Handheld Nonthermal Plasma Augmentation of Glass–Ceramic Spray Deposition on Zirconia Surface Characterization and MG-63/HGF-1 Cell Behavior: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Treatments
- (1)
- NT group: Ground only.
- (2)
- PL group: HNP (PiezoBrush® PZ3; relyon Plasma GmbH, Regensburg, Germany) at 18 W/50 kHz for 30 s.
- (3)
- AB group: Airborne-particle abrasion with 50 µm Al2O3 (Cobra; Renfert GmbH, Hilzingen, Germany) at 3 bar, 10 mm standoff, 10 s.
- (4)
- ABPL group: AB followed by HNP.
- (5)
- GE group: GCSD using a glass–ceramic powder (Biomic LiSi connector; Aidite) sprayed uniformly at 10 mm standoff, then processed in a dental furnace (AUSTROMAT 220; DEKEMA Dental-Keramiköfen GmbH, Freilassing, Germany): 450 °C drying for 1 min; heating at 80 °C/min to 895 °C with a 1.5 min hold; controlled cooling at 10 °C/min to 25 °C; etched with 4.5% hydrofluoric (HF) acid (IPS Ceramic Etching Gel; Ivoclar Vivadent, Schaan, Liechtenstein) for 100 s.
- (6)
- GEPL group: GCSD followed by HNP.
- (7)
- Ti group (baseline): Ground titanium.
2.3. Surface Morphology and Elemental Analyses
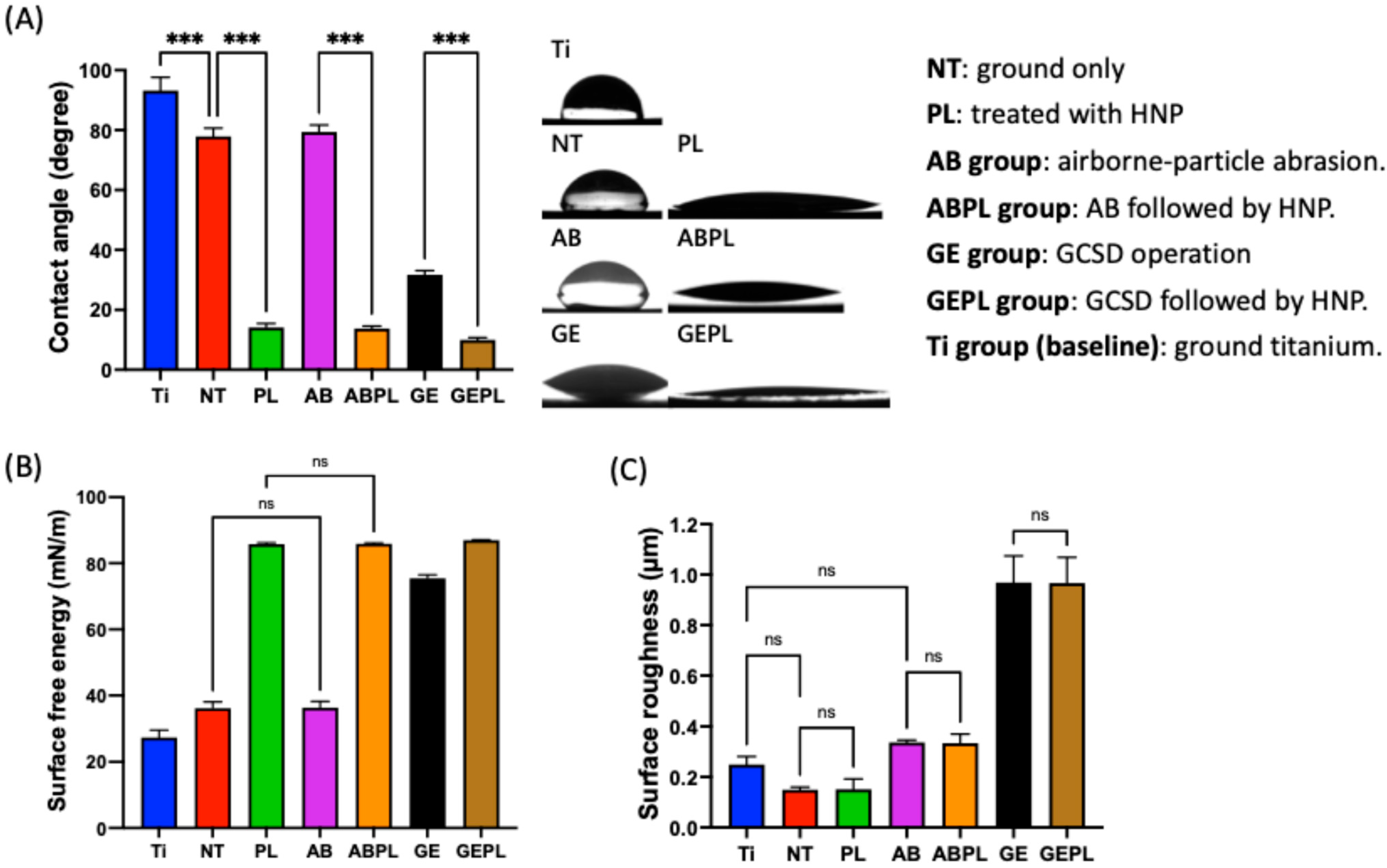
2.4. Surface Wettability and Roughness
2.5. Cell Culture
2.6. Cell Attachment
2.7. Cell Metabolic Activity
2.8. Cytotoxicity
2.9. Inflammatory Response
2.10. Statistical Analysis
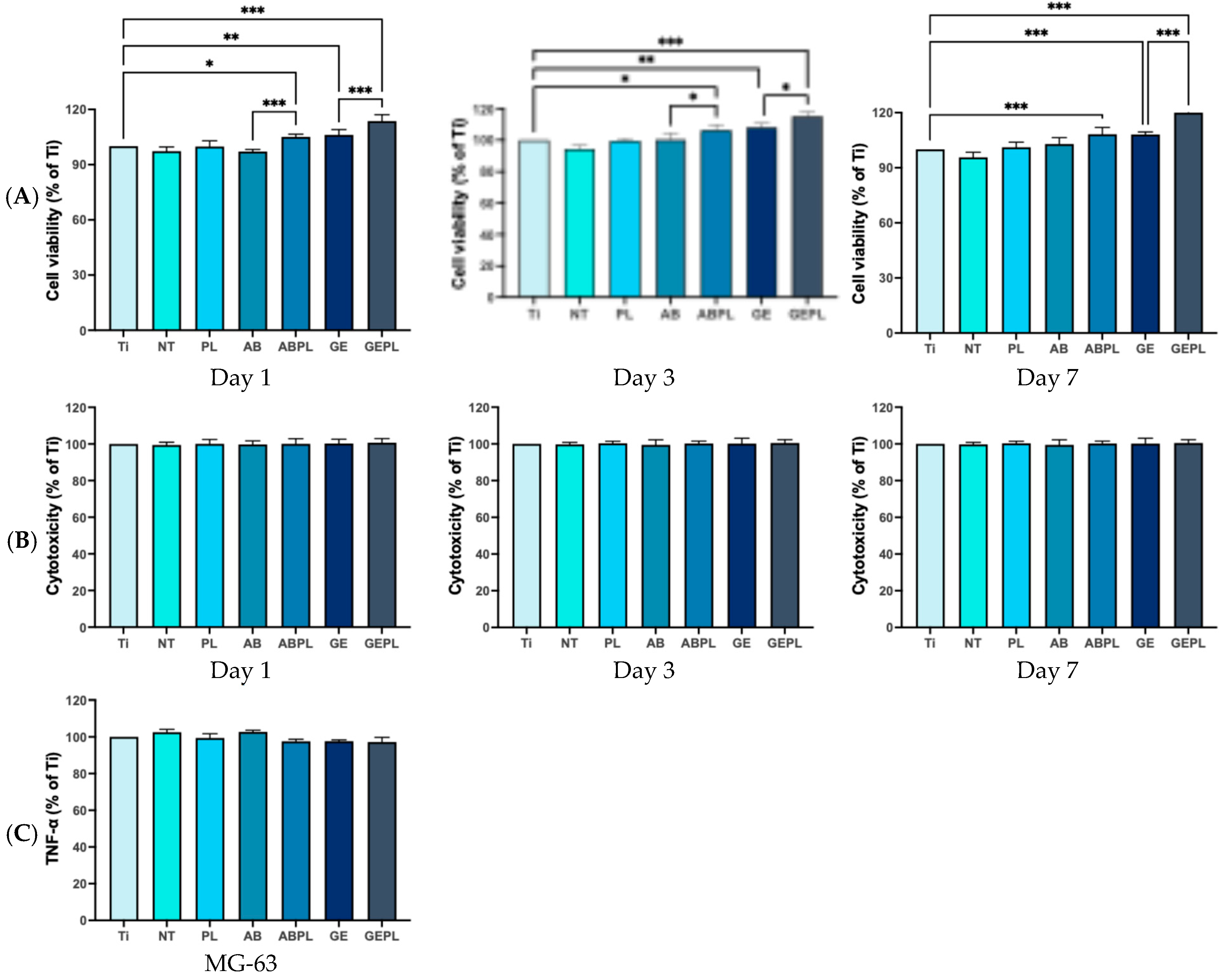
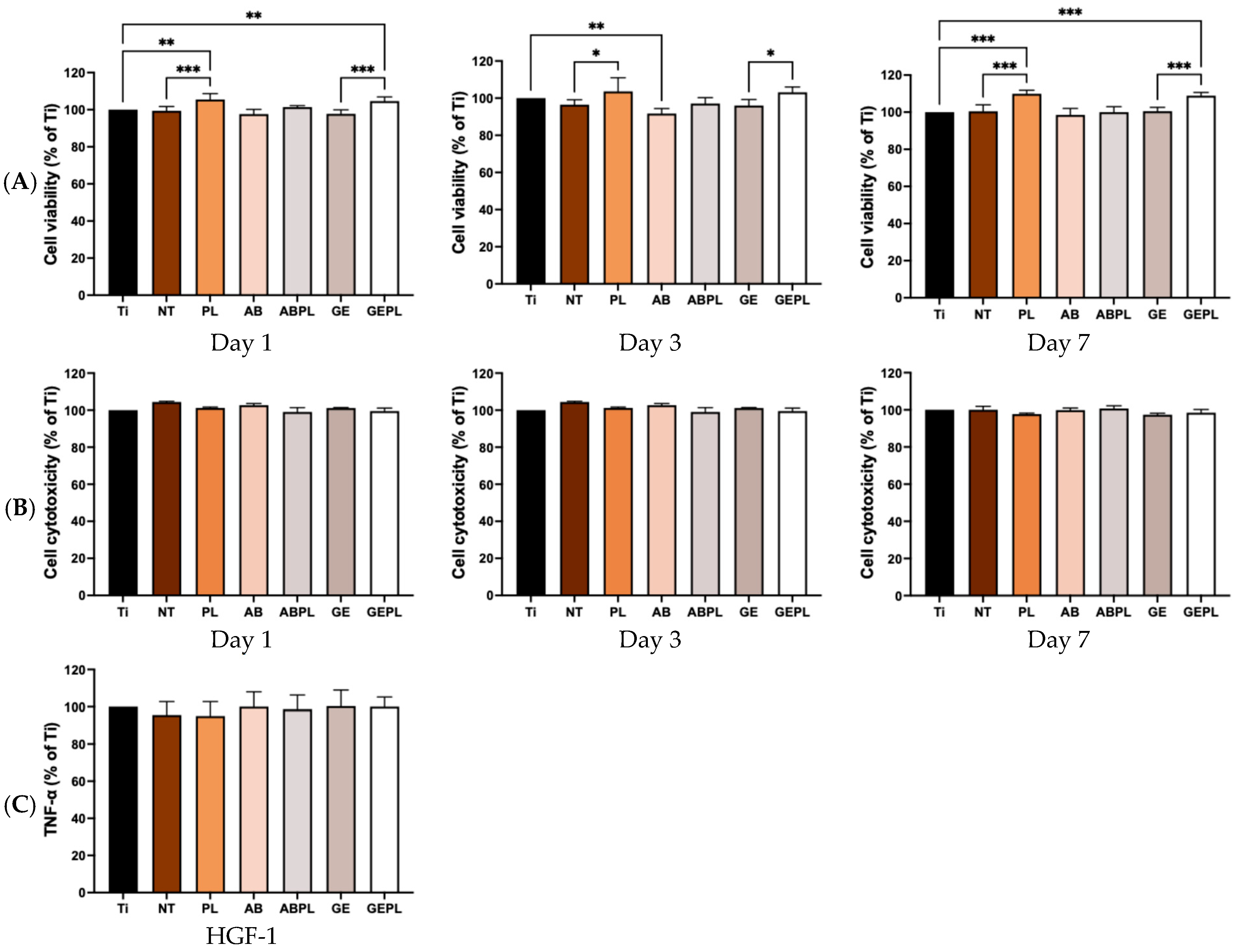
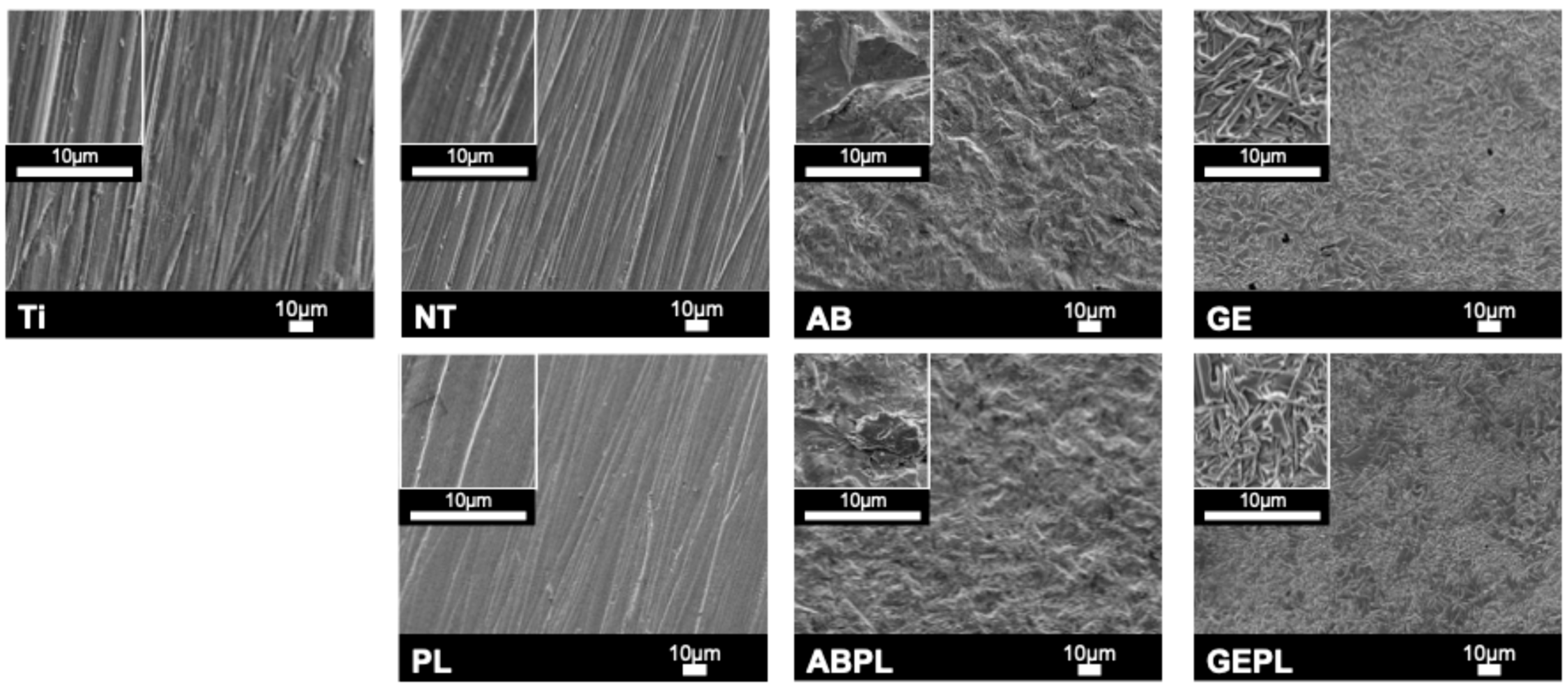
3. Results
3.1. Surface Characteristics
3.2. Cell Attachment and Spreading
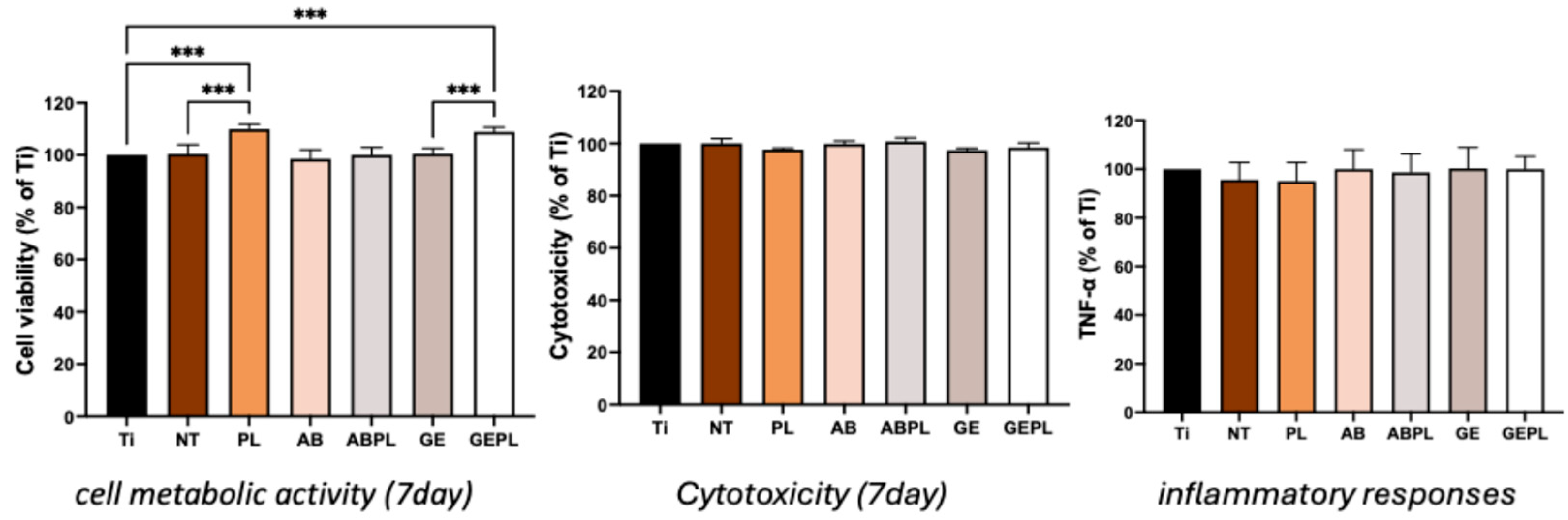
