The Effect of Titanium Dioxide Nanotubes and Graphene Compounds on the Proliferation and Osteogenic Differentiation of Rat BMSCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- Prepare the NT-GO intermediate phase solution: Dissolve 50 mg of graphite oxide dispersion in 100 mL of methanol and ethanol (volume ratio 1:4) under stirring conditions, to obtain an intermediate phase solution with a concentration of 0.5 mg/mL of graphite oxide dispersion.
- Prepare the NT-GQD intermediate phase solution: Dissolve 10 mg of graphene quantum dots in 100 mL of methanol and ethanol (volume ratio 1:4), to obtain an intermediate phase solution with a concentration of 0.1 mg/mL of graphene quantum dots.
- Add 20 mg potassium chloride to the above prepared intermediate phase solution. Stir at a constant temperature of 50 °C until it is completely dissolved to obtain an electrolyte containing 0.2 mg/mL of potassium chloride. After adding the conductive salt and additives, the conductivity of the electrolyte can be improved, enabling the electrodeposition process to proceed continuously and rapidly.
2.2. Assessment of Surface Characterization
2.3. Cell Spreading
2.4. Assessment of Cell Adhesion and Proliferation
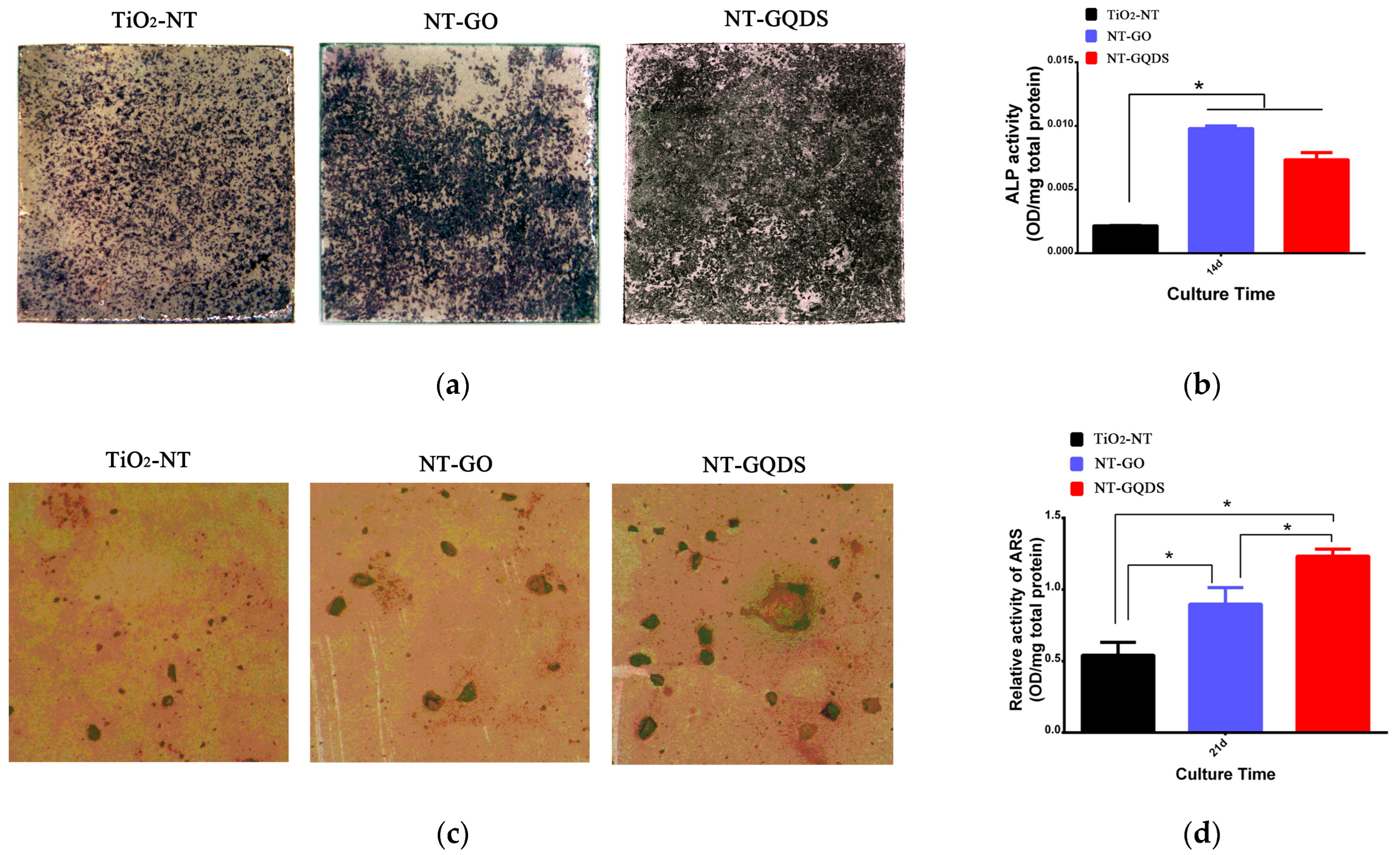
2.5. ALP Activity
2.6. Formation of Mineralized Nodules
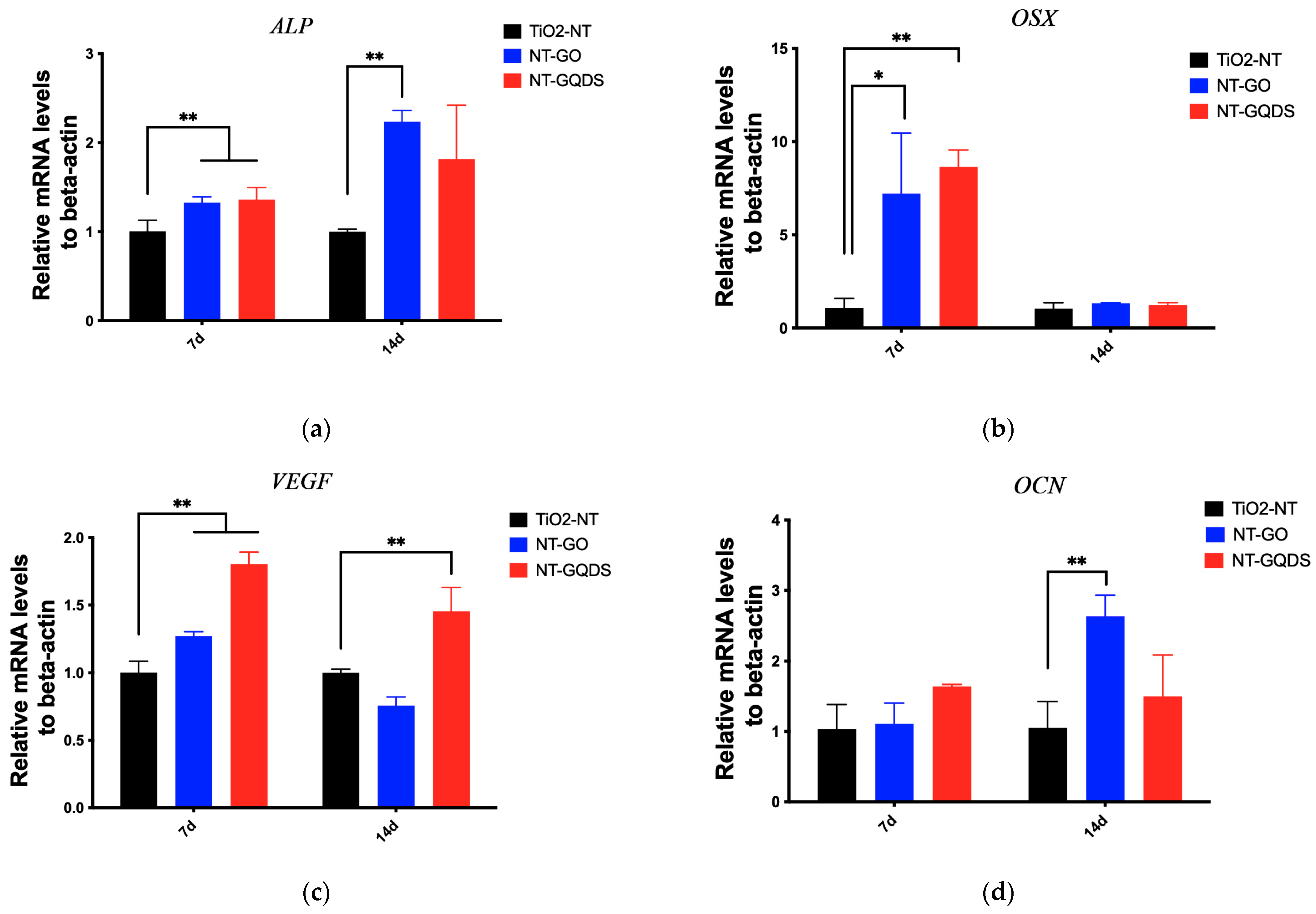
2.7. Real-Time PCR
2.8. Animal Experiments
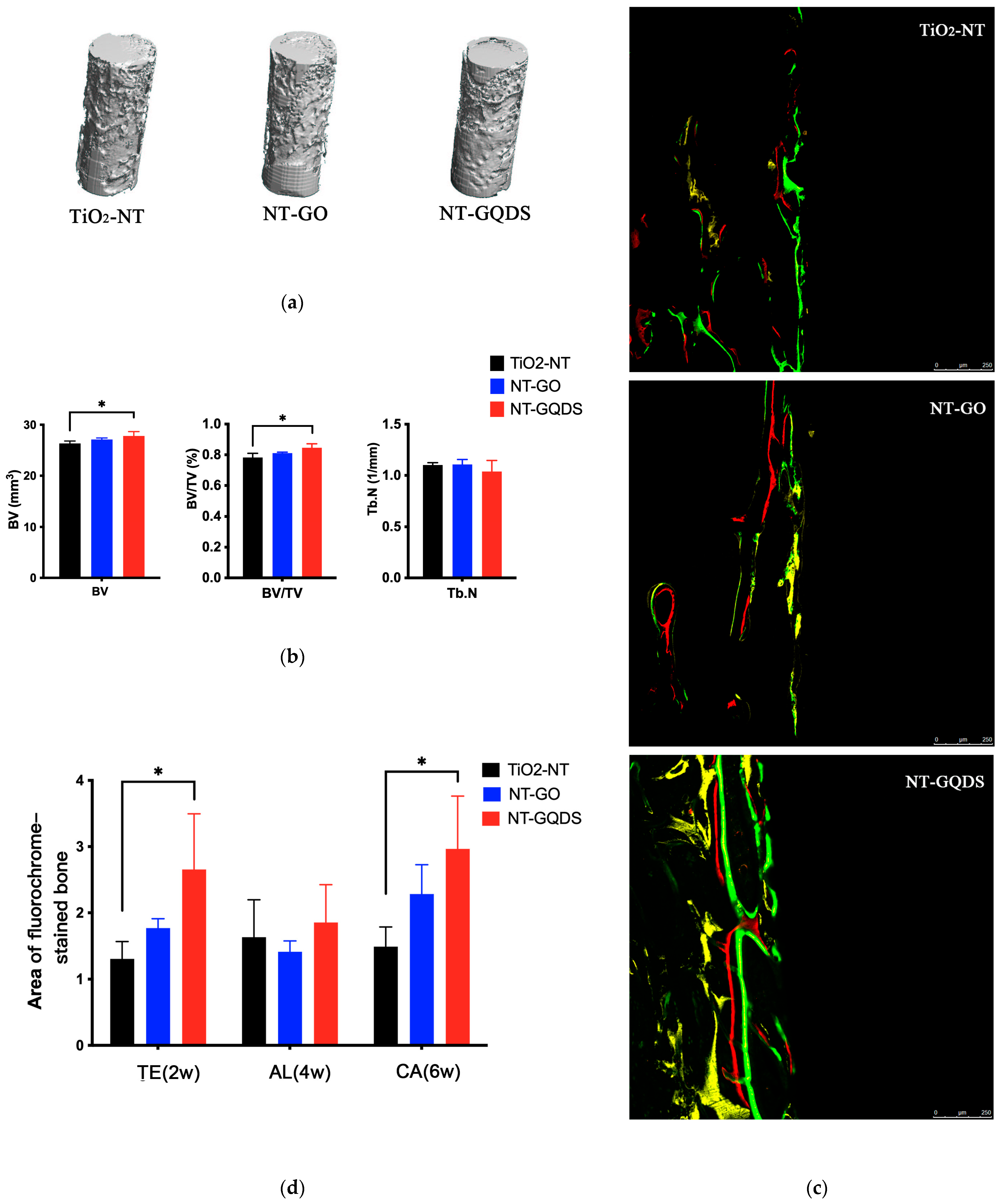
2.9. Micro-CT Scanning
2.10. Hard-Tissue Sections
2.11. Statistical Analysis
3. Results
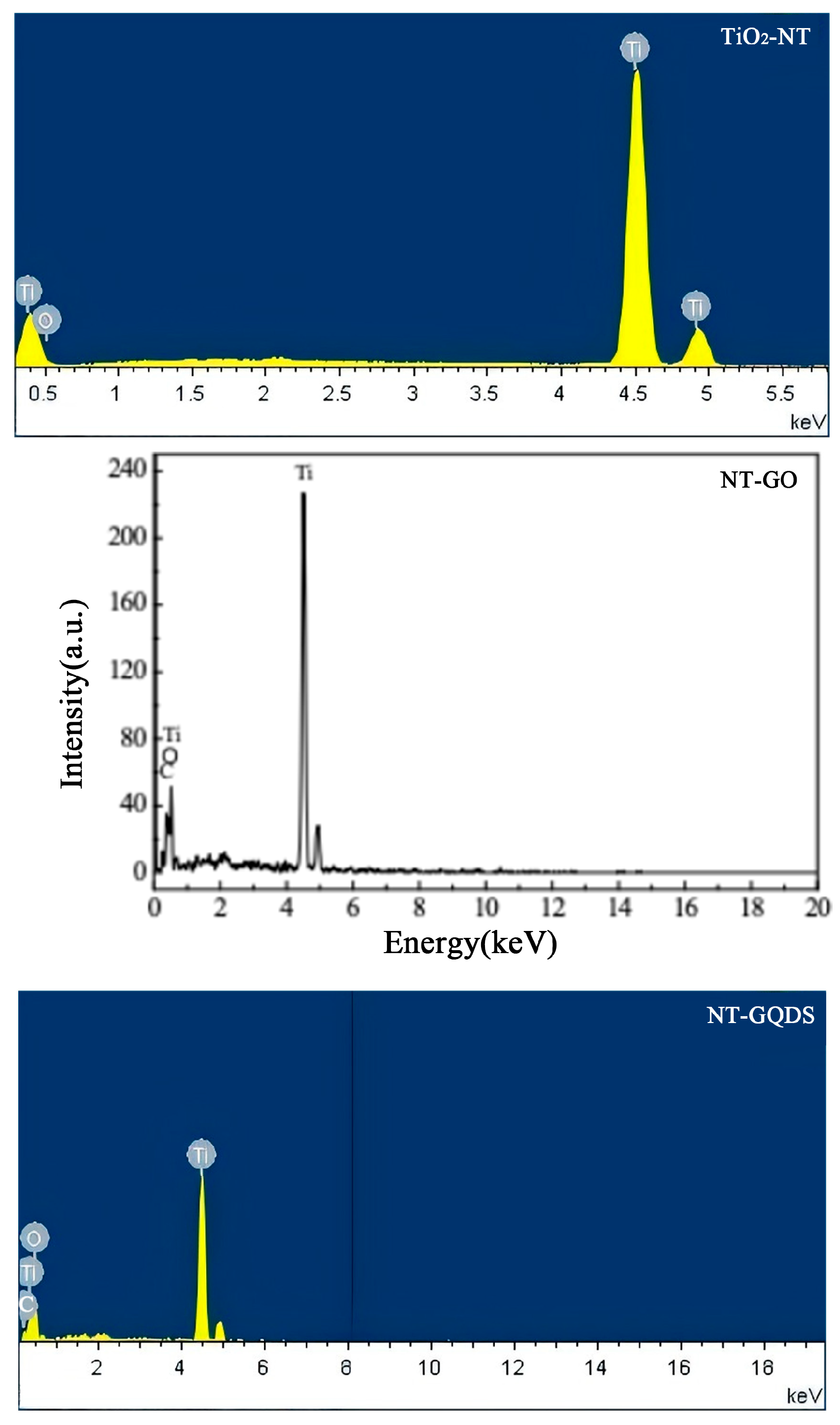
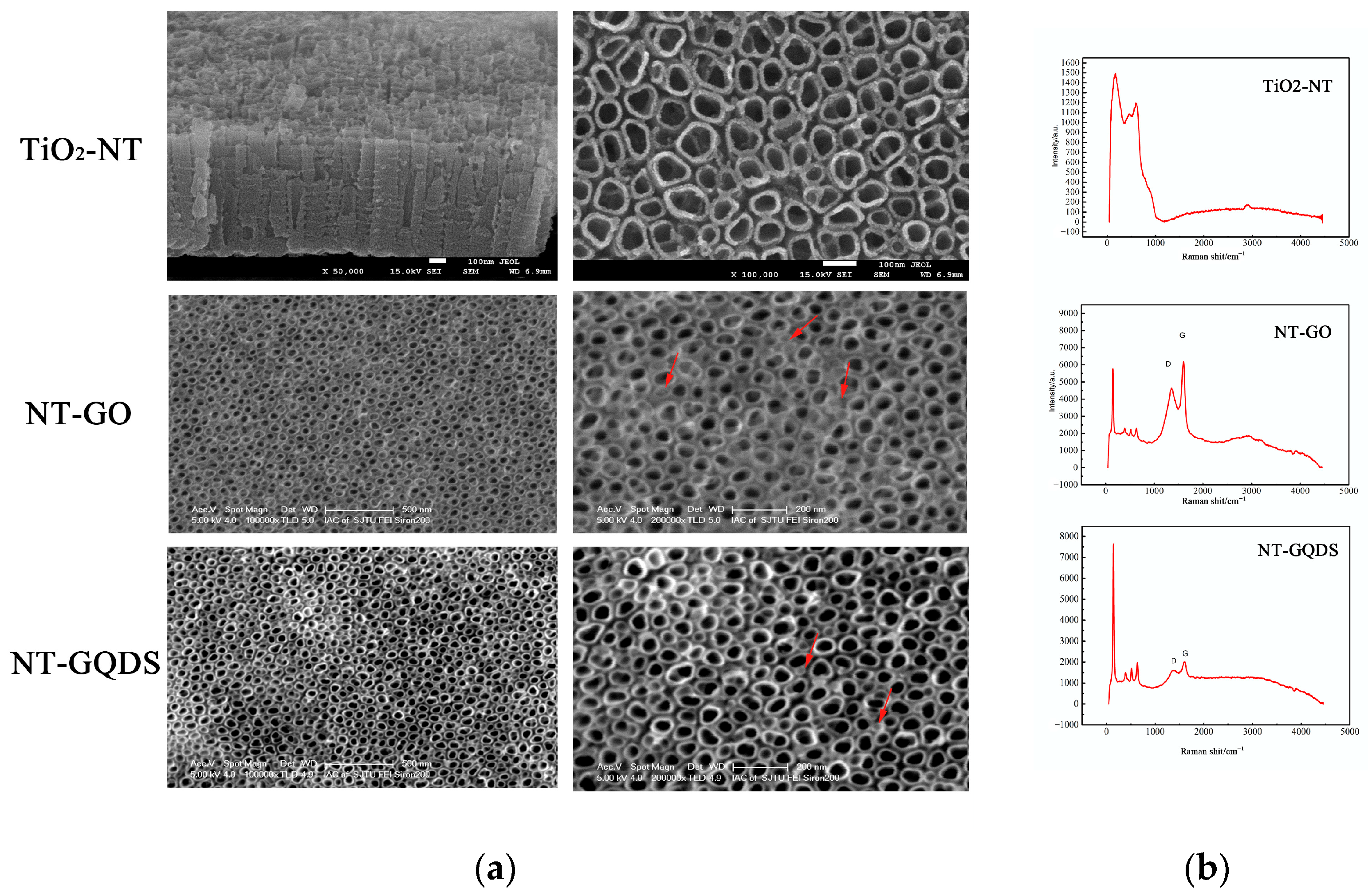
3.1. Surface Characterization
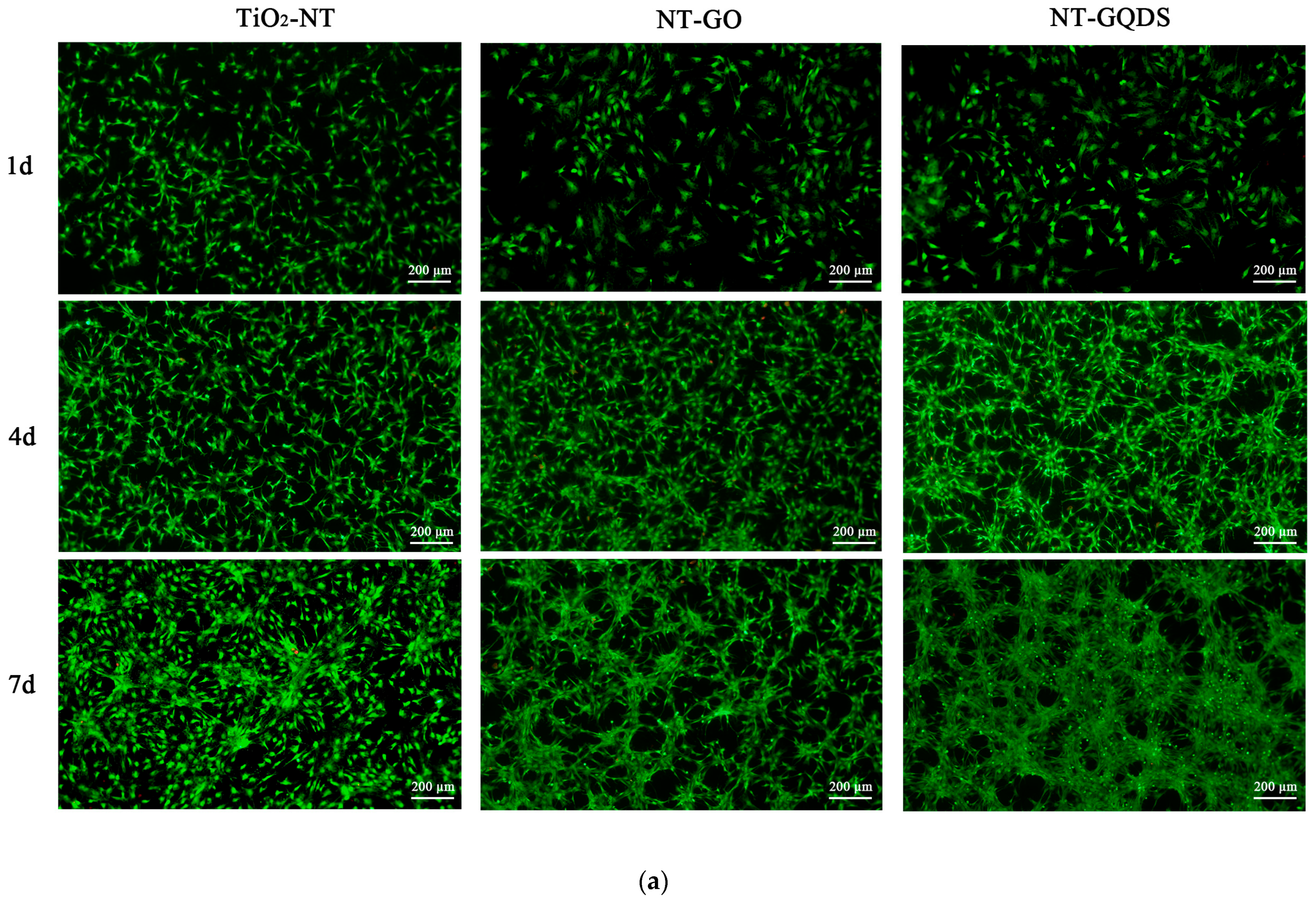
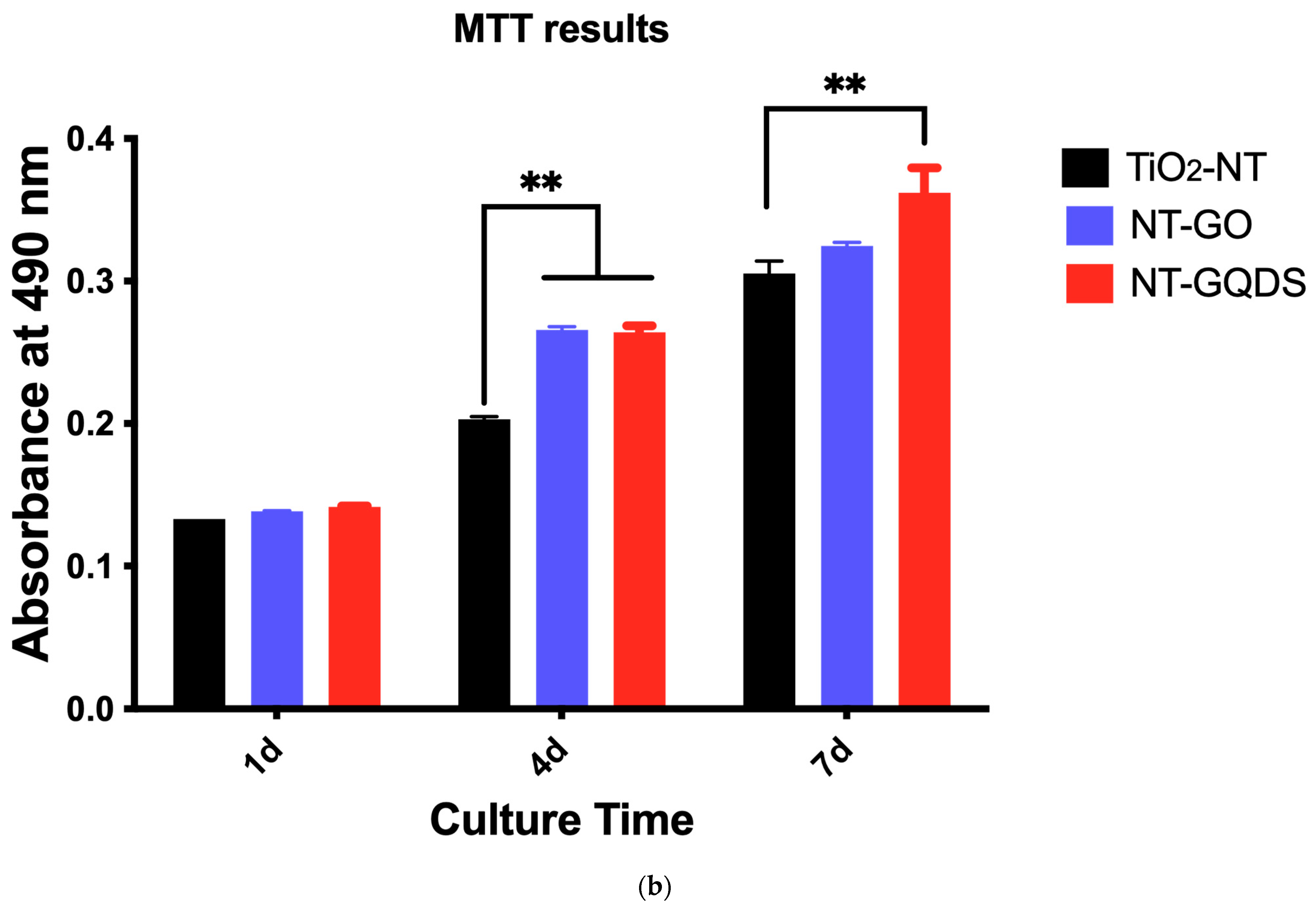
3.2. Cell Adhesion and Proliferation
3.3. Osteogenic Differentiation
