Surface Treatment of Dental Mini-Sized Implants and Screws: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Study Strategy and Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Included Studies Characteristics
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
3.2.3. Clinical Trials
3.3. Studied Outcomes and Chosen Tests
3.4. Risk of Bias
3.5. Meta-Analysis
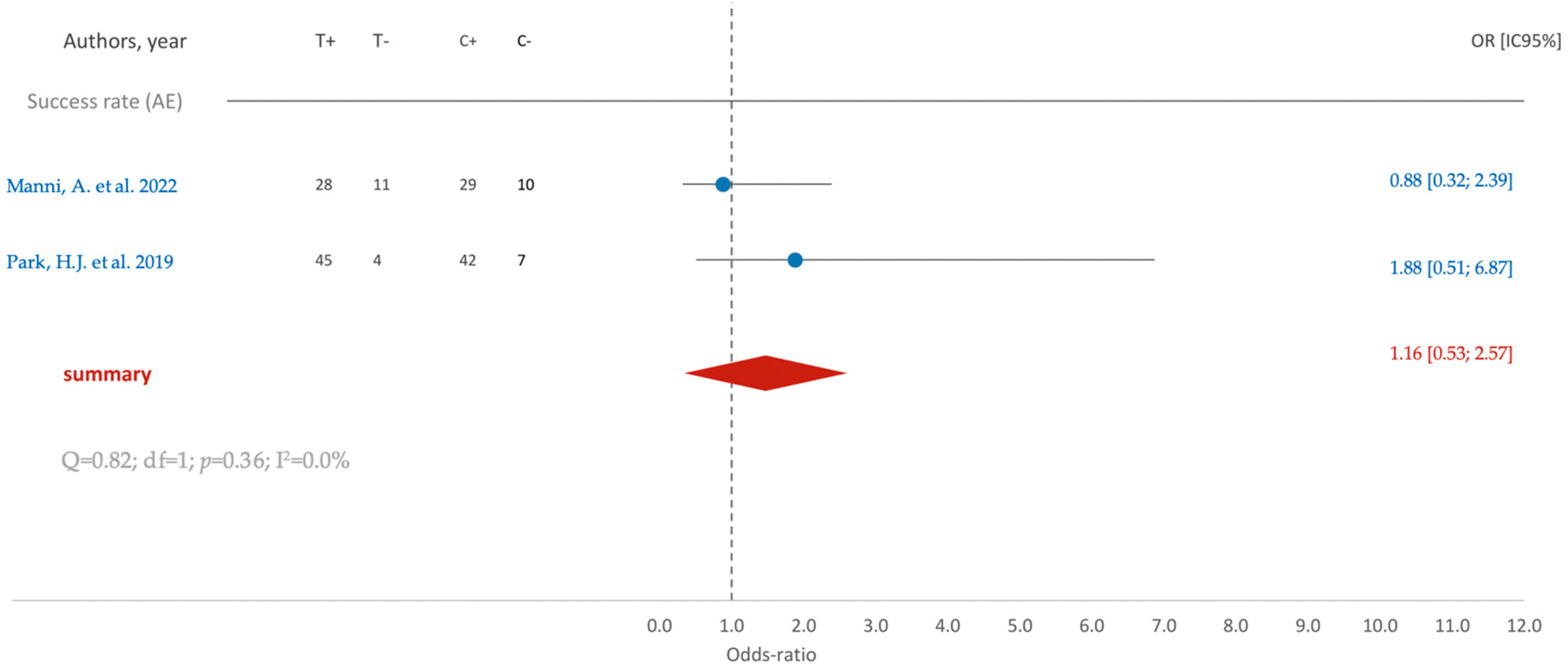
3.5.1. Clinical Studies
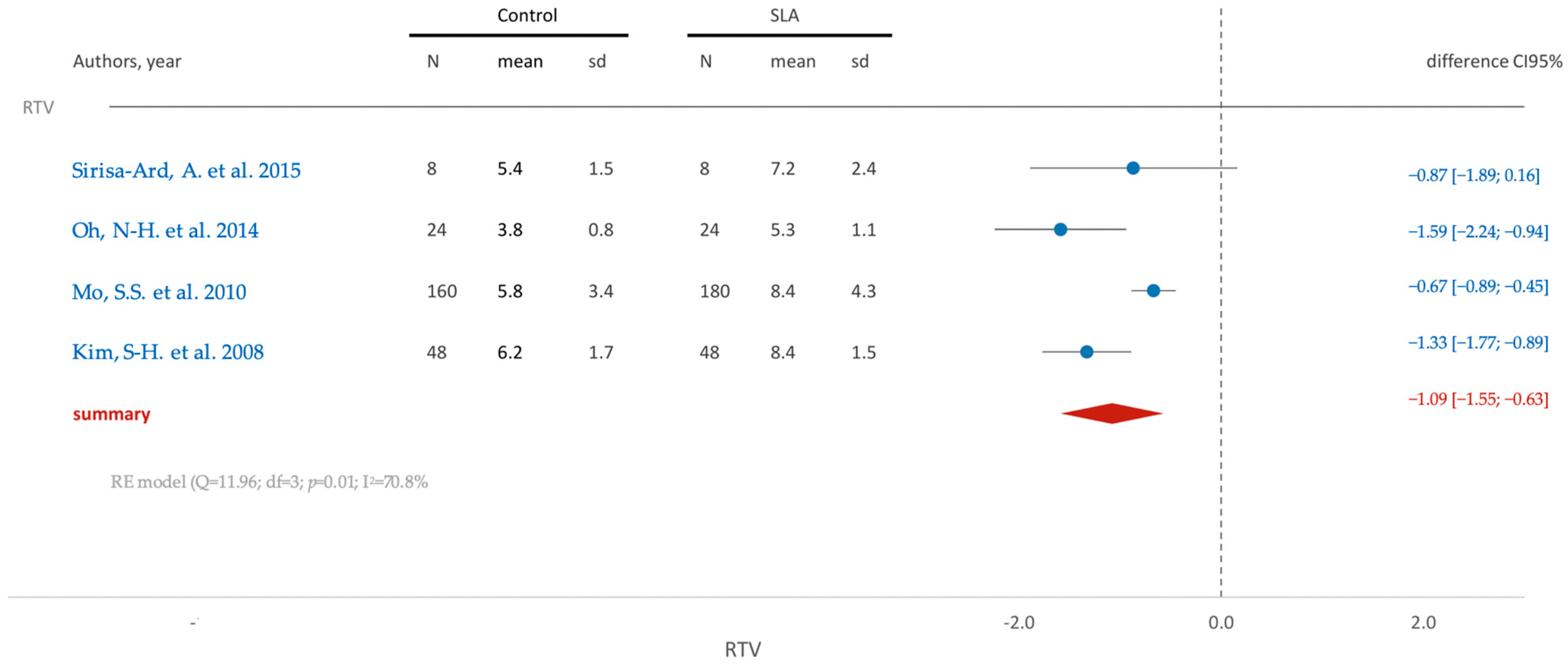
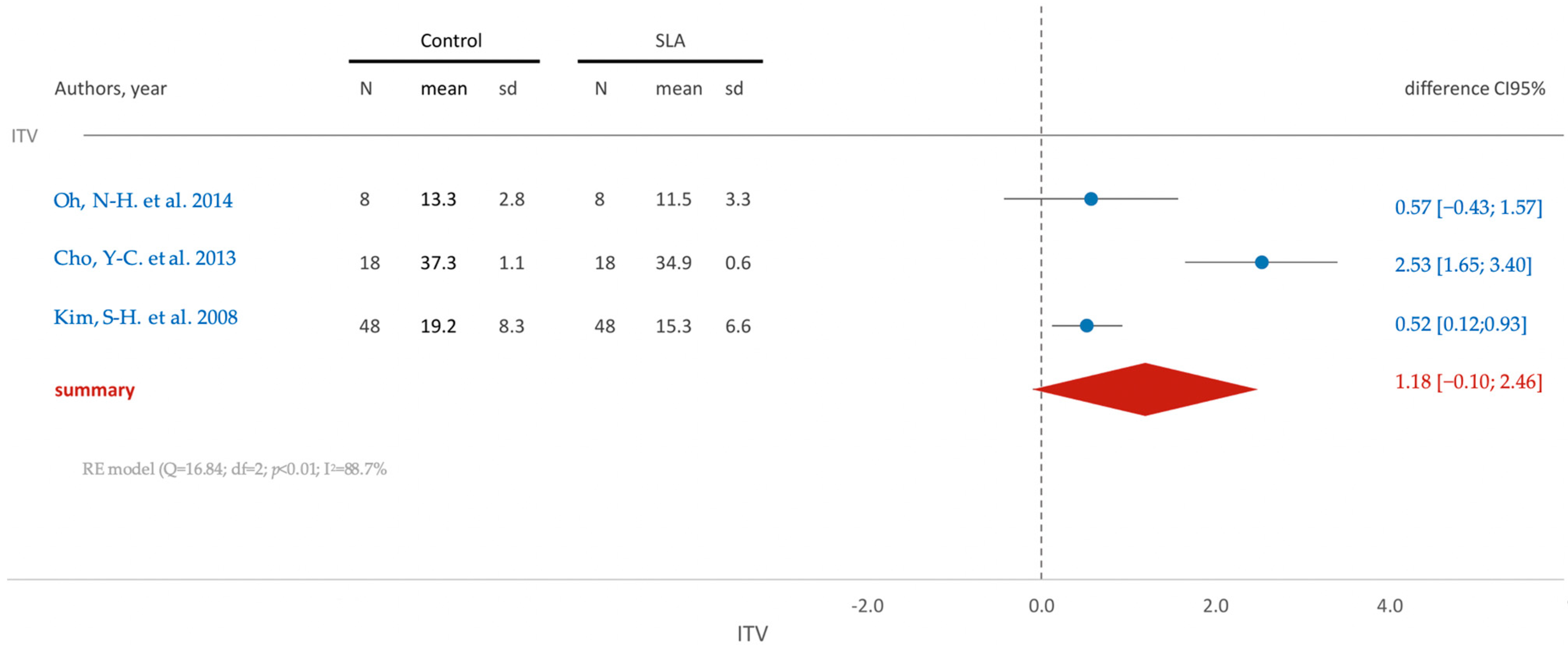
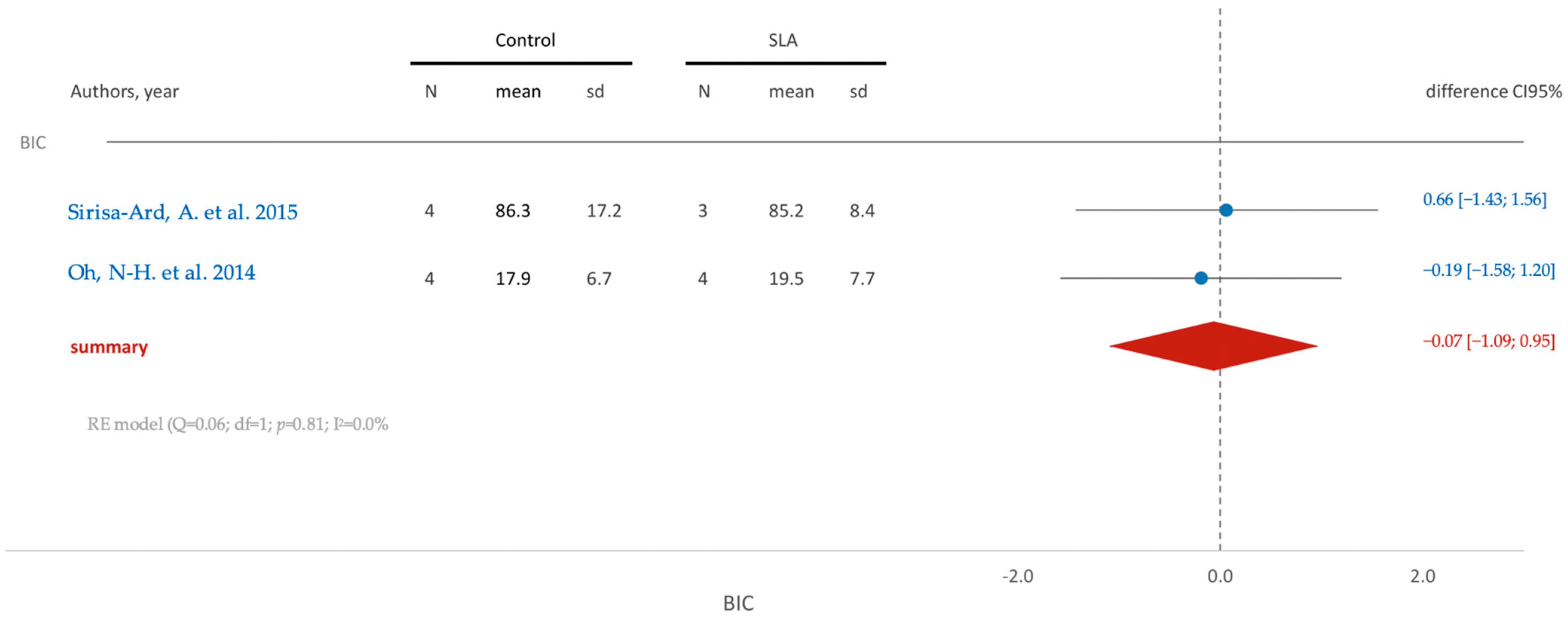
3.5.2. In Vivo Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Johany, S.S.; Al Amri, M.D.; Alsaeed, S.; Alalola, B. Dental Implant Length and Diameter: A Proposed Classification Scheme. J. Prosthodont. 2017, 26, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.C.; Suarez, F.; Chan, H.-L.; Padial-Molina, M.; Wang, H.-L. Implants for Orthodontic Anchorage: Success Rates and Reasons of Failures. Implant. Dent. 2014, 23, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Upendran, A.; Gupta, N.; Salisbury, H.G. Dental Mini-Implants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Papadopoulos, M.A.; Tarawneh, F. The Use of Miniscrew Implants for Temporary Skeletal Anchorage in Orthodontics: A Comprehensive Review. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2007, 103, e6–e15. [Google Scholar] [CrossRef] [PubMed]
- Proffit, R.F.; Larson, E.; Sarver, M.D. Contemporary Orthodontics, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Watanabe, K.; Mitchell, B.; Sakamaki, T.; Hirai, Y.; Kim, D.-G.; Deguchi, T.; Suzuki, M.; Ueda, K.; Tanaka, E. Mechanical Stability of Orthodontic Miniscrew Depends on a Thread Shape. J. Dent. Sci. 2022, 17, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Jasoria, G.; Kalra, A.; Jaggi, N.; Shamim, W.; Rathore, S.; Manchanda, M. Miniscrew Implants as Temporary Anchorage Devices in Orthodontics: A Comprehensive Review. J. Contemp. Dent. Pract. 2013, 14, 993–999. [Google Scholar] [CrossRef]
- Papadopoulos, M.A.; Papageorgiou, S.N.; Zogakis, I.P. Clinical Effectiveness of Orthodontic Miniscrew Implants: A Meta-Analysis. J. Dent. Res. 2011, 90, 969–976. [Google Scholar] [CrossRef]
- Inoue, M.; Kuroda, S.; Yasue, A.; Horiuchi, S.; Kyung, H.-M.; Tanaka, E. Torque Ratio as a Predictable Factor on Primary Stability of Orthodontic Miniscrew Implants. Implant. Dent. 2014, 28, 28–38. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Zogakis, I.P.; Papadopoulos, M.A. Failure Rates and Associated Risk Factors of Orthodontic Miniscrew Implants: A Meta-Analysis. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 577–595. [Google Scholar] [CrossRef]
- Giudice, A.L.; Rustico, L.; Longo, M.; Oteri, G.; Papadopoulos, M.A.; Nucera, R. Complications Reported with the Use of Orthodontic Miniscrews: A Systematic Review. Korean J. Orthod. 2021, 51, 199–216. [Google Scholar] [CrossRef]
- Al-Thomali, Y.; Basha, S.; Mohamed, R.N. Effect of Surface Treatment on the Mechanical Stability of Orthodontic Miniscrews. Angle Orthod. 2022, 92, 127–136. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.-J.; Cho, I.-S.; Kim, S.-K.; Kim, T.-W. Rotational Resistance of Surface-Treated Mini-Implants. Angle Orthod. 2009, 79, 899–907. [Google Scholar] [CrossRef]
- Rampurawala, A.; Patil, A.; Bhosale, V. Bone-Miniscrew Contact and Surface Element Deposition on Orthodontic Miniscrews After Ultraviolet Photofunctionalization. Int. J. Oral. Maxillofac. Implant. 2020, 35, 1090–1097. [Google Scholar] [CrossRef]
- Bratu, D.C.; Popa, G.; Petrescu, H.; Karancsi, O.L.; Bratu, E.A. Influence of Chemically-Modified Implant Surfaces on the Stability of Orthodontic Mini-Implants. Rev. Chim. 2014, 65, 1222–1225. [Google Scholar]
- Chaddad, K.; Ferreira, A.H.; Geurs, N.; Reddy, M.S.; Chaddada, A.H.F.K.; Bueno, R.C.; Basting, R.T.; Jung, S.-A.; Choi, Y.J.; Lee, D.-W.; et al. Influence of Surface Characteristics on Survival Rates of Mini-Implants. Angle Orthod. 2008, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Manni, A.; Drago, S.; Migliorati, M. Success Rate of Surface-Treated and Non-Treated Orthodontic Miniscrews as Anchorage Reinforcement in the Lower Arch for the Herbst Appliance: A Single-Centre, Randomised Split-Mouth Clinical Trial. Eur. J. Orthod. 2022, 44, 452–457. [Google Scholar] [CrossRef]
- Ekizer, A.; Türker, G.; Uysal, T.; Güray, E.; Taşdemir, Z. Light Emitting Diode Mediated Photobiomodulation Therapy Improves Orthodontic Tooth Movement and Miniscrew Stability: A Randomized Controlled Clinical Trial. Lasers Surg. Med. 2016, 48, 936–943. [Google Scholar] [CrossRef]
- Benalcázar Jalkh, E.B.; Parra, M.; Torroni, A.; Nayak, V.V.; Tovar, N.; Castellano, A.; Badalov, R.M.; Bonfante, E.A.; Coelho, P.G.; Witek, L. Effect of Supplemental Acid-Etching on the Early Stages of Osseointegration: A Preclinical Model. J. Mech. Behav. Biomed. Mater. 2021, 122, 104682. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.J.; Marques, R.G.; Elias, C.N. Influence of Acid Treatment on Surface Properties and in Vivo Performance of Ti6Al4V Alloy for Biomedical Applications. J. Mater. Sci. Mater. Med. 2017, 28, 164. [Google Scholar] [CrossRef]
- Moghaddam, S.F.; Mohammadi, A.; Behroozian, A. The Effect of Sandblasting and Acid Etching on Survival Rate of Orthodontic Miniscrews: A Split-Mouth Randomized Controlled Trial. Prog. Orthod. 2021, 22, 2. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and Acid Etched Titanium Dental Implant Surfaces Systematic Review and Confocal Microscopy Evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Faggion, C.M. Guidelines for Reporting Pre-Clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Espinar-Escalona, E.; Bravo-Gonzalez, L.-A.; Pegueroles, M.; Gil, F.J. Roughness and Wettability Effect on Histological and Mechanical Response of Self-Drilling Orthodontic Mini-Implants. Clin. Oral. Investig. 2016, 20, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Mattos, C.T.; Ruellas, A.C.D.O.; Elias, C.N. Is It Possible to Re-Use Mini-Implants for Orthodontic Anchorage? Results of an in Vitro Study. Mat. Res. 2010, 13, 521–525. [Google Scholar] [CrossRef][Green Version]
- Im, C.; Park, J.-H.; Jeon, Y.-M.; Kim, J.-G.; Jang, Y.-S.; Lee, M.-H.; Jeon, W.-Y.; Kim, J.-M.; Bae, T.-S. Improvement of Osseointegration of Ti–6Al–4V ELI Alloy Orthodontic Mini-Screws through Anodization, Cyclic Pre-Calcification, and Heat Treatments. Prog. Orthod. 2022, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.-M.; Jeon, J.; Jang, Y.-S.; Jeon, W.-Y.; Lee, M.-H.; Jeon, Y.-M.; Kim, J.-G.; Bae, T.-S. Evaluation of Osseointegration of Ti-6Al-4V Alloy Orthodontic Mini-Screws with Ibandronate-Loaded TiO2 Nanotube Layer. Dent. Mater. J. 2023, 42, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Gezer, P.; Yilanci, H. Comparison of Mechanical Stability of Mini-Screws with Resorbable Blasting Media and Micro-Arc Oxidation Surface Treatments under Orthodontic Forces: An in Vitro Biomechanical Study. Int. Orthod. 2023, 21, 100775. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, M.; Wei, L.; Werner, A.; Liu, Y. Biomimetic Calcium Phosphate Coating on Medical Grade Stainless Steel Improves Surface Properties and Serves as a Drug Carrier for Orthodontic Applications. Dent. Mater. 2023, 39, 152–161. [Google Scholar] [CrossRef]
- Baser, B.; Ozel, M.B. Comparison of Primary Stability of Used and Unused Self-Tapping and Self-Drilling Orthodontic Mini-Implants. Adv. Clin. Exp. Med. 2023, 33. [Google Scholar] [CrossRef] [PubMed]
- Mattos, C.T.; Ruellas, A.C.; Sant’Anna, E.F. Effect of Autoclaving on the Fracture Torque of Mini-implants Used for Orthodontic Anchorage. J. Orthod. 2011, 38, 15–20. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Brantley, W.A.; Yuasa, T.; Kyung, H.-M.; Mizoguchi, I. Effects of Sodium Fluoride Mouth Rinses on the Torsional Properties of Miniscrew Implants. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 588–593. [Google Scholar] [CrossRef]
- Cho, I.-S.; Kim, S.-K.; Chang, Y.-I.; Baek, S.-H. In Vitro and in Vivo Mechanical Stability of Orthodontic Mini-Implants: Effect of Sandblasted, Large-Grit, and Anodic-Oxidation vs. Sandblasted, Large-Grit, and Acid-Etching. Angle Orthod. 2012, 82, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Piemontese, M.; Ravanetti, F.; Lumetti, S.; Passeri, G.; Gandolfini, M.; Macaluso, G.M. Effect of Surface Treatment on Cell Responses to Grades 4 and 5 Titanium for Orthodontic Mini-Implants. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 705–714. [Google Scholar] [CrossRef]
- Noorollahian, S.; Alavi, S.; Monirifard, M. A Processing Method for Orthodontic Mini-Screws Reuse. Dent. Res. J. 2012, 9, 447–451. [Google Scholar]
- Akyalcin, S.; McIver, H.P.; English, J.D.; Ontiveros, J.C.; Gallerano, R.L. Effects of Repeated Sterilization Cycles on Primary Stability of Orthodontic Mini-Screws. Angle Orthod. 2013, 83, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Morais, L.; Elias, C.N.; Semenova, I.P.; Valiev, R.; Salimgareeva, G.; Pithon, M.; Lacerda, R. Nanostructured Severe Plastic Deformation Processed Titanium for Orthodontic Mini-Implants. Mater. Sci. Eng. C 2013, 33, 4197–4202. [Google Scholar] [CrossRef]
- Tozlu, M.; Nalbantgil, D.; Ozdemir, F. Effects of a Newly Designed Apparatus on Orthodontic Skeletal Anchorage. Eur. J. Dent. 2013, 7, S083–S088. [Google Scholar] [CrossRef]
- Estelita, S.; Janson, G.; Chiqueto, K.; Ferreira, E.S. Effect of Recycling Protocol on Mechanical Strength of Used Mini-Implants. Int. J. Dent. 2014, 2014, 424923. [Google Scholar] [CrossRef]
- Oh, E.-J.; Nguyen, T.-D.T.; Lee, S.-Y.; Jeon, Y.-M.; Bae, T.-S.; Kim, J.-G. Enhanced Compatibility and Initial Stability of Ti6Al4V Alloy Orthodontic Miniscrews Subjected to Anodization, Cyclic Precalcification, and Heat Treatment. Korean J. Orthod. 2014, 44, 246. [Google Scholar] [CrossRef]
- Miyawaki, S.; Tomonari, H.; Yagi, T.; Kuninori, T.; Oga, Y.; Kikuchi, M. Development of a Novel Spike-like Auxiliary Skeletal Anchorage Device to Enhance Miniscrew Stability. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, L.; Crismani, A.; Falkensammer, F.; Bantleon, H.-P.; Rausch-Fan, X.; Andrukhov, O. Behavior of Osteoblasts on TI Surface with Two Different Coating Designed for Orthodontic Devices. J. Mater. Sci. Mater. Med. 2015, 26, 10. [Google Scholar] [CrossRef]
