Rat Calvarial Guided Bone Regeneration Model: Preclinical Insights into Biomaterials, Barrier Design, and Systemic Modulators
Abstract
1. Introduction
2. Experimental Model
3. Bone Substitute
4. Growth Factor and Hormonal Modulator
5. Mechanical Barriers
6. Risk Factors and Systemic Conditions
7. Biological Potential of Regenerated Bone
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBR | Guided Bone Regeneration |
| HA | Hydroxyapatite |
| HA/Col | Hydroxyapatite/Collagen Composite |
| CO3Ap | Carbonate Apatite |
| AB | Autogenous Bone |
| DBBM | Deproteinized Bovine Bone Minera |
| BMD | Bone Mineral Density |
| BV/TV | Bone Volume/Total Volume |
| PDGF(-BB) | Platelet-Derived Growth Factor (-BB subtype) |
| rhFGF-2 | Recombinant Human Basic Fibroblast Growth Factor-2 |
| PTH | Parathyroid Hormone |
| TRAP | Tartrate-Resistant Acid Phosphatase |
| RUNX2 | Runt-Related Transcription Factor 2 |
| COL-I | Collagen Type I |
| PLACL | Poly-L-lactic acid/ε-caprolactone |
| OVX | Ovariectomized |
| micro-CT | Micro-Computed Tomography |
| ROI | Region of Interest |
References
- Retzepi, M.; Donos, N. Guided bone regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 203–215. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.D.; Frigério, P.B.; Chica, G.E.A.; Okamoto, R.; Buchaim, R.L.; Buchaim, D.V.; Messora, M.R.; Issa, J.P.M. Biomaterials for Guided Tissue Regeneration and Guided Bone Regeneration: A Review. Dent. J. 2025, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.K.; Shapira, L.; Wilensky, A. Membrane barriers for guided bone regeneration: An overview of available biomaterials. Periodontology 2000 2023, 93, 56–76. [Google Scholar] [CrossRef]
- Alqahtani, A.M.; Moorehead, R.; Asencio, I.O. Guided tissue and bone regeneration membranes: A review of biomaterials and techniques for periodontal treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef]
- Namanloo, R.A.; Ommani, M.; Abbasi, K.; Alam, M.; Badkoobeh, A.; Rahbar, M.; Arasteh, H.K.; Hajmohammadi, E.; Soufdoost, R.S.; Mosaddad, S.A. Biomaterials in Guided Bone and Tissue Regenerations: An Update. Adv. Mater. Sci. Eng. 2022, 2022, 2489399. [Google Scholar] [CrossRef]
- Donos, N.; Akcali, A.; Padhye, N.; Sculean, A.; Calciolari, E. Bone regeneration in implant dentistry: Which are the factors affecting the clinical outcome? Periodontology 2000 2023, 93, 26–55. [Google Scholar] [CrossRef]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided bone regeneration in implant dentistry: Basic principle, progress over 35 years, and recent research activities. Periodontology 2000 2023, 93, 9–25. [Google Scholar] [CrossRef]
- Blanc-Sylvestre, N.; Bouchard, P.; Chaussain, C.; Bardet, C. Pre-Clinical Models in Implant Dentistry: Past, Present, Future. Biomedicines 2021, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Marger, L.; Barone, A.; Martinelli-Kläy, C.P.; Schaub, L.; Strasding, M.; Mekki, M.; Sailer, I.; Scherrer, S.S.; Durual, S. Calvarial Model of Bone Augmentation in Rabbit for Assessment of Bone Growth and Neovascularization in Bone Substitution Materials. J. Vis. Exp. 2019, 150, e59976. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Oryan, A. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect. Tissue Res. 2015, 56, 175–194. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Sato, S.; Takayama, T.; Murai, M.; Suzuki, N.; Ito, K. Short-term effects of rhBMP-2-enhanced bone augmentation beyond the skeletal envelope within a titanium cap in rabbit calvarium. J. Periodontol. 2008, 79, 348–354. [Google Scholar] [CrossRef]