3.3. Cellular Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Takashi, M.; Takashi, N.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- Jitwirachot, K.; Rungsiyakull, P.; Holloway, J.A.; Jia-Mahasap, W. Wear behavior of different generations of zirconia: Present literature. Int. J. Dent. 2022, 2022, 9341616. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Candido, L.; Fais, L.; Ferreira, E.; Antonio, S.; Pinelli, L. Characterization of a Diamond Ground Y-TZP and Reversion of the Tetragonal to Monoclinic Transformation. Oper. Dent. 2017, 42, 407–417. [Google Scholar] [CrossRef]
- Agustín-Panadero, R.; Román-Rodríguez, J.L.; Ferreiroa, A.; Solá-Ruíz, M.F.; Fons-Font, A. Zirconia in fixed prosthesis. A literature review. J. Clin. Exp. Dent. 2014, 6, e66–e73. [Google Scholar] [CrossRef]
- Xue, M.; Liu, S.; Wang, X.; Jiang, K. High fracture toughness of 3Y-TZP ceramic over a wide sintering range. Mater. Chem. Phys. 2020, 244, 122693. [Google Scholar] [CrossRef]
- Takagi, S.; Inukai, K.; Kawai, N.; Nozawa, S.; Kyono, A.; Fukaya, R.; Adachi, S.-i.; Ichiyanagi, K. Visualization of transformation toughening of zirconia ceramics during dynamic fracture. Appl. Phys. Lett. 2021, 118, 231901. [Google Scholar] [CrossRef]
- Bocam, K.; Anunmana, C.; Eiampongpaiboon, T. Grain size, crystalline phase and fracture toughness of the monolithic zirconia. J. Adv. Prosthodont. 2022, 14, 285–293. [Google Scholar] [CrossRef]
- Tabatabaian, F. Color Aspect of Monolithic Zirconia Restorations: A Review of the Literature. J. Prosthodont. 2019, 28, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Manziuc, M.-M.; Gasparik, C.; Negucioiu, M.; Constantiniuc, M.; Burde, A.; Vlas, I.; Dudea, D. Optical properties of translucent zirconia: A review of the literature. EuroBiotech J. 2019, 3, 45–51. [Google Scholar] [CrossRef]
