling materials due to their aesthetic properties and ease of manipulation. However, their adhesion to tooth structures was inadequate, leading to marginal leakage and secondary caries. It was not until Buonocore introduced the acid-etch technique in 1955 that a significant leap was made in adhesive dentistry. By etching the enamel with phosphoric acid, Buonocore created a roughened surface that enhanced the mechanical retention of acrylic resins. This technique represented the first step towards the adhesive revolution in dentistry, setting the foundation for future advancements in the field [15].
The early development of dental resin adhesives was thus characterized by a gradual shift from mechanical retention strategies towards a more adhesive approach. These initial steps set the stage for extensive research and development, leading to the sophisticated adhesive systems used in contemporary dentistry.
2.2. Buonocore’s Acid-Etch Technique
The inception of Buonocore’s acid-etch technique in 1955 marked a significant breakthrough in adhesive dentistry. This technique involved the application of phosphoric acid on enamel surfaces and enhanced adhesion between restorative materials and tooth structure by creating micro-roughness for mechanical interlocking [15]. This seminal development improved the durability and efficacy of dental restorations and laid the groundwork for subsequent advancements in dental adhesives. The acid-etch technique introduced a new paradigm by demonstrating that chemical bonding could be more effective. By etching the enamel with phosphoric acid, Buonocore achieved an increased surface area for bonding, significantly improving the retention of acrylic-based restoratives [16]. This technique effectively addressed the limitations of existing restorative procedures, such as marginal leakage and secondary caries.
Buonocore’s work inspired further research into the nature of adhesion to both enamel and dentin. The success of the acid-etch technique underscored the potential of developing adhesive systems that could bond to the different substrates in the oral environment. The introduction of Bis-GMA resin by Bowen in 1962 marked a significant breakthrough. This resin, compatible with etched enamel surfaces, formed stronger and more durable bonds.
In the subsequent years, enamel etching was extended to dentin. Early attempts to bond to dentin were challenged due to its complex structure. However, by the 1980s, breakthroughs in understanding the histology and physiology of dentin led to the development of dentin bonding agents that could penetrate the collagen matrix and form stable bonds [17].
2.3. Discovery of the Hybrid Layer
The discovery of the hybrid layer represents a pivotal development in the evolution of dental adhesives, fundamentally enhancing our understanding of dentin bonding. This breakthrough, primarily attributed to the work of Nakabayashi and his team in the early 1980s, has profoundly impacted the field of adhesive dentistry [17,18].
The hybrid layer, a microscopic zone at the interface between the adhesive resin and dentin, was first described by Nakabayashi et al. in 1982 [18]. They observed that, when dentin is treated with phosphoric acid and then infiltrated with resin, a unique intermingling of demineralized collagen fibers and resin occurs. This zone, termed the hybrid layer, is crucial in establishing a strong bond between the resin material and dentin substrate [17,19]. The hybrid layer’s effectiveness lies in its ability to provide micromechanical interlocking that significantly improves bond strength and durability—characterizing the hybrid layer led to a deeper understanding of the complexities involved in bonding to dentin, which is inherently more challenging than bonding to enamel due to its organic composition and moisture content. The hybrid layer formation was shown to be crucial for creating practical and durable bonds in restorative procedures involving dentin [19,20].
Subsequent research by Fusayama et al. in 1979 [21] and Gwinnett in 1991 [22] further elucidated the properties and formation mechanisms of the hybrid layer. These studies highlighted the importance of proper etching and resin infiltration techniques in achieving optimal hybrid layer formation, influencing the development of new adhesive systems and application protocols. The discovery of the hybrid layer also influenced the shift towards more conservative restorative techniques. It allowed for the preservation of more tooth structure by enhancing the bonding efficacy to dentin, thus supporting the principles of minimally invasive dentistry [2]. Figure 1 demonstrates the significant milestones in dental adhesive development.
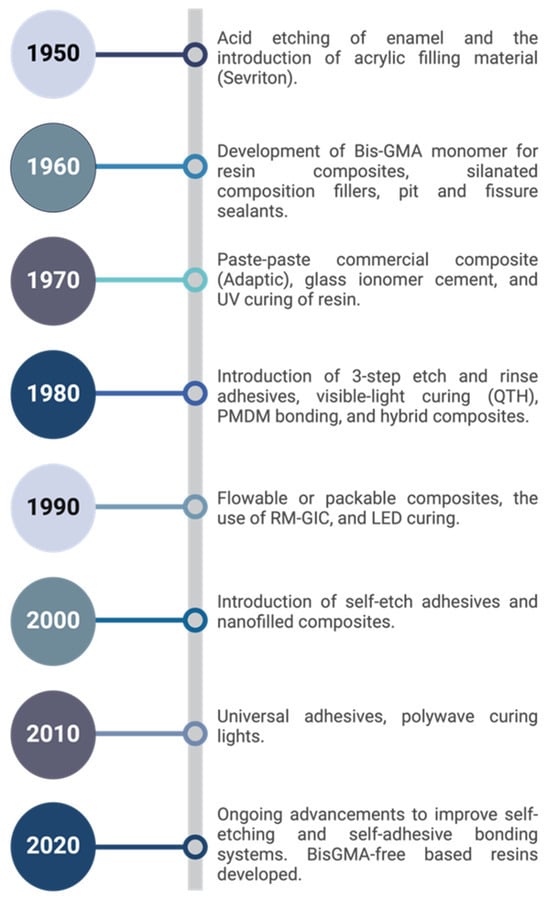
Figure 1.
Timeline of milestones in dental resin adhesive development [3,15,23,24,25,26,27,28,29].
2.4. From Total-Etch to Self-Etch Adhesive Systems
The progression from total-etch to self-etch adhesive systems in dentistry is a story of significant advancements in bond strength and the development of newer adhesive generations, each tailored to meet the evolving demands of clinical practice.
- Total-Etch Systems: Originating from Buonocore’s acid-etch technique, total-etch systems (fourth and fifth generations) involve a phosphoric acid application to both enamel and dentin, followed by a bonding agent. These systems typically achieve bond strengths ranging from 20 to 30 MPa, providing adequate adhesion but requiring careful techniques to avoid issues such as postoperative sensitivity. Despite their effectiveness, total-etch systems are more sensitive to contaminants like saliva and blood, affecting their bond strength [30,31,32].
- Self-Etch Systems: By contrast, self-etch adhesives (sixth, seventh, and eighth generations) integrate the etching and priming steps. These systems have bond strength values ranging from 18 to 35 MPa. They offer the advantage of reduced technique sensitivity and lower risk of postoperative sensitivity. Two-step self-etch adhesives have demonstrated higher shear bond strength than total-etch and multimode adhesives [33]. However, total-etch systems can exhibit higher bond strength in specific applications, such as when bonding to calcium silicate-based cement [34,35].
- Generational Progression and Comparison: Each generation of dental adhesives has aimed to improve bond strength and application ease. The fourth and fifth generations (total-etch) focused on maximizing bond strength but were more technique-sensitive. In contrast, the sixth to eighth generations (self-etch) emphasized ease of use and consistency in performance, often with slightly lower but more predictable bond strength. Recent developments, like universal adhesives, seek to combine the strengths of both systems, offering versatility in application as they can be used as total-etch or self-etch and enhanced performance across diverse clinical scenarios [36,37].
In summary, the shift from total-etch to self-etch adhesive systems has marked a significant advancement in adhesive dentistry. While total-etch systems offer high bond strength under optimal conditions, self-etch systems provide consistent results with reduced sensitivity to technique and moisture.
2.5. Current Generations of Dental Adhesives
- First Generation of Bonding Agents: A Swiss chemist, Oskar Hagger, who worked for DeTrey/Amalgamated Dental Company in the late 1940s, developed the first dental adhesive agent, Sevriton Cavity Seal [38]. This bonding agent had glycerolphosphoric acid dimethacrylate (NPG-GMA), and it was claimed to penetrate the dentin surface and prepare the surface for the chemically cured resin Sevriton [39]. Today, we call the resin-penetrated zone the hybrid zone/layer. These bonding agents preformed an ionic bond with the hydroxyapatite or a covalent bond to the collagen with a (hydrogen-bonding). However, this adhesive product had an overall poor clinical performance due to the high interfacial stress and thermal expansion caused by the methacrylate composites [40]. The bond strength was in the 1–3 MPa range [41]. The advent of first-generation dental adhesives had a profound impact on dental practices. It allowed for more conservative restorations since adhesives could effectively secure materials to a tooth structure with minimal preparation. This was a significant advancement over more invasive techniques requiring extensive tooth modification [42,43].
- Second Generation of Bonding Agents: The second generation was introduced in the late 1970s and utilized an ionic bond between calcium and chlorophosphnate groups. These adhesives primarily used polymerizable phosphonate added to Bis-GMA resins to promote bonds to calcium ions [41,44]. However, this ionic bond was susceptible to degradation, even by the water within the dentin structure, resulting in microleakage when submerged in water. This generation did not remove the smear layer, resulting in a weak, unreliable bond strength [41]. This generation is no longer used due to its bonding failures with the loosely bonded smear layer. Also, the presence of water in the formula led to concerns about hydrolytic stability and degradation over time [44,45]. The second-generation adhesives marked the beginning of incorporating primers into adhesive systems, which later evolved into the more sophisticated multi-step systems of the third generation [22]. They also helped set the stage for the development of aesthetic dentistry, as these adhesives were more compatible with the translucent properties of newer composite materials.
- Third Generation of Bonding Agents: This generation was introduced in the late 1970s and early 1980s. A revolution in this system was introducing the “total-etch” system to modify or partially remove the smear layer [41,46]. This allowed the penetration of the primer within the dentinal tubules after the acid was rinsed entirely away. Then, the primer will be added to the cavity, and an unfilled resin will be placed on dentin and enamel. The chemical composition of the primers and adhesives allowed for better interaction with the hydrophilic dentin and hydrophobic resin materials, improving the interface strength [19]. The weakness of this generation was the unfilled resins that did not infiltrate the smear layer [47]. While third-generation adhesives significantly improved bonding effectiveness, they were not without drawbacks. The total-etch technique increased the risk of post-operative sensitivity due to over-etching or incomplete sealing of dentin tubules. Additionally, the reliance on multiple application steps still posed a challenge regarding technique sensitivity [45]. Developing third-generation adhesives was a critical step towards the later introduction of simplified systems, such as the fourth and fifth generations, which combined the etching and priming steps or even included all steps in one application [48].
- Fourth Generation of Bonding Agents: Introduced in the 1980s and 1990s, fourth-generation adhesives were developed to optimize the bonding process by separating the etching, priming, and bonding steps. This generation is often considered the gold standard for dental adhesive systems due to its high efficacy and predictable results [3,41]. The total-etch technique used with these adhesives involved phosphoric acid to etch both enamel and dentin, which provided a more uniform etch and a reliable bonding surface. This generation protocol was to remove the smear layer entirely, and it is still considered the golden standard. It has three primary components: an acid etchant, a primer, and a bonding agent. These systems are very effective when correctly used as they are technique -sensitive. The enamel and dentin had to be etched with phosphoric acid for 15–20 s and then rinsed, and the surface must be left moist to avoid collagen collapse. Using a hydrophilic primer enhanced the infiltration into the collagen network and formed the hybrid layer [41]. Due to hybridization, the bond strength improved significantly compared to previous generations. It ranged from low to mid-20 MPa [15]. On the downside, these systems can be time-consuming and confusing with many bottles and application steps.
- Fifth Generation of Bonding Agents: The fifth generation of dental adhesives, which appeared in the late 1990s, introduced single-bottle systems combining the primer and adhesive in one solution. This generation aimed to simplify the bonding procedure while maintaining the high bond strengths of the fourth-generation systems. They are known to be the “one-bottle” or “one-step” systems [42]. The composition typically included a mixture of hydrophilic and hydrophobic monomers, solvents such as ethanol or acetone, and photoinitiators in a single solution. The consolidation into one bottle aimed to reduce variability in application and decrease the potential for technique-sensitive errors. The primer was combined with the bonding agent into one solution to be applied simultaneously after acid etching with 35–37% phosphoric acid, and this technique prevented collagen collapse and minimized postoperative sensitivity [41,45,49,50]. The single bottle etch-and-rinse adhesive type shows the same mechanical interlocking and comparable bond strengths to the 4th generation. However, they faced criticism for potential compromises in bond strength compared to their predecessors, particularly regarding long-term durability and susceptibility to hydrolytic degradation. They were more prone to water sorption and degradation over time than the 4th generation [51,52].
- Sixth Generation of Bonding Agents: The sixth generation was introduced in the latter part of the 1990s and early 2000s. A self-etching primer is applied to the tooth surface, followed by a bonding agent. The concept of self-etching was first introduced in a publication by Watanabe and Nakabayashi in 1993 [53]. The most significant advantage of this system is that it is less dependent on the hydration status of the dentin than the total-etch systems. Innovations in sixth-generation adhesives have focused on enhancing the chemical composition of the primers to improve their efficacy and compatibility with both enamel and dentin. Specifically, the introduction of functional monomers, such as methacryloyloxydecyl dihydrogen phosphate (MDP), enhanced the adhesive’s interaction with hydroxyapatite in the tooth structure. These modifications aim to improve the hybrid layer’s mechanical properties and increase the bond’s durability [54]. These systems form a bond that is stronger to dentin than enamel, which might be because their pH is not acidic enough to etch enamel [32]. To overcome this problem, it is recommended to utilize selective etching of enamel [51].
- Seventh Generation of Bonding Agents: This system was introduced in late 1999 and early 2005. The primary innovation of seventh-generation adhesives lies in their all-in-one application, significantly reducing procedure time and the potential for error associated with multiple-step systems. These adhesives use an acidic monomer that demineralizes and infiltrates the tooth substrate, creating a bond in one step [35,51]. These are considered acidic systems, and they are prone to hydrolysis and chemical breakdown [55]. In addition, they are more hydrophilic than self-etching primer, making them more susceptible to water sorption, limiting the depth of resin infiltration into the tooth, and creating more voids [56]. These systems have the lowest initial and long-term bond strength of any adhesive in the market [57]. Shear bond strength of the 7th generation ranges from 19.80 to 30.30 MPa [58]. The convenience and speed of application have made seventh-generation adhesives particularly popular for quick and less invasive procedures. Their ability to effectively bond in a moisture-rich environment makes them suitable for pediatric dentistry and for patients with limited cooperation [37]. Research continues to focus on enhancing the formulation of these adhesives by optimizing the ratio of acidic monomers and solvents, and by developing new monomers that can provide stronger and more durable bonds [59].
- Eighth Generation of Bonding Agents: Eighth-generation dental adhesives were introduced in the 2010s, and they are also known as “universal adhesives”, “multi-mode”, or “multi-purpose” because they may be used as self-etch (SE) adhesives, etch-and-rinse (ER) adhesives, or as SE adhesives on dentin and ER adhesives on enamel (a technique commonly referred to as “selective enamel etching”) [37]. As medical devices, universal adhesives are designed to bond to various substrates, such as enamel, dentin, and restorative materials, regardless of the application mode. However, their clinical performance depends on the chosen mode: the SE mode involves simultaneous etching and priming without a separate rinsing step, while the ER mode requires a separate phosphoric acid etching step followed by rinsing and applying the adhesive. Voco America introduced Voco Futurabond DC as the 8th generation of bonding agents in 2010. It contained nanosized fillers, which increased the monomer penetration and the hybrid layer thickness, providing better enamel and dentin bond strength, stress sorption, and longer shelf-life [60]. Shear bond strength of the 8th generation ranges from 22.10 to 37.10 MPa [58].
The primary innovation in eighth-generation adhesives is the inclusion of functional monomers such as 10-MDP, which chemically bond to tooth structure, and silane, which can bond to ceramic surfaces, making these adhesives suitable for various restorative materials. Using these multifunctional monomers simplifies the procedure while maximizing the bond strength. 10-MDP has mild-etching properties. It forms a stable ionic bond with calcium salts within the tooth structure. This bond was first proven by Yoshida et al. in 2004 using XPS (or X-ray photo-electron spectroscopy) [2]. In addition, a silane-containing universal bonding agent enhances adhesion between restorative materials (like composite resins and ceramics) and tooth structures by incorporating silane, which chemically bonds with silica-based ceramics and resin adhesives. These versatile adhesives work with various materials and etching techniques, improving bond strength, moisture tolerance, and reducing technique sensitivity for both direct and indirect restorations.
A systemic review and meta-analysis published in 2015 by Rosa et al [55]. concluded that selective etching of enamel before the application of a mild universal bonding agent improved the bonding integrity and durability, while prior etching of the dentin with phosphoric acid did not improve the bond strength and increased the risk of post-operative sensitivity [2,61]. In addition, universal adhesive systems showed the least cell cytotoxicity when compared to other systems [62,63]. Continuous advancements are being made to optimize the formulations of these adhesives to enhance their performance and ease of use. Recent innovations focus on improving solvent systems and photo initiators to enhance polymerization effectiveness and reduce technique sensitivity [64].
Table 1 summarizes the primary compositions and techniques of these eight generations. It also highlights their bonding mechanism and range of bond strength. In addition, the challenges of each generation and representative commercial products are listed. Although both the 7th and the 8th generations are “All in one bottle”, there are key differences between them that are represented in Table 2.

Table 1.
Generations of dental resin adhesives.

Table 2.
Key differences between the 7th and the 8th generations of resin adhesives: [2,25,56,68,69,70,71].
3. Fundamental Composition of Adhesive Materials
A traditional dental resin adhesive system includes etchants, primers, resin monomers, initiators, solvents, reinforcing fillers, and sometimes other functional ingredients such as antimicrobial agents [72,73,74]. This section focuses on the materials that make primers and adhesives in current commercially available dental resin adhesives. Methacrylate-based resins are the most popular contemporary dental primers and adhesives for direct restorations [75,76]. Table 3 lists the most common commercially available resin formulations. Additionally, we discuss the major components of dental resin adhesives, including base monomers, diluting monomers, initiators, and fillers. Given their increasing popularity, we specialize on adhesion-promoting functional monomers, vital components of 8th-generation dental adhesives. However, clinical evidence has highlighted certain shortcomings of these contemporary dental adhesives. We will also discuss these drawbacks, emphasizing the need to develop a new generation of dental adhesives.