- Yamada, Y.; Nanba, K.; Ito, K. Effects of occlusiveness of a titanium cap on bone generation beyond the skeletal envelope in the rabbit calvarium. Clin. Oral Implants Res. 2003, 14, 455–463. [Google Scholar] [CrossRef]
- Min, S.; Nguyen, T.; Kure, K.; Hasuike, A.; Sato, S.; Zadeh, H. Antibody Mediated Osseous Regeneration (AMOR) in Conjunction With Decortication in Rabbit Calvaria Model. J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35609. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Yamada, A.; Ninomiya, T.; Kato, T.; Masuda, Y. Micro-computed tomography newly developed for in vivo small animal imaging. Oral Radiol. 2005, 21, 14–18. [Google Scholar] [CrossRef]
- Ramanathan, S.; Lin, Y.C.; Thirumurugan, S.; Hu, C.C.; Duann, Y.F.; Chung, R.J. Poly(methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef]
- Han, F.; Liu, Z.; Wei, Q.; Ding, L.; Yu, L.; Wang, J.; Wang, H.; Zhang, W.; Yu, Y.; Zhao, Y.; et al. Minimally Invasive Implantable Biomaterials for Bone Reconstruction. Engineering 2025, 46, 23–46. [Google Scholar] [CrossRef]
- Kochi, G.; Sato, S.; Fukuyama, T.; Morita, C.; Honda, K.; Arai, Y.; Ito, K. Analysis on the guided bone augmentation in the rat calvarium using a microfocus computerized tomography analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e42–e48. [Google Scholar] [CrossRef] [PubMed]
- Kochi, G.; Sato, S.; Ebihara, H.; Hirano, J.; Arai, Y.; Ito, K. A comparative study of microfocus CT and histomorphometry in the evaluation of bone augmentation in rat calvarium. J. Oral Sci. 2010, 52, 203–211. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasuike, A.; Koshi, R.; Ozawa, Y.; Ozaki, M.; Kubota, T.; Sato, S. Influences of mechanical barrier permeability on guided bone augmentation in the rat calvarium. J. Oral Sci. 2018, 60, 453–459. [Google Scholar] [CrossRef]
- Kogure, K.; Hasuike, A.; Kurachi, R.; Igarashi, Y.; Idesawa, M.; Sato, S. Effect of a Recombinant Human Basic Fibroblast Growth Factor 2 (rhFGF-2)-Impregnated Atelocollagen Sponge on Vertical Guided Bone Regeneration in a Rat Calvarial Model. Dent. J. 2025, 13, 177. [Google Scholar] [CrossRef]
- Oginuma, T.; Sato, S.; Udagawa, A.; Saito, Y.; Arai, Y.; Ito, K. Autogenous bone with or without hydroxyapatite bone substitute augmentation in rat calvarium within a plastic cap. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114 (Suppl. S5), S107–S113. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Kubota, T.; Yamamoto, T.; Tsukune, N.; Koshi, R.; Nishida, T.; Asano, M.; Sato, S. Comparison of the bone augmentation ability of absorbable collagen sponge with that of hydroxyapatite/collagen composite. J. Oral Sci. 2018, 60, 514–518. [Google Scholar] [CrossRef]