- Nowicka, A.; El-Maghraby, H.; Švančárková, A.; Galusková, D.; Reveron, H.; Gremillard, L.; Chevalier, J.; Galusek, D. Corrosion and low temperature degradation of 3Y-TZP dental ceramics under acidic conditions. J. Eur. Ceram. Soc. 2020, 40, 6114–6122. [Google Scholar] [CrossRef]
- Lin, H.; Yin, C.; Mo, A. Zirconia based dental biomaterials: Structure, mechanical properties, biocompatibility, surface modification, and applications as implant. Front. Dent. Med. 2021, 2, 689198. [Google Scholar] [CrossRef]
- Jung, S.; Moser, M.M.; Kleinheinz, J.; Happe, A. Biocompatibility of Lithium Disilicate and Zirconium Oxide Ceramics with Different Surface Topographies for Dental Implant Abutments. Int. J. Mol. Sci. 2021, 22, 7700. [Google Scholar] [CrossRef]
- Wagner, G.; Eggers, B.; Duddeck, D.; Kramer, F.J.; Bourauel, C.; Jepsen, S.; Deschner, J.; Nokhbehsaim, M. Influence of cold atmospheric plasma on dental implant materials—An in vitro analysis. Clin. Oral Investig. 2022, 26, 2949–2963. [Google Scholar] [CrossRef]
- Staehlke, S.; Brief, J.; Senz, V.; Eickner, T.; Nebe, J.B. Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation. Materials 2023, 16, 4203. [Google Scholar] [CrossRef]
- Guo, L.; Zou, Z.; Smeets, R.; Kluwe, L.; Hartjen, P.; Gosau, M.; Henningsen, A. Attachment and Osteogenic Potential of Dental Pulp Stem Cells on Non-Thermal Plasma and UV Light Treated Titanium, Zirconia and Modified PEEK Surfaces. Materials 2022, 15, 2225. [Google Scholar] [CrossRef]
- Halim, F.C.; Pesce, P.; De Angelis, N.; Benedicenti, S.; Menini, M. Comparison of the clinical outcomes of titanium and zirconia implant abutments: A systematic review of systematic reviews. J. Clin. Med. 2022, 11, 5052. [Google Scholar] [CrossRef]