Table 3.
Most common existing commercially available resin formulations [2,25,56,68,69,70,71].
3.1. Common Composition of Current Dental Resin Adhesives
3.1.1. Matrix Resins
Matrix resins are critical components in dental adhesives, providing the necessary structural framework that binds the fillers and transfers stresses within the adhesive. The matrix resins determine the adhesive system’s mechanical properties, durability, and clinical efficacy.
- Base Monomers: Base monomers form the skeleton of the resin, determining its physical properties, such as flexural strength and compressive resistance. Two commonly used base monomers include Bisphenol A-Glycidyl Methacrylate (Bis-GMA) and Urethane Dimethacrylate (UDMA). These monomers provide excellent mechanical properties. However, these monomers must often be modified or diluted with other components due to their high viscosity to improve handling. For instance, Bis-GMA offers high strength and rigidity but requires dilution to enhance flow and manipulation properties [104]. Similarly, UDMA is used for its flexibility and toughness but requires diluting agents to achieve optimal handling properties [1,105].
- Diluting Monomers: Diluting monomers adjust dental resins’ viscosity and handling properties. These monomers are less viscous than base monomers, allowing for easier resin application in clinical settings. Diluting monomers such as TEGDMA or HEMA are incorporated into the formulation to reduce the viscosity of the resin, making it easier to handle and apply. In 1-step self-etching adhesives to prevent phase separation between water and the adhesive monomers [106]. In addition, they are vital in optimizing the degree of polymerization and conversion, affecting the material’s shrinkage and overall mechanical performance. However, their inclusion must be carefully balanced, as excessive use of diluting monomers can weaken the mechanical properties of the resin, potentially leading to reduced restoration longevity [4,107].
3.1.2. Initiators
Dental resin adhesives rely on initiators to catalyze the polymerization of resins into solid matrices that bond restorative materials to tooth structures. These initiator systems are categorized into three primary types: chemical-cure, light-cure, and dual-cure systems. The choice of initiator affects the adhesives’ properties, including degree of conversion, mechanical strength, and aesthetic stability. The amount of initiator added to adhesive systems depends on the type of initiator and the adhesive system but is usually in the range of 0.1–1 wt% [62].
- Chemical Initiator Systems: Chemical initiator systems, also known as self-cure or auto-cure systems, rely on a catalyst (usually benzoyl peroxide) and an activator (commonly an amine) to initiate polymerization without light. Valid areas where light cannot penetrate, such as deep cavities or post-core build-ups [13]. However, it has a shorter working time and potential for color instability [1,86,108].
- Photo-Initiator Systems: Photo-initiator systems use light to activate polymerization. The most common initiator is camphorquinone (CQ), which is activated by blue light. CQ is generally used with an amine co-initiator to accelerate the production of free radicals for polymerization. CQ/amine combination initiates polymerization upon exposure to visible light at approximately 465 nm [90]. However, CQ’s yellow color may affect aesthetics [90]. Combining CQ with Ivocerin or Lucirin TPO broadens the absorption spectrum and improves the depth of cure. It improves esthetics and enhances the depth of the cure [4]. Phenylpropanedione absorbs light at a similar wavelength to CQ but with slightly different optical and polymerization properties. It may be used as a substitute or in combination with CQ to reduce yellowing effects [86].
- Dual-Curing Systems: Dual-cure systems are designed to polymerize through both chemical and light-initiated mechanisms. These systems are beneficial when light penetration is limited, such as in deep cavities or beneath opaque restorations [109]. The most popular combination in dual cure systems is CQ with a tertiary amine for the light-curing component and benzoyl peroxide with an aromatic sulfonic acid salt for the chemical-curing component [13,86].
3.1.3. Fillers
Fillers are particularly noteworthy as they improve the adhesive’s mechanical strength, viscosity, and handling characteristics. Fillers influence the adhesive’s properties, including polymerization shrinkage, wear resistance, radiopacity, and handling characteristics. Silica fillers are among the most widely used traditional fillers in dental adhesives. They may be silanized and provide improved bonding in the resin matrix. However, these silanization agents are generally methacrylate-based compounds subject to hydrolytic degradation in the oral environment [110]. Traditional dental adhesives often incorporate aluminosilicate glass fillers, contributing to the material’s structural integrity and caries prevention through fluoride release [111]. These fillers have been used for many years due to their strength and anti-cariogenic properties. In older formulations, zinc oxide-polyacrylic acid-based fillers in dental cement show some adhesion to tooth tissues. However, they are often replaced by more advanced composite materials today [112].
3.2. Adhesion Promoting Functional Monomers in Dental Resin Adhesives
Functional monomers play a critical role in the bonding efficacy of dental adhesives, especially at the interface between the adhesive and tooth structure. Functional monomers establish chemical interactions with tooth tissue components. They are pivotal in achieving strong and durable bonds, which are crucial for the long-term success of dental restorations. Studies have shown that functional monomers, such as phosphate monomers 10-MDP and MF8P, the phosphate functional group, can form strong ionic bonds with hydroxyapatite. They improve bond durability in dental adhesives, ensuring the longevity of restorations [62,113] owing to the low solubility of the resulting calcium salts. Functional monomers also etch the enamel and dentin surfaces in self-etch adhesive systems. The impact of functional monomers in all-in-one adhesive systems on the formation of enamel/dentin acid-base resistant zones was investigated by Nikaido et al. Variations in thickness and morphology were observed between enamel and dentin interfaces, influenced by the specific functional monomers used in the adhesive systems [114]. For example, 10-MDP is one of the mild acidic monomers that enable a universal adhesive to be used with any etching technique in a pH ranging 2–3 [115]. This compound was invented by Kuraray (Osaka, Japan). Its dihydrogenphosphate group can dissociate in water and establish an intensive and stable chemical interaction with hydroxyapatite [116].
Stable MDP-calcium salts are formed during this reaction and deposited in self-assembled nano-layers, illustrated in Figure 2 [117]. Nano-layering of 10-MDP_Ca salts was documented to still exist after 1 year of water storage, and it was found to be aging-resistant due to the low dissolution rate [97,118]. This ionic bond makes the adhesive interface more resistant to biodegradation [37,67,116]. The bonding strength, e.g., micro-tensile bond strength (μTBS), was maintained after 1 year of aging [119]. The good in vitro and clinical outcomes of the Clearfil SE Bond, an adhesive containing 10-MDP, may be partly attributed to its intense chemical adhesion with tooth tissue [4]. It was proven that 10-MDP or 10-MDP-Ca salt inhibits the matrix metalloproteinases (MMPs) that accelerates the degradation of the hybrid layer, which in turn reduces nanoleakage of the bonding interface and improves its durability [120]. Van Landuyt et al. considered the most promising monomer for chemical adhesion to the hydroxyapatite to be 10-MDP. Feitosa et al. (2014) studied the influence of spacer carbon chains in acidic functional monomers on self-etch dental adhesives’ physical and chemical properties. They found that highly hydrophilic monomers with longer spacer carbon chains increased wettability and water sorption on dentine surfaces, affecting the ultimate tensile strength of one-step self-etch adhesives [121].
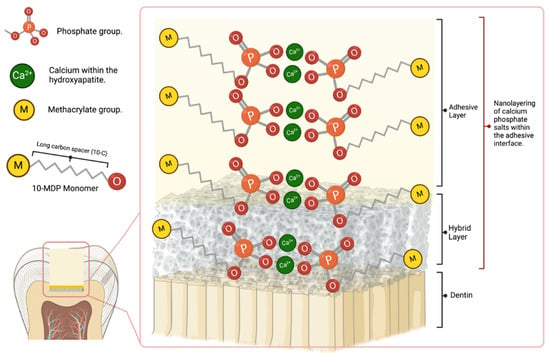
Figure 2.
Schematic explaining the formation of MDP-Ca salt and interfacial nanolayering.
However, combined use of 2-hydroxyethylmethacrylate (HEMA) and acidic functional monomer may compromise the bonding. Yoshida et al. (2012) [98] investigated the effect of 2-hydroxyethylmethacrylate (HEMA) on the nano-layering of the functional monomer 10-MDP at the interface with hydroxyapatite-based substrates. They discovered that adding HEMA inhibits the nano-layering of MDP at the interface and subsequently compromises the long-term durability of the bond to tooth tissue. In addition, using these acidic monomers may reduce the shelf-life of methacrylate-based resin adhesive due to ester hydrolysis. They also affect the polymerization efficiency, mainly when amine is used as the initiator [98].
3.3. Challenges of Current Dental Adhesives
There have been ongoing advances in adhesive dentistry for decades, particularly in the various elements of composition and classification. However, optimal performance has yet to be developed for direct adherence, bond strength, stability, and durability. Most commonly, adhesive interface failure results in clinical complications like sensitivity, stains, and secondary caries that ultimately compromise the restoration [122]. Current research has identified some of the following factors that affect the adhesive-dentine interface.
3.3.1. Biocompatibility and Toxicity of Dental Resins
Recently, the biocompatibility of resin monomers has come under significant scrutiny. Research indicates that residual monomers can leach into saliva after curing, and resin degradation can lead to additional chemical release into the human body. Many monomers and degraded products, particularly methacrylate-derivatives, have been found to exhibit cytotoxic effects. In addition to their cytotoxicity, concerns have been raised about the potential endocrine-disrupting effects of these monomers [123,124,125,126,127].
Despite the widespread use of Bisphenol A-glycidyl methacrylate (Bis-GMA) resin in dental composites, there are concerns about its potential toxicity, primarily due to its potential to leach out of the composite material over time. The main concern with Bis-GMA is BPA, a substance that has been linked to a variety of health problems, including hormonal disruptions and potential carcinogenic effects [82,83,128,129,130,131,132]. When Bis-GMA is used in dental composites, it can potentially release BPA as the initial raw materials might contain unreacted BPA or degrade over time [133].
Once released, BPA can be swallowed or absorbed into the oral tissues, leading to systemic exposure. A recent study showed absorption of BPA by the sublingual area in dogs, allowing its direct entry into the bloodstream, bypassing the digestive system and liver, and multiplying its bioavailability by a factor of 80 [134]. While the exact health effects of this exposure are still being studied, there is concern that it could contribute to hormonal disruptions or other health problems due to the estrogenic activity of BPA [129,130,132,135,136]. More research has reported the health effects of Bis-GMA. Kuan and his colleagues conducted an experiment that showed Bis-GMA demonstrated cytotoxicity to macrophages in a dose- and time-dependent manner. It also induced the production of Prostaglandin E2, a key regulator of immunopathology in inflammatory reactions [137]. Several additional studies were performed by Sadeghinejad et al., aimed at investigating the impact of Bis-GMA biodegradation products on cariogenic bacteria. The researchers discovered that bishydroxypropoxyphenyl-propane (BisHPPP), a Bis-GMA biodegradation product, had a significant effect on the gene expression and protein synthesis of cariogenic bacteria, which could potentially result in increased secondary caries around resin composite restorations [138]. Another team study found that triethylene glycol, a hydrophilic biodegradation product of Bis-GMA, stimulated the growth of Streptococcus mutans, a dominant cariogenic bacterium, and up-regulated genes linked to bacterial virulence [139]. Bis-GMA is also considered cytotoxic, which can alter the cell cycle and induce oxidative stress, leading to apoptosis and necrosis in a concentration-dependent manner. High concentrations of Bis-GMA resulted in cell death by necrosis, while low concentrations resulted in cell death by necrosis or late apoptosis [140,141]. The degradation of Bis-GMA and release of BPA can occur due to several factors: (1) During the curing process, not all of the Bis-GMA may fully polymerize, leaving residual monomers that can potentially leach out over time. (2) Over time, the composite material can degrade due to wear and tear, as well as exposure to the oral environment (e.g., changes in pH, temperature, and exposure to bacterial and oral enzymes). (3) Exposure to certain solvents, such as alcohol or other components of oral hygiene products [81,83].
HEMA (2-hydroxyethylmethacrylate) is a hydrophilic methacrylate monomer that is frequently added to dental adhesives due to its wetting enhancement effect and promotes the diffusion of co-monomers by expanding the demineralized collagen [142,143]. Apart from the main disadvantage HEMA poses of facilitating water uptake and the subsequent gradual hydrolytic degradation of the polymers, swelling, and resin staining [55]. Spagnuolo et al. also reported on the cytotoxicity of HEMA as unreacted HEMA increased levels of reactive oxygen species (ROS). They decreased the levels of antioxidants like glutathione, causing oxidative dysfunction and apoptosis of fibroblasts in the pulpal tissues [144,145]. Lee et al. also reported on the cytotoxicity of HEMA as it induced cell toxicity in RAW264.7 macrophages with a concentration-dependent matter. A higher HEMA concentration was associated with a higher level of apoptosis and genotoxicity [146].
3.3.2. Effects of Oral Conditions on Adhesive Durability
Oral bacteria metabolize dietary sugars and produce acids, primarily lactic acid, which challenges the stability of dental adhesives by lowering the pH at the adhesive-tooth interface. Lower pH accelerates the hydrolysis of the resin matrix and demineralization of tooth structure. This acidic environment poses a risk to both secondary caries, adhesive degradation, and eventually restoration failure [147].
Masticatory forces are unavoidable oral situations that affect the adhesive and tooth bonding interface. They usually impact adhesive bond strength and generate marginal leakage on the restoration surface. Furthermore, incomplete infiltration of adhesive triggers leakage formation. Simultaneously, formed leakages enhance the exposure of dentin collagen and accelerate oral fluid absorption, causing the degradation of adhesive and collagen matrix [148,149,150,151,152].
Generally, dentine’s intrinsic matrix protease activity is responsible for collagen degradation over time. At the same time, treated dentine with some enzyme inhibitors showed a notable decrease in collagenolytic and intrinsic dentine gelatinolytic activity [153,154,155]. Reports have shown that matrix metalloproteinases (MMPs) and cysteine cathepsins (CTs) are the best-known endogenous enzymes among all enzymes in dentine and are responsible for collagen degradation [156,157,158].
3.3.3. Properties and Performance of Dental Resin Adhesive That Need to Be Improved
Hydrolysis of the ester functional groups in the resin’s constituent monomers was reported to contribute to resin matrix degradation. They are readily hydrolyzed and degraded by oral enzymes and cariogenic bacteria, incomplete infiltration of the resin monomers into the dentinal tubules, and the high-water absorption of the resin network [159,160,161,162]. Moreover, ester bonds in methacrylate adhesives are also prone to chemical hydrolysis [154,163]. Reports showed that water incorporated in a hybrid resin layer acts as a medium to initiate hydrolysis. It also plays its role as a plasticizer within the adhesive polymer chains, accelerating the matrix degradation [164,165]. Additionally, cholesterol esterase and pseudocholinesterase in saliva facilitate methacrylate’s degradation. On the other hand, hydrophilic and hydrophobic-containing dental adhesives experience a phase separation phenomenon that leads to degradation [166].
Incomplete infiltration usually leaves a microleakage caused by either inadequate adhesive dispersion or the lack of function of acid etch [167,168,169]. This microleakage exposed dentin collagen and facilitated the activity of MMPs and CTs. Therefore, the bonding strength of the adhesive is affected more readily than completely sealed dentine. [154,170,171] A firm and complete hybrid layer is complex in such a condition. At some point, monomers take up space at the interface, which cannot protect collagen-bonded fluid flow. On the other hand, large monomers (Bis-GMA) may become trapped in inter-fibrillary spaces and cannot penetrate the hybrid layer. However, small monomers (HEMA) may penetrate but provide frail linear chains, eventually resulting in the collagen’s cyclic fatigue failure [170].
Various factors, including polymerization shrinkage, inadequate bonding, incomplete tooth preparation or defragment of restoration area, inconsistent adhesives application, and other associated compression stresses such as natural wear and tear that occur over time, are responsible for microleakage. Oral fluids and microbes can invade this microleakage and penetrate the adhesive interface. The acid produced by cariogenic bacteria and bacterial collagenases enhances adhesive degradation, leakages, and lease [172,173,174,175]. At the same time, it activates MMPs and CTs activity and synergistically increases the degradation of adhesives and collagens, thereby reducing the stability and durability of the resin-dentin bond [176,177,178].
4. Advances in Solving Challenges
The dental research community has widely acknowledged these challenges. Notably, the Dental Materials Innovation Workshop held in London in December 2012, and its subsequent publication in Advances in Dental Research (2013), emphasized the critical need for hydrolytically stable resin networks in dental restorations [179]. In response, the National Institutes of Health (NIH) in the United States funded six projects to develop next-generation dental resin restoratives. This recognition and support have inspired scientists to innovate and create new materials designed to address these challenges, ultimately improving the performance and longevity of dental resin adhesives. In this section, we highlight several new materials and technologies that have been developed to meet these goals.
4.1. Hydrolytically Stable Resin Networks
Table 4 highlights recent advancements in methacrylate-based resins for dental adhesives, summarizing their enhanced properties and limitations as reported in the literature.

Table 4.
Experimental resins to advance dental adhesives.
New monomers and polymerization mechanisms have been introduced as alternatives to hydrolyzable methacrylate monomers [206,207,208,209,210]. Among these advancements, a step-growth thiol-ene reaction has been proposed as a substitute for the conventional radical polymerization method [211,212,213,214]. This approach notably delays the gelation process, allowing higher degrees of conversion (DC), and reduces polymerization stress, a common cause of micro-leakage and tooth fractures. The high DCs achievable in these systems minimize the presence of unreacted monomers, significantly reducing the leaching of potentially toxic compounds [7,21,22,23]. Dental composites based on silorane, and those incorporating thiourethane oligomers, have demonstrated superior mechanical properties to traditional methacrylate-based composites [215,216,217]. Adding thiourethane oligomers further enhances the performance of resin composites [218,219].