- Senoo, M.; Hasuike, A.; Yamamoto, T.; Ozawa, Y.; Watanabe, N.; Furuhata, M.; Sato, S. Comparison of Macro-and Micro-porosity of a Titanium Mesh for Guided Bone Regeneration: An In Vivo Experimental Study. Vivo 2022, 36, 76–85. [Google Scholar] [CrossRef]
- Watanabe, T.; Hasuike, A.; Wakuda, S.; Kogure, K.; Min, S.; Watanabe, N.; Sakai, R.; Chaurasia, A.; Arai, Y.; Sato, S. Resorbable bilayer membrane made of L-lactide-ε-caprolactone in guided bone regeneration: An in vivo experimental study. Int. J. Implant Dent. 2024, 10, 1. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Chang, G.; Deng, S.; Chan, H.F. Bioactive glass in tissue regeneration: Unveiling recent advances in regenerative strategies and applications. Adv. Mater. 2025, 37, 2312964. [Google Scholar] [CrossRef] [PubMed]
- Gasson, S.B.; Dobson, L.K.; Pfau-Cloud, M.R.; Beltran, F.O.; Pool, R.R.; Gregory, C.A.; Grunlan, M.A.; Saunders, W.B. Biocompatibility and bone regeneration by shape memory polymer scaffolds. J. Biomed. Mater. Res. Part A 2025, 113, e37806. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Kantorski, K.Z.; Dubey, N.; Daghrery, A.; Fenno, J.C.; Mishina, Y.; Chan, H.-L.; Mendonça, G.; Bottino, M.C. Personalized and defect-specific antibiotic-laden scaffolds for periodontal infection ablation. ACS Appl. Mater. Interfaces 2021, 13, 49642–49657. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Morand, D.-N.; Thomas, L.; Bugueno, I.M.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a novel electrospun polycaprolactone scaffold functionalized with ibuprofen for periodontal regeneration: An in vitro andin vivo study. Materials 2018, 11, 580. [Google Scholar] [CrossRef]
- Deng, L.; Ai, L.; Li, R.; Xu, W.; Zheng, L.; Wang, C.; Huang, H. Animal Experimental Study on Delayed Implantation in a Severely Atrophic Alveolar Ridge Reconstructed Using a 3D-Printed Bioactive Glass Scaffold: A Pilot Study. J. Funct. Biomater. 2025, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Sato, S.; Kigami, R.; Yoshimaki, T.; Arai, Y.; Ito, K. Effects of Platelet-Derived Growth Factor on Enhanced Bone Augmentation beyond the Skeletal Envelope within a Plastic Cap in the Rat Calvarium. J. Hard Tissue Biol. 2013, 22, 221–226. [Google Scholar] [CrossRef]
- Tsunori, K.; Sato, S.; Hasuike, A.; Manaka, S.; Shino, H.; Sato, N.; Kubota, T.; Arai, Y.; Ito, K.; Miyazaki, M. Effects of intermittent administration of parathyroid hormone on bone augmentation in rat calvarium. Implant Dent. 2015, 24, 142–148. [Google Scholar] [CrossRef]
- Karipidou, N.; Gorley, J.P.M.; Katrilaka, C.; Manglaris, C.; Tzavellas, A.N.; Pitou, M.; Cheva, A.; Michailidis, N.; Tsiridis, E.E.; Choli-Papadopoulou, T.; et al. A Critical Review of Commercial Collagen-Based Scaffolds in Bone Regeneration: Functional Properties and Clinical Evidence from Infuse® Bone Graft. J. Funct. Biomater. 2025, 16, 313. [Google Scholar] [CrossRef]
- Thoma, D.S.; Lim, H.C.; Sapata, V.M.; Yoon, S.R.; Jung, R.E.; Jung, U.W. Recombinant bone morphogenetic protein-2 and platelet-derived growth factor-BB for localized bone regeneration. Histologic and radiographic outcomes of a rabbit study. Clin. Oral Implants Res. 2017, 28, e236–e243. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Z.; Yun, J.; Liu, R.; Li, J.; Chen, Y.; Cai, H.; Jiang, H.B.; Lee, E.S.; Han, J.; et al. Effect of Different Membranes on Vertical Bone Regeneration: A Systematic Review and Network Meta-Analysis. BioMed Res. Int. 2022, 2022, 7742687. [Google Scholar] [CrossRef] [PubMed]