- Pumnil, S.; Rungsiyakull, P.; Rungsiyakull, C.; Elsaka, S. Effect of different customized abutment types on stress distribution in implant-supported single crown: A 3D finite element analysis. J. Prosthodont. 2022, 31, e2–e11. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-Y.; Kang, C.-M.; Feng, S.-W.; Hung, C.-Y.; Iwaguro, S.; Lin, D.-J. Effects of glass-ceramic spray deposition manipulation on the surface characteristics of zirconia dental restorations. Ceram. Int. 2022, 48, 29873–29881. [Google Scholar] [CrossRef]
- Liang, S.; Yuan, F.; Chen, H.; Sun, Y. Digital evaluation of the effect of nanosilica-lithium spray coating on the internal and marginal fit of high translucent zirconia crowns. J. Dent. 2023, 132, 104503. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wang, H.; Shi, Y.; Su, Z.; Hannig, M.; Fu, B. The effect of surface treatments on zirconia bond strength and durability. J. Funct. Biomater. 2023, 14, 89. [Google Scholar] [CrossRef]
- Kang, C.-M.; Lin, D.-J.; Feng, S.-W.; Hung, C.-Y.; Iwaguro, S.; Peng, T.-Y. Innovation glass-ceramic spray deposition technology improving the adhesive performance for zirconium-based dental restorations. Int. J. Mol. Sci. 2022, 23, 12783. [Google Scholar] [CrossRef]
- Jin, C.; Wang, J.; Huang, Y.; Yu, P.; Xiong, Y.; Yu, H.; Gao, S. Effects of hydrofluoric acid concentration and etching time on the bond strength to ceramic-coated zirconia. J. Adhes. Dent. 2022, 24, 125–136. [Google Scholar] [CrossRef]