3.4. In Vivo Osseointegration of Implant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMSCs | Bone marrow-derived mesenchymal stem cells |
| NT | Nanotubes |
| GO | Graphene oxide |
| GQDs | Graphene quantum dots |
| GDs | Graphene and its derivatives |
| SEM | Scanning electron microscope |
| CV | Cyclic voltammetry |
| TiO2 | Titanium dioxide |
Appendix A
| Genes | Primer Sequence |
|---|---|
| β-actin | F: CACCCGCGAGTACAACCTTC |
| R: CCCATACCCACCATCACACC | |
| ALP | F: CACGTTGACTGTGGTTACTGCTGA |
| R: CCTTGTAACCAGGCCCGTTG | |
| Osterix | F: CTATGCCAATGACTACCCACCC |
| R: CTGCCCACCACCTAACCAA | |
| VEGF | F: TTGAGTTGGGAGGAGGATGT |
| R: TGGCAGGCAAACAGACTTC | |
| OCN | F: GCCCTGACTGCATTCTGCCTCT |
| R: TCACCACCTTACTGCCCTCCTG |
| Items | Effect Size (Cohen’s f) | Power (1-β Err Prob) |
|---|---|---|
| BV | 1.28 | 75.13% |
| BV/TV | 1.482 | 86.51% |
| Area of TE | 1.36 | 80.16% |
| Area of CA | 1.31 | 77.09% |
Appendix B
References
- Huang, S.; Zhong, Y.; Fu, Y.; Zheng, X.; Feng, Z.; Mo, A. Graphene and Its Derivatives: “One Stone, Three Birds” Strategy for Orthopedic Implant-Associated Infections. Biomater. Sci. 2023, 11, 380–399. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.; Pi-Anfruns, J.; Moshaverinia, A.; Sim, D.; Grogan, T.; Hadaya, D. The Effects of Systemic Diseases and Medications on Implant Osseointegration: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, s35–s49. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Amato, A.; Chiacchio, A.; Sisalli, L.; Giordano, F. Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review. Dent. J. 2023, 11, 146. [Google Scholar] [CrossRef]
- Bokobza, L. On the Use of Nanoparticles in Dental Implants. Materials 2024, 17, 3191. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Qian, C.; Jiang, X.; Zhang, F.; Weng, W. Mechanisms of Stem Cell Osteogenic Differentiation on TiO2 Nanotubes. Colloids Surf. B Biointerfaces 2015, 136, 779–785. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Chowdhury, M.A.; Alam, A. Advances of Nanoparticles Employment in Dental Implant Applications. Appl. Surf. Sci. Adv. 2022, 12, 100341. [Google Scholar] [CrossRef]
