Preclinical Performance of a Novel Dental Implant Design Reducing Mechanical Stress in Cortical Bone
Abstract
1. Introduction
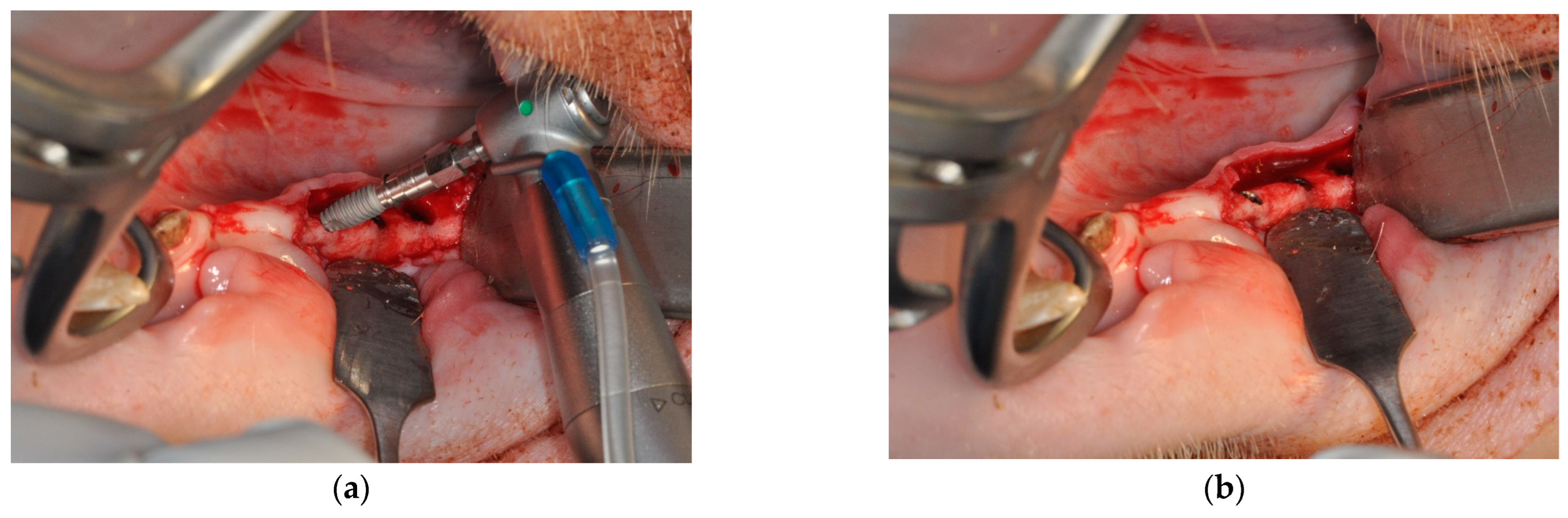
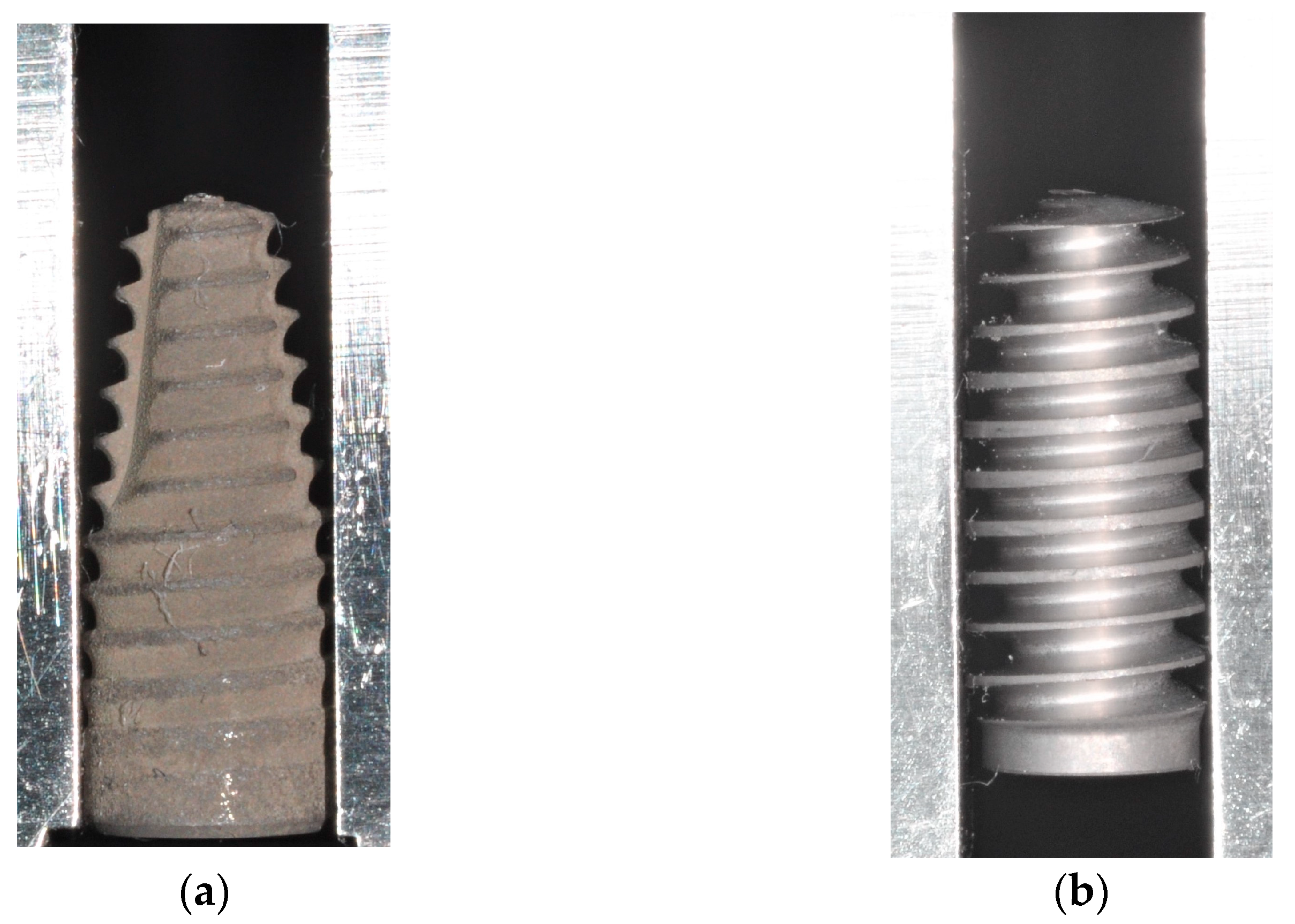
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MBL | marginal bone loss |
| BIC | bone-to-implant contact |
References
- Abboud, M.; Rugova, S.; Orentlicher, G. Immediate Loading: Are Implant Surface and Thread Design More Important Than Osteotomy Preparation? Compend. Contin. Educ. Dent. 2020, 41, 384–386. [Google Scholar] [PubMed]
- Heuzeroth, R.; Pippenger, B.E.; Sandgren, R.; Bellón, B.; Kühl, S. Thermal exposure of implant osteotomies and its impact on osseointegration-A preclinical in vivo study. Clin. Oral Implant. Res. 2021, 32, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Ikar, M.; Grobecker-Karl, T.; Karl, M.; Steiner, C. Mechanical stress during implant surgery and its effects on marginal bone: A literature review. Quintessence Int. 2020, 51, 142–150. [Google Scholar]
- Aghvami, M.; Brunski, J.B.; Serdar Tulu, U.; Chen, C.H.; Helms, J.A. A Thermal and Biological Analysis of Bone Drilling. J. Biomech. Eng. 2018, 140, 1010101. [Google Scholar] [CrossRef]
- Orgev, A.; Gonzaga, L.; Martin, W.; Morton, D.; Lin, W.S. Addition of an irrigation channel to a surgical template to facilitate cooling during implant osteotomy. J. Prosthet. Dent. 2021, 126, 164–166. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Bettach, R.; Cayron, B.; Boukhris, G.; Dedavid, B.A.; Frutos, J.C.P. Development of a New Drill Design to Improve the Temperature Control during the Osteotomy for Dental Implants: A Comparative In Vitro Analysis. Biology 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Kuster, M.; Mukaddam, K.; Zitzmann, N.U.; Filippi, A.; Kühl, S. Influence of a Novel Drill Design on Heat Generation During Conventional and Guided Implant Osteotomy. Int. J. Oral Maxillofac. Implants 2021, 36, e31–e41. [Google Scholar] [CrossRef]
- Karaca, F.; Aksakal, B.; Kom, M. Influence of orthopaedic drilling parameters on temperature and histopathology of bovine tibia: An in vitro study. Med. Eng. Phys. 2011, 33, 1221–1227. [Google Scholar] [CrossRef]
- Strbac, G.D.; Unger, E.; Donner, R.; Bijak, M.; Watzek, G.; Zechner, W. Thermal effects of a combined irrigation method during implant site drilling. A standardized in vitro study using a bovine rib model. Clin. Oral Implants Res. 2014, 25, 665–674. [Google Scholar] [CrossRef]