Gonzalez-Bonet et. al. successfully synthesized and characterized multiple ether-based monomers that may replace conventional resins. Specifically, triethylene glycol divinylbenzyl ether (TEG-DVBE) and its homopolymer showed no degradation under the challenges of PBS and esterase [195]. Yang et al. further discovered that the equimolar UDMA/TEG-DVBE mixtures were photo-polymerized in a controlled fashion. As a result, the styrene functional groups (ether-based TEG-DVBE) and methacrylate functional groups (ester-based UDMA) were packed alternatively. Such alternative packing protects ester functional groups from hydrolysis by hydrophobic styrene. The resulting copolymers are analogs of vinyl ester resin, a hybrid resin network made from copolymerization of styrene and methacrylate derivatives. The VERs are hydrolysis/corrosion-resistant materials that are superior to hydrolyzable polyesters. Moreover, they demonstrated the extraordinary biostability of poly-UDMA/TEG-DVBE in comparison to traditional ester-based co-polymers, poly-UDMA/TEGDMA using nanoimprint lithography Nano-scale line-and-space patterns (line height 110 nm; line width 135 nm) imprinted on poly-U/T were eradicated entirely within 72 h under esterase enzyme challenges (PCE 15 units/mL in PBS) while the same nanoscale patterns imprinted on poly-U/V are unaltered (Figure 3). The disappearance of nanopatterns on poly-U/T in only 3 days signifies the need to replace these hydrolyzable traditional resin networks. Furthermore, the distinguished poly-UDMA/TEG-DVBE biostability undeniably demonstrated its potential as a durable material in oral environments.
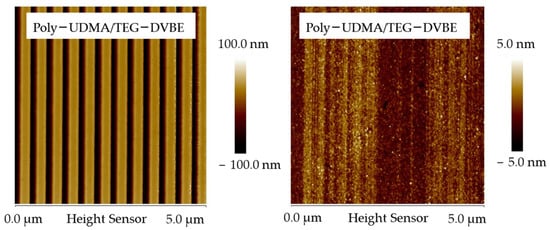
Figure 3.
Biostability Comparison of Resin Networks. Atomic force microscope (AFM) scans illustrate the biostability contrast between a hybrid resin network, poly-UDMA/TEG-DVBE (left), and a methacrylate-based resin network, poly-UDMA/TEGDMA (right). Nano-scale line-and-space patterns (line-height: 110 nm; line width: 135 nm) were imprinted onto the two resin networks using nanoimprint lithography. The images show the patterns after exposure to the enzyme pseudocholine esterase for 3 days, highlighting the differential resistance to enzymatic degradation.
Ester-based and ether-based resins work differently in terms of stability and performance in dental applications. Ester-based resins (i.e., Bis-GMA) are commonly used for desirable properties, such as high viscosity and low volatility, that form a strong and durable polymer. However, ester groups are prone to hydrolysis in the moist oral environment. Hydrolysis leads to the degradation of the resin over time, which decreases its mechanical properties and durability and ultimately leads to the failure of the restoration [220,221]. Conversely, ether groups are resistant to hydrolysis. Therefore, they are more stable and durable in the oral conditions [221]. The report showed that hybrid resins containing both ether and ester groups have desirable properties when combined with both resins. The ester groups contribute to strength and durability, while the ether groups inhibit hydrolysis, enhancing the overall stability of the resin [195]. Introduction of TEG-DVBE (Triethylene Glycol Divinylbenzyl Ether), a monomer that forms a hydrophobic cross-linked polymer network, resists hydrolysis and water absorption. Thus, maintain the integrity of dental restorations in the moist oral environment [195].
4.2. Resin Matrix Reinforcement
Fillers, nanoparticles, and nanocomposites are widely considered to be used as reinforcing agents for adhesives [222]. The incorporation of carbon nanotubes [223], silicon dioxide nanoparticles [224], titanium oxide nanoparticles [225], and zirconia nanoparticles [226] were reported to improve the mechanical properties of adhesives. In addition, they facilitate elasticity on the adhesive-tooth interface that plays a role in counteracting stress caused by polymerization shrinkage.
4.3. Improving Adhesive Compatibility with Collagens
Reports demonstrated that adding MMPs and CTs inhibitors to adhesives improves the durability of adhesives [227]. Inhibitors reduce the degradation of the collagen fibrils [154]. Recently, various crosslinking agents were incorporated into adhesives to prevent the degradation of collagen fibers, where they were added as primers into the adhesive composition [228,229]. Chlorhexidine (CHX) [230,231,232,233], glutaraldehyde [234,235,236,237], and zinc-doped adhesives [238,239] were predominantly added to the dental adhesive to inhibit collagen degradation. At the same time, some natural anti-MMPs, CTs, and collagen crosslinking agents were also reported to be incorporated in the dental adhesive to prevent collagen degradation, such as grape seed extract (GSE) [240,241,242], hesperidin (HPN) [243,244,245], and quercetin [246,247,248]. Pretreatment of the restoration site was also reported to inhibit MMPs activity. Pretreatment is the last step before adhesive application on the restoration site. Based on required properties improvement various application agents were reported. Carbodiimides were found to be applied to inhibit MMPs [249,250]. Ethylenediaminetetraacetic acid (EDTA) was used to inhibit MMPs activity and remove the smear layers that reduce the chances of incomplete infiltration of adhesives. In addition, EDTA application enhances mechanical retention [251]. Galardin application was also reported to be used for the inhibition of MMPs activity [252]. Besides the incorporation of natural agents in adhesives, some of them were also reported to be used in the pretreatment of restorations for MMPs inhibition, including Epigallocatechin-3-gallate (EGCG) [253], grape seed extract (GSE), and quercetin [247].
4.4. Functionalization of Adhesives for Remineralization
The prevalence of secondary caries formation, incomplete penetration of adhesion, marginal leakage, and collagen degeneration always increases the risk of demineralization at the adhesive tooth interface. Ongoing research focuses on increasing the remineralization of the interface to serve sustainable restorations. Reports have demonstrated that the remineralization process replaces water from the interface of the hybrid layer with apatite deposition. Thus, it reduces hydrolytic activity and maintains strong bonds [254,255,256]. The functionalization of adhesive with remineralization agents benefits the stability of adhesive-tooth bonding, enhances mechanical properties, and prevents the exposure of MMPs and CTs [257,258]. Several agents were reported to be incorporated into adhesives to add remineralizing properties, including amorphous calcium phosphate nanoparticles (NACP) [259,260], bioactive glass (BAG) [261,262,263], Cu-doped BAG [258], dentin phosphoproteins analogs [264,265], and hydroxyapatite [266,267].
4.5. Providing Adhesives with Antibacterial Activity
The antibacterial effects of dental adhesives are advantageous in inhabiting residual bacteria in restoration surfaces. At the same time, it prevents secondary bacterial innovation in adhesive-tooth interface through marginal microleakage [268,269,270]. Adding an antibacterial ingredient to dental adhesive may have additional benefits against oral bacteria and biofilms that attack the adhesive-tooth interface, leading to resin degradation and secondary caries’ formation [271]. Chitosan [272], fluoride [273], quaternary ammonium salts [274,275], silver nanoparticles [276], and surface pre-reacted glass ionomers [277] have been tested in dental adhesives to add antimicrobial activity.
Recent dental adhesive developments incorporate various functional fillers and additives to enhance their properties. Carbon nanoparticles, chitosan, iron oxide, and titania nanoparticles improve bond strength, while copper and zinc oxide nanoparticles also reduce bacteria, biofilm formation, and enzymatic degradation, stabilizing the adhesive layer. Hydroxyapatite and wollastonite promote mineralization and bonding durability, while wollastonite also enhances mechanical properties over time [12,147,222,275,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294].
Antibacterial agents such as silver nanoparticles, chlorhexidine, doxycycline, and others (e.g., benzyl dimethyl dodecyl ammonium chloride, triclosan) target biofilm and secondary caries prevention. Novel materials like boron nitride nanotubes (BNNTs) and graphene nanoplatelets show promise for mechanical stability and anti-biofilm effects. Pre-reacted glass ionomer (PRG) fillers enhance fluoride release and bonding longevity. Compounds like resveratrol and 4-formylphenyl acrylate also focus on improving bond strength, demonstrating the potential for future innovation in adhesive materials. Table 5 lists experimental fillers and additives used in the resin network to provide additional functions in dental resin adhesives.

Table 5.
Properties of experimental functional fillers and additives in dental adhesives.
The evolution of dental resin adhesives has significantly improved restorative outcomes, shifting from traditional mechanically retained materials to advanced chemical bonding systems. Other adhesives, such as zinc phosphate and glass ionomer cement (GICs), primarily relied on mechanical interlocking for retention, often necessitating extensive tooth preparation to create macro-retentive features. Although GICs offered fluoride release, their bond strength ranged between 5 and 15 MPa, which resulted in higher microleakage and increased secondary caries risk over time [325]. In contrast, modern adhesives, particularly self-etch and universal systems, incorporate functional monomers such as 10-MDP, which establish durable chemical bonds with hydroxyapatite, significantly improving bond strength to over 30 MPa [326]. Moreover, antibacterial monomers like MDPB and nanoparticle-reinforced adhesives have strengthened modern adhesive systems by inhibiting bacterial growth and promoting dentin remineralization, reducing the incidence of secondary caries and adhesive degradation [315]. Additionally, while traditional adhesives require precise moisture control and etching procedures, making them highly technique-sensitive, universal adhesives offer simplified, more predictable application protocols, allowing for self-etch, selective-etch, or total-etch approaches with improved clinical outcomes [79]. The transition from traditional to modern adhesives has resulted in higher bond strength, reduced microleakage, and enhanced ease of application, making universal and bioactive adhesives the preferred choice in contemporary restorative dentistry. Table 6 illustrates examples of market-available functionalized adhesives.

Table 6.
Market-available dental adhesives that incorporate remineralizing agents, antibacterial properties, or functional innovations.
5. The Next Generation of Dental Adhesives
The field of dental adhesives is undergoing significant transformation, driven by emerging technologies, advanced biomaterials, and the integration of digital and AI-powered solutions. Innovative pretreatment techniques enhance adhesive performance, with universal adhesives, laser treatments, and novel bonding protocols offering improved bond strength, durability, and stability. Concurrently, advancements in biomaterials are addressing key challenges in resin restoratives, introducing multifunctional, biocompatible materials that mimic natural tooth structures while preventing degradation and secondary caries. As digital dentistry revolutionizes restorative workflows, dental adhesives adapt to ensure precise bonding for CAD/CAM and 3D-printed restorations. Meanwhile, AI and machine learning accelerate adhesive development by optimizing formulations, personalizing treatments, and predicting clinical outcomes, heralding a new era of intelligent and efficient dental care solutions.
5.1. Emerging Technologies in Pretreatment
Emerging technologies, particularly the development of universal adhesives, are a focus area. These adhesives are designed to function effectively across total-etch, self-etch, or selective-etch modes, offering versatility and enhanced bond strength. Simultaneously, researchers suggested modifications to existing protocols. Laser pretreatment was reported to be beneficial for adhesive application. Laser pretreatment to acid-etched dentin softens and occludes orifices of dentinal tubules and reduces dentin permeability [343]. At the same time, laser treatment of the bonding agent before curing improves the adhesive and tooth surface interface bonding. Moreover, increased temperature due to laser application was also reported to be beneficial for adhesive infiltration and evaporation of unwanted fluids and solvents [343,344]. Non-thermal atmospheric plasma treatment was also reported to increase hydrophobicity, thus enhancing the adhesive penetration and interfacial bond strength [345]. Some studies suggested modifying the bonding protocols. An approach known as Dimethyl Sulfoxide (DMSO) wet bonding was reported to improve adhesive-tooth interface stability. In this approach, DMSO facilitates the removal of water from dentin, thereby increasing the bond strength of etch-and-rinse and self-etch adhesives, even after aging. Additionally, DMSO can stabilize the demineralized dentin matrix, inhibit matrix metalloproteinases (MMPs), and improve adhesive infiltration. This protocol could be promising in achieving the desired adhesive-tooth bonding interface to incomplete infiltration and microleakage and preventing secondary caries, thereby encompassing the durability of adhesives [346,347,348]. In wet bonding, water can be a reason for phase separation. In addition, water-suspended dentin collagen acts as a barrier. As a result, incomplete infiltration may occur. Ethanol-wet bonding protocol was reported using ethanol instead of water in etch-and-rinse procedures for adhesive application. [349,350] This process prevents hydrolysis and inhibits MMP activities [351].
5.2. Advancements in Biomaterials
As the amalgam phases out, resin restorative becomes a promising replacement. The advanced biomaterials will be easy to apply, error-forgiving, biocompatible, and high-performance. These materials focus on biocompatibility while being functional and durable. They maintain the integrity of the collagen structure and enhance restorations’ aesthetic and functional outcomes [352,353].
Next-generation dental adhesives will be multifunctional, preventing the key challenges that induce secondary caries. These challenges include hydrolysis of the resin network, cariogenic bacteria attack, and stresses related to polymerization shrinkage and mechanical forces. In addition to the new hydrolytically stable resins discussed in the previous section, antimicrobial potential is desirable. Moreover, self-healing or self-repairing functions that may recognize microcracks and other defects and repair them autonomously without human intervention will be helpful. Innovative materials have been proposed to achieve self-healing functions through double network chemistry, self-healing design and bioactive fillers [354]. These new developments will also correct the suboptimal placement of resin restoration, thus significantly enhancing resin restorative’s applicability in underdeveloped areas with limited resources and inadequately skilled health professionals.
Biomimetic materials are the new era for designing and developing dental adhesives [355,356]. Biomimetic materials in dentistry are designed to mimic the natural structure and function of tooth tissues, such as enamel and dentin. These materials aim to replicate the mechanical, chemical, and biological properties of natural teeth to restore function and aesthetics while promoting integration with the surrounding tissues [357,358]. For example, mussels’ moisture-resistant adhesion properties and firm surface coating ability have impressed dental research. A recent study investigated mussel-functionalized catechol-thiol-based dental adhesives, a catechol-functionalized copolymer, poly (dopamine-methacrylate-co2-methoxyetheyl acrylate) (pDMA-MEA) was analyzed as a primer for an etch-and-rinse adhesive and reported to improve the bond strength in a saliva-contaminated condition [359]. Also, Biomimetic composites that mimic the layered structure of enamel and dentin [357,360,361,362,363].
However, controversies and challenges remain. Some studies question the long-term effectiveness of ion release and remineralization potential, as the bioactive properties may diminish over time [363,364]. Balancing bioactivity with mechanical strength and durability is another significant challenge, as these materials often exhibit inferior mechanical properties compared to traditional resins, limiting their use in high-stress areas [1,3]. Additionally, there is a need for more long-term clinical studies to validate the benefits and safety of bioactive adhesive resins, as current evidence is often limited to in vitro or short-term in vivo studies [365]. Addressing these challenges is crucial for widely adopting these materials in clinical practice.
5.3. Dental Adhesives in Digital Dentistry
Dental resin adhesives are critical in enhancing digital dentistry by ensuring strong and durable bonding for CAD/CAM and 3D-printed restorations [79,366]. The key characteristic of dental adhesives for digital dentistry is their versatility and reliability in bonding to a wide range of materials used in digitally fabricated restorations, such as ceramics, composites, and 3D-printed resins. These adhesives must provide strong and durable bonds, ensuring long-lasting adhesion and precise marginal sealing to prevent microleakage. They support fast curing times to streamline workflows for same-day procedures and offer aesthetic stability by maintaining color and translucency. Universal formulations simplify application, while bioactive or antibacterial properties enhance tooth health. Together, these features ensure efficient, precise, and predictable outcomes in digital dentistry.
5.4. AI and Machine Learning for Dental Adhesive Development
Machine learning (ML) and artificial intelligence (AI) will significantly advance the development of dental adhesives by optimizing formulations, accelerating testing, enhancing performance, and personalizing treatments. By leveraging existing data on methacrylate-based dental adhesives, materials informatics (MI) in dental materials research applies computational techniques, particularly ML, to analyze and predict material properties, streamlining the discovery and development of dental restorative materials like resin composites, glass ceramics, and luting cement. Utilizing supervised learning models, MI algorithms identify optimal chemical compositions and enhance bonding strength, durability, and biocompatibility properties. Despite its potential, MI faces challenges, including the complexity of synthesizing new materials and limited integration with manufacturing processes. Future efforts aim to combine MI with process informatics (PI) and autonomous systems to optimize material design and production, supported by open-access databases and advanced robotics [367,368]. AI- and ML-powered virtual testing methods can simulate and forecast adhesive behavior under various clinical conditions, minimizing the need for time-consuming trial-and-error experiments. AI-driven research can also uncover new bioactive or antibacterial agents that enhance tooth health and help prevent secondary caries [367]. Additionally, AI can enable the customization of adhesives to individual patient needs by considering factors such as tooth structure, material properties, and clinical performance [369]. This data-driven approach accelerates innovation, shortens development timelines, and ensures the production of more reliable, efficient adhesives tailored to the demands of modern digital dentistry workflows [369,370]. Future directions emphasize the creation of sustainable, open-access databases and leveraging autonomous systems to advance both material discovery and production [368].