- Shokri, M.; Kharaziha, M.; Tafti, H.A.; Eslaminejad, M.B.; Aghdam, R.M. Synergic Role of Zinc and Gallium Doping in Hydroxyapatite Nanoparticles to Improve Osteogenesis and Antibacterial Activity. Biomater. Adv. 2022, 134, 112684. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and Its Derivatives: Opportunities and Challenges in Dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Mohammadrezaei, D.; Golzar, H.; Rezai Rad, M.; Omidi, M.; Rashedi, H.; Yazdian, F.; Khojasteh, A.; Tayebi, L. In Vitro Effect of Graphene Structures as an Osteoinductive Factor in Bone Tissue Engineering: A Systematic Review. J. Biomed. Mater. Res. A 2018, 106, 2284–2343. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Hou, L.; Li, T.; Gong, Z.; Huang, H.; Wang, G.; Chen, X.; Li, X. A Dual Role of Graphene Oxide Sheet Deposition on Titanate Nanowire Scaffolds for Osteo-Implantation: Mechanical Hardener and Surface Activity Regulator. Sci. Rep. 2015, 5, 18266. [Google Scholar] [CrossRef]
- Guo, J.; Cao, G.; Wei, S.; Han, Y.; Xu, P. Progress in the Application of Graphene and Its Derivatives to Osteogenesis. Heliyon 2023, 9, e21872. [Google Scholar] [CrossRef]
- Tabish, T.A.; Scotton, C.J.; Ferguson, D.C.; Lin, L.; der Veen, A.V.; Lowry, S.; Ali, M.; Jabeen, F.; Ali, M.; Winyard, P.G.; et al. Biocompatibility and Toxicity of Graphene Quantum Dots for Potential Application in Photodynamic Therapy. Nanomedicine 2018, 13, 1923–1937. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Wu, J.; Fu, Y.; Li, S.; Hou, W.; Lin, L.; Li, P.; Yu, D.; Zhao, W. Effects of Low-Concentration Graphene Oxide Quantum Dots on Improving the Proliferation and Differentiation Ability of Bone Marrow Mesenchymal Stem Cells through the Wnt/β-Catenin Signaling Pathway. ACS Omega 2022, 7, 13546–13556. [Google Scholar] [CrossRef] [PubMed]