- Elad, A.; Rider, P.; Rogge, S.; Witte, F.; Tadić, D.; Kačarević, Ž.P.; Steigmann, L. Application of Biodegradable Magnesium Membrane Shield Technique for Immediate Dentoalveolar Bone Regeneration. Biomedicines 2023, 11, 744. [Google Scholar] [CrossRef]
- Luo, Q.; Gao, K.; Li, Y.; Zhang, Z.; Chen, S.; Zhou, J. Osteogenesis Activity and Porosity Effect of Biodegradable Mg-Ga Alloys Barrier Membrane for Guided Bone Regeneration: An in Vitro and in Vivo Study in Rabbits. Biomedicines 2025, 13, 1940. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, J.; Schmidt, F.; Dai, J.; Li, J.; Xu, S.; Li, A.; Yu, Z.; Witte, F. Magnesium-based Barrier Membrane for Guided Bone Regeneration: From Bedside to Bench and Back again. Biomaterials 2025, 328, 123783. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, S.; Oginuma, T.; Saito, Y.; Arai, Y.; Ito, K. Effects of nicotine on guided bone augmentation in rat calvarium. Clin. Oral Implants Res. 2013, 24, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Hasuike, A.; Tsukune, N.; Ozawa, Y.; Yamamoto, T.; Min, S.; Naito, M.; Sato, S. Influence of estrogen deficiency on guided bone augmentation: Investigation of rat calvarial model and osteoblast-like MC3T3-E1 cells. Eur. J. Oral Sci. 2018, 126, 206–213. [Google Scholar] [CrossRef]
- Kubota, T.; Hasuike, A.; Naito, M.; Tsunori, K.; Min, S.; Sato, S. Enhancement of Bone Augmentation in Osteoporotic Conditions by the Intermittent Parathyroid Hormone: An Animal Study in the Calvarium of Ovariectomized Rat. Int. J. Oral Maxillofac. Implants 2018, 33, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Calciolari, E.; Wall, I.; Lewis, M.P.; Donos, N. The effect of experimental diabetes and glycaemic control on guided bone regeneration: Histology and gene expression analyses. Clin. Oral Implants Res. 2018, 29, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Retzepi, M.; Petrie, A.; Hakimi, A.R.; Schwarz, F.; Donos, N. The effect of diabetes on bone formation following application of the GBR principle with the use of titanium domes. Clin. Oral Implants Res. 2013, 24, 28–35. [Google Scholar] [CrossRef]
- Kubota, T.; Hasuike, A.; Ozawa, Y.; Yamamoto, T.; Tsunori, K.; Yamada, Y.; Sato, S. Regenerative capacity of augmented bone in rat calvarial guided bone augmentation model. J. Periodontal Implant Sci. 2017, 47, 77–85. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and histomorphometric analysis of bone tissue after guided bone regeneration with non-resorbable membranes vs resorbable membranes and titanium mesh. Clin. Implant Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef]