6. Limitations and Conclusions
This review has several limitations. First, a significant portion of the evidence presented is derived from in vitro studies, which may not fully replicate the complex oral environment. For instance, laboratory studies have demonstrated the hydrolytic stability of TEG-DVBE-based resins and the antibacterial efficacy of silver nanoparticle-doped adhesives. However, long-term clinical trials need to validate these findings to assess their performance under real-world conditions. Second, the rapid pace of innovation in dental adhesives means that some emerging technologies, such as AI-driven adhesive development and bioinspired adhesives, are still in their early stages. While AI has shown promise in optimizing adhesive formulations and predicting clinical outcomes, its application in dentistry is limited by the lack of large, high-quality datasets and the potential for algorithmic bias [368]. Similarly, bioinspired adhesives, such as those mimicking mussel adhesion proteins, offer exciting possibilities but require further research to address challenges related to scalability and biocompatibility [359]. Third, the review primarily focuses on methacrylate-based adhesives dominating the current market. However, alternative materials, such as siloranes and thiourethanes, have shown potential for improving bond durability and reducing polymerization shrinkage. Future studies should explore these materials in greater depth, particularly in the context of digital dentistry and 3D-printed restorations. Finally, the review highlights the importance of functional monomers, such as 10-MDP, in enhancing bond strength and durability. However, the long-term stability of these monomers in the oral environment remains a concern, as hydrolytic degradation and enzymatic activity can compromise the adhesive interface over time [36]. Addressing these challenges will require a multidisciplinary approach, combining advances in materials science, digital technologies, and clinical research.
In conclusion, the development of dental adhesives reflects a dedication to continuous innovation and improved patient care, paving the way for more effective, durable, and predictable dental restorations in the future. By addressing the limitations outlined above and embracing emerging technologies, researchers and clinicians can further advance the field of adhesive dentistry, ultimately benefiting patients through enhanced oral health outcomes.
Author Contributions
Conceptualization, W.K.A., M.Z.I.N., H.H.K.X. and J.S.; methodology, W.K.A., M.Z.I.N. and J.S.; validation, W.K.A., M.Z.I.N., H.H.K.X. and J.S.; formal analysis, W.K.A., M.Z.I.N. and J.S.; investigation, W.K.A., M.Z.I.N. and J.S.; resources, H.H.K.X. and J.S.; data curation, H.H.K.X. and J.S.; writing—original draft preparation, W.K.A.; writing—review and editing, W.K.A., M.Z.I.N., H.H.K.X. and J.S.; visualization, W.K.A. and J.S.; supervision, J.S.; project administration, J.S.; funding acquisition, W.K.A., M.Z.I.N., H.H.K.X. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institute of Dental and Craniofacial Research, grant number [RM1DE034233 and R01DE033442A], and also by the ADA Forsyth Institute, grant number [NIH R90DE027638].
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Acronym and Abbreviation List
| 10-MDP: 10-methacryloyloxydecyl dihydrogenphosphate A174: γ-methacryloxypropyltrimethoxysilane Coupling factor A174: g-methacryloxypropyltrimethoxysilane 2EMATE-BDI: 2-hydroxy-1-ethyl methacrylate 4-AETA: 4-acryloyloxyethyl trimellitate anhydride 4-AET: 4-acryloylethyl trimellitic acid 4-META: 4-methacryloyloxyethyl trimellitate anhydride 4-MET: 4-methacryloyloxyethyl trimellitic acid AMPS: 2-acrylamido-2-methyl-1-propanesulfonic acid BAADA: N,N′-bis(acrylamido) 1,4-diazepane Bis-EMA: ethoxylated bisphenol A glycol dimethacrylate Bis-GMA: bisphenol A diglycidyl methacrylate Bis-MEP: bis[2-(methacryloyloxy)ethyl] phosphate BMAAPMA: N,N-Bis[(3-methylaminoacryl)propyl]methylamine BPDM: biphenyl dimethacrylate or 4,40-dimethacryloyloxyethyloxycarbonylbiphenyl-3,30-dicarboxylic acid BPO: benzoyl peroxide (redox initiator) BS acid: benzenesulfinic acid sodium salt (redox initiator) CQ: camphorquinone or camphoroquinone or 1.7.7-trimethylbicyclo-[2,2,1]-hepta-2,3-dione (photo-initiator) DC: Double bond conversion DEBAAP: N,N-Diethyl-1,3-bis(acrylamido)propane Di-HEMA phosphate: di-2-hydroxyethyl methacryl hydrogenphosphate DMAEMA: dimethylaminoethyl methacrylate EAEPA: ethyl 2-[4-(dihydroxyphosphoryl)-2-oxabutyl]acrylate EGDMA: ethyleneglycol dimethacrylate EM: Elastic modulus F-PRG: full reaction type pre-reacted glass-ionomer fillers FBMA: fluorinated diluent 1 H,1 H-heptafluorobutyl methacrylate FDMA: fluorinated dimethacrylate FM: Flexural modulus FS: Flexural strength GDMA: glycerol dimethacrylate GPDM: glycerol phosphate dimethacrylate HDDMA: 1,6-hexanediol dimethacrylate HEMA: 2-hydroxyethyl methacrylate IBMA: isobornyl methacrylate MA: methacrylic acid MAC-10: 11-methacryloyloxy-1,10-undecanedicarboxylic acid MAEPA: 2,4,6 trimethylphenyl 2-[4-(dihydroxyphosphoryl)-2-oxabutyl]acrylate MDPB: methacryloyloxydodecylpyridinium bromide MF8P: 6-methacryloxy-2,2,3,3,4,4,5,5-octafluorohexyl dihydrogen phosphate NaF: sodium fluoride Na2SiF6: disodium hexafluorosilicate NPG-GMA: N-phenylglycine glycidyl methacrylate NTG-GMA: N-tolylglycine glycidyl methacrylate or N-(2-hydroxy-3-((2-methyl-1-oxo-2-propenyl)oxy)propyl)-N-tolyl glycine PEGDMA: polyethylene glycol dimethacrylate PEM-F: pentamethacryloyloxyethylcyclohexaphosphazene monofluoride PENTA: dipentaerythritol pentaacrylate monophosphate Phenyl-P: 2-(methacryloyloxyethyl)phenyl hydrogenphosphate PMDM: pyromellitic diethylmethacrylate or 2,5-dimethacryloyloxyethyloxycarbonyl-1,4-benzenedicarboxylic acid PMGDM: pyromellitic glycerol dimethacrylate or 2,5-bis(1,3-dimethacryloyloxyprop-2-yloxycarbonyl)benzene-1,4-dicarboxylic acid POSS nano-particulates: polyhedral oligomer silsesquioxanes QAUDMA-m: quaternary ammonium urethane-dimethacrylate derivative (QAUDMA-m, where m was 8, 10, 12, 14, 16, 18, and corresponded to the number of carbon atoms in the N-alkyl substituent) SBS: Shear bond strength SiO2 nanofiber fillers: silicon dioxide nanofibers TBS: Tensile bond strength TE-EGDMA: tetraethylene glycol dimethacrylate TEG-DVBE: triethylene glycol divinylbenzyl ether TEGDMA: triethylene glycol dimethacrylate TMAAEA: Tris[(2-methylaminoacryl)ethyl]amine TMPTMA: trimethylolpropane trimethacrylate UDMA: urethane dimethacrylate or 1,6-di(methacryloyloxyethylcarbamoyl)-3,30,5-trimethylhexane |
| VS: Volumetric shrinkage WS: Water sorption WSL: Water solubility |
References
- Ferracane, J.L. Resin composite--state of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Heintze, S.D.; Loguercio, A.D.; Hanzen, T.A.; Reis, A.; Rousson, V. Clinical efficacy of resin-based direct posterior restorations and glass-ionomer restorations—An updated meta-analysis of clinical outcome parameters. Dent. Mater. 2022, 38, e109–e135. [Google Scholar] [CrossRef]
- Hofsteenge, J.W.; Scholtanus, J.D.; Özcan, M.; Nolte, I.M.; Cune, M.S.; Gresnigt, M.M.M. Clinical longevity of extensive direct resin composite restorations after amalgam replacement with a mean follow-up of 15 years. J. Dent. 2023, 130, 104409. [Google Scholar] [CrossRef]
- Scholz, K.J.; Tabenski, I.M.; Vogl, V.; Cieplik, F.; Schmalz, G.; Buchalla, W.; Hiller, K.A.; Federlin, M. Randomized clinical split-mouth study on the performance of CAD/CAM-partial ceramic crowns luted with a self-adhesive resin cement or a universal adhesive and a conventional resin cement after 39 months. J. Dent. 2021, 115, 103837. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; De Munck, J.; Maruo, Y.; Nishigawa, G.; Minagi, S.; Osaka, A.; et al. Adhesive interfacial interaction affected by different carbon-chain monomers. Dent. Mater. 2013, 29, 888–897. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; Yoshida, Y.; Van Meerbeek, B. Chemical interaction of glycero-phosphate dimethacrylate (GPDM) with hydroxyapatite and dentin. Dent. Mater. 2018, 34, 1072–1081. [Google Scholar] [CrossRef]
- Sebold, M.; André, C.B.; Sahadi, B.O.; Breschi, L.; Giannini, M. Chronological history and current advancements of dental adhesive systems development: A narrative review. J. Adhes. Sci. Technol. 2021, 35, 1941–1967. [Google Scholar] [CrossRef]
- Thomé, T.; Erhardt, M.C.; Leme, A.A.; Al Bakri, I.; Bedran-Russo, A.K.; Bertassoni, L.E. Emerging polymers in dentistry. Adv. Polym. Med. 2015, 265–296. [Google Scholar] [CrossRef]
- Zhang, K.; Baras, B.; Lynch, C.D.; Weir, M.D.; Melo, M.A.S.; Li, Y.; Reynolds, M.A.; Bai, Y.; Wang, L.; Wang, S.; et al. Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth. Materials 2018, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Mount, G.J.; Hume, W.R.; Ngo, H.C.; Wolff, M.S. Preservation and Restoration of Tooth Structure; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Buonocore, M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef]
- Retief, D.H. Effect of conditioning the enamel surface with phosphoric acid. J. Dent. Res. 1973, 52, 333–341. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Nakamura, M.; Yasuda, N. Hybrid layer as a dentin-bonding mechanism. J. Esthet. Dent. 1991, 3, 133–138. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Pashley, D.H. Hybridization of Dental Hard Tissues; Quintessence Publishing Company: Berlin, Germany, 1998. [Google Scholar]
- Kanca, J., 3rd. Improving bond strength through acid etching of dentin and bonding to wet dentin surfaces. J. Am. Dent. Assoc. 1992, 123, 35–43. [Google Scholar] [CrossRef]
- Fusayama, T.; Nakamura, M.; Kurosaki, N.; Iwaku, M. Non-pressure adhesion of a new adhesive restorative resin. J. Dent. Res. 1979, 58, 1364–1370. [Google Scholar] [CrossRef]
- Gwinnett, A.J. Moist versus dry dentin: Its effect on shear bond strength. Am. J. Dent. 1992, 5, 127–129. [Google Scholar]
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Current trends in dental composites. Crit. Rev. Oral Biol. Med. 1995, 6, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Swift, E.J., Jr. Universal Adhesives. J. Esthet. Restor. Dent. 2015, 27, 331–334. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef]
- Almeida, R.; Manarte-Monteiro, P.; Domingues, J.; Falcao, C.; Herrero-Climent, M.; Rios-Carrasco, B.; Lemos, B.F. High-Power LED Units Currently Available for Dental Resin-Based Materials—A Review. Polymers 2021, 13, 2165. [Google Scholar] [CrossRef]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An application of nanotechnology in advanced dental materials. J. Am. Dent. Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 2008, 29, 1127–1137. [Google Scholar] [CrossRef]
- Dey, S.; Shenoy, A.; Kundapur, S.S.; Das, M.; Gunwal, M.; Bhattacharya, R. Evaluation of the effect of different contaminants on the shear bond strength of a two-step self-etch adhesive system, one-step, self-etch adhesive system and a total-etch adhesive system. J. Int. Oral Health 2016, 8, 378. [Google Scholar] [CrossRef]
- Armstrong, S.R.; Vargas, M.A.; Fang, Q.; Laffoon, J.E. Microtensile bond strength of a total-etch 3-step, total-etch 2-step, self-etch 2-step, and a self-etch 1-step dentin bonding system through 15-month water storage. J. Adhes. Dent. 2003, 5, 47–56. [Google Scholar]
- De Munck, J.; Van Meerbeek, B.; Yoshida, Y.; Inoue, S.; Vargas, M.; Suzuki, K.; Lambrechts, P.; Vanherle, G. Four-year water degradation of total-etch adhesives bonded to dentin. J. Dent. Res. 2003, 82, 136–140. [Google Scholar] [CrossRef]
- Valsan, D.; Bhaskaran, S.; Mathew, J.; Hari, K.; Joy, J. Comparative Evaluation of the Bonding Efficacy of Multimode Adhesive, Two-Step Self-Etch Adhesive, and a Total-Etch System to Pulpal Floor Dentin—An In vitro Study. Contemp. Clin. Dent. 2023, 14, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Mutluay, A.T.; Mutluay, M. Characterisation of the calcium silicate-based cement–composite interface and the bonding strength with total-etch or single/two-stage self-etch adhesive systems. Aust. Endod. J. 2022, 48, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Makishi, P.; Ayres, A.P.; Vermelho, P.M.; Fronza, B.M.; Nikaido, T.; Tagami, J. Self-etch adhesive systems: A literature review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s pioneering acid-etch technique to self-adhering restoratives. A status perspective of rapidly advancing dental adhesive technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- McLean, J.W. The pioneers of enamel and dentin bonding. J. Adhes. Dent. 1999, 1, 185–187. [Google Scholar]
- Kramer, I.; McLean, J. The response of the human pulp to self-polymerising acrylic. Br. Dent. J. 1952, 93, 150–153. [Google Scholar]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kugel, G.; Ferrari, M. The science of bonding: From first to sixth generation. J. Am. Dent. Assoc. 2000, 131, 20S–25S. [Google Scholar] [CrossRef]
- Swift, E.J., Jr.; Perdigao, J.; Heymann, H.O. Bonding to enamel and dentin: A brief history and state of the art, 1995. Quintessence Int. 1995, 26, 95–110. [Google Scholar]
- Buonocore, M.G.; Matsui, A.; Gwinnett, A.J. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch. Oral Biol. 1968, 13, 61–70. [Google Scholar] [CrossRef]
- Broome, J.; Duke, E.; Norling, B. Shear bond strengths of composite resin with three dentin adhesives. J. Dent. Res. 1985, 64, 244. [Google Scholar]
- Perdigao, J.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G.; Lopes, A.L. Field emission SEM comparison of four postfixation drying techniques for human dentin. J. Biomed. Mater. Res. 1995, 29, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Kanca, J., 3rd. Resin bonding to wet substrate. 1. Bonding to dentin. Quintessence Int. 1992, 23, 39–41. [Google Scholar] [PubMed]
- Tao, L.; Pashley, D.H.; Boyd, L. Effect of different types of smear layers on dentin and enamel shear bond strengths. Dent. Mater. 1988, 4, 208–216. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between bond-strength tests and clinical outcomes. Dent. Mater. 2010, 26, e100–e121. [Google Scholar] [CrossRef]
- De Munck, J.d.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Leinfelder, K.F. Dentin adhesives for the twenty-first century. Dent. Clin. N. Am. 2001, 45, 1–6. [Google Scholar] [CrossRef]
- Alex, G. Adhesive considerations in the placement of direct composite restorations. Oral Health 2008, 98, 109–119. [Google Scholar]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Fernandes, C.A.; Tay, F. The microtensile bond test: A review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar]
- Watanabe, I.; Nakabayashi, N.; Pashley, D.H. Bonding to ground dentin by a phenyl-P self-etching primer. J. Dent. Res. 1994, 73, 1212–1220. [Google Scholar] [CrossRef]
- Hashimoto, M.; Fujita, S.; Endo, K.; Ohno, H. In vitro degradation of resin-dentin bonds with one-bottle self-etching adhesives. Eur. J. Oral. Sci. 2009, 117, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Have dentin adhesives become too hydrophilic? J.-Can. Dent. Assoc. 2003, 69, 726–732. [Google Scholar] [PubMed]
- Yaseen, S.M.; Reddy, V.S. Comparative evaluation of shear bond strength of two self-etching adhesives (sixth and seventh generation) on dentin of primary and permanent teeth: An in vitro study. J. Indian. Soc. Pedod. Prev. Dent. 2009, 27, 33. [Google Scholar] [CrossRef]
- Mishra, A.; Koul, M.; Upadhyay, V.K.; Abdullah, A. A Comparative Evaluation of Shear Bond Strength of Seventh- and Eighth-Generation Self-etch Dentin Bonding Agents in Primary Teeth: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2020, 13, 225–229. [Google Scholar] [CrossRef]
- Sanares, A.M.; Itthagarun, A.; King, N.M.; Tay, F.R.; Pashley, D.H. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent. Mater. 2001, 17, 542–556. [Google Scholar] [CrossRef]
- Nair, M.; Paul, J.; Kumar, S.; Chakravarthy, Y.; Krishna, V. Comparative evaluation of the bonding efficacy of sixth and seventh generation bonding agents: An In-Vitro study. J. Conserv. Dent. JCD 2014, 17, 27. [Google Scholar] [CrossRef]