- Kashte, S.B.; Kadam, S.; Maffulli, N.; Potty, A.G.; Migliorini, F.; Gupta, A. Osteoinductive Potential of Graphene and Graphene Oxide for Bone Tissue Engineering: A Comparative Study. J. Orthop. Surg. Res. 2024, 19, 527. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zou, L.; Jiang, L.; Zhao, Z.; Liu, J. Osteoinductive and Antimicrobial Mechanisms of Graphene-Based Materials for Enhancing Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2021, 15, 915–935. [Google Scholar] [CrossRef]
- Cao, G.; Yan, J.; Ning, X.; Zhang, Q.; Wu, Q.; Bi, L.; Zhang, Y.; Han, Y.; Guo, J. Antibacterial and Antibiofilm Properties of Graphene and Its Derivatives. Colloids Surf. B Biointerfaces 2021, 200, 111588. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cataldi, A.; Zara, S.; Gallorini, M. Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials 2022, 15, 2229. [Google Scholar] [CrossRef]
- Guo, J.; Cao, G.; Wang, X.; Tang, W.; Diwu, W.; Yan, M.; Yang, M.; Bi, L.; Han, Y. Coating CoCrMo Alloy with Graphene Oxide and ϵ-Poly-L-Lysine Enhances Its Antibacterial and Antibiofilm Properties. Int. J. Nanomed. 2021, 16, 7249–7268. [Google Scholar] [CrossRef]
- Li, K.; Yan, J.; Wang, C.; Bi, L.; Zhang, Q.; Han, Y. Graphene Modified Titanium Alloy Promote the Adhesion, Proliferation and Osteogenic Differentiation of Bone Marrow Stromal Cells. Biochem. Biophys. Res. Commun. 2017, 489, 187–192. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Du, F.; Gu, M.; Zhang, P.; Zhang, X.; Liu, J.; Longwei, L.; Xiong, C.; Zhou, Y. Single-Layer Graphene Enhances the Osteogenic Differentiation of Human Mesenchymal Stem Cells In Vitro and In Vivo. J. Biomed. Nanotechnol. 2016, 12, 1270–1284. [Google Scholar] [CrossRef]