- Matsushita, Y.; Noguchi, A.; Ono, W.; Ono, N. Multi-omics analysis in developmental bone biology. Jpn. Dent. Sci. Rev. 2023, 59, 412–420. [Google Scholar] [CrossRef]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 2023, 24, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, X.; Ma, M.; Dou, T.; Wei, Y.; Rux, D.; Qin, L.; Yang, Y.; Zhu, Y.; Yao, L. Integrating spatial and single-cell transcriptomics to characterize mouse long bone fracture healing process. Commun. Biol. 2025, 8, 887. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, P.; Wang, J.; Lv, H.; Han, J.; Hou, Z.; Xu, R.; Chen, W. Advances in spatial transcriptomics and its application in the musculoskeletal system. Bone Res. 2025, 13, 54. [Google Scholar] [CrossRef]
- Yang, F.; Jia, J.; Xiao, Y.; Feng, P. Advances in 3D/4D printing of bone scaffolds and their shape/properties adaptability. Rev. Mater. Res. 2025, 1, 100088. [Google Scholar] [CrossRef]
- Di Spirito, F.; Giordano, F.; Di Palo, M.P.; Ferraro, C.; Cecere, L.; Frucci, E.; Caggiano, M.; Lo Giudice, R. Customized 3D-printed mesh, membrane, bone substitute, and dental implant applied to guided bone regeneration in oral implantology: A narrative review. Dent. J. 2024, 12, 303. [Google Scholar] [CrossRef]
- Khorasani, E.; Vahidi, B. 3D-Printed Scaffolds for Cranial Bone Regeneration: A Systematic Review of Design, Materials, and Computational Optimization. Biotechnol. Bioeng. 2025, 122, 1982–2008. [Google Scholar] [CrossRef]
- Xue, C.; Chen, L.; Wang, N.; Chen, H.; Xu, W.; Xi, Z.; Sun, Q.; Kang, R.; Xie, L.; Liu, X. Stimuli-responsive hydrogels for bone tissue engineering. Biomater. Transl. 2024, 5, 257–273. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Y.; Lin, C.; Chen, X.; Zhao, F. Stimulus-responsive smart bioactive glass composites for repair of complex tissue defects. Theranostics 2025, 15, 1760–1786. [Google Scholar] [CrossRef]
- Riosalido, P.M.; Arango-Ospina, M.; Velasquez, P.; Murciano, A.; Boccaccini, A.R.; De Aza, P.N. Bioactive scaffolds harnessing ionic modifications to promote osteogenesis and angiogenesis in bone regeneration. Boletín Soc. Española Cerámica Vidr. 2025, 64, 100447. [Google Scholar] [CrossRef]
- Farbehi, N.; Alinejad-Rokny, H.; Chtanova, T.; Rnjak-Kovacina, J. Spatial and single-cell transcriptomics unravel the complex interplay between the body and medical implants. Cell Biomater. 2025, 1, 100099. [Google Scholar] [CrossRef]
| Study (Author, Year) | Bone Substitute(s) | Micro-CT Findings | Histological Findings | Key Conclusion |
|---|---|---|---|---|
| Kochi et al., 2009 [20] | HA vs. Empty | The HA group showed significant time-dependent bone volume increase; new bone reached the cap top. Control had bone at ~half height new bone formed beyond the skeletal envelope. In contrast, there was no bone formation beyond the skeletal envelope at the control site. | HA bridged defect edges; no vertical augmentation in empty control. | HA performed as a scaffold for vertical bone augmentation beyond the skeletal envelope. |
| Kochi et al., 2010 [21] | HA vs. Empty | Micro-CT and histology showed consistent regeneration; strong correlation. | The HA group had more mineralized tissue than control. At 12 weeks, histology showed that the HA group had significantly higher percentages and height of newly generated and mineralized tissue than the control. | Micro-CT is reliable for longitudinal bone regeneration analysis. |