- Ganzorig, K.; Kuroda, S.; Maeda, Y.; Mansjur, K.; Sato, M.; Nagata, K.; Tanaka, E. Low-Intensity Pulsed Ultrasound Enhances Bone Formation around Miniscrew Implants. Arch. Oral. Biol. 2015, 60, 902–910. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Xu, J.; Li, X.; Li, X.; Yan, Y.; Qi, M.; Hu, M. Strontium Coating by Electrochemical Deposition Improves Implant Osseointegration in Osteopenic Models. Exp. Ther. Med. 2015, 9, 172–176. [Google Scholar] [CrossRef][Green Version]
- Tabuchi, M.; Ikeda, T.; Hirota, M.; Nakagawa, K.; Park, W.; Miyazawa, K.; Goto, S.; Ogawa, T. Effect of UV Photofunctionalization on Biologic and Anchoring Capability of Orthodontic Miniscrews. Int. J. Oral. Maxillofac. Implant. 2015, 30, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Upadhyay, M.; Roberts, W.E. Biomechanical and Histomorphometric Properties of Four Different Mini-Implant Surfaces. Eur. J. Orthod. 2015, 37, 627–635. [Google Scholar] [CrossRef]
- Kang, H.-K.; Chu, T.-M.; Dechow, P.; Stewart, K.; Kyung, H.-M.; Liu, S.S.-Y. Laser-Treated Stainless Steel Mini-Screw Implants: 3D Surface Roughness, Bone-Implant Contact, and Fracture Resistance Analysis. Eur. J. Orthod. 2016, 38, 154–162. [Google Scholar] [CrossRef]
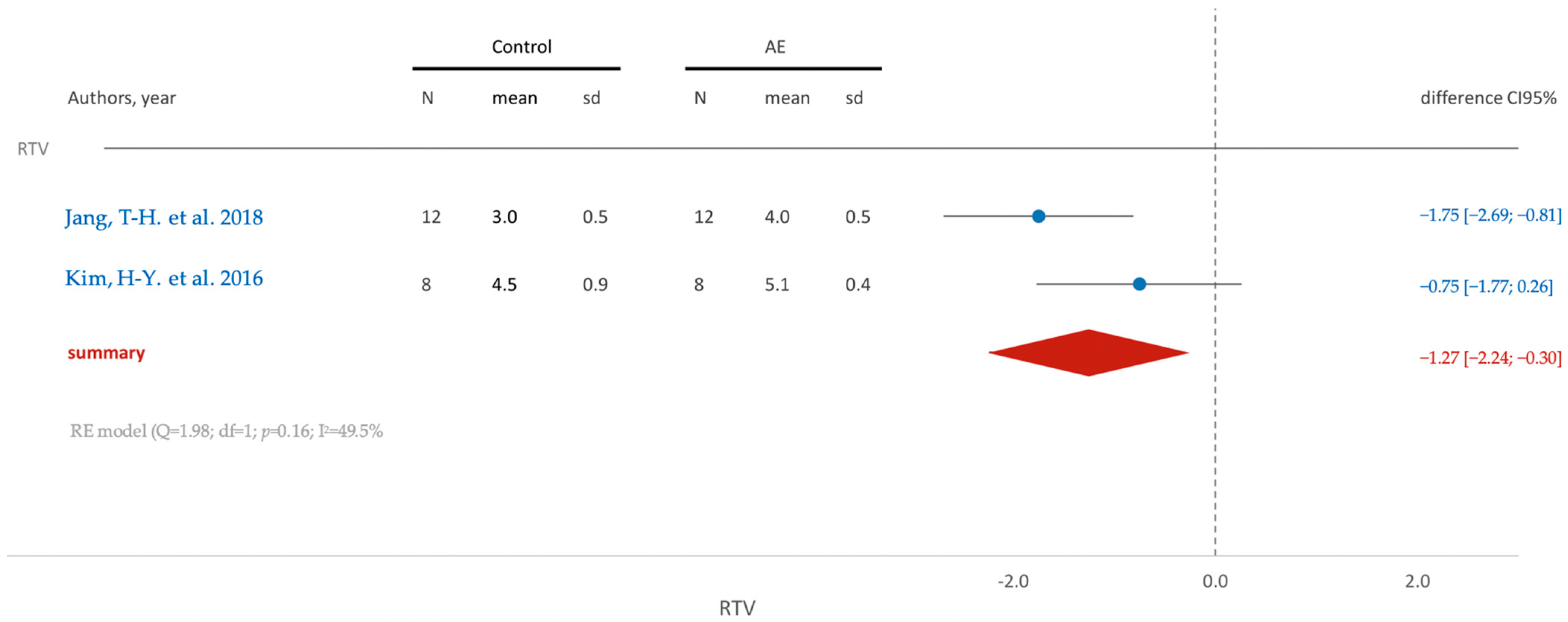
- Kim, H.-Y.; Kim, S.-C. Bone Cutting Capacity and Osseointegration of Surface-Treated Orthodontic Mini-Implants. Korean J. Orthod. 2016, 46, 386. [Google Scholar] [CrossRef]
- Pop, S.I.; Bratu, D.C.; Chiorean, R.; Balan, R.A.; Bud, A.; Petrescu, H.P.; Simon, C.P.; Dudescu, M. Biochemical Characteristics of Mini-Implants Sterilised by Different Chemical and Physical Procedures. Mater. Plast. 2017, 54, 253–256. [Google Scholar] [CrossRef]
- Tejani, H.; Venugopal, A.; Yu, W.; Kyung, H.M. Effects of UV Treatment on Orthodontic Microimplant Surface after Autoclaving. Korean J. Dent. Mater. 2017, 44, 119–127. [Google Scholar] [CrossRef]
- Kaci, N.; Hakem, K.; Laraba, S.; Benrekaa, N.; Le Gall, M. Micrographic Study and Torsional Strength of Grade 23 Titanium Mini-Implants Recycled for Orthodontic Purposes. Int. Orthod. 2018, 16, 246–257. [Google Scholar] [CrossRef]
- Pop, S.I.; Chiorean, R.; Bratu, D.C.; Pacurar, M.; Merie, V.; Manuc, D.; Bechir, E.S.; Teodorescu, E.; Tarmure, V.; Dudescu, M. Surface Properties and Maximum Insertion Energy of Sterilized Orthodontic Mini-Implants with Different Chemical Materials. Rev. Chim. 2018, 69, 3218–3220. [Google Scholar] [CrossRef]
- Hergel, C.A.; Acar, Y.B.; Ateş, M.; Küçükkeleş, N. In-Vitro Evaluation of the Effects of Insertion and Sterilization Procedures on the Mechanical and Surface Characteristics of Mini Screws. Eur. Oral. Res. 2019, 53, 25–31. [Google Scholar] [CrossRef]
- Iodice, G.; Perinetti, G.; Ludwig, B.; Polishchuk, E.V.; Polishchuk, R.S. Biological Effects of Anodic Oxidation on Titanium Miniscrews: An In Vitro Study on Human Cells. Dent. J. 2019, 7, 107. [Google Scholar] [CrossRef]
- Jongwannasiri, C.; Charasseangpaisarn, T.; Watanabe, S. Preliminary Testing for Reduction of Insertion Torque of Orthodontic Mini-Screw Implant Using Diamond-like Carbon Films. J. Phys. Conf. Ser. 2019, 1380, 012062. [Google Scholar] [CrossRef]
- Ly, N.T.K.; Shin, H.; Gupta, K.C.; Kang, I.K.; Yu, W. Bioactive Antibacterial Modification of Orthodontic Microimplants Using Chitosan Biopolymer. Macromol. Res. 2019, 27, 504–510. [Google Scholar] [CrossRef]
- Oga, Y.; Tomonari, H.; Kwon, S.; Kuninori, T.; Yagi, T.; Miyawaki, S. Evaluation of Miniscrew Stability Using an Automatic Embedding Auxiliary Skeletal Anchorage Device. Angle Orthod. 2019, 89, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, A.; Perissinotto, F.; Turco, G.; Contardo, L.; Stjepan, S. Do Chlorhexidine and Probiotics Solutions Provoke Corrosion of Orthodontic Mini-Implants? An In Vitro Study. Int. J. Oral. Maxillofac. Implant. 2019, 34, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Asadi, F.; Raji, S.H.; Samie, S. Effect of Steam and Dry Heat Sterilization on the Insertion and Fracture Torque of Orthodontic Miniscrews. Dent. Res. J. 2020, 17, 219. [Google Scholar] [CrossRef]
- Giri, M.; Sabapathy, K.; Govindasamy, B.; Rajamurugan, H. Evaluation of Insertion Torque and Surface Integrity of Zirconia-Coated Titanium Mini Screw Implants. J. World Fed. Orthod. 2020, 9, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Iwanami-Kadowaki, K.; Uchikoshi, T.; Uezono, M.; Kikuchi, M.; Moriyama, K. Development of Novel Bone-like Nanocomposite Coating of Hydroxyapatite/Collagen on Titanium by Modified Electrophoretic Deposition. J. Biomed. Mater. Res. 2021, 109, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Zogheib, T.; Walter-Solana, A.; De La Iglesia, F.; Espinar, E.; Gil, J.; Puigdollers, A. Do Titanium Mini-Implants Have the Same Quality of Finishing and Degree of Contamination before and after Different Manipulations? An In Vitro Study. Metals 2021, 11, 245. [Google Scholar] [CrossRef]
- Li, M.; Wu, G.; Wang, M.; Hunziker, E.B.; Liu, Y. Crystalline Biomimetic Calcium Phosphate Coating on Mini-Pin Implants to Accelerate Osseointegration and Extend Drug Release Duration for an Orthodontic Application. Nanomaterials 2022, 12, 2439. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.; Evans, C.A.; Zaki, A.M.; George, A. Use of Bone Morphogenetic Protein-2 and Dentin Matrix Protein-1 to Enhance the Osteointegration of the Onplant System. Connect. Tissue Res. 2003, 44, 30–41. [Google Scholar] [CrossRef]
- Nishioka-Sakamoto, K.; Hotokezaka, H.; Hotokezaka, Y.; Nashiro, Y.; Funaki, M.; Ohba, S.; Yoshida, N. Fixation of an Orthodontic Anchor Screw Using Beta-Tricalcium Phosphate in a Screw-Loosening Model in Rats. Angle Orthod. 2023, 93, 341–347. [Google Scholar] [CrossRef]
- Okawa, K.; Matsunaga, S.; Kasahara, N.; Kasahara, M.; Tachiki, C.; Nakano, T.; Abe, S.; Nishii, Y. Alveolar Bone Microstructure Surrounding Orthodontic Anchor Screws with Plasma Surface Treatment in Rats. J. Funct. Biomater. 2023, 14, 356. [Google Scholar] [CrossRef]
- Yamagata, K.; Oga, Y.; Kwon, S.; Maeda-Iino, A.; Ishikawa, T.; Miyawaki, S. A Novel Auxiliary Device Enhances Miniscrew Stability under Immediate Heavy Loading Simulating Orthopedic Treatment. Angle Orthod. 2023, 93, 71–78. [Google Scholar] [CrossRef]
- Aoki, T.; Ogawa, K.; Miyazawa, K.; Kawai, T.; Goto, S. The Use of Bioabsorbable Implants as Orthodontic Anchorage in Dogs. Dent. Mater. J. 2005, 24, 628–635. [Google Scholar] [CrossRef][Green Version]
- Kim, S.-H.; Cho, J.-H.; Chung, K.-R.; Kook, Y.-A.; Nelson, G. Removal Torque Values of Surface-Treated Mini-Implants after Loading. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Baek, S.-H.; Kim, J.-W.; Chang, Y.-I. Effects of Microgrooves on the Success Rate and Soft Tissue Adaptation of Orthodontic Miniscrews. Angle Orthod. 2008, 78, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Lee, T.; Chang, C.; Liu, J. The Effect of Microrough Surface Treatment on Miniscrews Used as Orthodontic Anchors. Clin. Oral. Implant. Res. 2009, 20, 1178–1184. [Google Scholar] [CrossRef]
- Niwa, K.; Ogawa, K.; Miyazawa, K.; Aoki, T.; Kawai, T.; Goto, S. Application of α-Tricalcium Phosphate Coatings on Titanium Subperiosteal Orthodontic Implants Reduces the Time for Absolute Anchorage: A Study Using Rabbit Femora. Dent. Mater. J. 2009, 28, 477–486. [Google Scholar] [CrossRef]
- Mo, S.-S.; Kim, S.-H.; Kook, Y.-A.; Jeong, D.-M.; Chung, K.-R.; Nelson, G. Resistance to Immediate Orthodontic Loading of Surface-Treated Mini-Implants. Angle Orthod. 2010, 80, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-S.; Kim, T.-W.; Ahn, S.-J.; Yang, I.-H.; Baek, S.-H. Effects of Insertion Angle and Implant Thread Type on the Fracture Properties of Orthodontic Mini-Implants during Insertion. Angle Orthod. 2013, 83, 698–704. [Google Scholar] [CrossRef]
- Choi, S.-H.; Cha, J.-Y.; Joo, U.-H.; Hwang, C.-J. Surface Changes of Anodic Oxidized Orthodontic Titanium Miniscrew. Angle Orthod. 2012, 82, 522–528. [Google Scholar] [CrossRef]
- Karmarker, S.; Yu, W.; Kyung, H.-M. Effect of Surface Anodization on Stability of Orthodontic Microimplant. Korean J. Orthod. 2012, 42, 4. [Google Scholar] [CrossRef]
- Omasa, S.; Motoyoshi, M.; Arai, Y.; Ejima, K.-I.; Shimizu, N. Low-Level Laser Therapy Enhances the Stability of Orthodontic Mini-Implants via Bone Formation Related to BMP-2 Expression in a Rat Model. Photomed. Laser Surg. 2012, 30, 255–261. [Google Scholar] [CrossRef]
- Uysal, T.; Ekizer, A.; Akcay, H.; Etoz, O.; Guray, E. Resonance Frequency Analysis of Orthodontic Miniscrews Subjected to Light-Emitting Diode Photobiomodulation Therapy. Eur. J. Orthod. 2012, 34, 44–51. [Google Scholar] [CrossRef]
- Cho, Y.-C.; Cha, J.-Y.; Hwang, C.-J.; Park, Y.-C.; Jung, H.-S.; Yu, H.-S. Biologic Stability of Plasma Ion-Implanted Miniscrews. Korean J. Orthod. 2013, 43, 120. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Pinto, M.; Dos Santos, R.L.; Pithon, M.M.; De Souza Araújo, M.T.; Braga, J.P.V.; Nojima, L.I. Influence of Low-Intensity Laser Therapy on the Stability of Orthodontic Mini-Implants: A Study in Rabbits. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 115, e26–e30. [Google Scholar] [CrossRef] [PubMed]
- Cuairán, C.; Campbell, P.M.; Kontogiorgos, E.; Taylor, R.W.; Melo, A.C.; Buschang, P.H. Local Application of Zoledronate Enhances Miniscrew Implant Stability in Dogs. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Motoyoshi, M.; Inaba, M.; Iwai, H.; Karasawa, Y.; Shimizu, N. A Preliminary Study of the Effects of Low-Intensity Pulsed Ultrasound Exposure on the Stability of Orthodontic Miniscrews in Growing Rats. Eur. J. Orthod. 2014, 36, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.-H.; Kim, E.-Y.; Paek, J.; Kook, Y.-A.; Jeong, D.-M.; Cho, I.-S.; Nelson, G. Evaluation of Stability of Surface-Treated Mini-Implants in Diabetic Rabbits. Int. J. Dent. 2014, 2014, 838356. [Google Scholar] [CrossRef] [PubMed]
- El-Wassefy, N.; El-Fallal, A.; Taha, M. Effect of Different Sterilization Modes on the Surface Morphology, Ion Release, and Bone Reaction of Retrieved Micro-Implants. Angle Orthod. 2015, 85, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Goymen, M.; Isman, E.; Taner, L.; Kurkcu, M. Histomorphometric Evaluation of the Effects of Various Diode Lasers and Force Levels on Orthodontic Mini Screw Stability. Photomed. Laser Surg. 2015, 33, 29–34. [Google Scholar] [CrossRef]
- Jang, I.; Shim, S.-C.; Choi, D.-S.; Cha, B.-K.; Lee, J.-K.; Choe, B.-H.; Choi, W.-Y. Effect of TiO2 Nanotubes Arrays on Osseointegration of Orthodontic Miniscrew. Biomed. Microdevices 2015, 17, 76. [Google Scholar] [CrossRef]
- Sirisa-Ard, A.; Michael, S.N.W.; Ahmed, K.; Dunstan, C.R.; Pearce, S.G.; Bilgin, A.A.; Dalci, O.; Darendeliler, M.A. Histomorphological and Torque Removal Comparison of 6 Mm Orthodontic Miniscrews with and without Surface Treatment in New Zealand Rabbits. Eur. J. Orthod. 2015, 37, 578–583. [Google Scholar] [CrossRef]