- Ji, M.-K.; Kim, H.; Jeong, G.; Kim, W.-J.; Ryu, J.-H.; Cho, H.; Lim, H.-P. Effects of TiO2 Nanotubes and Reduced Graphene Oxide on Streptococcus Mutans and Preosteoblastic Cells at an Early Stage. Int. J. Mol. Sci. 2024, 25, 1351. [Google Scholar] [CrossRef]
- Zhu, C.; Lv, Y.; Qian, C.; Qian, H.; Jiao, T.; Wang, L.; Zhang, F. Proliferation and Osteogenic Differentiation of Rat BMSCs on a Novel Ti/SiC Metal Matrix Nanocomposite Modified by Friction Stir Processing. Sci. Rep. 2016, 6, 38875. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Zeng, D.; Zhang, S.; Zhang, F.; Yu, W. PLGA Film/Titanium Nanotubues as a Sustained Growth Factor Releasing System for Dental Implants. J. Mater. Sci. Mater. Med. 2018, 29, 141. [Google Scholar] [CrossRef] [PubMed]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef]
- Sun, X.-D.; Liu, T.-T.; Wang, Q.-Q.; Zhang, J.; Cao, M.-S. Surface Modification and Functionalities for Titanium Dental Implants. ACS Biomater. Sci. Eng. 2023, 9, 4442–4461. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhao, Y.; Bai, Z.; Yan, J.; Qin, D.; Peng, H.; Liu, Y.; Tong, J.; Sun, L.; Wu, X.; et al. The Effect of Antibacterial-Osteogenic Surface Modification on the Osseointegration of Titanium Implants: A Static and Dynamic Strategy. ACS Biomater. Sci. Eng. 2024, 10, 4093–4113. [Google Scholar] [CrossRef]
- Tadyszak, K.; Musiał, A.; Ostrowski, A.; Wychowaniec, J.K. Unraveling Origins of EPR Spectrum in Graphene Oxide Quantum Dots. Nanomaterials 2020, 10, 798. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Chen, G.; Wei, C.; Zhang, X.; Zeng, B.; Yi, C.; Wang, C.; Yu, D. Concentration-Dependent Cellular Behavior and Osteogenic Differentiation Effect Induced in Bone Marrow Mesenchymal Stem Cells Treated with Magnetic Graphene Oxide. J. Biomed. Mater. Res. A 2020, 108, 50–60. [Google Scholar] [CrossRef]