- Daguano, J.; Milesi, M.T.B.; Rodas, A.C.D.; Weber, A.F.; Sarkis, J.E.S.; Hortellani, M.A.; Zanotto, E.D. In vitro biocompatibility of new bioactive lithia-silica glass-ceramics. Mater. Sci. Eng. C 2019, 94, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-C.; Cha, T.-Y.; Yao, Y.-C.; Kang, C.-M.; Wu, S.-H.; Mine, Y.; Tseng, C.-F.; Lee, I.-T.; Lin, D.-J.; Peng, T.-Y. Promoting surface energy and osteoblast viability on zirconia implant abutments through glass–ceramic spray deposition technology. J. Funct. Biomater. 2025, 16, 288. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Swain, M. A novel polymer infiltrated ceramic dental material. Dent. Mater. 2011, 27, 527–534. [Google Scholar] [CrossRef]
- Donmez, M.B.; Diken Turksayar, A.A.; Olcay, E.O.; Sahmali, S.M. Fracture Resistance of Single-Unit Implant-Supported Crowns: Effects of Prosthetic Design and Restorative Material. J. Prosthodont. 2022, 31, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Thirumaran, D.; Leoney, A.; Fayeez, A.; Yazhini Shanmya, P. Effect of different occlusal materials on peri-implant stress distribution with different osseointegration condition: A finite element analysis. J. Indian Prosthodont. Soc. 2024, 24, 292–299. [Google Scholar] [CrossRef]
- Tseng, C.F.; Lee, I.T.; Wu, S.H.; Chen, H.M.; Mine, Y.; Peng, T.Y.; Kok, S.H. Effects of handheld nonthermal plasma on the biological responses, mineralization, and inflammatory reactions of polyaryletherketone implant materials. J. Dent. Sci. 2024, 19, 2018–2026. [Google Scholar] [CrossRef]
- Lee, S.K.; Ji, M.K.; Jo, Y.J.; Park, C.; Cho, H.; Lim, H.P. Effect of Non-Thermal Plasma Treatment of Contaminated Zirconia Surface on Porphyromonas gingivalis Adhesion and Osteoblast Viability. Materials 2022, 15, 5348. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lai, S.-Y.; Lee, F.-T.; Wu, Y.-C.; Feng, S.-W.; Nikawa, H.; Peng, T.