- Tabuchi, M.; Ikeda, T.; Nakagawa, K.; Hirota, M.; Park, W.; Miyazawa, K.; Goto, S.; Ogawa, T. Ultraviolet Photofunctionalization Increases Removal Torque Values and Horizontal Stability of Orthodontic Miniscrews. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 274–282. [Google Scholar] [CrossRef]
- Vilani, G.N.L.; Ruellas, A.C.D.O.; Elias, C.N.; Mattos, C.T. Stability of Smooth and Rough Mini-Implants: Clinical and Biomechanical Evaluation—An in Vivostudy. Dent. Press. J. Orthod. 2015, 20, 35–42. [Google Scholar] [CrossRef][Green Version]
- Bayani, S.; Masoomi, F.; Aghaabbasi, S.; Farsinejad, A. Evaluation of the Effect of Platelet-Released Growth Factor and Immediate Orthodontic Loading on the Removal Torque of Miniscrews. Int. J. Oral Maxillofac. Implant. 2016, 31, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.-K.; Choi, D.-S.; Jang, I.; Choe, B.-H.; Choi, W.-Y. Orthodontic Tunnel Miniscrews with and without TiO2 Nanotube Arrays as a Drug-Delivery System: In Vivo Study. Bio-Medical Mater. Eng. 2016, 27, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Kim, S.-J.; Lee, K.-J.; Sung, S.-J.; Chun, Y.-S.; Hwang, C.-J. Stress Distributions in Peri-Miniscrew Areas from Cylindrical and Tapered Miniscrews Inserted at Different Angles. Korean J. Orthod. 2016, 46, 189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gansukh, O.; Jeong, J.-W.; Kim, J.-W.; Lee, J.-H.; Kim, T.-W. Mechanical and Histological Effects of Resorbable Blasting Media Surface Treatment on the Initial Stability of Orthodontic Mini-Implants. BioMed Res. Int. 2016, 2016, 7520959. [Google Scholar] [CrossRef]
- Takahashi, M.; Motoyoshi, M.; Inaba, M.; Hagiwara, Y.; Shimizu, N. Enhancement of Orthodontic Anchor Screw Stability Under Immediate Loading by Ultraviolet Photofunctionalization Technology. Int. J. Oral. Maxillofac. Implant. 2016, 31, 1320–1326. [Google Scholar] [CrossRef][Green Version]
- Jang, I.; Choi, D.-S.; Lee, J.-K.; Kim, W.-T.; Cha, B.-K.; Choi, W.-Y. Effect of Drug-Loaded TiO2 Nanotube Arrays on Osseointegration in an Orthodontic Miniscrew: An in-Vivo Pilot Study. Biomed. Microdevices 2017, 19, 94. [Google Scholar] [CrossRef]
- Maino, B.G.; Di Blasio, A.; Spadoni, D.; Ravanetti, F.; Galli, C.; Cacchioli, A.; Katsaros, C.; Gandolfini, M. The Integration of Orthodontic Miniscrews under Mechanical Loading: A Pre-Clinical Study in Rabbit. Eur. J. Orthod. 2017, 39, 519–527. [Google Scholar] [CrossRef]
- Yun, S.-D.; Choi, S.-H.; Cha, J.-Y.; Yu, H.-S.; Kim, K.-M.; Kim, J.; Hwang, C.-J. Effects of Recycling on the Biomechanical Characteristics of Retrieved Orthodontic Miniscrews. Korean J. Orthod. 2017, 47, 238. [Google Scholar] [CrossRef]
- Jang, T.-H.; Park, J.-H.; Moon, W.; Chae, J.-M.; Chang, N.-Y.; Kang, K.-H. Effects of Acid Etching and Calcium Chloride Immersion on Removal Torque and Bone-Cutting Ability of Orthodontic Mini-Implants. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 108–114. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Hoang, P.; Fathi, A.; Foley, M.; Dunstan, C.; Dalci, O.; Papadopoulou, A.K.; Darendeliler, M.A. A Comparative Histomorphological and Micro Computed Tomography Study of the Primary Stability and the Osseointegration of The Sydney Mini Screw; a Qualitative Pilot Animal Study in New Zealand Rabbits. Eur. J. Orthod. 2019, 41, 360–369. [Google Scholar] [CrossRef]
- Yücesoy, T.; Seker, E.; Cenkcı, E.; Yay, A.; Alkan, A. Histologic and Biomechanical Evaluation of Osseointegrated Miniscrew Implants Treated with Ozone Therapy and Photobiomodulation at Different Loading Times. Int. J. Oral. Maxillofac. Implant. 2019, 34, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-C.; Hung, W.-C.; Lan, W.-C.; Saito, T.; Huang, B.-H.; Lee, C.-H.; Tsai, H.-Y.; Huang, M.-S.; Ou, K.-L. Anodized Biomedical Stainless-Steel Mini-Implant for Rapid Recovery in a Rabbit Model. Metals 2021, 11, 1575. [Google Scholar] [CrossRef]
- Choi, S.-H.; Shin, J.; Cha, J.-K.; Kwon, J.-S.; Cha, J.-Y.; Hwang, C.-J. Evaluation of Success Rate and Biomechanical Stability of Ultraviolet-Photofunctionalized Miniscrews with Short Lengths. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 158–166. [Google Scholar] [CrossRef]
- Auciello, O.; Renou, S.; Kang, K.; Tasat, D.; Olmedo, D. A Biocompatible Ultrananocrystalline Diamond (UNCD) Coating for a New Generation of Dental Implants. Nanomaterials 2022, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Seker, E.D.; Yavuz, I.; Yucesoy, T.; Cenkci, E.; Yay, A. Comparison of the Stability of Sandblasted, Large-Grit, and Acid-Etched Treated Mini-Screws with Two Different Surface Roughness Values: A Histomorphometric Study. J. Craniofacial Surg. 2022, 33, 41–47. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Liou, E.J.-W.; Chen, S.-W. Comparison between Microporous and Nanoporous Orthodontic Miniscrews: An Experimental Study in Rabbits. J. Orofac. Orthop. 2024, 85, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Durrani, O.K. Comparison of in Vivo Failure of Precipitation-Coated Hydroxyapatite Temporary Anchorage Devices with That of Uncoated Temporary Anchorage Devices over 18 Months. Am. J. Orthod. Dentofac. Orthop. 2023, 163, 520–525. [Google Scholar] [CrossRef]
- Ravi, J.; Duraisamy, S.; Rajaram, K.; Kannan, R.; Arumugam, E. Survival Rate and Stability of Surface-Treated and Non-Surface-Treated Orthodontic Mini-Implants: A Randomized Clinical Trial. Dent. Press J. Orthod. 2023, 28, e2321345. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, J.-H.; Chung, K.-R.; Nelson, G. Do Sand Blasted with Large Grit and Acid Etched Surface Treated Mini-Implants Remain Stationary under Orthodontic Forces? Angle Orthod. 2012, 82, 304–312. [Google Scholar] [CrossRef]
- Calderón, J.H.; Valencia, R.M.; Casasa, A.A.; Sánchez, M.A.; Espinosa, R.; Ceja, I. Biomechanical Anchorage Evaluation of Mini-Implants Treated with Sandblasting and Acid Etching in Orthodontics. Implant. Dent. 2011, 20, 273–279. [Google Scholar] [CrossRef]
- Matys, J.; Flieger, R.; Gedrange, T.; Janowicz, K.; Kempisty, B.; Grzech-Leśniak, K.; Dominiak, M. Effect of 808 Nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study. Materials 2020, 13, 2265. [Google Scholar] [CrossRef]
- Flieger, R.; Gedrange, T.; Grzech-Leśniak, K.; Dominiak, M.; Matys, J. Low-Level Laser Therapy with a 635 Nm Diode Laser Affects Orthodontic Mini-Implants Stability: A Randomized Clinical Split-Mouth Trial. J. Clin. Med. 2019, 9, 112. [Google Scholar] [CrossRef]
- Schätzle, M.; Männchen, R.; Balbach, U.; Hämmerle, C.H.F.; Toutenburg, H.; Jung, R.E. Stability Change of Chemically Modified Sandblasted/Acid-etched Titanium Palatal Implants. A Randomized-controlled Clinical Trial. Clin. Oral. Implant. Res. 2009, 20, 489–495. [Google Scholar] [CrossRef]
- Park, H.-J.; Choi, S.-H.; Choi, Y.J.; Park, Y.-B.; Kim, K.-M.; Yu, H.-S. A Prospective, Split-Mouth, Clinical Study of Orthodontic Titanium Miniscrews with Machined and Acid-Etched Surfaces. Angle Orthod. 2019, 89, 411–417. [Google Scholar] [CrossRef]
- Xuereb, M.; Camilleri, J.; Attard, N. Systematic Review of Current Dental Implant Coating Materials and Novel Coating Techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Jang, S.-H.; Cha, J.-Y.; Hwang, C.-J. Evaluation of the Surface Characteristics of Anodic Oxidized Miniscrews and Their Impact on Biomechanical Stability: An Experimental Study in Beagle Dogs. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Nagay, B.E.; Dini, C.; Borges, G.A.; Mesquita, M.F.; Cavalcanti, Y.W.; Magno, M.B.; Maia, L.C.; Barão, V.A.R. Clinical Efficacy of Anodized Dental Implants for Implant-supported Prostheses after Different Loading Protocols: A Systematic Review and Meta-analysis. Clin. Oral. Implant. Res. 2021, 32, 1021–1040. [Google Scholar] [CrossRef]
- Dini, C.; Nagay, B.E.; Magno, M.B.; Maia, L.C.; Barão, V.A.R. Photofunctionalization as a Suitable Approach to Improve the Osseointegration of Implants in Animal Models—A Systematic Review and Meta-analysis. Clin. Oral. Implant. Res. 2020, 31, 785–802. [Google Scholar] [CrossRef] [PubMed]
| Surface Treatment | Advantages | Disadvantages |
|---|---|---|
| Sandblasting, large-grit and acid-etching [15,16] |
|
|
| Acid-etching [17] |
|
|
| Photofunctionalization with UV-light [14] |
|
|
| Photobiomodulation with LED [18] |
|
|
| Author, Year | Sample | Type and Sample Size | Surface Treatment (Type, Time, Dose and Protocol) | Experimental Group | Control Group | Period Follow-Up | Test Used to Evaluate Outcomes | Primary Outcome | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Mattos, CT et al., 2010 [29] | Porcine femur cortical bone | Ti6Al4V (SIN, Brazil), 8 mm × 1.4 mm, n = 40 | n = 20 Autoclave sterilization: STERMAX, one cycle, 30 min, 121 °C, n = 30 Ms recovered from 19 orthodontic patients and cleaned ultrasonically (Maxiclean 1400A) in an enzymatic detergent solution. | n = 20, autoclave sterilization. n = 30, ultrasonic cleaning | n = 20, no treatment | SEM at 20 kV: surface morphology analysis; Fracture Torque Tests (Digital Measurement). | Stability and risk of fracture | SEM: recovered with surface showing signs of use and marks; corrosion and defects were absent in the sterilized and recovered groups, as in the control. Fracture torque: significant difference between control group and recovered group; recovered with greater variation in values. | Recovered Ms have altered surfaces and a greater variance of FTVs. Reusing these is not recommended. The use of new Ms sterilized by autoclave is a recommended practice. | |
| Mattos, CT et al., 2011 [35] | Porcine cortical bone in segments | Ti6Al4V: Neodent, SIN and Titanium Fix; n = 100 | Five groups, one of each type of Ms; n = 10 × 5. Sterilization in STERMAX autoclave, 30-min cycle at 121 °C. | n = 50 Autoclave sterilization | n = 50, new ones | - | Tests fracture torque digital. | Stability | The effect of autoclave sterilization is not a significant factor affecting the variance of results (p = 0.4113), unlike the manufacturer of the Ms (p < 0.0001), with the differences in stability observed being due to this factor. | Autoclave sterilization of Ms did not cause pronounced effects on their fracture resistance, with the manufacturer being the most responsible factor for differences. |