- Liao, T.T.; Deng, Q.Y.; Wu, B.J.; Li, S.S.; Li, X.; Wu, J.; Leng, Y.X.; Guo, Y.B.; Huang, N. Dose-Dependent Cytotoxicity Evaluation of Graphite Nanoparticles for Diamond-like Carbon Film Application on Artificial Joints. Biomed. Mater. 2017, 12, 015018. [Google Scholar] [CrossRef]
- Du, Z.; Wang, C.; Zhang, R.; Wang, X.; Li, X. Applications of Graphene and Its Derivatives in Bone Repair: Advantages for Promoting Bone Formation and Providing Real-Time Detection, Challenges and Future Prospects. Int. J. Nanomed. 2020, 15, 7523–7551. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Barajaa, M.; Tahmasbi Rad, A.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2021, 10, 2001414. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Y.; Pang, D.W.-P.; Hwang, S.-M.; Tuan, H.-Y.; Hu, Y.-C. A Graphene-Based Platform for Induced Pluripotent Stem Cells Culture and Differentiation. Biomaterials 2012, 33, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Wu, N.; Gao, H.; Wang, X.; Pei, X. Surface Modification of Titanium Implants by Metal Ions and Nanoparticles for Biomedical Application. ACS Biomater. Sci. Eng. 2023, 9, 2970–2990. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, H.; Moghaddam, M.M.; Naderi-Manesh, H.; Taheri, R.A. Single-Layer Graphene Oxide Nanosheets Induce Proliferation and Osteogenesis of Single-Cell hBMSCs Encapsulated in Alginate Microgels. Sci. Rep. 2024, 14, 25272. [Google Scholar] [CrossRef]