-Y. Influence of handheld nonthermal plasma on shear bond strength of polyaryletherketone to resin-matrix cement. J. Dent. Sci. 2024, 19, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-Y.; Lin, S.-I.; Chang, C.-W.; Shen, Y.-R.; Mine, Y.; Lin, Z.-C.; Fang, M.-L.; Sung, C.-C.; Tseng, C.-F.; Peng, T.-Y.; et al. Comparative evaluation of dental clinical surface treatments for polyetheretherketone with airborne-particle abrasion, hydrofluoric acid etching, and handheld nonthermal plasma activation on long-term bond performance. Polymers 2025, 17, 1448. [Google Scholar] [CrossRef]
- ISO 1997: 2018; Granulated Cork and Cork Powder—Classification, Properties and Packing. International Organization for Standardization: Geneva, Switzerland, 2018.
- Brehm, M.; Scheiger, J.M.; Welle, A.; Levkin, P.A. Reversible surface wettability by silanization. Adv. Mater. Interfaces 2020, 7, 1902134. [Google Scholar] [CrossRef]
- Lai, S.-Y.; Wei, C.; Peng, T.-Y. Enhancing bonding and durability of polyaryletherketone (PAEK) restorations with nonthermal plasma activation and monomer-based priming. J. Prosthet. Dent. 2025; in press. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; da Silva, E.V.F.; Vechiato-Filho, A.J.; Cesar, P.F.; Rangel, E.C.; da Cruz, N.C.; Goiato, M.C. Aging effect of atmospheric air on lithium disilicate ceramic after nonthermal plasma treatment. J. Prosthet. Dent. 2016, 115, 780–787. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Liu, L. Zirconia materials for dental implants: A literature review. Front. Dent. Med. 2021, 2, 687983. [Google Scholar] [CrossRef]
- Jia, F.; Wang, S.; Xu, S.; Wu, W.; Zhou, L.; Zeng, J. The role of titanium surface micromorphology in MG-63 cell motility during osteogenesis. Sci. Rep. 2022, 12, 9971. [Google Scholar] [CrossRef]