| Muguruma, T. et al., 2011 [36] | - | Teeth, Ti-6Al-4V, 12 mm × 1.4 mm; n = 25 | n = 20; Ultrasonic cleaning in ethanol and dimethyl ketone + immersion in solutions of 450 ppm F (G1 and G3) or 900 ppm F (G2 and G4) for 1 and 24 h, respectively. | n = 5 × 4; G1: 0.1% NaF 1 h; G2: 0.2% NaF 1 h; G3: 0.1% NaF 24 h; G4: 0.2% NaF 24 h | n = 5, no treatment | 1 and 24 h | Torsion test: torques and fracture angles. | Stability | Torsion test: greater variations in fracture angle between groups than in fracture torques (similar between groups), but without significant differences compared to controls. | Use of these solutions as mouthwashes should not cause a decrease in the stability of the Ms and their clinical performance. |
| Cho, IS et al., 2012 [37] | Polyurethane sponge block | Ti6Al4V (Biomaterials Korea, Seoul, Republic of Korea), 8 mm × 1.45 mm, n = 54 = 18 + 36 | (n = 18) SLA: 100 µm aluminum particles, 2 min, 2 bar + immersion 30% HCl, 60% H2SO4 and diluting solution. (n = 18) SLAO: SLA + oxidative anodizing (calcium acetate and β-glycerolphosphate) 3 min, 250 V. | n = 6 + 12 SLA; n = 6 + 12 SLAO | n = 6 + 12, no treatment | 8 weeks | Digital measurement of ITV and RTV: analysis of maximum torque, total energy and peak energy. | Stability | Higher MITV in control (p < 0.01), but without differences in insertion TEV and NPE, or in all removal values. | SLAO treatment can be an effective way to reduce the damage due to the insertion of Ms, both in the tissue and in the device, and can also improve its stability. |
| Galli, C. et al., 2012 [38] | MC3T3 cells mouse | Titanium discs grade IV and grade V (HDC Company, Sarcedo, Italy) 10 mm × 1.5 mm, n = 6 | Grade IV AE with hydrochloric acid. Grade V: AO by electrochemical protocol. | Grade IV G1: AE; G2: AE + calcium phosphate. Grade V: G1: AO; G2: AO + calcium phosphate | n = 2, without treatment in both grades | 3 days | SEM:Cellular and surface morphology; Cell viability and organization analysis; PCR: expression analysis genetics. | Stability | Greater cell proliferation on smooth surfaces in grade IV compared to rough surfaces, and without differences in grade V. Calcium phosphate in grade IV increased the amount of messenger RNA for osteocalcin and alkaline phosphatase. Greater osteoblastic markers on grade V control surfaces than on rough ones, similar to grade IV titanium acid conditioning. | Ti grade IV with calcium phosphate obtained higher differentiation value in vitro. Machined Ti grade V had good cell proliferation and bone matrix synthesis, as well as greater expression of differentiating markers, which makes it an option for orthodontic Ms. |
| Noorollahian, S. et al., 2012 [39] | - | 1.4 mm × 8 mm, 32: n = 16 new, n = 16 used for 3 years | H2O and drying + phosphoric acid gel 37% 1 mL + immersion in NaOCl 5.25 mL (10 mL) for 30 min. | NP1: irrigation + drying; P1: irrigation+ drying+ phosphoric acid + NaOCl (n = 16) | New Ms, C1: irrigation + drying (n = 16) | - | AAS: % calcium ion on the surface. | Amount Ca2 ion | NP1: 4.7 ppm; P1: 0.43 ppm; C: 0.02 ppm. NP1 significantly higher than other groups (p = 0.000), but P1 and C1 without significant differences between them. | Treatment reduces remaining tissue to manufacturing levels, allowing its use in processing used Ms. |
| Akyalcin, S. et al., 2013 [40] | - | Vector TAS, Aarhus, Dual-Top, Ortho Anchor 8 mm × 1.4 mm–1.5 mm (Aarhus system, American Orthodontics, Sheboygan, WI, USA); n = 120 = 30 × 4 | n = 80; Autoclave sterilization (SciCan Statim 500) at 132 °C, 6 min. Insertion into synthetic bone blocks for all groups. | n = 20 per brand; G1 (n = 10): 5 sterilization cycles; G2 (n = 10): 10 sterilization cycles | n = 10 per brand; G0: 1 sterilization cycle | - | Maximum insertion torques and side displacement force. | Stability | ITVs: differences found between the four brands and between the three sterilization cycles and between them (p < 0.05). Lateral displacement: significantly affected by the brand, but not by the number of sterilization cycles; Arhus group with differences for the remaining brands (p < 0.05). | Depending on the manufacturer, each Ms has its own behavior. The values obtained for the stability of Ms indicate that sterilization up to and including 10 cycles does not affect the clinical stability. |
| Serra, G. et al., 2013 [41] | - | Prosthetic Systems Connection (Conexão, São Paulo, Brazil) 6 mm × 2 mm, n = 15 | Surface nanostructuring (n Ti) by severe plastic deformation: grade 4 Ti pressed at 450 °C in longitudinal axis rotation (ECAP) + forging and drawing at 80% deformation + reheating at 300–350 °C for 1 h. | G2: Ti6Al4V; G3: nTi. Machined + clean + HNO3 | G1: Pure Ti | - | Tensile strength test. Maximum Endurance Torque (MTR) Tests. SEM. | Stability | Elastic strength: G3 with fatigue resistance almost 80% greater than G1. MTR with increased values in G3 compared to control (p < 0.05), and no differences between G3 and G2. SEM: G3 with smooth morphology and transgranular fracture appearance; G3 with the roughest surface of all the groups. | nTi Ms bring together the biocompatibility of pure Titanium and the mechanical resistance of Ti6Al4V. They have greater torsional resistance than pure Ti and equal that of grade V titanium alloy. |
| Tozlu, M. et al., 2013 [42] | Bone model of bovine ilium (iliosacral joint), with cortical 0.5–2.5 mm, in strips | Ti-6Al-4V grade V cylindrical TM, Trimed, 9 mm × 1.6 μm; n = 48 | n = 24 MI ring (MIR) device with four spikes which come into contact with the bone cortex when placing the ring on the Ms with a manual instrument, in a cavity in the Ms head, in order to support the ring. | n = 24 Mini Ti ring (Ti6Al4V grade V) MIR with spikes, fitted to the micro. | n = 24 without treatment | - | Mechanical test: anchoring strength; insertion and removal torque tests; measurement of cortical bone thickness. | Stability | AFR: experimental group showed significantly higher anchoring force values than the control (p < 0.001); the resistance of the control groups was influenced by the cortical thickness (p < 0.05). ITVs: significantly higher in the experimental groups (p < 0.01); higher insertion torque values the greater the thickness, for both groups (p < 0.01). RTVs: no significant influence of MIR. | The new MIR coupler device increased the anchoring force and insertion torque, therefore increasing the primary stability and resistance of the Ms under study. This device had no influence, however, on the removal torque and mobility values. |
| Estelita, S. et al., 2014 [43] | Porcine iliac bone in segmentsSR | n = 200 | G1: insertion into high-density bone and removal; G2: initial protocol G1, ultrasonic cleaning 40 kHz, 25 °C, 20 min in detergent + irrigation with ultrasound + autoclave sterilization; G3: G1 protocol, but inclusion of sandblasting Al2O3, 90 µm, 60 psi, 20 min (n = 150). | G1, G2, G3 | untreated high-density artificial bone inserted and removed (n = 50) | - | Tests fracture torque digital; sample weighing: mass loss analysis. | Stability | Similar fracture torques, not influenced by previous insertion into bone and sterilizing treatments. Ms diameter increases fracture torque with every 1 mm added, in any group. G3 with less weight compared to the others. Linear regression analysis showed that only the Ms diameter influences the variability of fracture torque, by 97%. | Ms do not suffer from decreased stability due to previous use or sterilization treatments. Sandblasting causes loss of mass, but without loss of torque. Differences of 0.1 mm in diameter influence removal torque, and therefore the stability of Ms. |
| Oh, EJ. et al., 2014 [44] | - | Ti6Al4V (Plates Kobe Steel Ltd., Japan) 10 × 10 × 2 mm | n = 16 APH treatment: TiO2 nanotubes in solution Glycerol/H2O/NH4F, 20 V, 1 h + cyclic calcification for incorporation of CaP and HA by repeated immersion in NaH2PO4(0.05 M), 80 °C and Ca(OH)2 (100 °C) 1 min/cycle for 30 cycles + heat treatment 500 °C, 2 h. Immersion in simulated body fluid (SBF). | APH: anodizing + pre-calcification + heat; AH: anodizing + heat | UT: no treatment | 3 days, 3 and 6 weeks | FE-SEM; surface roughness test; microscopy assessment of hydrophilicity; measurement of removal torques; EDS/XRD; histological analysis of %BIC. | Stability and osteointegration | Morphology: APH group with an organized and compact arrangement of nanotubes, dense HA precipitate that fills all the empty spaces in the nanotubes. Bioactivity (EDS): APH group covered in HA protuberances; % Ca and P similar to HA, indicating good bioactivity. | APH treatment accelerated the formation of Hydroxyapatite, improved bone formation and presented surfaces with bioactivity and biocompatibility, which will allow an improvement in the initial stability of Ms. Indication for poor quality bone, where rapid healing and osseointegration is required. |
| Miyawaki, S. et al., 2015 [45] | - | Ti 6 mm × 1.6 mm, n = 4 | SpikeAnchor, Ti6Al4V, two portions: portion that receives forces and portion with peaks that contact the cortical bone. | Implantation in the femur with SpikeAnchor | No Spike | 4 weeks | Lateral displacement test: mechanical retention forces; visual analysis of device insertion. | Stability | The retention force was significantly greater in the experimental group at different displacements (p < 0.05). The smaller the displacement, the greater differences in the forces applied in the experiment. Spikes were implanted at 0.3 mm after application of compressive forces. | SpikeAnchor allowed automatic implantation over time into cortical bone. There was an increase in Ms stability of 3–5 times when compared to the experimental group and the control group. |
| Fleischmann, L. et al., 2015 [46] | Cells Osteoblast -likeMG-63 | Discs Ti-6Al-4V 14.8 mm × 0.6 mm | G1: Discs coated in TiN with TiN plasma spray, 6 µm; G2: Discs coated with PTFE powder + oven heating = 40 µm PTFE. | G1: TiN plasma; G2: PTFE | G3: no treatment; G4: plastic cells | 48, 120, 168 h | MTT test. Time-lapse microscopy Apoptosis test by flow cytometry; alkaline phosphatase activity and PCR for osteogenesis markers. | Stability | Viability/Proliferation: 168 h: PTFE and G4 groups showed greater cell viability than the other groups (p < 0.05). Cell behavior: at 12.5 h adhesion to G3 surfaces, 50% of cells to adhere in the PTFE group. Apoptosis: G1 and G2 have a lower apoptotic rate (p < 0.05) than G4. Gene expression: alkaline phosphatase and osteocalcin mRNA higher in the PTFE group than in G3 (p < 0.05). Osteoprotegerin: higher in PTFE than in G3 (p < 0.05). | Surface treatment with PTFE on titanium showed better biocompatibility, both in relation to the non-use of treatment and the use of TiN. It is suggested that this promotes osteointegration and hydrophilicity, with less microbial adhesion. More in vivo studies are needed. |