- Flanagan, D. Heat generated during seating of dental implant fixtures. J. Oral Implantol. 2014, 40, 174–181. [Google Scholar] [CrossRef]
- Sekura, K.; Erbel, C.; Karl, M.; Grobecker-Karl, T. Determinants of temperature development during dental implant surgery. Prosthesis 2024, 6, 657–669. [Google Scholar] [CrossRef]
- Coyac, B.R.; Leahy, B.; Salvi, G.; Hoffmann, W.; Brunski, J.B.; Helms, J.A. A preclinical model links osseo-densification due to misfit and osseo-destruction due to stress/strain. Clin. Oral Implants Res. 2019, 30, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Shahdad, S.; Bosshardt, D.; Patel, M.; Razaghi, N.; Patankar, A.; Roccuzzo, M. Benchmark performance of anodized vs. sandblasted implant surfaces in an acute dehiscence type defect animal model. Clin. Oral Implant. Res. 2022, 33, 1135–1146. [Google Scholar]
- Li, Z.; Arioka, M.; Liu, Y.; Aghvami, M.; Tulu, S.; Brunski, J.B.; Helms, J.A. Effects of condensation and compressive strain on implant primary stability: A longitudinal, in vivo, multiscale study in mice. Bone Jt. Res. 2020, 9, 60–70. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Hoffmann, W.; Gasser, A.; Brunski, J.B.; Helms, J.A. Mechanical and Biological Advantages of a Tri-Oval Implant Design. J. Clin. Med. 2019, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Tokuc, B.; Kan, B. The effect of triangular cross-section neck design on crestal bone stability in the anterior mandible: A randomized, controlled, split-mouth clinical trial. Clin. Oral Implants Res. 2021, 32, 1241–1250. [Google Scholar] [CrossRef]
- Li Manni, L.; Lecloux, G.; Rompen, E.; Aouini, W.; Shapira, L.; Lambert, F. Clinical and radiographic assessment of circular versus triangular cross-section neck Implants in the posterior maxilla: A 1-year randomized controlled trial. Clin. Oral Implants Res. 2020, 31, 814–824. [Google Scholar] [CrossRef]
- Fabbri, G.; Staas, T.; Urban, I. A Retrospective Observational Study Assessing the Clinical Outcomes of a Novel Implant System with Low-Speed Site Preparation Protocol and Tri-Oval Implant Geometry. J. Clin. Med. 2022, 11, 4859. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implant. Res. 2010, 21, 129–136. [Google Scholar] [CrossRef]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.D.; Herman, B.C.; Laudier, D.M.; Majeska, R.J.; Sun, H.B.; Schaffler, M.B. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 2012, 50, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Hansson, S.; Werke, M. The implant thread as a retention element in cortical bone: The effect of thread size and thread profile: A finite element study. J. Biomech. 2003, 36, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Ruffoni, D.; Wirth, A.J.; Steiner, J.A.; Parkinson, I.H.; Müller, R.; van Lenthe, G.H. The different contributions of cortical and trabecular bone to implant anchorage in a human vertebra. Bone 2012, 50, 733–738. [Google Scholar] [CrossRef]
- Yang, B.; Landa, A.I.; Heuberger, P.; Ploeg, H.L. Effects of dental implant diameter and tapered body design on stress distribution in rigid polyurethane foam during insertion. Med. Eng. Phys. 2024, 129, 104181. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implants 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Schulz, A.; Klär, V.; Grobecker-Karl, T.; Karl, M. Biomechanical rationale for a novel implant design reducing stress on buccal bone. Appl. Sci. 2023, 13, 666. [Google Scholar] [CrossRef]
- Klär, V.; Zimmerer, R.; Schulz, A.; Lethaus, B. Biomechanical performance of a novel implant design in simulated extraction sites and sinuslift procedures. Appl. Sci. 2023, 13, 7541. [Google Scholar] [CrossRef]
- Pawlowsky, K.; Ernst, L.; Steitz, J.; Stopinski, T.; Kögel, B.; Henger, A.; Kluge, R.; Tolba, R. The Aachen Minipig: Phenotype, Genotype, Hematological and Biochemical Characterization, and Comparison to the Göttingen Minipig. Eur. Surg. Res. 2017, 58, 193–203. [Google Scholar] [CrossRef]
- Steiner, C.; Karl, M.; Laschke, M.W.; Schupbach, P.; Venturato, A.; Gasser, A. Comparison of extraction sites versus artificial defects with xenogenic bone substitute in minipigs. Clin. Exp. Dent. Res. 2021, 7, 490–501. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Palarie, V.; Nacu, V.; Al-Nawas, B.; Kämmerer, P.W. Vertical osteoconductive characteristics of titanium implants with calcium-phosphate-coated surfaces—A pilot study in rabbits. Clin. Implant Dent. Relat. Res. 2014, 16, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Palarie, V.; Bicer, C.; Lehmann, K.M.; Zahalka, M.; Draenert, F.G.; Kämmerer, P.W. Early outcome of an implant system with a resorbable adhesive calcium-phosphate coating—A prospective clinical study in partially dentate patients. Clin. Oral Investig. 2012, 16, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Aghvami, M.; Brunski, J.; Helms, J. Biophysical regulation of osteotomy healing: An animal study. Clin. Implant Dent. Relat. Res. 2017, 19, 590–599. [Google Scholar] [CrossRef]