- Kearns, V.R.; Williams, R.L.; Mirvakily, F.; Doherty, P.J.; Martin, N. Guided gingival fibroblast attachment to titanium surfaces: An in vitro study. J. Clin. Periodontol. 2013, 40, 99–108. [Google Scholar] [CrossRef]
- Liang, Y.; Leng, Y.; Zhang, J. Influence of clinical zirconia surface treatments on microscopic characteristics and adhesion-proliferation behavior of human gingival fibroblasts. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101564. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Lu, R.; Gao, S.; Ling, Y.; Chen, S. Responses of human gingival fibroblasts to superhydrophilic hydrogenated titanium dioxide nanotubes. Colloids Surf. B Biointerfaces 2021, 198, 111489. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Oster, P.; Seemann, S.; Kruse, F.; Brief, J.; Nebe, B. Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation. Materials 2022, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Chen, H.; Tian, Z. Effects of Surface Treatment and Dental Preparation on the Fracture and Debonding of High-Translucency Zirconia Occlusal Veneers. J. Esthet. Restor. Dent. 2025, 37, 2430–2439. [Google Scholar] [CrossRef] [PubMed]




| C | O | Al | Si | Y | Zr | Ti | V | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Ti | 6.48 | 0.00 | 5.85 | 0.00 | 0.00 | 0.00 | 85.06 | 2.60 | 100.00 |
| NT | 12.02 | 23.44 | 0.00 | 0.00 | 4.34 | 60.21 | 0.00 | 0.00 | 100.00 |
| PL | 4.76 | 24.59 | 0.00 | 0.00 | 4.60 | 66.05 | 0.00 | 0.00 | 100.00 |
| AB | 12.30 | 25.94 | 1.65 | 0.00 | 3.40 | 56.72 | 0.00 | 0.00 | 100.00 |
| ABPL | 8.08 | 28.20 | 2.11 | 0.00 | 3.43 | 58.18 | 0.00 | 0.00 | 100.00 |
| GE | 11.60 | 52.98 | 0.87 | 31.67 | 0.00 | 2.87 | 0.00 | 0.00 | 100.00 |