| Oginuma et al., 2012 [24] | AB vs. AB + HA | Both increased bone volume similarly. | AB-only group had significantly higher mineralized tissue (27.6%) than AB+HA (45.5%, p < 0.05). | HA may interfere with early mineralization when mixed with AB. |
| Ozawa et al., 2018 [25] | HA/Collagen Composite vs. Collagen sponge | The HA/Collagen Composite group reached approximately twice compared with collagen sponge. | HA/Collagen induced dense trabeculae; collagen sponge showed sparse bone with voids. | The HA/Collagen composite is superior to collagen sponge for bone augmentation. |
| Senoo et al., 2022 [26] | DBBM vs. CO3Ap | Similar bone volume (DBBM: 34.0%, CO3Ap: 35.7%), but BMD was higher in CO3Ap (1.92 g/cm3 vs. 1.85 g/cm3, p < 0.05). | CO3Ap: uniform trabecular bone; DBBM: fibrous encapsulation between particles. | CO3Ap supports better bone integration and remodeling. |
| Watanabe et al., 2024 [27] | DBBM or CO3Ap + PLACL or Collagen Membrane | CO3Ap + PLACL showed favorable height and space maintenance at 24 weeks. | CO3Ap + PLACL induced thick non-calcified tissue, no inflammation. | CO3Ap effective long-term; PLACL membrane ensures prolonged barrier function. |
| Study (Author, Year) | Bioactive Agent | Delivery Method (Scaffold Included) | Micro-CT Findings | Histological Findings | Key Conclusion |
|---|---|---|---|---|---|
| Tsuchiya et al. (2013) [34] | PDGF-BB (0.01%, 0.03%) | Local (with collagen sponge) | Increased BV; plastic cap full at 4 w (0.03%) and at 12 w (0.01%); 1/3 of the plastic cap full at 12 w (control) | BV: 71.8% (0.01%), 62.4% (0.03%) 34.7% (control); height: 95.3% (0.01%), 90.9% (0.03%), 48.4% (control) | PDGF-BB promotes bone formation beyond the skeletal envelope. |
| Kogure et al. (2025) [23] | rhFGF-2 (0.3%) | Local (with collagen sponge) | Increased BV; a rapid increase started at 8 w; by 12 w, approximately half of the ROIs were within the caps | Increased BV; 0.3% rhFGF-2: 35.6% (area) and 41.9% height at 12 w; control: 9.1% (area) and 13.4% height at 12 w | With proper space maintenance, rhFGF-2 can effectively promote vertical bone regeneration without requiring additional bone mineral particles. |
| Tsunori et al. (2023) [35] | PTH (35, 105 µg/kg) | Systemic (intraperitoneal injection without scaffold) | Dose-dependent increase: ~1/3 (control), ~1/2 (PTH-35), ~2/3 (PTH-105) | Thicker lamellar bone; more Runx2+ osteoblasts in PTH groups | Intermittent PTH systemically enhances osteogenesis in a dose-dependent manner without a scaffold. |
| Study (Author, Year) | Barrier Type | Micro-CT Findings | Histological Findings | Key Conclusion |
|---|---|---|---|---|
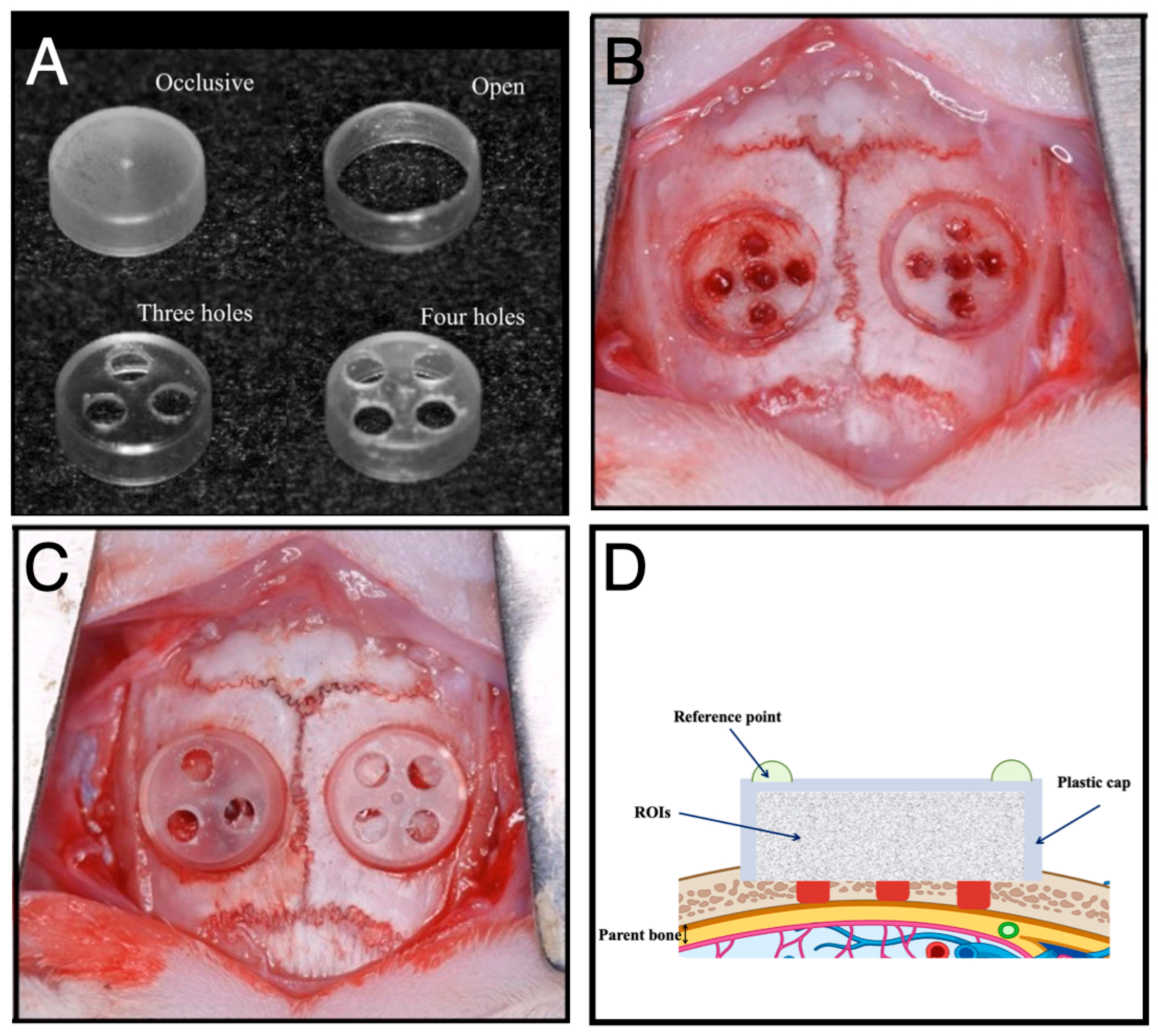
| Yamamoto et al., 2018 [22] | Plastic caps: Occlusive/Open/3-hole/4-hole | Bone volume inversely correlated with permeability. Occlusive > 3-hole > 4-hole > Open. | The 3-hole group exhibited the highest level of collagen maturation (Masson’s trichrome). | Moderate permeability (e.g., 3-hole) optimizes balance between soft tissue exclusion and biological signaling. |
| Senoo et al., 2022 [26] | Titanium mesh: Microporous (20 μm) vs. Macroporous (1–2 mm) | Microporous: ~38–40% new bone; Macroporous: ~31.5%. | Microporous: Less fibrous ingrowth, rich microvasculature Macroporous: Fibrous ingrowth | Microporous barriers provide optimal vascular entry while preventing fibrous invasion. |