- Cecchinato, D.; Marino, M.; Håkansson, J.; Lindhe, J.; Derks, J. Occurrence of complications in patients restored with implants. Clin. Oral Implant. Res. 2022, 33, 913–920. [Google Scholar] [CrossRef]



| Drill Temperature [°C] | Undersizing [mm] | Insertion Torque [Ncm] | BIC [%] | MBL [mm] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Control (Straumann BLT®) | 8 | 27.6 | 2.3 | 8 | 0.15 | 0.28 | 6 | 61.5 | 21.4 | 8 | 50.00 | 18.71 | 16 | 2.80 | 1.67 |
| Test Porous (AlfaGate MT®) | 7 | 26.0 | 1.0 | 7 | 0.33 | 0.08 | 6 | 63.8 | 20.8 | 6 | 55.83 | 23.69 | 14 | 2.76 | 2.07 |
| Test Bioactive (AlfaGate MT®) | 9 | 26.5 | 1.1 | 10 | 0.34 | 0.08 | 10 | 76.1 | 8.9 | 9 | 48.11 | 22.89 | 14 | 4.07 | 3.35 |
| Drill Temperature | Undersizing | Insertion Torque | BIC | MBL | |
|---|---|---|---|---|---|
| Levené | 0.82 | 0.31 | 0.16 | 0.91 | 0.09 |
| Shapiro | 0.000 * | 0.000 * | 0.001 * | 0.600 | 0.009 * |
| Kruskal–Wallis | 0.08 | 0.09 | 0.70 | 0.60 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erbel, C.; Laschke, M.W.; Grobecker-Karl, T.; Karl, M. Preclinical Performance of a Novel Dental Implant Design Reducing Mechanical Stress in Cortical Bone. J. Funct. Biomater. 2025, 16, 102. https://doi.org/10.3390/jfb16030102
Erbel C, Laschke MW, Grobecker-Karl T, Karl M. Preclinical Performance of a Novel Dental Implant Design Reducing Mechanical Stress in Cortical Bone. Journal of Functional Biomaterials. 2025; 16(3):102. https://doi.org/10.3390/jfb16030102
Chicago/Turabian StyleErbel, Carolin, Matthias W. Laschke, Tanja Grobecker-Karl, and Matthias Karl. 2025. "Preclinical Performance of a Novel Dental Implant Design Reducing Mechanical Stress in Cortical Bone" Journal of Functional Biomaterials 16, no. 3: 102. https://doi.org/10.3390/jfb16030102
APA StyleErbel, C., Laschke, M. W., Grobecker-Karl, T., & Karl, M. (2025). Preclinical Performance of a Novel Dental Implant Design Reducing Mechanical Stress in Cortical Bone. Journal of Functional Biomaterials, 16(3), 102. https://doi.org/10.3390/jfb16030102