- Gao, S.; Chen, B.; Gao, M.; Xu, Y.; Yang, X.; Yang, C.; Pan, S. Substrate Stiffness of Bone Microenvironment Controls Functions of Pre-Osteoblasts and Fibroblasts In Vitro. Biomimetics 2023, 8, 344. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Lee, P.S.; Hess, R.; Haenchen, V.; Basu, B.; Scharnweber, D. Interplay of Substrate Conductivity, Cellular Microenvironment, and Pulsatile Electrical Stimulation toward Osteogenesis of Human Mesenchymal Stem Cells in Vitro. ACS Appl. Mater. Interfaces 2015, 7, 23015–23028. [Google Scholar] [CrossRef]
- Zancanela, D.C.; Simão, A.M.S.; Francisco, C.G.; De Faria, A.N.; Ramos, A.P.; Gonçalves, R.R.; Matsubara, E.Y.; Rosolen, J.M.; Ciancaglini, P. Graphene Oxide and Titanium: Synergistic Effects on the Biomineralization Ability of Osteoblast Cultures. J. Mater. Sci. Mater. Med. 2016, 27, 71. [Google Scholar] [CrossRef]
- Halim, A.; Liu, L.; Ariyanti, A.D.; Ju, Y.; Luo, Q.; Song, G. Low-Dose Suspended Graphene Oxide Nanosheets Induce Antioxidant Response and Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells via JNK-Dependent FoxO1 Activation. J. Mater. Chem. B 2019, 7, 5998–6009. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced Graphene Oxide Coating Enhances Osteogenic Differentiation of Human Mesenchymal Stem Cells on Ti Surfaces. Biomater. Res. 2021, 25, 4. [Google Scholar] [CrossRef]
- Shin, Y.C.; Bae, J.-H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.-B.; Han, D.-W. Enhanced Osseointegration of Dental Implants with Reduced Graphene Oxide Coating. Biomater. Res. 2022, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior Antibacterial Activity of Zinc Oxide/Graphene Oxide Composites Originating from High Zinc Concentration Localized around Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]

| Graphene | Substrate | Coating Method | Outcomes | Mechanism |
|---|---|---|---|---|
| GO | (SLA)-treated Ti | Ultrasonic atomization spraying technique | Promoted cell proliferation, adhesion, diffusion, and osteogenic differentiation | FAK/P38 signaling pathways [42] |