| Ganzorig, K. et al., 2015 [47] | MC3T3-E1 cells | 33 mm discs (Nishimura Metal, Japan) Ti6Al4V and 1.6 mm × 1.5 mm SS, n = 140 | LIPUS Osteotron_D IB, Ito Co; 1.5 MHz, 30 mW/cm2, pulse ratio 1:4; 24 h after insertion, 20 min/day in the right tibia (1×/15 min in the cells). | n = 80, LIPUS | n = 80, no treatment | 0–14 days | Alkaline phosphatase test. Light microscopy; analysis of the presence of minerals; PCR. | Stability and osteogenesis | From day 3 to day 14, BIC gradually increased in all groups. LIPUS increased BD density, CBT and bone formation rate after insertion (p < 0.05). Increase in GLIPUS of ALP regulation in vitro on the third day (p < 0.05). | It is suggested that the application of LIPUS improves bone formation around titanium alloy and stainless steel Ms and may therefore improve their initial stability and success rate throughout orthodontic treatment. |
| Liang, Y. et al., 2015 [48] | - | Ti grade 4: 10 × 10 × 2 mm boards | Strontium (Sr) application: polishing + HCl + CaCl2 + ultrasonic cleaning in acetone, 10 min + H2O + drying + ECD (electrochemical deposition with platinum mesh). | n = 12 G1: without ECD, ovariectomy; n = 12 G2: ECD with Sr, ovariectomy | n = 12, no treatment + no ovariectomy | 2 and 4 weeks | XRD: analysis of the chemical composition; FE-SEM. | Stability | XRD: coating with SrHPO4, with peak intensity higher than other crystals. FE-SEM: thickness 25 µm; morphology of lamellar crystals in clusters. | Strontium treatment is easily performed by ECD. This has a promoting effect on the osteointegration of Ms in animals with osteopenia and may be a new protocol in patients with osteoporosis. |
| Tabuchi, M. et al., 2015 [49] | Bone marrow cells from 8-week-old Sprague-Dawley rats | 6 mm × 1.4 mm (Jeil Medical, Guro-gu, Republic of Korea), n = 12; Ti6Al4V discs (n = 18) 20 mm × 1.5 mm | Before implantation and placement in culture, the Ms and discs, respectively, were treated with ultraviolet light for 12 min with a TheraBeam Super Osseo device. | UV Light | No treatment | 3 weeks | SEM; EDX; ELISA; CLSM: growth and cell adhesion; alkaline phosphatase activity; mechanical Tests. | Stability | Number of osteoblasts adhered to the experimental surface at 3 and 24 h is significantly higher than in the control (p < 0.05); experimental group with higher expression of vinculin and actin (p < 0.001 and p < 0.01, respectively); positive area for alkaline phosphatase (ALP) 80% greater in the functionalized groups, and equally in its activity and calcium deposition (p < 0.001). | The displacement of photofunctionalized Ms was 30–40% less than untreated ones. It is suggested that photofunctionalization increases the bioactivity of the Ms under study, as well as their anchoring capacity and stability, without changing the mechanical morphology itself. |
| Yadav, S. et al., 2015 [50] | - | Circular discs 3 mm × 3 mm Ti6Al4V grade V (Dentaurum Co., Ispringen, Germany) | G2 (n = 32): AE 0.11 mol/L HCl 65 °C for 20 min + oven drying for 24 h; G3 (n = 32): gritblasting with 25–50 µm alumina; G4 (n = 32): gritblasting with alumina 25–50 µm + AE 0.11 mol/L HCl 65 °C for 20 min + oven drying for 24 h. | G2: AE (n = 32); G3: gritblasting (n = 32); G4: gritblasting + acid-etching (n = 32) | n = 32, G1: no treatment | 8 weeks | Optical profilometer: surface roughness; goniometer: contact angle measurement; removal torque test; histological analysis (toluidine blue): BIC. | Biological integration | Surfaces: G1: irregularities in 1 direction; G2: thin and hard surface with elevations and depressions; G3: highly irregular surface and cavities; G4: uniform surface, with smaller cavities than G3. Hardness: G3 < G4. Wettability: G4 lower with blood and sodium chloride (p < 0.0002); G2 and G3 smaller than G1 (p < 0.0003); for DMSO and water, there were differences between all groups. RTV: no significant interaction between bone type and Ms surface; G4 with significantly greater torque than G3, G2 and G1. Contact: significantly higher in G4 than G3, G2 and G1. | Surface hardness was shown to be higher in gritblasted Ms than in others, following this coupled with acid-etching with HCl. Contact with liquids was greater for the control group and lower for gritblasted with conditioning. The removal torque in both the tibia and femur was greater in the gritblasted with conditioning group. Greater bone/Ms contact on rougher surfaces than on machined surfaces alone. |
| Fleischmann, L. et al., 2015 [46] | Cells Osteoblast -likeMG-63 | Discs Ti6Al4V 14.8 mm × 0.6 mm | G1: Discs coated in TiN with TiN plasma spray, 6 µm; G2: Discs coated with PTFE powder + oven heating = 40 µm PTFE. | G1: TiN plasma; G2: PTFE | G3: no treatment; G4: plastic cells | 48, 120, 168 h | MTT test. Time-lapse microscopy Apoptosis test by flow cytometry; alkaline phosphatase activity and PCR for osteogenesis markers. | Stability | Viability/Proliferation: 168 h: PTFE and G4 groups showed greater cell viability than the other groups (p < 0.05). Cell behavior: at 12.5 h adhesion to G3 surfaces, 50% of cells to adhere in the PTFE group. Apoptosis: G1 and G2 have a lower apoptotic rate (p < 0.05) than G4. Gene expression: alkaline phosphatase and osteocalcin mRNA higher in the PTFE group than in G3 (p < 0.05). Osteoprotegerin: higher in PTFE than in G3 (p < 0.05). | Surface treatment with PTFE on titanium showed better biocompatibility, both in relation to the non-use of treatment and the use of TiN. It is suggested that this promotes osteointegration and hydrophilicity, with less microbial adhesion. More in vivo studies are needed. |
| Espinar-Escalona, E. et al., 2016 [28] | - | Ti pure (HDC Company, Sarcedo, Italy), 9 mm × 2 mmn = 20 | AE: immersion in 0.35 M HF, 15 s, 25 °C. Gritblasting: 600 µm alumina particles, 0.25 MPa until roughness saturation. GBA: combination of previous techniques. Acetone cleaning protocol, 15 min + H2O + drying in N2. | n = 5 AE: conditioning acid; n = 5 GB—alumina beam; n = 5 GBA—AE + GB | n = 5, no treatment | 10 weeks | FE-SEM: analysis of the O/M interface. Manual measurement of removal torque. Analysis of %BIC. | Stability | Roughness of GB and GBA significantly higher than control and AE. GB and GBA significantly more hydrophobic than the rest. %BIC: GBA 79% and GB 75% (p < 0.05), AE 26% and control with 19%. No differences in removal torque between AE and control groups, but higher values for GB and GBA. Greater roughness and wettability in treatments than in control, leading to higher %BIC and higher RTV. | Surface treatments on Ms such as AE, GB and GBA are effective in modifying the surface and improving osseointegration and stability of the device. These create removal torques that do not compromise stability and do not promote fractures. Wettability was the parameter that showed the most influence on torque. |
| Kang, H.K. et al., 2016 [51] | - | 316 SS stainless steel 6 mm × 1.2–1.3 mm, n = 48 | Nd-YAG 1064 nm treatment Q-switched. | n = 12, Nd-YAG laser Q-switched | n = 12, no treatment | 0 and 8 weeks | (ZeGage): surface roughness measurement x3; SEM; fracture torque tests (digital measurement). | Stability | All Ms with mobility <1 mm. No flaws. Surface roughness: higher values in the treated group (p < 0.05), triple increase; No differences in fracture torque and 2D and 3D BIC between groups. | Surface roughness increased by more than three times due to surface reactions created by the laser. There was no increase in fracture resistance, despite an increase in surface roughness. |
| Kim, H.Y. et al., 2016 [52] | - | OSSH1606 (Osstem Implant, Seul, Republic of Korea) 1.6 mm × 6 mm Ti6Al4V, n = 150 | G1: hydrochloric and nitric AE; G2: RBM, calcium phosphate beam and acid wash—removed from in vivo experiment due to fractures at placement; G3: hybrid: 75 µm calcium phosphate beam, except for cutting one-third and acid washing. | G1, G2 and G3 | G0: no treatment | 1, 2, 4 and 8 weeks | Insertion and removal torque tests; optical microscopy; SEM: surface changes; EDS: quantitative analysis of surface composition. | Stability | Cutting capacity: more superficial insertion in the RBM group than other groups (p < 0.05). Osseointegration: at 4 weeks, the removal torque in the control group decreased significantly, but was increased in G1 (p < 0.05 in both); G4 with increased values at 2 weeks, and with higher values compared to the other Gs at 8 weeks (p < 0.05). There was infiltration of calcium and phosphorus on the surface; bone was detected in the G4 group. | Partial/hybrid RBM group with greater stability compared to the other groups, without a reduction in cutting capacity. |
| Fernandes, D.J. et al., 2017 [20] | - | Ti6Al4V Discs (ASTM grade V), 6.35 mm | Polishing with 0.05 µm alumina + washing with acetone, alcohol and water. AE: (HNO3 + H2O + H2SO4) under magnetic stirring + HNO3. | n = 24 AE (1, 4 and 8 s) | n = 24, no treatment (1, 4 and 8 s) | 1, 4 and 8 weeks | FE-SEM; SPM: thickness and roughness of the TiO2 layer. Goniometer; Panalytical X’Pert PRO: investigation of the crystal structure. XRF: surface composition. | Stability | No fractures or infections. Surface: intercommunicating micropores in the experimental group; micro and sub-microscopic roughness; TiO layer two greater in Ms with treatment, and with lower % of Al and V. Insertion and removal torque: higher values for the treated group; ITVs > RTVs. Histological analysis: dense Ca/P particles with proliferating osteoblasts at the O/M interface of the group treated at 4 weeks, new bone formed at 8 weeks. Blood analysis: % Al and V decreased in all follow-ups in the treated group. | Acid-etching treatment of the surface of Ms improves surface morphology and mechanical stability, with early signs of osseointegration. It also allowed a reduction in the release of Al and V ions. |