| Watanabe et al., 2024 [27] | Resorbable bilayer membrane: PLACL vs. Collagen | Bone volume increased over time in all groups, with no significant differences among them. | Total tissue height and non-calcified tissue height: Significantly higher in the PLACL group than in the Collagen group. | The PLACL membrane is expected to not only promote bone augmentation through long-term space maintenance but also enhance soft-tissue formation. |
| Study (Author, Year) | Systemic Factor | Experimental Group | Micro-CT Findings | Histological Findings | Key Conclusion |
|---|---|---|---|---|---|
| Saito et al., 2012 [42] | Nicotine | Nicotine injection (systemic) | Significantly reduced bone volume and height gains, with delayed and diminished radiopacity in the augmented space | Thinner lamellar bone, fewer osteoblast-like cells, fewer microvessels | Nicotine compromises but does not abolish bone formation via inhibition of osteogenesis and angiogenesis. |
| Kubota et al., 2018 [43] | Estrogen Deficiency (OVX) | OVX rats | Significantly reduced bone volume in augmented space compared to controls | Sparse, thinning trabeculae; abundant non-calcified areas; reduced RUNX2+ and COL-I+ osteoblast activity | Estrogen deficiency impairs osteoblast differentiation and ECM production, compromising bone augmentation. |
| Kubota et al., 2018 [44] | Estrogen Deficiency + Rescue Therapy (PTH) | OVX rats + intermittent PTH | Bone volume significantly higher than untreated OVX and healthy controls | 50% bone fill (OVX + PTH) vs. 12% (untreated OVX) and 23.6% (sham); abundant RUNX2+ cells; mild increase in TRAP cells | Intermittent PTH restores bone regeneration in OVX rats, indicating strong anabolic osteogenic effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasuike, A.; Watanabe, T.; Wakuda, S.; Suzuki, T.; Kikuchi, S.; Min, S.; Arai, Y.; Sato, S. Rat Calvarial Guided Bone Regeneration Model: Preclinical Insights into Biomaterials, Barrier Design, and Systemic Modulators. J. Funct. Biomater. 2025, 16, 438. https://doi.org/10.3390/jfb16120438
Hasuike A, Watanabe T, Wakuda S, Suzuki T, Kikuchi S, Min S, Arai Y, Sato S. Rat Calvarial Guided Bone Regeneration Model: Preclinical Insights into Biomaterials, Barrier Design, and Systemic Modulators. Journal of Functional Biomaterials. 2025; 16(12):438. https://doi.org/10.3390/jfb16120438
Chicago/Turabian StyleHasuike, Akira, Taito Watanabe, Shin Wakuda, Tomoe Suzuki, Shuto Kikuchi, Seiko Min, Yoshinori Arai, and Shuichi Sato. 2025. "Rat Calvarial Guided Bone Regeneration Model: Preclinical Insights into Biomaterials, Barrier Design, and Systemic Modulators" Journal of Functional Biomaterials 16, no. 12: 438. https://doi.org/10.3390/jfb16120438
APA StyleHasuike, A., Watanabe, T., Wakuda, S., Suzuki, T., Kikuchi, S., Min, S., Arai, Y., & Sato, S. (2025). Rat Calvarial Guided Bone Regeneration Model: Preclinical Insights into Biomaterials, Barrier Design, and Systemic Modulators. Journal of Functional Biomaterials, 16(12), 438. https://doi.org/10.3390/jfb16120438