- Rosa, W.L.; Piva, E.; Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Bedran-Russo, A.; Leme-Kraus, A.A.; Vidal, C.M.P.; Teixeira, E.C. An Overview of Dental Adhesive Systems and the Dynamic Tooth-Adhesive Interface. Dent. Clin. N. Am. 2017, 61, 713–731. [Google Scholar] [CrossRef]
- Elias, S.T.; Santos, A.F.d.; Garcia, F.C.; Pereira, P.N.; Hilgert, L.A.; Fonseca-Bazzo, Y.M.; Guerra, E.N.; Ribeiro, A.P.D. Cytotoxicity of universal, self-etching and etch-and-rinse adhesive systems according to the polymerization time. Braz. Dent. J. 2015, 26, 160–168. [Google Scholar] [CrossRef]
- Perdigao, J.; Kose, C.; Mena-Serrano, A.P.; De Paula, E.A.; Tay, L.Y.; Reis, A.; Loguercio, A.D. A new universal simplified adhesive: 18-month clinical evaluation. Oper. Dent. 2014, 39, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R. Adhesive bonding of various materials to hard tooth tissues. II. Bonding to dentin promoted by a surface-active comonomer. J. Dent. Res. 1965, 44, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Janani, A.; Majidinia, S.; Sadeghi, R.; Shirazi, A.S. Are self-etch adhesives reliable for primary tooth dentin? A systematic review and meta-analysis. J. Conserv. Dent. 2018, 21, 243–250. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Meerbeek, B.V. Self-assembled nano-layering at the adhesive interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef]
- Wagner, A.; Wendler, M.; Petschelt, A.; Belli, R.; Lohbauer, U. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014, 42, 800–807. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Dental adhesives of the future. J. Adhes. Dent. 2002, 4, 91. [Google Scholar]
- Fehrenbach, J.; Lacerda-Santos, R.; Machado, L.S.; Miotti, L.L.; de Carvalho, F.G.; Munchow, E.A. Which self-etch acidic composition may result in higher dental bonds at the long-term? A network meta-analysis review of in vitro studies. J. Dent. 2022, 126, 104283. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Pashley, D.H.; Sano, H.; Ciucchi, B.; Yoshiyama, M.; Carvalho, R.M. Adhesion testing of dentin bonding agents: A review. Dent. Mater. 1995, 11, 117–125. [Google Scholar] [CrossRef]
- Haller, B. Recent developments in dentin bonding. Am. J. Dent. 2000, 13, 44–50. [Google Scholar]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjaderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, J.W.; Sunnegardh-Gronberg, K.; Lindberg, A. Clinical long-term retention of etch-and-rinse and self-etch adhesive systems in non-carious cervical lesions. A 13 years evaluation. Dent. Mater. 2007, 23, 1101–1107. [Google Scholar] [CrossRef]
- Nishitani, Y.; Yoshiyama, M.; Donnelly, A.M.; Agee, K.A.; Sword, J.; Tay, F.R.; Pashley, D.H. Effects of resin hydrophilicity on dentin bond strength. J. Dent. Res. 2006, 85, 1016–1021. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Swift, E.J., Jr. Dentin/enamel adhesives: Review of the literature. Pediatr. Dent. 2002, 24, 456–461. [Google Scholar]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- Pulgar, R.; Olea-Serrano, M.F.; Novillo-Fertrell, A.; Rivas, A.; Pazos, P.; Pedraza, V.; Navajas, J.-M.; Olea, N. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000, 108, 21–27. [Google Scholar] [CrossRef]
- Soderholm, K.J.; Mariotti, A. BIS-GMA--based resins in dentistry: Are they safe? J. Am. Dent. Assoc. 1999, 130, 201–209. [Google Scholar] [CrossRef]
- Arenholt-Bindslev, D.; Breinholt, V.; Preiss, A.; Schmalz, G. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin. Oral. Investig. 1999, 3, 120–125. [Google Scholar] [CrossRef]
- Schmalz, G.; Preiss, A.; Arenholt-Bindslev, D. Bisphenol-A content of resin monomers and related degradation products. Clin. Oral Investig. 1999, 3, 114–119. [Google Scholar] [CrossRef]
- Izumi, Y.; Yamaguchi, K.; Ishikawa, T.; Ando, M.; Chiba, K.; Hashimoto, H.; Shiotani, M.; Fujisawa, M. Molecular changes induced by bisphenol-A in rat Sertoli cell culture. Syst. Biol. Reprod. Med. 2011, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Massaro, H.; Zambelli, L.F.A.; Britto, A.A.; Vieira, R.P.; Ligeiro-de-Oliveira, A.P.; Andia, D.C.; Oliveira, M.T.; Lima, A.F. Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials 2019, 12, 2750. [Google Scholar] [CrossRef] [PubMed]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. Structure-property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dent. Mater. 2009, 25, 1082–1089. [Google Scholar] [CrossRef]
- Finger, W.J.; Fritz, U.B. Resin bonding to enamel and dentin with one-component UDMA/HEMA adhesives. Eur. J. Oral Sci. 1997, 105, 183–186. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Influence of UEDMA BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent. Mater. 1998, 14, 51–56. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Polikowski, A.; Krasowski, M.; Fronczek, M.; Sokolowski, J.; Bociong, K. The Influence of Low-Molecular-Weight Monomers (TEGDMA, HDDMA, HEMA) on the Properties of Selected Matrices and Composites Based on Bis-GMA and UDMA. Materials 2022, 15, 2649. [Google Scholar] [CrossRef]
- Becher, R.; Kopperud, H.M.; Al, R.H.; Samuelsen, J.T.; Morisbak, E.; Dahlman, H.J.; Lilleaas, E.M.; Dahl, J.E. Pattern of cell death after in vitro exposure to GDMA, TEGDMA, HEMA and two compomer extracts. Dent. Mater. 2006, 22, 630–640. [Google Scholar] [CrossRef]
- Araujo-Neto, V.G.; Nobre, C.F.A.; De Paula, D.M.; Souza, L.C.; Silva, J.C.; Moreira, M.M.; Picanco, P.R.B.; Feitosa, V.P. Glycerol-dimethacrylate as alternative hydrophilic monomer for HEMA replacement in simplified adhesives. J. Mech. Behav. Biomed. Mater. 2018, 82, 95–101. [Google Scholar] [CrossRef]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Yoshioka, M.; Snauwaert, J.; Abe, Y.; Lambrechts, P.; Vanherle, G.; Okazaki, M. Adhesion to and decalcification of hydroxyapatite by carboxylic acids. J. Dent. Res. 2001, 80, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, Y.; Tay, F.R. Bonding ability of 4-META self-etching primer used with 4-META/MMA-TBB resin to enamel and dentine: Primary vs permanent teeth. J. Dent. 2014, 42, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Nagakane, K.; Yoshida, Y.; Hirata, I.; Fukuda, R.; Nakayama, Y.; Shirai, K.; Ogawa, T.; Suzuki, K.; Van Meerbeek, B.; Okazaki, M. Analysis of chemical interaction of 4-MET with hydroxyapatite using XPS. Dent. Mater. J. 2006, 25, 645–649. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Matsumoto, T.; Minagi, S.; Osaka, A.; Van Landuyt, K.; Van Meerbeek, B. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J. Dent. Res. 2012, 91, 1060–1065. [Google Scholar] [CrossRef]
- Nurrohman, H.; Nakashima, S.; Takagaki, T.; Sadr, A.; Nikaido, T.; Asakawa, Y.; Uo, M.; Marshall, S.J.; Tagami, J. Immobilization of phosphate monomers on collagen induces biomimetic mineralization. Biomed. Mater. Eng. 2015, 25, 89–99. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yoshinari, M.; Sakae, T.; Nemoto, K. Calcium phosphate formation on the phosphorylated dental bonding agent in electrolyte solution. J. Oral. Rehabil. 2004, 31, 67–73. [Google Scholar] [CrossRef]
- Teshima, I. Degradation of 10-methacryloyloxydecyl dihydrogen phosphate. J. Dent. Res. 2010, 89, 1281–1286. [Google Scholar] [CrossRef]
- Andre, C.B.; Gomes, B.P.; Duque, T.M.; Stipp, R.N.; Chan, D.C.; Ambrosano, G.M.; Giannini, M. Dentine bond strength and antimicrobial activity evaluation of adhesive systems. J. Dent. 2015, 43, 466–475. [Google Scholar] [CrossRef]
- Inoue, S.; Koshiro, K.; Yoshida, Y.; De Munck, J.; Nagakane, K.; Suzuki, K.; Sano, H.; Van Meerbeek, B. Hydrolytic stability of self-etch adhesives bonded to dentin. J. Dent. Res. 2005, 84, 1160–1164. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Achilias, D.S. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.J.; Dickens, S.H. Network structure of Bis-GMA- and UDMA-based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.; De Munck, J.; Snauwaert, J.; Coutinho, E.; Poitevin, A.; Yoshida, Y.; Inoue, S.; Peumans, M.; Suzuki, K.; Lambrechts, P. Monomer-solvent phase separation in one-step self-etch adhesives. J. Dent. Res. 2005, 84, 183–188. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y.; Walker, M.P.; Wieliczka, D.M.; Swafford, J.R. Interfacial chemistry of the dentin/adhesive bond. J. Dent. Res. 2000, 79, 1458–1463. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Dynamic thermomechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent. Mater. 2008, 24, 737–743. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Stansbury, J.; Burke, F.J.T. Self-adhesive resin cements–chemistry, properties and clinical considerations. J. Oral Rehabil. 2011, 38, 295–314. [Google Scholar] [CrossRef]
- Elshereksi, N.W.; Ghazali, M.; Muchtar, A.; Azhari, C.H. Review of titanate coupling agents and their application for dental composite fabrication. Dent. Mater. J. 2017, 36, 539–552. [Google Scholar] [CrossRef]
- Ikemura, K.; Tay, F.R.; Endo, T.; Pashley, D.H. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dent. Mater. J. 2008, 27, 315–339. [Google Scholar] [CrossRef]
- Paffenbarger, G.C. Dental cements, direct filling resins, composite and adhesive restorative materials: A resume. J. Biomed. Mater. Res. 1972, 6, 363–393. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K. Clinical recipe for durable dental bonding: Why and how? J. Adhes. Dent. 2014, 16, 94. [Google Scholar]
- Nikaido, T.; Ichikawa, C.; Li, N.; Takagaki, T.; Sadr, A.; Yoshida, Y.; Suzuki, K.; Tagami, J. Effect of functional monomers in all-in-one adhesive systems on formation of enamel/dentin acid-base resistant zone. Dent. Mater. J. 2011, 30, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Han, F.; Yuan, X.; Chen, C. Effects of Solvents and pH Values on the Chemical Affinity of 10-Methacryloyloxydecyl Dihydrogen Phosphate toward Hydroxyapatite. ACS Omega 2021, 6, 19183–19193. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Kamenoue, S.; Okihara, T.; Ogawa, T.; Nakamura, M.; Osaka, A.; Van Meerbeek, B. Novel fluoro-carbon functional monomer for dental bonding. J. Dent. Res. 2014, 93, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Atomic level observation and structural analysis of phosphoric-acid ester interaction at dentin. Acta Biomater. 2019, 97, 544–556. [Google Scholar] [CrossRef]
- Tian, F.C.; Wang, X.Y.; Huang, Q.; Niu, L.N.; Mitchell, J.; Zhang, Z.Y.; Prananik, C.; Zhang, L.; Chen, J.H.; Breschi, L.; et al. Effect of nanolayering of calcium salts of phosphoric acid ester monomers on the durability of resin-dentin bonds. Acta Biomater. 2016, 38, 190–200. [Google Scholar] [CrossRef]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Ogliari, A.O.; Yoshihara, K.; Zanchi, C.H.; Correr-Sobrinho, L.; Sinhoreti, M.A.; Correr, A.B.; Watson, T.F.; et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent. Mater. 2014, 30, e317–e323. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Q.; Zhao, Q.; Bai, Z.; Chen, C.; Xie, H. Inhibition of matrix metalloproteinase-9 by 10-MDP and its calcium salt contributes to improved dentin-bonding durability. Int. J. Adhes. Adhes. 2023, 120, 103302. [Google Scholar] [CrossRef]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Stansbury, J.W.; Carpenter, G.H.; Watson, T.F.; Sinhoreti, M.A.; Correr, A.B. The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J. Dent. 2014, 42, 565–574. [Google Scholar] [CrossRef]
- Mokeem, L.S.; Garcia, I.M.; Melo, M.A. Degradation and Failure Phenomena at the Dentin Bonding Interface. Biomedicines 2023, 11, 1256. [Google Scholar] [CrossRef]
- Engelmann, J.; Janke, V.; Volk, J.; Leyhausen, G.; von Neuhoff, N.; Schlegelberger, B.; Geurtsen, W. Effects of BisGMA on glutathione metabolism and apoptosis in human gingival fibroblasts in vitro. Biomaterials 2004, 25, 4573–4580. [Google Scholar] [CrossRef]
- Geurtsen, W.; Lehmann, F.; Spahl, W.; Leyhausen, G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J. Biomed. Mater. Res. 1998, 41, 474–480. [Google Scholar] [CrossRef]
- Geurtsen, W.; Spahl, W.; Muller, K.; Leyhausen, G. Aqueous extracts from dentin adhesives contain cytotoxic chemicals. J. Biomed. Mater. Res. 1999, 48, 772–777. [Google Scholar] [CrossRef]
- Lewis, J.B.; Rueggeberg, F.A.; Lapp, C.A.; Ergle, J.W.; Schuster, G.S. Identification and characterization of estrogen-like components in commercial resin-based dental restorative materials. Clin. Oral. Investig. 1999, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Ishibashi, H.; Miyahara, M.; Shinohara, R.; Shiraishi, F.; Arizono, K. Effects of dental resin metabolites on estrogenic activity in vitro. J. Mater. Sci. Mater. Med. 2003, 14, 307–310. [Google Scholar] [CrossRef]
- De Nys, S.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Bisphenol A as degradation product of monomers used in resin-based dental materials. Dent. Mater. 2021, 37, 1020–1029. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.M.; Birnbaum, L.S.; Crain, D.A.; Eriksen, M.; Farabollini, F.; Guillette, L.J., Jr.; Hauser, R.; Heindel, J.J.; et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007, 24, 131–138. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Zhang, L.; Weir, M.D.; Chow, L.C.; Antonucci, J.M.; Chen, J.; Xu, H.H. Novel rechargeable calcium phosphate dental nanocomposite. Dent. Mater. 2016, 32, 285–293. [Google Scholar] [CrossRef]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J., Jr. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef]
- Joskow, R.; Barr, D.B.; Barr, J.R.; Calafat, A.M.; Needham, L.L.; Rubin, C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J. Am. Dent. Assoc. 2006, 137, 353–362. [Google Scholar] [CrossRef]
- Gayrard, V.; Lacroix, M.Z.; Collet, S.H.; Viguié, C.; Bousquet-Melou, A.; Toutain, P.-L.; Picard-Hagen, N. High bioavailability of bisphenol A from sublingual exposure. Environ. Health Perspect. 2013, 121, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Koin, P.J.; Kilislioglu, A.; Zhou, M.; Drummond, J.L.; Hanley, L. Analysis of the degradation of a model dental composite. J. Dent. Res. 2008, 87, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.-H.; Huang, F.-M.; Lee, S.-S.; Li, Y.-C.; Chang, Y.-C. Bisgma stimulates prostaglandin E2 production in macrophages via cyclooxygenase-2, cytosolic phospholipase A2, and mitogen-activated protein kinases family. PLoS ONE 2013, 8, e82942. [Google Scholar] [CrossRef] [PubMed]
- Sadeghinejad, L.; Cvitkovitch, D.G.; Siqueira, W.L.; Merritt, J.; Santerre, J.P.; Finer, Y. Mechanistic, genomic and proteomic study on the effects of BisGMA-derived biodegradation product on cariogenic bacteria. Dent. Mater. 2017, 33, 175–190. [Google Scholar] [CrossRef]
- Sadeghinejad, L.; Cvitkovitch, D.G.; Siqueira, W.L.; Santerre, J.P.; Finer, Y. Triethylene Glycol Up-Regulates Virulence-Associated Genes and Proteins in Streptococcus mutans. PLoS ONE 2016, 11, e0165760. [Google Scholar] [CrossRef]
- Li, Y.C.; Kuan, Y.H.; Huang, F.M.; Chang, Y.C. The role of DNA damage and caspase activation in cytotoxicity and genotoxicity of macrophages induced by bisphenol-A-glycidyldimethacrylate. Int. Endod. J. 2012, 45, 499–507. [Google Scholar] [CrossRef]
- Chang, M.C.; Chen, L.I.; Chan, C.P.; Lee, J.J.; Wang, T.M.; Yang, T.T.; Lin, P.S.; Lin, H.J.; Chang, H.H.; Jeng, J.H. The role of reactive oxygen species and hemeoxygenase-1 expression in the cytotoxicity, cell cycle alteration and apoptosis of dental pulp cells induced by BisGMA. Biomaterials 2010, 31, 8164–8171. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Watanabe, A.; Gendusa, N. Dentin adhesion of “modified” 4-META/MMA-TBB resin: Function of HEMA. Dent. Mater. 1992, 8, 259–264. [Google Scholar] [CrossRef]
- Nakaoki, Y.; Nikaido, T.; Pereira, P.; Inokoshi, S.; Tagami, J. Dimensional changes of demineralized dentin treated with HEMA primers. Dent. Mater. 2000, 16, 441–446. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Mauro, C.; Leonardi, A.; Santillo, M.; Paterno, R.; Schweikl, H.; Avvedimento, E.V.; Rengo, S. NF-kappaB protection against apoptosis induced by HEMA. J. Dent. Res. 2004, 83, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; D’Anto, V.; Cosentino, C.; Schmalz, G.; Schweikl, H.; Rengo, S. Effect of N-acetyl-L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials 2006, 27, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Ho, Y.C.; Lee, S.S.; Li, Y.C.; Lai, M.Y.; Kuan, Y.H. Cytotoxicity and Apoptotic Mechanism of 2-Hydroxyethyl Methacrylate via Genotoxicity and the Mitochondrial-Dependent Intrinsic Caspase Pathway and Intracellular Reactive Oxygen Species Accumulation in Macrophages. Polymers 2022, 14, 3378. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Albaladejo, A.; Aguilera, F.S.; Osorio, E. Differential effect of in vitro degradation on resin-dentin bonds produced by self-etch versus total-etch adhesives. J. Biomed. Mater. Res. A 2006, 77, 128–135. [Google Scholar] [CrossRef]