| Pop, S. et al., 2017 [53] | Synthetic pig bone, high density | Linkfrom MIS™ (MIS Implants Technologies, HaZafon, Israel) and Yesanchor (Orlus™, Ortholution, Seoul, Republic of Korea) both 1.6 × 8 mm, n = 100 | G1 (n = 10): ultrasonic cleaning 40 kHz, 25 °C, double + autoclave 121°, 15 psi for 20 min; G2 (n = 10): chemical cleaning (37% phosphoric acid gel) 10 min + cleaning, drying and 5.25% NaOCl 30 min + cleaning + autoclave as G1; G3 (n = 10): insertion and removal protocol G1 + ultrasonic detergent cleaning 8 min + cleaning distilled water + sandblasting Al2O3-90 µm 60 psi + cleaning and ultrasonic bath 20 min + autoclave; G4 (n = 10): distilled water + autoclave. | n = 40, G1: ultrasonic cleaning and autoclave; G2: chemical cleaning + autoclave; G3: ultrasonic cleaning + sandblasting + autoclave; G4: cleaning distilled water + autoclave | n = 10 new ones, unused, untreated | - | Maximum Insertion Torque Test. | Stability | Average ITV ranged: Link 22.40 Ncm—26.94 Ncm; Yesanchor 36.46 Ncm—42.37 Ncm. Significant differences between G0 and G4 (p = 0.0177), G2 and G3 (p = 0.0402) and G3 and G4 (p = 0.0135), G3 with greater torque than G2 and G4; no differences between groups Between Link and Yesanchor groups, there were statistically significant differences (p < 0.001). | Differences in maximum insertion torque specific to the brands of Ms used were found. Different types of chemical and mechanical cleaning of Ms create variable effects on torque values, differences that are more pronounced in Link Ms. More studies are needed in order to find other modifications and their influence on other parameters, such as surface topography, or fracture and removal torque. |
| Tejani, H. et al., 2017 [54] | - | Teeth, Ti-6Al-4V, n = 120 | n = 2 × 30. New group and 11-year-old group; sterilization with autoclave + UV light treatment with 5 × 8 W bactericidal lamp in a 254 nm tube, 12 min, with carbon deposition measurements following both treatments. | n = 90; Sterilization + UV Light G1: 6 years of archive; G2: 9 years of archive; G3: 11 years of archive | n = 30; G0. Autoclave + UV Light, new manufacturing | - | XPS: carbon content of tested titanium surfaces; water contact angle test. | Stability | Archive time: the longer it is, the higher the carbon content. Sterilization: increase in carbon content in G0. UV light: significantly decontaminated surfaces (lower % carbon); G4: with differences in carbon load due to UV light and not autoclave sterilization; increased hydrophilicity of samples by UV treatment, but without increased cellular activity. | Ms with a longer archive time will have a greater amount of carbon contamination on their surface. Steam sterilization with an autoclave increased the carbon content on the surface. UV light reduced the carbon content. However, it did not modify the osteoblastic differentiation. |
| Kaci, N. et al., 2018 [55] | - | Ti grade 23, 8 mm × 2 mm, n = 52; CPT Pure Ti 10 mm × 2 mm | Reuse of Ms for four different periods: G1 0 days of use; G2 2 months of use; G3 1 year of use; G4 14 months of use. Reinsertion in the upper posterior region. | G1: n = 6; G2: n = 6; G3 n = 20; G4 n = 20 | G0: CPT new 10 mm × 2 mm | - | Image polarized optics: surface analysis. Torsional Mechanical Tests: Fracture Resistance. | Stability and fracture resistance | Surface characteristics for minor uses revealed no defects at the micron level, and with fracture torques of around 53 N/cm2. G3 and G4 with evident surface changes, mainly where there was an interface with the gingiva, and with fracture torque of 42–39 N/cm2, respectively. | Grade 23 titanium is a good compromise between pure titanium and stainless steel. Mechanically, the reuse of Ms that have been used for 0–2 months is possible, but it is not recommended for those with longer use, as there is a decrease in their resistance. |
| Pop, S. et al., 2018 [56] | Pork jaw; artificial bone 1 cm3 | YesAnchor (Orlus, Ortholution, Seoul, Republic of Korea) 8 mm × 1.6 mm, n = 50 | Ultrasonic cleaning + autoclave sterilization; cleaning with 37% phosphoric gel, 10 min + immersion in NaOCl 5.25%, 30 min + autoclave sterilization; insertion and removal + cleaning + Al2O3 +autoclave sterilization; insertion and removal + cleaning H2O + sterilization in autoclave. All groups subsequently inserted into artificial bone (including YA0). | n = 10 × 4 YA1; YA2; YA3; YA4 | n = 10, YA0: new, without treatment or insertion | - | Insertion energy measurement. Microscopy: analysis of the degree of morphology change. | Stability | YA1 group with the highest average value of insertion energy. Only significant differences in maximum insertion energy between YA1 and YA3 (p = 0.04). | No significant differences in the behavior of Ms in groups YA1, YA3 and YA4. Decrease in total insertion energy in the YA3 group due to the use of sandblasting. Sterilization by autoclave followed by cleaning with distilled water did not promote total elimination of the organic tissue remaining on the surface of the YA4 group. Presence of chemical corrosion if used chemical cleaning. |
| Miyawaki, S. et al., 2015 [45] | - | Ti 6 mm × 1.6 mm, n = 4 | SpikeAnchor, Ti6Al4V, two portions: portion that receives forces and portion with peaks that contact the cortical bone. | Implantation in the femur with SpikeAnchor | No Spike | 4 weeks | Lateral displacement test: mechanical retention forces; visual analysis of device insertion. | Stability | The retention force was significantly greater in the experimental group at different displacements (p < 0.05). The smaller the displacement, the greater differences in the forces applied in the experiment. Spikes were implanted at 0.3 mm after application of compressive forces. | SpikeAnchor allowed automatic implantation over time into cortical bone. There was an increase in Ms stability of 3–5 times when compared to the experimental group and the control group. |
| Hergel, C. et al., 2019 [57] | Sawbone Artificial Bone | Dual-Top 8 mm × 1.6 mm (Jeil Medical, Republic of Korea); Ortho-Easy 8 mm × 1.7 mm (FORESTADENT, Germany), n = 140 = 70 × 2 | n = 60 + 60. Insertion into Sawbone artificial bone, followed by removal. Ultrasonic cleaning 30 min, 1LH2O, 5 mL Endozyme + sterilization in an autoclave at 135 °C. 10 min + Statim 7000 drying, 55 min. | G2: insertion + Sterilization + insertion; G3: (insert + autoclave) × 2 + insertion | n = 10 + 10; without insertion and treatment | 1 month | ITVs and RTVs. Vertical and horizontal resistance Tests. SEM; Fracture Torque Tests. | Stability | Significantly higher MITV in G1 (p < 0.05). No differences were found in MRTVS and the remaining VH Tests and torsional strength. SEM: atrophy of the coils of the Ms used was detected, + in the apical region; oxidized layer disappeared in some places in G2 and 3. | Although some wear and atrophy of the Ms used has been proven, their primary stability and fracture torque values did not show significant differences after a second insertion. |
| Iodice, G. et al., 2019 [58] | Osteoblast cells -like Osteosarc oma Saos-2 | Grade V Titanium Plates Orthoeasy® (FORESTADENT, Germany) 10 mm × 2 m; n = 272 (68/type) | AO treatment (different thicknesses of titanium oxide on the surface of titanium plates—pink, gold and rosé groups). | Thickness TiO2 n = 68 each: G.pink 40–50 nm; G.golden 130 nm; G. rose 140 nm | n = 68, Gray group: without layer TiO2 | 12, 24, 40 and 48 h | CLSM: cell growth, Live/Dead™ Viability/Cytot oxicity Kit: cell viability analysis; Hoechst and α-tubulin staining. | Stability | Growth: higher in Procollagen I control (p = 0.019), Rosé with higher concentrations. Viability: differences in live cells (p = 0.016), but absence in dead cells. Migration: differences in cell-free areas at 12 h, 24 h and 40 h, with no free areas at 48 h, lower values in control and with larger ones in rosé at 24 h and 40 h, therefore showing faster and slower migration, respectively. | Anodization to obtain TiO2 produces minor effects on cell viability, although greater in the initial population times. There is no clear relationship between thickness and cellular response to Ms. |
| Jongwannasiri, C. et al., 2019 [59] | Porcine mandibular bone | Ti6Al4V (Osstem Implant, Seul, Republic of Korea), 10 mm × 1.8 mm | DLC (diamond-like carbon): thin film of diamond carbon reinforced by Fluorine or Silica, through a mixture of gases containing C2H2, CF4 and Si(CH3)4 or tetramethylsilane(TMS) in a vacuum chamber, 2 Pa, 5 kV. | G DLC, without F or Si, G F-DLC, G Si-DLC | No treatment | - | Friction tests: dry air and ambient air at 20 °C. Modified Dulbecco method. Measurement of torque insertion. | Stability and healing | 0%RH and 40%RH, F-DLC influenced the friction coefficients and Si-DLC significantly influenced the morphology of the carbon films (less friction the higher Si), these being less cytotoxic than in the other groups. Lower insertion torques for groups F and Si. | These treatments can be considered to improve the performance of orthodontic Ms. |
| Kim, H.Y. et al., 2016 [52] | - | OSSH1606 (Osstem Implant, Seul, Republic of Korea), 1.6 mm × 6 mm Ti6Al4V, n = 150 | G1: hydrochloric and nitric AE; G2: RBM, calcium phosphate beam and acid wash—removed from in vivo experiment due to fractures at placement; G3: hybrid: 75 µm calcium phosphate beam, except for cutting one-third and acid washing. | G1, G2 and G3 | G0: no treatment | 1, 2, 4 and 8 weeks | Insertion and removal torque tests; optical microscopy; SEM: surface changes; EDS: quantitative analysis of surface composition. | Stability | Cutting capacity: more superficial insertion in the RBM group than other groups (p < 0.05). Osseointegration: at 4 weeks, the removal torque in the control group decreased significantly, but was increased in G1 (p < 0.05 in both); G4 with increased values at 2 weeks, and with higher values compared to the other Gs at 8 weeks (p < 0.05). There was infiltration of calcium and phosphorus on the surface; bone was detected in the G4 group. | Partial/hybrid RBM group with greater stability compared to the other groups, without a reduction in cutting capacity. |
| Ly, N. et al., 2019 [60] | MC3T3-E1 cells of mouse | Ti6Al4V AbsoAnchor (DENTOS Inc., Daegu, Republic of Korea) + TI disks (Ti-6Al-4V) | Chitosan covalent bond; aminofunctionalization (APTES—PM, 221.37 g/mol) in deionized water, 4 h + cleaning with 70% ethanol and drying + grafting with spacer: succinic acid (SA—PM, 118.09 g/mol) or polyacrylic acid (AA—PM, 150,000 g/mol), 6 h + drying + binding of 0.5% chitosan (low molecular weight, 50,000 g/mol, ADI, 75%) with 2% acetic acid, 8 h + saline solution wash. | Treatment Covalent bonding of chitosan (succinic acid spacer or polyacrylic acid) | No treatment | 24 and 72 h | FE-SEM: cell adhesion test; ELISA: cell proliferation test; CLSM: feasibility test; absorbance test at 595 nm: biofilm formation. | Stability and cellular response | Lower contact angle when using succinic acid. Greater contact with samples with chitosan and succinic acid. FE-SEM: at 72 h greater cell adhesion in the SA experimental group than in the control; at 24 h, no significant difference; at 72 h, significant greater proliferation with the use of chitosan (p < 0.05). CLSM: at 24 h similar viability between control and SA-CH; at 72 h, experimental group has no dead cells and an increase in live cells. Antibacterial activity: effective reduction of the biofilm created in both microbial samples through the action of chitosan (and SA) (p < 0.05). | The use of succinic acid spacer was more advantageous than polyacrylic acid. Surfaces modified by chitosan, with SA spacer, presented hydrophilic nanostructures, which promoted cell adhesion, proliferation and cell viability, as well as reduction of biofilms by S. mutans and S. sobrinus by 53% and 31%, respectively. It is suggested that treatment with chitosan may promote the stability and antibacterial properties. |