| rGO | Ti | Meniscus-dragging deposition | Promoted cell proliferation, ALP activity, and matrix mineralization | Decrease in the surface roughness and contact angle [43] |
| rGO | (SLA)-treated Ti | Peptide bonds | Promoted osteogenesis | Absorption of exogenous proteins [44] |
| Graphene films | Ti6Al4V | Physical adsorption | Promoted the adhesion and early extension of BMSCs | Surface morphology [20] |
| rGO | TiO2 nanotube | Atmospheric plasma deposition | Increased the initial adhesion and proliferation of pre-osteoblastic cells | Increase in contact angle [22] |
| GQDS | (SLA)-treated Ti6Al4V | Immersion | Accelerated new bone formation | Activation of the Wnt/β-catenin signaling pathway [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Deng, Y.; Xu, J.; Wen, J.; Huang, Q.; Yu, W. The Effect of Titanium Dioxide Nanotubes and Graphene Compounds on the Proliferation and Osteogenic Differentiation of Rat BMSCs. J. Funct. Biomater. 2025, 16, 413. https://doi.org/10.3390/jfb16110413
Zhu C, Deng Y, Xu J, Wen J, Huang Q, Yu W. The Effect of Titanium Dioxide Nanotubes and Graphene Compounds on the Proliferation and Osteogenic Differentiation of Rat BMSCs. Journal of Functional Biomaterials. 2025; 16(11):413. https://doi.org/10.3390/jfb16110413
Chicago/Turabian StyleZhu, Chenyuan, Yuwei Deng, Jing Xu, Jin Wen, Qingfeng Huang, and Weiqiang Yu. 2025. "The Effect of Titanium Dioxide Nanotubes and Graphene Compounds on the Proliferation and Osteogenic Differentiation of Rat BMSCs" Journal of Functional Biomaterials 16, no. 11: 413. https://doi.org/10.3390/jfb16110413
APA StyleZhu, C., Deng, Y., Xu, J., Wen, J., Huang, Q., & Yu, W. (2025). The Effect of Titanium Dioxide Nanotubes and Graphene Compounds on the Proliferation and Osteogenic Differentiation of Rat BMSCs. Journal of Functional Biomaterials, 16(11), 413. https://doi.org/10.3390/jfb16110413