| GEPL | 6.67 | 53.35 | 1.29 | 34.36 | 0.00 | 4.34 | 0.00 | 0.00 | 100.00 |
| Ti | NT | PL | AB | ABPL | GE | GEPL | |
|---|---|---|---|---|---|---|---|
| MG-63 | 3.25 ± 0.37 | 2.32 ± 0.53 | 7.88 ± 0.55 | 6.94 ± 1.11 | 10.63 ± 1.57 | 19.35 ± 1.37 | 32.76 ± 1.25 |
| HGF-1 | 8.78 ± 0.58 | 19.31 ± 0.97 | 22.19 ± 1.39 | 11.01 ± 0.62 | 14.70 ± 1.07 | 19.12 ± 1.16 | 23.70 ± 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-H.; Lai, S.-Y.; Lee, I.-T.; Mine, Y.; Huang, H.-Y.; Peng, T.-Y. Handheld Nonthermal Plasma Augmentation of Glass–Ceramic Spray Deposition on Zirconia Surface Characterization and MG-63/HGF-1 Cell Behavior: An In Vitro Study. J. Funct. Biomater. 2025, 16, 421. https://doi.org/10.3390/jfb16110421
Wu S-H, Lai S-Y, Lee I-T, Mine Y, Huang H-Y, Peng T-Y. Handheld Nonthermal Plasma Augmentation of Glass–Ceramic Spray Deposition on Zirconia Surface Characterization and MG-63/HGF-1 Cell Behavior: An In Vitro Study. Journal of Functional Biomaterials. 2025; 16(11):421. https://doi.org/10.3390/jfb16110421
Chicago/Turabian StyleWu, Sheng-Han, Szu-Yu Lai, I-Ta Lee, Yuichi Mine, Huei-Yu Huang, and Tzu-Yu Peng. 2025. "Handheld Nonthermal Plasma Augmentation of Glass–Ceramic Spray Deposition on Zirconia Surface Characterization and MG-63/HGF-1 Cell Behavior: An In Vitro Study" Journal of Functional Biomaterials 16, no. 11: 421. https://doi.org/10.3390/jfb16110421
APA StyleWu, S.-H., Lai, S.-Y., Lee, I.-T., Mine, Y., Huang, H.-Y., & Peng, T.-Y. (2025). Handheld Nonthermal Plasma Augmentation of Glass–Ceramic Spray Deposition on Zirconia Surface Characterization and MG-63/HGF-1 Cell Behavior: An In Vitro Study. Journal of Functional Biomaterials, 16(11), 421. https://doi.org/10.3390/jfb16110421