| Oga, Y. et al., 2019 [61] | - | Dual-Top (Jeil Medical, Republic of Korea), 6 mm × 1.6 mm + auxiliary built-in device Ti6Al4V, ASTM F136-96, PCT + Durometer silicone rings; n = 42 | n = 22 Ti auxiliary built-in device Ti6Al4V with two portions: capture of compressive forces + three peaks inserted into the bone cortex. | n = 11 4 s; n = 11 8 s; placement with assistive device | n = 9 4 s; n = 11 8 s; No treatment | 4 and 8 weeks | Lateral displacement test with compression test machine: mechanical retention analysis. | Stability | Cortical thickness: at 4 s, auxiliary group (GA) 1.33 m and control 1.41 mm; at 8 s, GA 1.41 mm and control 1.44 mm (both follow-ups p > 0.05). Peak insertion depth: GA 0.28 mm 4 s and 0.37 mm 8 s. Lateral displacement: GA had significant effectsts on lateral displacement (p < 0.01), not taking time into account. Retention force: GA in 4 s and 8 s greater than controls in all displacements. | The automatic anchoring auxiliary device coupled to the Ms increased its stability, on average, by 1.6 to 2.8×. It may be possible to allow the use of Ms smaller in length and diameter, fundamental characteristics in substrates that are more difficult to insert. |
| Pavlic, A. et al., 2019 [62] | Lactobacillus us reuteri | 316 Stainless steel (SS) Ti grade V OrthoEasy (FORESTADENT, Germany); Ti grade 23 (Unitec 3M, Monrovia, CA, USA), n = 12 × 5 = 60 | Immersion in: artificial saliva (AS) 1.5 g/L KCl, 1.5 g/L NaHCO3, 0.5 g/L NaH2PO4 × H2O, 0.5 g/L KSCN, 0.9 g/L lactic acid, pH 4.8; probiotic bacteria Lactobacillus reuteridiluted in AS ph4.8 1:1 in 30 mL; Oral antiseptic chlorhexidine-digloconate 0.05% with 0.05% sodium fluoride with AS pH 4.8 of 1:1. | G1: artificial saliva; G2: Probiotic; G3: Chlorhexidine. For each type of microimplant five times | No treatment | 28 days | AFM: roughness of surface; Vickers method: microhardness analysis. SEM: qualitative analysis of topography. | Stability | Roughness: lower in untreated SS than in both Ti Ms (p < 0.001); lower in Ti grade 5 than grade 23 for average roughness parameter (p < 0.002); Ti grade 23 with greater roughness, except in chlorhexidine; Ti grade 5 with greater roughness in probiotics than in other media. SS group loses chlorhexidine treatment; greater roughness in Ti grade 23 in saliva; marked corrosion in the Ti grade 5 probiotic group; less corrosion in group Ti grade 23 than in others. Hardness: equal between Ti groups, and lower in SS groups (p < 0.001), AS and probiotics provide similar hardness in all groups. | Grade 5 titanium Ms showed an increase in surface roughness in the presence of probiotics. SS show increase when treated with chlorhexidine. SA increased roughness only in grade 23 Ti. Guidance on the use of chlorhexidine in patients with titanium Ms and probiotics in SS. |
| Alavi, S. et al., 2020 [63] | - | Jeil (Jeil Medical, Republic of Korea); Hubit; 8 mm × 1.6 m, n = 72 = 6 × 12 | G1: steam sterilization (MELAG autoclave, Euroklab) at 121 °C, 15 psi, 15 min; G2: sterilization by dry heat at 161 °C, 2 h. | n = 4 × 12 G1: steam sterilization with autoclave; G2: dry heat sterilization | n = 12 × 2; G = 0 without treatment | - | Insertion and fracture torque tests. | Stability | Jeil: differences in insertion torques between G2 and G0 (p < 0.001); differences between groups in fracture torque (p < 0.001). Hubit: no differences. There were significant differences between the torque values between the two manufacturers. | Steam sterilization did not have any adverse effect on the stability of Ms. Dry heat sterilization interfered with its mechanical properties. |
| Giri, M. et al., 2020 [64] | Bull femur | Ti (DENTOS Inc., Daegu, Republic of Korea); 8 mm/10 mm × 1.3 mm conical or cylindrical; n = 24 | n = 20 Radio frequency current, Vacuum sputtering unit model BC-300, Yttrium stabilized Zirconia deposition. | n = 20 = 4 × 5 Zirconia in four groups | n = 4; You without treatment | ITVs tests; SEM: surface analysis; XRD: structure of the layer of zirconia. | Stability | Maximum ITV: no significant differences between experimental and control values; values in all groups quite similar. | Insertion changes increased cylindrical Ms, as well as those with greater length. Ms treated with coatingzirconia maintained its structural integrity. | |
| Iwanami-Kadowaki, K. et al., 2021 [65] | Cells Osteoblast -likeMG-63 | Grade II titanium plates (Kobe Steel, Ltd., Kobe, Japan), 20 × 50 × 0.5 mm3, n = 24 | 1 g modified Hap/Col powder + 100 mL 2-propanol suspension, 2 mL glycerol, 1–50 mg hydrated magnesium nitrate, 2 mL H2O + dispersion ultrasonic 10 min + voltage 20–60 V/cm 2–6 min applied to Ti plates (cathode) covered with stainless steel plates (anode) + cleaning with 2-propanol + drying. | Plates with hydroxyapatite and collagen treatment at different follow-ups | Untreated Ti board | 1, 3, 5 and 7 days | Zeta potential and sedimentation analysis; SEM and laser microscopy; EDS; Tape test: adhesive strength of the coating. | Stability | Successful creation of surface treatment, and the thickness increases with treatment time and voltage and presence of cracks and fractures. Two min at 20 V seemed to be the protocol with the smoothest and flattest thickness. The existence of cracks did not influence the adhesion of the HAp/Col layer to the titanium. Cell viability was identical between the control and experimental groups (p < 0.01). | It was demonstrated that hydroxyapatite and collagen surface treatment could be achieved with controlled thickness and high adhesion strength on titanium surfaces. It also showed excellent biological properties. |
| Zogheib, T. et al., 2021 [66] | Rib of cow | Dual Top® (Jeil Medical, Republic of Korea), Spider Screw® (HDC Company, Sarcedo, Italy), Absoanchor ® (DENTOS Inc., Daegu, Republic of Korea),Microdent® (Barcelona, Espanha) 6–8 mm × 1.4–1.6 mm, n = 96 | G1: (n = 12) OA with 0.1 mM/L H2SO4 at 5V for 20 min G2: (n = 12) Sandblasting (SB) by alumina projection 50-µm, 20 psi, 1 min, 1 cm distance, 45° angle + ultrasonic cleaning 5 min in acetone +drying; G3: (n = 12) Sandblasting G1 and AO (SBAO) with 0.1 mM/L H2SO4 at 5V for 20 min. | G1: AO (n = 8); G2: (n = 12) Sandblasting (SB); G3: (n = 12) Sandblasting and oxidative anodizing | SEM of n = 8 for surface analysis and G0: n = 4 untreated (one each brand) | - | SEM and EDX: surface and chemical composition; optical profilometer: surface roughness; Sessile drop method: contact angle static. | Stability | Control with irregular surface contaminants originating from materials inherent to production. Handling also showed other contaminantsSR. Higher surface roughness in the SB group, and higher contact angle in the SBAO group. Evidence of bone contamination with particles from the surface of microimplants is observed, which increases with preparation for clinical procedures. | Surface treatments increased surface roughness and the bone/Ms contact angle, which could promote osseointegration. The insertion and removal of these devices leaves contaminating particles in the bone. The use of gauze with 0.12% chlorhexidine is recommended when handling Ms. Required more studies. |
| Im, C. et al., 2022 [30] | MC3T3 pre-osteoblastic cells mouse | Ti–6Al–4V ELI Boards (Kobe Steel Ltd., Kobe, Japan) 20 mm × 10 mm × 1 mm; n = 18 | AO (HNO3 + HF + H2O) 10 s + H2O+ 1.4 wt% NH4F) 20 V, 60 min + P: 0.5 vol% silicate, 5 min + drying 1 h + 20 × (0.05 M NaH2PO4 and Ca(OH)2, 90 °C, 1 min interval). Heat: electric furnace at 500 °C, increase 10 °C/min, 2 h. | AH: anodizing and heat treatment; APH: anodizing, pre-calcification and heat | UT: no treatment | 2, 3 and 4 days | SBF tests: analysis of bioactivity by EDS; WST-8 test: cytotoxicity assessment. | Stability | Surface: AH—dense and aligned formation of nanotubes, with protrusions; APH—presence of Ca granular precipitate Ca3(PO4)2. Bioactivity: presence of Ca in the AH and APH groups, absence in the UT; FE-SEM: removal causes fractures not only at the interface (UT), but also in places of attached bone tissue, mainly in APH. | The surface created with APH obtained well-aligned nanotubes with a dense structure. Precipitates of calcium phosphate and hydroxyapatite were obtained in clusters. Compared to the control, the experimental groups showed significantly higher removal torque. |
| Li, M. et al., 2022 [67] | - | Mini-pin (original SLA) 5.0 mm × 1.1 mm, n = 144 | Immersion in simulated body fluid (SBF) 24 h, 37 °C for BioCaP formation + immersion in SBF 5 × 24 h, 37 °C for deposition of amorphous layer + 20 mL calcium phosphate solution (BSA introduction for SEM reading). | G2—no treatment, bath in PBS + BSA; G3—amorphous treatment; G4—amorphous treatment + BSA; G5—crystalline treatment; G6—crystalline treatment + BSA | G1: no treatment or BSA | 3 days, 1, 2 and 4 weeks post-surgery | SEM: analysis of surfaces with and without BSA; FTIR; CLSM; spectrometry; alkaline phosphatase activity and %BIC. | Stability | The thickness of crystalline layers is seven times greater than that of amorphous ones. Treatment by crystalline BioCaP allowed pharmacological transport. There was an increase in bone/Ms contact in the 1st week in G4, but in other groups this increase occurred at 2 and 4 weeks. | The pharmacological transport capacity was 10 times greater in the BioCaP treatment than in the amorphous ones. The contact between bone and Ms increased after the 1st week in the crystalline group, unlike the others, suggesting that this treatment is a technique that can increase stability and increase the success rate of Ms. |