- Osorio, R.; Pisani-Proenca, J.; Erhardt, M.C.; Osorio, E.; Aguilera, F.S.; Tay, F.R.; Toledano, M. Resistance of ten contemporary adhesives to resin-dentine bond degradation. J. Dent. 2008, 36, 163–169. [Google Scholar] [CrossRef]
- Ozer, F.; Blatz, M.B. Self-etch and etch-and-rinse adhesive systems in clinical dentistry. Compend. Contin. Educ. Dent. 2013, 34, 12–14, 16, 18; quiz 20, 30. [Google Scholar]
- Reis, A.; Carrilho, M.; Breschi, L.; Loguercio, A.D. Overview of clinical alternatives to minimize the degradation of the resin-dentin bonds. Oper. Dent. 2013, 38, E103–E127. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Albaladejo, A.; Aguilera, F.S.; Tay, F.R.; Ferrari, M. Effect of cyclic loading on the microtensile bond strengths of total-etch and self-etch adhesives. Oper. Dent. 2006, 31, 25–32. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying adhesive materials to improve the longevity of resinous restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [PubMed]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.M.; Tjäderhane, L. The role of matrix metalloproteinases in the oral environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, D.; Baldion, P.; Castellanos, J. Resin-dentin bonding interface: Mechanisms of degradation and strategies for stabilization of the hybrid layer. Int. J. Biomater. 2019, 2019, 5268342. [Google Scholar] [CrossRef]
- Lehmann, N.; Debret, R.; Roméas, A.; Magloire, H.; Degrange, M.; Bleicher, F.; Sommer, P.; Seux, D. Self-etching increases matrix metalloproteinase expression in the dentin-pulp complex. J. Dent. Res. 2009, 88, 77–82. [Google Scholar] [CrossRef]
- Nascimento, F.; Minciotti, C.; Geraldeli, S.; Carrilho, M.; Pashley, D.H.; Tay, F.; Nader, H.; Salo, T.; Tjäderhane, L.; Tersariol, I. Cysteine cathepsins in human carious dentin. J. Dent. Res. 2011, 90, 506–511. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Huang, B.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018, 71, 330–338. [Google Scholar] [CrossRef]
- Yiu, C.K.; King, N.M.; Pashley, D.H.; Suh, B.I.; Carvalho, R.M.; Carrilho, M.R.; Tay, F.R. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials 2004, 25, 5789–5796. [Google Scholar] [CrossRef]
- Armstrong, S.R.; Keller, J.C.; Boyer, D.B. The influence of water storage and C-factor on the dentin-resin composite microtensile bond strength and debond pathway utilizing a filled and unfilled adhesive resin. Dent. Mater. 2001, 17, 268–276. [Google Scholar] [CrossRef]
- Manappallil, J.J. Basic Dental Materials; JP Medical Ltd.: London, UK, 2015. [Google Scholar]
- de Moraes, I.Q.S.; do Nascimento, T.G.; da Silva, A.T.; de Lira, L.M.S.S.; Parolia, A.; de Moraes Porto, I.C.C. Inhibition of matrix metalloproteinases: A troubleshooting for dentin adhesion. Restor. Dent. Endod. 2020, 45, e31. [Google Scholar] [CrossRef]
- Hosaka, K.; Nishitani, Y.; Tagami, J.; Yoshiyama, M.; Brackett, W.W.; Agee, K.; Tay, F.; Pashley, D.H. Durability of resin-dentin bonds to water-vs. ethanol-saturated dentin. J. Dent. Res. 2009, 88, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Wang, Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J. Biomed. Mater. Res. 2002, 620, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.M.; Chersoni, S.; Frankenberger, R.; Pashley, D.H.; Prati, C.; Tay, F.R. A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occurs in self-etch adhesives. Biomaterials 2005, 26, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lueckel, H.; Paris, S. Improved resin infiltration of natural caries lesions. J. Dent. Res. 2008, 87, 1112–1116. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tay, F.R.; Svizero, N.R.; de Gee, A.J.; Feilzer, A.J.; Sano, H.; Kaga, M.; Pashley, D.H. The effects of common errors on sealing ability of total-etch adhesives. Dent. Mater. 2006, 22, 560–568. [Google Scholar] [CrossRef]
- Amin, F.; Fareed, M.A.; Zafar, M.S.; Khurshid, Z.; Palma, P.J.; Kumar, N. Degradation and stabilization of resin-dentine interfaces in polymeric dental adhesives: An updated review. Coatings 2022, 12, 1094. [Google Scholar] [CrossRef]
- Zhang, S.c.; Kern, M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int. J. Oral Sci. 2009, 1, 163–176. [Google Scholar] [CrossRef]
- Arias, V.G.; Campos, I.T.; Pimenta, L.A.F. Microleakage study of three adhesive systems. Braz. Dent. J. 2004, 15, 194–198. [Google Scholar] [CrossRef]
- Cavalcanti, A.N.; Lavigne, C.; Fontes, C.M.; Mathias, P. Microleakage at the composite-repair interface: Effect of different adhesive systems. J. Appl. Oral. Sci. 2004, 12, 219–222. [Google Scholar] [CrossRef]
- Fortin, D.; Swift, E.J., Jr.; Denehy, G.E.; Reinhardt, J.W. Bond strength and microleakage of current dentin adhesives. Dent. Mater. 1994, 10, 253–258. [Google Scholar] [CrossRef]
- Silveira de Araújo, C.; Incerti da Silva, T.; Ogliari, F.; Meireles, S.; Piva, E.; Demarco, F. Microleakage of seven adhesive systems in enamel and dentin. J. Contemp. Dent. Pract. 2006, 7, 26–33. [Google Scholar] [PubMed]
- Dung, S.-Z.; Gregory, R.; Li, Y.; Stookey, G. Effect of lactic acid and proteolytic enzymes on the release of organic matrix components from human root dentin. Caries Res. 1995, 29, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Chaussain, C.; Boukpessi, T.; Khaddam, M.; Tjaderhane, L.; George, A.; Menashi, S. Dentin matrix degradation by host matrix metalloproteinases: Inhibition and clinical perspectives toward regeneration. Front. Physiol. 2013, 4, 308. [Google Scholar] [CrossRef]
- Femiano, F.; Femiano, R.; Femiano, L.; Jamilian, A.; Rullo, R.; Perillo, L. Dentin caries progression and the role of metalloproteinases: An update. Eur. J. Paediatr. Dent. 2016, 17, 243–247. [Google Scholar]
- Pitts, N.B.; Drummond, J.; Guggenberger, R.; Ferrillo, P.; Johnston, S. Incorporating new materials and techniques into clinical practice. Adv. Dent. Res. 2013, 25, 33–40. [Google Scholar] [CrossRef]
- Daood, U.; Sauro, S.; Pichika, M.R.; Omar, H.; Liang Lin, S.; Fawzy, A.S. Novel riboflavin/VE-TPGS modified universal dentine adhesive with superior dentine bond strength and self-crosslinking potential. Dent. Mater. 2020, 36, 145–156. [Google Scholar] [CrossRef]
- Eusufzai, S.Z.; Barman, A.; Jamayet, N.B.; Ahmad, W.; Mahdi, S.S.; Sheikh, Z.; Daood, U. Effects of Riboflavin Collagen Crosslinker on Dentin Adhesive Bonding Efficiency: A Systematic Review and Meta-Analysis. Materials 2023, 16, 1701. [Google Scholar] [CrossRef]
- Park, J.G.; Ye, Q.; Topp, E.M.; Kostoryz, E.L.; Wang, Y.; Kieweg, S.L.; Spencer, P. Preparation and Properties of Novel Dentin Adhesives with Esterase Resistance. J. Appl. Polym. Sci. Symp. 2008, 107, 3588–3597. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Z.; Jiang, H.; Duan, Y.; Li, J.; Liu, X.; Hong, L.; Zhao, C. Preparation and evaluation of novel bio-based Bis-GMA-free dental composites with low estrogenic activity. Dent. Mater. 2022, 38, 281–293. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhao, C.; Bu, W.; Zhang, Y.; Na, H. Synthesis, characterization and evaluation of a fluorinated resin monomer with low water sorption. J. Mech. Behav. Biomed. Mater. 2018, 77, 446–454. [Google Scholar] [CrossRef]
- Liang, X.; Liu, F.; He, J. Synthesis of none Bisphenol A structure dimethacrylate monomer and characterization for dental composite applications. Dent. Mater. 2014, 30, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Kerby, R.E.; Knobloch, L.A.; Schricker, S.; Gregg, B. Synthesis and evaluation of modified urethane dimethacrylate resins with reduced water sorption and solubility. Dent. Mater. 2009, 25, 302–313. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Y.; Wang, L.; Zhang, H.; Na, H.; Zhang, Z. Synthesis and characterization of a novel resin monomer with low viscosity. J. Dent. 2017, 59, 11–17. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Hass, V.; Peng, Z.; Wang, Y. Methacrylate-functionalized proanthocyanidins as novel polymerizable collagen cross-linkers—Part 2: Effects on polymerization, microhardness and leaching of adhesives. Dent. Mater. 2021, 37, 1193–1201. [Google Scholar] [CrossRef]
- Hass, V.; Li, Y.; Wang, R.; Nguyen, D.; Peng, Z.; Wang, Y. Methacrylate-functionalized proanthocyanidins as novel polymerizable collagen cross-linkers—Part 1: Efficacy in dentin collagen bio-stabilization and cross-linking. Dent. Mater. 2021, 37, 1183–1192. [Google Scholar] [CrossRef]
- Chroszcz-Porebska, M.; Kazek-Kesik, A.; Chladek, G.; Barszczewska-Rybarek, I. Novel mechanically strong and antibacterial dimethacrylate copolymers based on quaternary ammonium urethane-dimethacrylate analogues. Dent. Mater. 2023, 39, 659–664. [Google Scholar] [CrossRef]
- Fugolin, A.P.; de Paula, A.B.; Dobson, A.; Huynh, V.; Consani, R.; Ferracane, J.L.; Pfeifer, C.S. Alternative monomer for BisGMA-free resin composites formulations. Dent. Mater. 2020, 36, 884–892. [Google Scholar] [CrossRef]
- Gotti, V.B.; Correr, A.B.; Lewis, S.H.; Feitosa, V.P.; Correr-Sobrinho, L.; Stansbury, J.W. Influence of nanogel additive hydrophilicity on dental adhesive mechanical performance and dentin bonding. Dent. Mater. 2016, 32, 1406–1413. [Google Scholar] [CrossRef]
- Yuca, E.; Xie, S.X.; Song, L.; Boone, K.; Kamathewatta, N.; Woolfolk, S.K.; Elrod, P.; Spencer, P.; Tamerler, C. Reconfigurable Dual Peptide Tethered Polymer System Offers a Synergistic Solution for Next Generation Dental Adhesives. Int. J. Mol. Sci. 2021, 22, 6552. [Google Scholar] [CrossRef]
- Perez-Mondragon, A.A.; Cuevas-Suarez, C.E.; Suarez Castillo, O.R.; Gonzalez-Lopez, J.A.; Herrera-Gonzalez, A.M. Evaluation of biocompatible monomers as substitutes for TEGDMA in resin-based dental composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 80–87. [Google Scholar] [CrossRef]
- Yamauchi, S.; Wang, X.; Egusa, H.; Sun, J. High-Performance Dental Adhesives Containing an Ether-Based Monomer. J. Dent. Res. 2020, 99, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.P.; Navarro, O.; Logan, M.G.; Huynh, V.; Franca, C.M.; Ferracane, J.L.; Pfeifer, C.S. Synthesis of di- and triacrylamides with tertiary amine cores and their evaluation as monomers in dental adhesive interfaces. Acta Biomater. 2020, 115, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Zeuner, F.; Angermann, J.; Fischer, U.K.; Rheinberger, V. Monomers for adhesive polymers, 4. Macromol. Mater. Eng. 2003, 288, 621–628. [Google Scholar] [CrossRef]
- Yin, M.; Liu, F.; He, J. Preparation and characterization of Bis-GMA free dental resin system with synthesized dimethacrylate monomer TDDMMA derived from tricyclo [5.2.1.0(2,6)]-decanedimethanol. J. Mech. Behav. Biomed. Mater. 2016, 57, 157–163. [Google Scholar] [CrossRef]
- Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. Int. J. Mol. Sci. 2016, 17, 2014. [Google Scholar] [CrossRef]
- He, J.; Stenhagen, I.S.R.; Dragland, I.S.; Kopperud, H.M. Preparation of a fluorinated dental resin system and its anti-adhesive properties against S. mutans. Dent. Mater. 2023, 39, 402–409. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, M.; Liu, F.; Huang, X.; Mai, S.; He, J. Preparation of Bis-GMA free dental resin composites with anti-adhesion effect against Streptococcus mutans using synthesized fluorine-containing methacrylate (DFMA). J. Mech. Behav. Biomed. Mater. 2022, 131, 105263. [Google Scholar] [CrossRef]
- Yin, M.; Guo, S.; Liu, F.; He, J. Synthesis of fluorinated dimethacrylate monomer and its application in preparing Bis-GMA free dental resin. J. Mech. Behav. Biomed. Mater. 2015, 51, 337–344. [Google Scholar] [CrossRef]
- Ashraf, U.; Tsoi, J.K.; He, J.; Shah, A.T.; Khan, A.S.; Pow, E.H.; Matinlinna, J.P. Effect of novel FUDMA-TEGDMA resin on resin-dentine adhesion strength. Int. J. Adhes. Adhes. 2022, 118, 103229. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Wang, H.; Zhu, J.; Wang, H.; Zheng, Y.; Zhu, S.; Cui, Z. Synthesis of an urushiol derivative and its use for hydrolysis resistance in dentin adhesive. RSC Adv. 2021, 11, 18448–18457. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Q.; Zhang, X.; Wei, Y.; Xu, M.; Yang, X.; Ma, Q.; Cheng, Y.; Deng, X. Improved performance of Bis-GMA/TEGDMA dental composites by net-like structures formed from SiO2 nanofiber fillers. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Jandt, K.D.; Sigusch, B.W. Future perspectives of resin-based dental materials. Dent. Mater. 2009, 25, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Urbas, A.; Gonzalez-Bonet, A.; Sheridan, R.J.; Seppala, J.E.; Beers, K.L.; Sun, J.R. A composition-controlled cross-linking resin network through rapid visible-light photo-copolymerization. Polym. Chem. 2016, 7, 5023–5030. [Google Scholar] [CrossRef]
- Huyang, G.; Sun, J. Clinically Applicable Self-Healing Dental Resin Composites. MRS Adv. 2016, 1, 547–552. [Google Scholar] [CrossRef]
- Huyang, G.; Debertin, A.E.; Sun, J. Design and development of self-healing dental composites. Mater. Des. 2016, 94, 295–302. [Google Scholar] [CrossRef]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of dental resins resistant to enzymatic and hydrolytic degradation in oral environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef]
- Xi, W.X.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef]
- Boulden, J.E.; Cramer, N.B.; Schreck, K.M.; Couch, C.L.; Bracho-Troconis, C.; Stansbury, J.W.; Bowman, C.N. Thiol-ene-methacrylate composites as dental restorative materials. Dent. Mater. 2011, 27, 267–272. [Google Scholar] [CrossRef]
- Cramer, N.B.; Couch, C.L.; Schreck, K.M.; Carioscia, J.A.; Boulden, J.E.; Stansbury, J.W.; Bowman, C.N. Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials. Dent. Mater. 2010, 26, 21–28. [Google Scholar] [CrossRef]
- Bacchi, A.; Pfeifer, C.S. Rheological and mechanical properties and interfacial stress development of composite cements modified with thio-urethane oligomers. Dent. Mater. 2016, 32, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Palin, W.M.; Fleming, G.J.P.; Nathwani, H.; Burke, F.J.T.; Randall, R.C. In vitro cuspal deflection and microleakage of maxillary premolars restored with novel low-shrink dental composites. Dent. Mater. 2005, 21, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, W.; Thalacker, C.; Guggenberger, R. Siloranes in dental composites. Dent. Mater. 2005, 21, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, A.; Consani, R.L.; Martim, G.C.; Pfeifer, C.S. Thio-urethane oligomers improve the properties of light-cured resin cements. Dent. Mater. 2015, 31, 565–574. [Google Scholar] [CrossRef]
- Bacchi, A.; Dobson, A.; Ferracane, J.L.; Consani, R.; Pfeifer, C.S. Thio-urethanes Improve Properties of Dual-cured Composite Cements. J. Dent. Res. 2014, 93, 1320–1325. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Photopolymerization of phosphoric acid ester-based self-etch dental adhesives. Dent. Mater. J. 2013, 32, 10–18. [Google Scholar] [CrossRef]
- Wang, X.; Yamauchi, S. Improve Dentin Bonding Performance Using a Hydrolytically Stable, Ether-Based Primer. J. Funct. Biomater. 2022, 13, 128. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Malaquias, P.; Matos, T.P.; Szesz, A.; Souza, S.; Bermudez, J.; Reis, A.; Loguercio, A.D.; Farago, P.V. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent. Mater. 2017, 33, 309–320. [Google Scholar] [CrossRef]
- Wernik, J.; Meguid, S. On the mechanical characterization of carbon nanotube reinforced epoxy adhesives. Mater. Des. 2014, 59, 19–32. [Google Scholar] [CrossRef]
- Rodríguez-Quirós, H.A.; Casanova-Yepes, H.F. Effect of the functionalization of silica nanoparticles as a reinforcing agent on dental composite materials. Rev. Fac. De Ing. Univ. De Antioq. 2015, 36–45. [Google Scholar] [CrossRef]
- Assery, M.K.; Ajwa, N.; Alshamrani, A.; Alanazi, B.J.; Durgesh, B.H.; Matinlinna, J.P. Titanium dioxide nanoparticles reinforced experimental resin composite for orthodontic bonding. Mater. Res. Express 2019, 6, 125098. [Google Scholar] [CrossRef]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.-D.; Grabow, J.; Müller, F.A. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef] [PubMed]
- Almahdy, A.; Koller, G.; Sauro, S.; Bartsch, J.; Sherriff, M.; Watson, T.; Banerjee, A. Effects of MMP inhibitors incorporated within dental adhesives. J. Dent. Res. 2012, 91, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.H.; Swetha, B.; Priya, B.D.; Mohan, T.M.; Malini, D.L.; Sravya, M.S. Effect of collagen cross-linking agents on the depth of penetration of bioceramic sealer and release of hydroxyproline: An in vitro study. J. Conserv. Dent. Endod. 2024, 27, 170–174. [Google Scholar] [CrossRef]
- Hardan, L.; Daood, U.; Bourgi, R.; Cuevas-Suarez, C.E.; Devoto, W.; Zarow, M.; Jakubowicz, N.; Zamarripa-Calderon, J.E.; Radwanski, M.; Orsini, G.; et al. Effect of Collagen Crosslinkers on Dentin Bond Strength of Adhesive Systems: A Systematic Review and Meta-Analysis. Cells 2022, 11, 2417. [Google Scholar] [CrossRef]
- Dionysopoulos, D. Effect of digluconate chlorhexidine on bond strength between dental adhesive systems and dentin: A systematic review. J. Conserv. Dent. Endod. 2016, 19, 11–16. [Google Scholar] [CrossRef]
- Kalagi, S.; Feitosa, S.A.; Münchow, E.A.; Martins, V.M.; Karczewski, A.E.; Cook, N.B.; Diefenderfer, K.; Eckert, G.J.; Geraldeli, S.; Bottino, M.C. Chlorhexidine-modified nanotubes and their effects on the polymerization and bonding performance of a dental adhesive. Dent. Mater. 2020, 36, 687–697. [Google Scholar] [CrossRef]
- Yiu, C.K.; Hiraishi, N.; Tay, F.R.; King, N.M. Effect of chlorhexidine incorporation into dental adhesive resin on durability of resin-dentin bond. J. Adhes. Dent. 2012, 14, 355. [Google Scholar]
- Stanislawczuk, R.; Reis, A.; Loguercio, A.D. A 2-year in vitro evaluation of a chlorhexidine-containing acid on the durability of resin-dentin interfaces. J. Dent. 2011, 39, 40–47. [Google Scholar] [CrossRef]
- Lee, J.; Sabatini, C. Glutaraldehyde collagen cross-linking stabilizes resin–dentin interfaces and reduces bond degradation. Eur. J. Oral Sci. 2017, 125, 63–71. [Google Scholar] [CrossRef]
- Boksman, L.; Swift, J.; Edward, J. Current usage of glutaraldehyde/HEMA. J. Esthet. Restor. Dent. 2011, 23, 410–416. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Zehnder, M.; Göhring, T.N.; Waltimo, T.M. Glutaraldehyde in bonding systems disinfects dentin in vitro. J. Adhes. Dent. 2004, 6, 61. [Google Scholar] [PubMed]
- Cilli, R.; Prakki, A.; de Araújo, P.A.; Pereira, J.C. Influence of glutaraldehyde priming on bond strength of an experimental adhesive system applied to wet and dry dentine. J. Dent. 2009, 37, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Román, J.S.; Toledano, M. Zinc-doped dentin adhesive for collagen protection at the hybrid layer. Eur. J. Oral Sci. 2011, 119, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Cabello, I.; Toledano, M. Bioactivity of zinc-doped dental adhesives. J. Dent. 2014, 42, 403–412. [Google Scholar] [CrossRef]
- Green, B.; Yao, X.; Ganguly, A.; Xu, C.; Dusevich, V.; Walker, M.P.; Wang, Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J. Dent. 2010, 38, 908–915. [Google Scholar] [CrossRef]
- Liu, Y.; Dusevich, V.; Wang, Y. Addition of Grape Seed Extract Renders Phosphoric Acid a Collagen-stabilizing Etchant. J. Dent. Res. 2014, 93, 821–827. [Google Scholar] [CrossRef]
- Khaddam, M.; Salmon, B.; Le Denmat, D.; Tjaderhane, L.; Menashi, S.; Chaussain, C.; Rochefort, G.Y.; Boukpessi, T. Grape seed extracts inhibit dentin matrix degradation by MMP-3. Front. Physiol. 2014, 5, 425. [Google Scholar] [CrossRef]
- Ghorab, S.; Ibraheim, A. Effect of hesperidin on antibacterial activity and adhesive properti es of an etch-and-rinse adhesive system. Egypt. Dent. J. 2018, 64, 3801–3812. [Google Scholar] [CrossRef]
- Ahmed, O.Q.; Alkassar, A.A.H.; Gabbar, A.A.; Jameel, M.I.; Kamel, M.F.; Mohammed, B.S. Antimicrobial Property and Cytotoxicity of Hesperidin Incorporated. J. Popul. Ther. Clin. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Hiraishi, N.; Maruno, T.; Tochio, N.; Sono, R.; Otsuki, M.; Takatsuka, T.; Tagami, J.; Kobayashi, Y. Hesperidin interaction to collagen detected by physico-chemical techniques. Dent. Mater. 2017, 33, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, K.; Yan, H.; Liu, S.; Wang, Y.; Huang, C. High-performance therapeutic quercetin-doped adhesive for adhesive–dentin interfaces. Sci. Rep. 2017, 7, 8189. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, H.; Yan, H.; Sun, Y.; Chen, X.; Guo, J.; Yue, J.; Huang, C. Quercetin as a simple but versatile primer in dentin bonding. RSC Adv. 2017, 7, 36392–36402. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, X.; Wang, X.; Yang, S.; Mao, J.; Gong, S. Quercetin as an auxiliary endodontic irrigant for root canal treatment: Anti-biofilm and dentin collagen-stabilizing effects in vitro. Materials 2021, 14, 1178. [Google Scholar] [CrossRef]
- Mazzoni, A.; Apolonio, F.; Saboia, V.; Santi, S.; Angeloni, V.; Checchi, V.; Curci, R.; Di Lenarda, R.; Tay, F.; Pashley, D.H. Carbodiimide inactivation of MMPs and effect on dentin bonding. J. Dent. Res. 2014, 93, 263–268. [Google Scholar] [CrossRef]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Comba, A.; Cadenaro, M.; Radovic, I.; Sebold, M.; Turco, G.; Breschi, L.; Mazzoni, A. The effect of carbodiimide on push-out bond strength of fiber posts and endogenous enzymatic activity. BMC Oral Health 2023, 23, 399. [Google Scholar] [CrossRef]
- Thompson, J.M.; Agee, K.; Sidow, S.J.; McNally, K.; Lindsey, K.; Borke, J.; Elsalanty, M.; Tay, F.R.; Pashley, D.H. Inhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acid. J. Endod. 2012, 38, 62–65. [Google Scholar] [CrossRef]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; Dorigo, E.D.S. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater. 2010, 26, 571–578. [Google Scholar] [CrossRef]
- Du, X.; Huang, X.; Huang, C.; Wang, Y.; Zhang, Y. Epigallocatechin-3-gallate (EGCG) enhances the therapeutic activity of a dental adhesive. J. Dent. 2012, 40, 485–492. [Google Scholar] [CrossRef]
- Ma, S.; Niu, L.; Li, F.; Fang, M.; Zhang, L.; Tay, F.R.; Imazato, S.; Chen, J. Adhesive materials with bioprotective/biopromoting functions. Curr. Oral Health Rep. 2014, 1, 213–221. [Google Scholar] [CrossRef]
- Niu, L.-N.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.-H.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef]
- Yue, S.; Wu, J.; Zhang, Q.; Zhang, K.; Weir, M.D.; Imazato, S.; Bai, Y.; Xu, H.H. Novel dental adhesive resin with crack self-healing, antimicrobial and remineralization properties. J. Dent. 2018, 75, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Mai, S.; Mazzoni, A.; Liu, Y.; Tezvergil-Mutluay, A.; Takahashi, K.; Zhang, K.; Pashley, D.H.; Tay, F.R. Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices–implications in the aging of resin–dentin bonds. Acta Biomater. 2010, 6, 3729–3739. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Fronza, B.M. The use of bioactive particles and biomimetic analogues for increasing the longevity of resin-dentin interfaces: A literature review. Dent. Mater. J. 2020, 39, 62–68. [Google Scholar] [CrossRef]
- Tao, S.; He, L.; Xu, H.H.; Weir, M.D.; Fan, M.; Yu, Z.; Zhang, M.; Zhou, X.; Liang, K.; Li, J. Dentin remineralization via adhesive containing amorphous calcium phosphate nanoparticles in a biofilm-challenged environment. J. Dent. 2019, 89, 103193. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive glass for dentin remineralization: A systematic review. Mater. Sci. Eng. C 2017, 76, 1369–1377. [Google Scholar] [CrossRef]
- Rifane, T.O.; Cordeiro, K.E.M.; Silvestre, F.A.; Souza, M.T.; Zanotto, E.D.; Araújo-Neto, V.G.; Giannini, M.; Sauro, S.; de Paula, D.M.; Feitosa, V.P. Impact of silanization of different bioactive glasses in simplified adhesives on degree of conversion, dentin bonding and collagen remineralization. Dent. Mater. 2023, 39, 217–226. [Google Scholar] [CrossRef]
- Oltramare, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Short-and long-term dentin bond strength of bioactive glass-modified dental adhesives. Nanomaterials 2021, 11, 1894. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Influence of phosphoproteins’ biomimetic analogs on remineralization of mineral-depleted resin–dentin interfaces created with ion-releasing resin-based systems. Dent. Mater. 2015, 31, 759–777. [Google Scholar] [CrossRef]
- Mai, S.; Kim, Y.K.; Toledano, M.; Breschi, L.; Ling, J.Q.; Pashley, D.H.; Tay, F.R. Phosphoric acid esters cannot replace polyvinylphosphonic acid as phosphoprotein analogs in biomimetic remineralization of resin-bonded dentin. Dent. Mater. 2009, 25, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Kavrik, F.; Kucukyilmaz, E. Effect of Nano-sized Hydroxyapatite Filler on Remineralization Efficacy of an Adhesive Resin: An in vitro study. J. Adhes. Sci. Technol. 2024, 38, 1334–1352. [Google Scholar] [CrossRef]
- Jardim, R.N.; Rocha, A.A.; Rossi, A.M.; de Almeida Neves, A.; Portela, M.B.; Lopes, R.T.; Dos Santos, T.M.P.; Xing, Y.; da Silva, E.M. Fabrication and characterization of remineralizing dental composites containing hydroxyapatite nanoparticles. J. Mech. Behav. Biomed. Mater. 2020, 109, 103817. [Google Scholar] [CrossRef] [PubMed]
- Slutzky, H.; Matalon, S.; Weiss, E.I. Antibacterial surface properties of polymerized single-bottle bonding agents: Part II. Quintessence Int. 2004, 35, 275. [Google Scholar]
- Lopes, S.R.; Matuda, A.G.N.; Campos, R.P.; Mafetano, A.; Barnabe, A.H.M.; Chagas, G.S.; Barcellos, D.C.; Niu, L.N.; Tay, F.R.; Pucci, C.R. Development of an Antibacterial Dentin Adhesive. Polymers 2022, 14, 2502. [Google Scholar] [CrossRef]
- Alhussein, A.; Alsahafi, R.; Wang, X.; Mitwalli, H.; Filemban, H.; Hack, G.D.; Oates, T.W.; Sun, J.; Weir, M.D.; Xu, H.H.K. Novel Dental Low-Shrinkage-Stress Composite with Antibacterial Dimethylaminododecyl Methacrylate Monomer. J. Funct. Biomater. 2023, 14, 335. [Google Scholar] [CrossRef]
- Tebyaniyan, H.; Hussain, A.; Vivian, M. Current antibacterial agents in dental bonding systems: A comprehensive overview. Future Microbiol. 2023, 18, 825–844. [Google Scholar] [CrossRef]
- Elsaka, S.E. Antibacterial activity and adhesive properties of a chitosan-containing dental adhesive. Quintessence Int. 2012, 43, 603. [Google Scholar]
- Korbmacher, H.M.; Huck, L.; Kahl-Nieke, B. Fluoride-releasing adhesive and antimicrobial self-etching primer effects on shear bond strength of orthodontic brackets. Angle Orthod. 2006, 76, 845–850. [Google Scholar]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Zalega, M.; Bociong, K. Antibacterial Agents Used in Modifications of Dental Resin Composites: A Systematic Review. Appl. Sci. 2024, 14, 3710. [Google Scholar] [CrossRef]
- Peralta, L.C.F.; Almeida, N.L.M.; Pontes, F.M.L.; Rinaldo, D.; Carneiro, C.A.; Neppelenbroek, K.H.; Lara, V.S.; Porto, V.C. Silver nanoparticles in denture adhesive: An antimicrobial approach against Candida albicans. J. Dent. 2023, 131, 104445. [Google Scholar] [CrossRef] [PubMed]
- Miki, S.; Kitagawa, H.; Kitagawa, R.; Kiba, W.; Hayashi, M.; Imazato, S. Antibacterial activity of resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 2016, 32, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Alsunbul, H.; Alfawaz, Y.F.; Alhamdan, E.M.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of carbon and graphene oxide nanoparticle on the adhesive properties of dentin bonding polymer: A SEM, EDX, FTIR study. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231159238. [Google Scholar] [CrossRef]
- Almutairi, B.; Kattan, H.F.; BinMahfooz, A.M.; Qutub, O.A.; Basunbul, G.; ArRejaie, A.S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion. A scanning electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared, micro-Raman, and bond strength study. Microsc. Res. Tech. 2021, 84, 2082–2094. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Bioactive Dental Composites and Bonding Agents Having Remineralizing and Antibacterial Characteristics. Dent. Clin. N. Am. 2017, 61, 669–687. [Google Scholar] [CrossRef]
- Wu, J.; Weir, M.D.; Melo, M.A.; Xu, H.H. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J. Dent. 2015, 43, 317–326. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Bermudez, J.; Dávila-Sánchez, A.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Hernández, M.; Astorga, J.; Reis, A.; Loguercio, A.D.; Farago, P.V.; et al. Zinc oxide and copper nanoparticles addition in universal adhesive systems improve interface stability on caries-affected dentin. J. Mech. Behav. Biomed. Mater. 2019, 100, 103366. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Bermudez, J.; Dávila-Sánchez, A.; Buvinic, S.; Hernández-Moya, N.; Reis, A.; Loguercio, A.D.; Farago, P.V.; et al. Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J. Dent. 2019, 82, 45–55. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Malaquias, P.; Hass, V.; Matos, T.P.; Lourenço, L.; Reis, A.; Loguercio, A.D.; Farago, P.V. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef]
- Vidal, O.; de Paris Matos, T.; Núñez, A.; Méndez-Bauer, L.; Sutil, E.; Ñaupari-Villasante, R.; Souta, M.C.; Pitlovanciv, M.; Gutiérrez, M.F.; Loguercio, A.D. A universal adhesive containing copper nanoparticles improves the stability of hybrid layer in a cariogenic oral environment: An in situ study. J. Mech. Behav. Biomed. Mater. 2022, 126, 105017. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Z.; Guo, R.; Peng, W.; Yang, H.; Huang, C. Epigallocatechin-3-gallate/nanohydroxyapatite platform delivery approach to adhesive-dentin interface stability. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111918. [Google Scholar] [CrossRef] [PubMed]
- Gonapa, P.; Sajjan, G.S.; Bhupathi, A.; Podugu, U.K.; Sundar, S.; Gondi, D.; Rathod, R.T. Evaluation of Bond Durability, Surface Morphology, and Remineralization at the Adhesive Interface with Dentin Bonding Agents Modified with Silica-doped Nanohydroxyapatite. Contemp. Clin. Dent. 2022, 13, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.H.; André, C.B.; Fronza, B.M.; Giannini, M.; Rosalen, P.L.; Consani, S.; França, R. Physicochemical properties, metalloproteinases inhibition, and antibiofilm activity of doxycycline-doped dental adhesive. J. Dent. 2021, 104, 103550. [Google Scholar] [CrossRef]
- Kharouf, N.; Eid, A.; Hardan, L.; Bourgi, R.; Arntz, Y.; Jmal, H.; Foschi, F.; Sauro, S.; Ball, V.; Haikel, Y.; et al. Antibacterial and Bonding Properties of Universal Adhesive Dental Polymers Doped with Pyrogallol. Polymers 2021, 13, 1538. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Lan, Y.; Simionato, A.; Ibrahim, M.S.; Weir, M.D.; Masri, R.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Magnetic motion of superparamagnetic iron oxide nanoparticles-loaded dental adhesives: Physicochemical/biological properties, and dentin bonding performance studied through the tooth pulpal pressure model. Acta Biomater. 2021, 134, 337–347. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Antibacterial response of oral microcosm biofilm to nano-zinc oxide in adhesive resin. Dent. Mater. 2021, 37, e182–e193. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of zinc oxide quantum dots in the antibacterial activity and cytotoxicity of an experimental adhesive resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wang, S.; Wang, B.; Wang, X.; Mi, Z.; Fu, J.; Zhang, Z.; Yan, W. Enhancing the Stability of the Resin-Dentin Bonding Interface with Ag+- and Zn2+-Exchanged Zeolite A. ACS Biomater. Sci. Eng. 2022, 8, 1717–1725. [Google Scholar] [CrossRef]
- Gomes, T.G. Effect of Bioactive Restorative Materials on Tooth Demineralization: An In Vitro and Comparative Study. Master’s Thesis, The University of Alabama at Birmingham, Birmingham, AL, USA, 2024. [Google Scholar]
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Wang, L.; Xu, H.H.K. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 2018, 72, 44–52. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Chen, Y.; Xing, X.; Wang, Y.; Wu, G. Evaluation of Chitosan-Oleuropein Nanoparticles on the Durability of Dentin Bonding. Drug Des. Devel Ther. 2023, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, H.E.; Vieira-Junior, W.F.; França, F.M.; Amaral, F.L.; Basting, R.T. Bonding strategy of a universal adhesive system containing chitosan: Influence on dentin permeability, and effect on adhesive layer micromorphology. Acta Odontol. Latinoam. 2022, 35, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Boutsiouki, C.; Frankenberger, R.; Lücker, S.; Krämer, N. Effect of Chlorhexidine-containing Etch-and-Rinse Adhesives on Dentin Microtensile Bond Strength after Biological Loading. J. Adhes. Dent. 2023, 25, 13–22. [Google Scholar] [CrossRef]
- Stanislawczuk, R.; Pereira, F.; Muñoz, M.A.; Luque, I.; Farago, P.V.; Reis, A.; Loguercio, A.D. Effects of chlorhexidine-containing adhesives on the durability of resin-dentine interfaces. J. Dent. 2014, 42, 39–47. [Google Scholar] [CrossRef]
- Meng, Y.C.; Huang, F.; Wang, S.L.; Li, M.W.; Lu, Y.; Pei, D.D. [Effect of hydroxyapatite based agents on the bonding properties of universal adhesives]. Chin. J. Stomatol. 2022, 57, 173–181. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, F.; Wang, S.; Huang, X.; Lu, Y.; Pei, D. Bonding properties of mild universal adhesives to dentin pretreated with hydroxyapatite-based desensitizing agents. West China J. Stomatol. 2022, 40, 668–675. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, W.; Liang, J.; Ran, S. Influence of silver nanoparticles on the resin-dentin bond strength and antibacterial activity of a self-etch adhesive system. J. Prosthet. Dent. 2022, 128, 1363.e1–1363.e10. [Google Scholar] [CrossRef]
- Jenabi, N.; Sadeghian, S.; Karimzadeh, F.; Pour, M.S.; Rakhshan, V. Antibacterial activity and shear bond strength of fiber-reinforced composites and bonding agents containing 0.5%, 1%, 2.5%, and 5% silver nanoparticles. Dent. Res. J. 2023, 20, 23. [Google Scholar] [CrossRef]
- Tristán-López, J.D.; Niño-Martínez, N.; Kolosovas-Machuca, E.S.; Patiño-Marín, N.; De Alba-Montero, I.; Bach, H.; Martínez-Castañón, G.A. Application of Silver Nanoparticles to Improve the Antibacterial Activity of Orthodontic Adhesives: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 1401. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Deng, J.; Nie, R.; Meng, X. Antibacterial one-step self-etching dental adhesive with silver nanoparticles synthesized in situ. J. Dent. 2023, 129, 104411. [Google Scholar] [CrossRef]
- Aguiar, J.D.; Pedrosa, M.D.S.; Toma, S.H.; Araki, K.; Marques, M.M.; Medeiros, I.S. Antibacterial effect, cytotoxicity, and bond strength of a modified dental adhesive containing silver nanoparticles. Odontology 2023, 111, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef]
- Jia, H.; Hou, W.; Wei, L.; Xu, B.; Liu, X. The structures and antibacterial properties of nano-SiO2 supported silver/zinc-silver materials. Dent. Mater. 2008, 24, 244–249. [Google Scholar] [CrossRef]
- Welch, K.; Cai, Y.; Engqvist, H.; Strømme, M. Dental adhesives with bioactive and on-demand bactericidal properties. Dent. Mater. 2010, 26, 491–499. [Google Scholar] [CrossRef]
- Stürmer, M.; Garcia, I.M.; Souza, V.S.; Visioli, F.; Scholten, J.D.; Samuel, S.M.W.; Leitune, V.C.B.; Collares, F.M. Titanium dioxide nanotubes with triazine-methacrylate monomer to improve physicochemical and biological properties of adhesives. Dent. Mater. 2021, 37, 223–235. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Leitune, V.C.B.; Samuel, S.M.W.; Collares, F.M. Boron nitride nanotubes as novel fillers for improving the properties of dental adhesives. J. Dent. 2017, 62, 85–90. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 1–13. [Google Scholar] [CrossRef]
- Szebeni, D.; Told, R.; Kunsagi-Mate, S.; Szalma, J.; Maroti, P.; Boddi, K.; Lempel, E. Monomer elution and shrinkage stress analysis of addition-fragmentation chain-transfer-modified resin composites in relation to the curing protocol. Dent. Mater. 2024, 40, 1611–1623. [Google Scholar] [CrossRef]
- Martini Garcia, I.; Jung Ferreira, C.; de Souza, V.S.; Castelo Branco Leitune, V.; Samuel, S.M.W.; de Souza Balbinot, G.; de Souza da Motta, A.; Visioli, F.; Damiani Scholten, J.; Mezzomo Collares, F. Ionic liquid as antibacterial agent for an experimental orthodontic adhesive. Dent. Mater. 2019, 35, 1155–1165. [Google Scholar] [CrossRef]
- Tziafas, D.; Koliniotou-Koumpia, E.; Tziafa, C.; Papadimitriou, S. Effects of a new antibacterial adhesive on the repair capacity of the pulp-dentine complex in infected teeth. Int. Endod. J. 2007, 40, 58–66. [Google Scholar] [CrossRef]
- Imazato, S.; Ebi, N.; Takahashi, Y.; Kaneko, T.; Ebisu, S.; Russell, R.R. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials 2003, 24, 3605–3609. [Google Scholar] [CrossRef] [PubMed]
- Bendary, I.M.; Garcia, I.M.; Collares, F.M.; Takimi, A.; Samuel, S.M.W.; Leitune, V.C.B. Wollastonite as filler of an experimental dental adhesive. J. Dent. 2020, 102, 103472. [Google Scholar] [CrossRef] [PubMed]
- Mokeem, L.S.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Melo, M.A.S. Benzyldimethyldodecyl Ammonium Chloride Doped Dental Adhesive: Impact on Core’s Properties, Biosafety, and Antibacterial/Bonding Performance after Aging. J. Funct. Biomater. 2022, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations. Dent. Mater. 2017, 33, e239–e254. [Google Scholar] [CrossRef]
- Leyva del Rio, D.; Sartori, N.; Tomblin, N.B.; Phark, J.-H.; Pardi, V.; Murata, R.M.; Duarte, S., Jr. Bioactive dental adhesive system with tt-farnesol: Effects on dental biofilm and bonding properties. Front. Bioeng. Biotechnol. 2020, 8, 865. [Google Scholar] [CrossRef]
- Silvestrin, L.B.; Garcia, I.M.; Visioli, F.; Collares, F.M.; Leitune, V.C.B. Physicochemical and biological properties of experimental dental adhesives doped with a guanidine-based polymer: An in vitro study. Clin. Oral Investig. 2022, 26, 3627–3636. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, J.; Zhu, X.; Tian, Z.; Zhu, S. Triclosan to Improve the Antimicrobial Performance of Universal Adhesives. Polymers 2023, 15, 304. [Google Scholar] [CrossRef]
- Guo, R.; Peng, W.; Yang, H.; Yao, C.; Yu, J.; Huang, C. Evaluation of resveratrol-doped adhesive with advanced dentin bond durability. J. Dent. 2021, 114, 103817. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zhang, J.H.; Li, K.X.; Chen, H.; Wang, H.M.; He, X.; Shi, Z.S.; Zhu, S.; Cui, Z.C. A Novel Chemical Binding Primer to Improve Dentin Bonding Durability. J. Dent. Res. 2022, 101, 777–784. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjaderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Chen, H.; Feng, S.; Jin, Y.; Hou, Y.; Zhu, S. Comparison of bond strength of universal adhesives using different etching modes: A systematic review and meta-analysis. Dent. Mater. J. 2022, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, H.; Inoue, G.; Chen, X.; Ikeda, M.; Shimada, Y. Acid resistance and bond strength of calcium-containing adhesive on ename. Int. Dent. J. 2025. In press. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suarez, C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, A.; Fischer, N.G.; Barkmeier, W.W.; Latta, M.A. Bond Durability of Two-Step HEMA-Free Universal Adhesive. J. Funct. Biomater. 2022, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Geraldeli, S. Bonding characteristics of self-etching adhesives to intact versus prepared enamel. J. Esthet. Restor. Dent. 2003, 15, 32–41; discussion 42. [Google Scholar] [CrossRef]
- Andre, C.B.; Chan, D.C.; Giannini, M. Antibacterial-containing dental adhesives’ effects on oral pathogens and on Streptococcus mutans biofilm: Current perspectives. Am. J. Dent. 2018, 31, 37B–41B. [Google Scholar]
- Kameyama, A.; Tsumori, M.; Ushiki, T.; Muto, Y.; Koga, H.; Matsukubo, T.; Hirai, Y. Fluoride release from newly developed dental adhesives. Bull. Tokyo Dent. Coll. 2002, 43, 193–197. [Google Scholar] [CrossRef]
- Burrer, P.; Simoni, S.; Zwicky, P.; Attin, T.; Tauböck, T.T. Effect of different adhesive application approaches on bond strength in over-etched dentin. Am. J. Dent. 2023, 36, 118–122. [Google Scholar]
- Neves, P.; Pires, S.; Marto, C.M.; Amaro, I.; Coelho, A.; Sousa, J.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E.; Abrantes, A.M. Evaluation of microleakage of a new bioactive material for restoration of posterior teeth: An in vitro radioactive model. Appl. Sci. 2022, 12, 11827. [Google Scholar] [CrossRef]
- Kitahara, S.; Shimizu, S.; Takagaki, T.; Inokoshi, M.; Abdou, A.; Burrow, M.F.; Nikaido, T. Dentin Bonding Durability of Four Different Recently Introduced Self-Etch Adhesives. Materials 2024, 17, 4296. [Google Scholar] [CrossRef]
- Brkanović, S.; Sever, E.K.; Vukelja, J.; Ivica, A.; Miletić, I.; Krmek, S.J. Comparison of different universal adhesive systems on dentin bond strength. Materials 2023, 16, 1530. [Google Scholar] [CrossRef]
- Rizk, M.; Pohle, A.; Dieckmann, P.; Tauböck, T.T.; Biehl, R.; Wiegand, A. Mineral precipitation, polymerization properties and bonding performance of universal dental adhesives doped with polyhedral oligomeric silsesquioxanes. Int. J. Adhes. Adhes. 2020, 100, 102573. [Google Scholar] [CrossRef]
- Whitworth, S.A. Self-assembling peptide nano-apatite hybrid material for dentine mineralisation. Ph.D. Thesis, University of Leeds, Leeds, England, 2018. [Google Scholar]
- Conti, G.; Veneri, F.; Amadori, F.; Garzoni, A.; Majorana, A.; Bardellini, E. Evaluation of antibacterial activity of a bioactive restorative material versus a Glass-Ionomer cement on Streptococcus mutans: In-vitro study. Dent. J. 2023, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, H.; Honda, K.; Arita, A.; Kumagai, T. Shear Bond Strength of G-Premio BOND to Dentin under Moist Conditions. J. Adhes. Dent. 2019, 21, 453. [Google Scholar]
- Shin, J.; Hwang, E.; Kim, J. An Evaluation of shear bond strength of new dentin bonding agents. J. Korean Acad. Pediatr. Dent. 2017, 44, 358–364. [Google Scholar] [CrossRef]
- Hegde, N.; Attavar, S.; Hegde, M.N.; Hegde, N.D. Comparative analysis of bond strength and microleakage of newer generation bonding agents to enamel and dentin: An: In vitro: Study. J. Conserv. Dent. 2020, 23, 593–597. [Google Scholar] [CrossRef]
- Sellan, P.L.B.; Zanatta, R.F.; Torres, C.R.G.; Tian, F.-c.; Bergeron, B.E.; Niu, L.-n.; Pucci, C.R. Effects of calcium-phosphate, laser and adhesive on dentin permeability and bond strength. Heliyon 2020, 6, e03925. [Google Scholar] [CrossRef]
- Batista, G.R.; Barcellos, D.C.; Gomes Torres, C.R.; Damião, Á.J.; de Oliveira, H.P.M.; de Paiva Gonçalves, S.E. Effect of Nd: YAG laser on the solvent evaporation of adhesive systems. Int. J. Esthet. Dent. 2015, 10, 598. [Google Scholar]
- Zhang, Y.; Yu, Q.; Wang, Y. Non-thermal atmospheric plasmas in dental restoration: Improved resin adhesive penetration. J. Dent. 2014, 42, 1033–1042. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, J.; Yao, C.; Yang, H.; Huang, C. New perspective to improve dentin–adhesive interface stability by using dimethyl sulfoxide wet-bonding and epigallocatechin-3-gallate. Dent. Mater. 2020, 36, 1452–1463. [Google Scholar] [CrossRef]
- Stape, T.H.S.; Tjäderhane, L.; Tezvergil-Mutluay, A.; Yanikian, C.R.F.; Szesz, A.L.; Loguercio, A.D.; Martins, L.R.M. Dentin bond optimization using the dimethyl sulfoxide-wet bonding strategy: A 2-year in vitro study. Dent. Mater. 2016, 32, 1472–1481. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, K.; Yang, H.; Yu, J.; Huang, C. The influence of dimethyl sulfoxide on resin–dentin bonding: A systematic review. Int. J. Adhes. Adhes. 2022, 113, 103037. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.-Y.; Zhang, L.; Kang, J.-J.; Chen, J.-H. Ethanol-wet bonding technique may enhance the bonding performance of contemporary etch-and-rinse dental adhesives. J. Adhes. Dent. 2012, 14, 113. [Google Scholar] [PubMed]
- Grégoire, G.; Sharrock, P.; Delannée, M.; Delisle, M.-B. Depletion of water molecules during ethanol wet-bonding with etch and rinse dental adhesives. Mater. Sci. Eng. C 2013, 33, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sadek, F.; Braga, R.R.; Muench, A.; Liu, Y.; Pashley, D.H.; Tay, F. Ethanol wet-bonding challenges current anti-degradation strategy. J. Dent. Res. 2010, 89, 1499–1504. [Google Scholar] [CrossRef]
- Eick, J.D.; Robinson, S.J.; Byerley, T.J.; Chappelow, C.C. Adhesives and nonshrinking dental resins of the future. Quintessence Int. 1993, 24, 632. [Google Scholar]
- Moszner, N.; Hirt, T. New polymer-chemical developments in clinical dental polymer materials: Enamel–dentin adhesives and restorative composites. J. Polym. Sci. Part. A Polym. Chem. 2012, 50, 4369–4402. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Garcia, I.M.; Mokeem, L.; Weir, M.D.; Xu, H.H.K.; Montoya, C.; Orrego, S. Developing Bioactive Dental Resins for Restorative Dentistry. J. Dent. Res. 2023, 102, 1180–1190. [Google Scholar] [CrossRef]
- Bazos, P.; Magne, P. Bio-emulation: Biomimetically emulating nature utilizing a histo-anatomic approach; structural analysis. Eur. J. Esthet. Dent. 2011, 6, 8–19. [Google Scholar]
- Bazos, P.; Magne, P. Bio-Emulation: Biomimetically emulating nature utilizing a histoanatomic approach; visual synthesis. Int. J. Esthet. Dent. 2014, 9, 330–352. [Google Scholar]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Bae, H.; Ahn, J.; Kang, T.; Seo, D.-G.; Hwang, D.S. Catechol-thiol-based dental adhesive inspired by underwater mussel adhesion. Acta Biomater. 2020, 103, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yucesoy, D.T.; Fong, H.; Hamann, J.; Hall, E.; Dogan, S.; Sarikaya, M. Biomimetic Dentin Repair: Amelogenin-Derived Peptide Guides Occlusion and Peritubular Mineralization of Human Teeth. ACS Biomater. Sci. Eng. 2023, 9, 1486–1495. [Google Scholar] [CrossRef]
- Chang, R.; Liu, Y.J.; Zhang, Y.L.; Zhang, S.Y.; Han, B.B.; Chen, F.; Chen, Y.X. Phosphorylated and Phosphonated Low-Complexity Protein Segments for Biomimetic Mineralization and Repair of Tooth Enamel. Adv. Sci. 2022, 9, e2103829. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.T.; Rao, C.Y.; Li, T.; Zhou, R.H. [Research Progress in Biomimetic Synthesis of Nano-Hydroxyapatite in Bone Tissue Engineering]. Sichuan Da Xue Xue Bao Yi Xue Ban 2021, 52, 740–746. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Biomimetic remineralization of resin-bonded acid-etched dentin. J. Dent. Res. 2009, 88, 719–724. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.A.S.; Weir, M.D.; Zhou, X.D.; Bai, Y.X.; Reynolds, M.A.; Xu, H.H.K. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Song, L.; Parthasarathy, R.; Boone, K.; Misra, A.; Tamerler, C. Threats to adhesive/dentin interfacial integrity and next generation bio-enabled multifunctional adhesives. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2673–2683. [Google Scholar] [CrossRef]
- Reymus, M.; Roos, M.; Eichberger, M.; Edelhoff, D.; Hickel, R.; Stawarczyk, B. Bonding to new CAD/CAM resin composites: Influence of air abrasion and conditioning agents as pretreatment strategy. Clin. Oral Investig. 2019, 23, 529–538. [Google Scholar] [CrossRef]
- Revilla-Leon, M.; Gomez-Polo, M.; Vyas, S.; Barmak, A.B.; Ozcan, M.; Att, W.; Krishnamurthy, V.R. Artificial intelligence applications in restorative dentistry: A systematic review. J. Prosthet. Dent. 2022, 128, 867–875. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Li, H.; Imazato, S. Materials informatics for developing new restorative dental materials: A narrative review. Front. Dent. Med. 2023, 4, 1123976. [Google Scholar] [CrossRef] [PubMed]
- Mallineni, S.K.; Sethi, M.; Punugoti, D.; Kotha, S.B.; Alkhayal, Z.; Mubaraki, S.; Almotawah, F.N.; Kotha, S.L.; Sajja, R.; Nettam, V.; et al. Artificial Intelligence in Dentistry: A Descriptive Review. Bioengineering 2024, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Suwardi, A.; Wang, F.; Xue, K.; Han, M.Y.; Teo, P.; Wang, P.; Wang, S.; Liu, Y.; Ye, E.; Li, Z.; et al. Machine Learning-Driven Biomaterials Evolution. Adv. Mater. 2022, 34, e2102703. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).