| Baser, B. et al., 2023 [34] | Polyurethane foam—artificial bone block | Cylindric (BioMaterials Korea, Seoul, Republic of Korea), 8 mm × 1.5 mm, n = 96 (self-tapping and self-drilling) | n = 46 retrieved from patients in previous study + sterilization n = 46 as received from manufacturer All inserted into artificial bone. | n = 46 reused n = 23 self-tapping, n = 23 self-drilling | n = 46 New, n = 23 self-tapping, n = 23 self-drilling | Periotest (mobility test). Torque value tests: maximum insertion. Pull out strength tests. | Primary stability | MITV: used 17.3 Ncm; new 18.9 Ncm; higher values for new self-drilling then for the used. The type of insertion had statistically significant differences. Periotest: only differences in the self-tapping group, p = 0.001. Pull-out strength test: 35 of the 46 self-tapping detached from the head, and self-drilling only had plastic deformation. | Used orthodontic MIs showed poor performance compared to unused implants inserted under in vitro conditions. | |
| Byeon, S. et al., 2023 [31] | - | Ti-6Al-4V ELI alloy rods (Fort Wayne Metals Research Products, Fort Wayne, IN, USA) | APH: (Nitric acid (HNO3) + HF: H2O in the ratio of 12:7:81 for 10 s)+ ultrasonic cleaning in distilled water for 5 min + dryer at 50 °C for 24 h. After, TNT layer was formed: DC eletrostatic device (Inverter tech) 1 h, 20 V, 20 mA/cm2 in eletrolyte solution +ultrasonic cleaning + heat treatment. SBF group: immersion in 10 mL of SBF for 5 days and 10 days, at 37 °C. Ibandronate groups: immersion for 10 min or 60 min in ibandronate ar −0.05 MpPa e and lyophilized with N2 gas + cleaning H2O + repeat for 7 days. | Two groups: AN—immersion in SBF; IB—immersion in ibandronate | UN: untreated, machined | 5, 7 and 10 days | FE-SEM | Stability and bioactivity | The nanotubes produced by electrochemically anodizing were fully self-aligned and had a dense structure. Each of the nanotubes had a hollow and independent tube structure with the outer walls attached to adjacent tubes. In the method of continuous immersion of ibandronate for 60 min, the amount of release decreased rapidly. The method of treating six times of 10 min-immersion showed stable release for 7 days. | TNT was formed on the surface of the Ti6Al-4V orthodontic Ms by anodization, and then ibandronate was loaded six times for 10 min to ensure continuous release. It was concluded that this method must be surface treatment to enhance the bioactivity and osseointegration. However, in clinical application, it is necessary to study the biostability and biofunctionality on the surface of Ms according to the concentration of the drug using the sustained release method of the drug, which is the present study. |
| Gezer, P. et al., 2023 [32] | Polyurethane foam—artificial bone block | TiAl6V4-ELI grade 23 (BioInfinity Orthodontic Screw, Istanbul, Turkiye) (Avrupa Implant, Istanbul, Turkiye) 8 mm × 1.6 mm, n = 36 | MAO: Ms as anode and stainless steel plate as the cathode in an aqueous electrolytic bath with phosphoric acid, for 5 min and at 250 V. RBM: calcium phosphate and hydroxyapatite mixture + acid rinse. | n = 12, MAO group: aqueous eletrolytic bath; n = 12, RBM group. | n = 12, no treatment, machined | 0 days, 1, 2, 4, 8 and 12 weeks | Torque tests: MIT and MRT. | Stability | Significant differences in MIT were observed between all groups. MRT: only the difference between MS group and RBM-treated group was significant; highest value in the RBM-treated group, lowest MS. A positive significant correlation was found between MIT and MRT in all groups. | RBM-treated group was significantly higher than the Ms group in MIT and MRT values. RBM-treated group was found to be significantly more stable than the MAO-treated group at weeks 8 and 12. |
| Li, M. et al., 2023 [33] | - | SLA treated Ti plates and smooth SSL plates (diameter: 5 mm, thickness: 1 mm) | Biocap coating: Ti discs were immersed in a biomimetic modified Tyrode (BMT) solution—NaCl: 55.15 mM; CaCl2·2H2O: 0.94 mM; MgCl2·2H2O: 0.56 mM; NaHCO3: 1.58 mM; Na2HPO4·2H2O: 0.38 mM (4.6 mL BMT per plate) at 37 °C for 24 h. SSL discs immersed for 0, 12, 24, 36 and 48 h, respectively. All were sterilized and immersed in a supersaturated calcium phosphate solution (CPS), namely, NaCl: 10.23 mM; CaCl2·2H2O: 0.30 mM; Na2HPO4·2H2O: 0.15 mM), buffered with TRIS (3.76 mM; pH 7.4) at 37 °C for 48 h to form a crystalline layer, composed of octacalcium phosphate (OCP). | (2) Ti 24 h BMT CPS group, (4) SSL 0 h SBF BMT group, (5) SSL 12 h BMT CPS group, (6) SSL 24 h BMT CPS group, (7) SSL 36 h BMT CPS group, and (8) SSL 48 h BMT CPS group. | (1) Ti group, (3) SSL group | 0, 12, 24 and 48 h | SEM, EDS, surface roughness meter, ELISA, contact angle detection for wettability. | Stability: surface roughness and wettability + surface properties | The morphology, chemical composition and drug loading capacity of the BioCaP coating on smooth SSL were confirmed. This coating improved roughness and wettability of SSL surface. In vitro, with the extension of BMT coating period, the cell seeding efficiency, cell spreading area and cell proliferation on the BioCaP coating were increased. | The BioCaP coating can be applied on the medical grade SSL surface and serve as a carrier system for bioactive agents. The surface properties of medical grade SSL surface such as biocompatibility, osteoconductivity and osteoinductivity by incorporation of osteoinductive agents can be improved by the BioCaP coating. |
| Author, Year | Species, Sex and Age | n | MI Type and Number | Surface Treatment (Type, Time, Dose and Protocol) | Experimental Group | Control Group | Follow -Up | Test Used to Evaluate Outcomes | Primary Outcome | Secondary Outcomes | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hassan, A. et al., 2003 [68] | New Zealand rabbit, M, mature | 9 | Onplant System (Nobel Biocare, Kloten, Suwiter), n = 54 | 100 µL of rhBMP-2 and 100 µL DMP-1 overnight + 100 µL neutral solution, 5 days | rBMP-2; rDMP-1; B + D | Without PBS immersion treatment | 6 s | Description biomechanics: strength assessment. Histological analysis | Stability | Surface properties | Group BMP2 with greater bone formation. Significant bone formation at the O/M interface in groups BMP-2 and B + D; mechanical characterization: BMP-2 with resistance to forces of 3.4–5 kg; B + D 3 kg; DMP-1 and control 0–1.3 kg. | Recommended treatments show potential to improve the osseointegration of implants in immediate orthodontic loading protocols. |
| Aoki, T, et al., 2005 [72] | Beagle dog, M, 8–10 months | 8 | PLLA (Fixsorb-MX, China), 8.00 mm × 2.9 mm, n = 16 | Poly-L-lactic acid in the constitution and on the surface (PLLA), molecular weight 200,000: bio absorbable material | n = 8, PLLA with load 100 gf and without load, 3 months and 6 months | n = 8, PLLA no charge (0 months) | 3 and 6 months | SEM. Tensile strength tests. Histological analysis. Analysis from BA and BIC. Molecular weight measurement | Stability | Surface properties. Tooth movement. Bioactivity | BA was higher in the 3-month group than in the 0-month group (p < 0.01); higher BIC in the 3 months than in the control (p < 0.001); BA and BIC with no differences between 3 and 6 months. Fracture strength: increased over time, with and without load; greater at 3 months (p < 0.001) and at 6 months (p < 0.01) than the control; no differences with or without load. Molecular weight: significant decrease at 3 months and at 6 months (p < 0.001 in both). | It is suggested that bioabsorbable implants have good biocompatibility and fracture resistance, being a protocol capable of clinical use in orthodontic treatments. |
| Kim, SH. et al., 2008 [73] | 9 M/28 F; 24.53 ± 7.61 years | 37 | Ti8.5 mm × 1.8 mm (C-implant, Seoul, Republic of Korea), n = 64 | Source SLA | G1: delayed load (0–3, 3–6, 6+ months) | G2: Immediate load | 0–22 months | Digital measurement of removal torque | Stability | Average RTV of 16.4 N/cm. No influence of age, malocclusion and load. From 6 months: RTVs significantly higher, 23.7 N (unloaded) vs. 15.1 N (0–3 months) vs. 20.7 N (3–6 month). | The longer treatment time, the greater the RTV. Treatment offers stability and resistance, without difficulty in removal. | |
| Kim, TW. et al., 2008 [74] | Beagle dog, M and F, 20 and 14 months | Two (one M and one F) | Jeil (Jeil Medical, Republic of Korea), 6.0 mm × 1.6 m; n = 2 × 40 = 80 | n = 40 10 µm microgrooves on the external surface | n = 40 (2 in buccal, 8 in palatal/lingual in the right maxilla and mandible) | n = 40; no treatment | 16 s | Success rate analysis; BIC and BA analysis | Stability | Bone area of pressure and tension | BIC: no differences between groups, except for the pressure site, which was greater in the group with microgrooves (p < 0.01); higher pressure sites in the control and lower than tension sites (p < 0.01), with no differences in the experimental group. Histology: samples with osteointegration present: Haversian systems, lamellar and new bone. | The Ms with microgrooves had a perpendicular arrangement of the connective fibers, while the control had parallel ones. Microgrooves can have effects on the way the connective gingival tissue is positioned, which can positively affect bone adaptation. |
| Chang, CS. et al., 2009 [75] | New Zealand White rabbit, adult | 24 | AbsoAnch (DENTOS Inc., Daegu, Republic of Korea), 1.3 mm diameter, n = 144 | SLA: Al2O3 particles, 355–425 µm, 4 kg/cm3+ AE HCl/H2SO4 70%, 80 °C, 30 min. SL/NaOH: SL+ immersion 5 M NaOH 60 °C, 24 h + cleaning + drying + heating at 600 °C in a furnace | n = 48, SLA; n = 48 SL/NaOH | n = 48, no treatment | 2, 4, 8 and 12 s | Digital measurement of removal torque. FE-SEM: %BIC | Stability | Surface properties | RTVs: higher in SLA and SL/NaOH. SEM: SLA with microscopic roughness at two levels. SL/NaOH with macroscopic roughness. RTVs: SLA increased significantly after 4 weeks; SL/NaOH increased after 8 weeks and at 12 weeks there were different (higher) SLA values (p < 0.05); after 12 weeks, %BIC was higher in the experimental groups than in the control group. There is a correlation between the %BIC and RTVs. | SLA and SL/NaOH treatments can increase removal torque under orthodontic loading. SLA allowed faster improvements in RTVs at 4 weeks, while SL/NaOH achieved increases only after 8 and 12 weeks. The expression of %BIC was different than that of RTVs, with a correlation between the two. |
| Kim, SH. et al., 2009 [13] | Dog, M, 7–11 months | 12 | 8.5 mm × 1.8 mm (C-implant, Seoul, Republic of Korea), n = 96 | SLA | n = 48, SLA | n = 48, Without treatment | 3 and 8 s | Success rate. Measurement of torque values: analysis of total absorbed energy (TIEV and TREV) | Stability | - | Success rate 50% (33 lost): Control 52.1% and SLA 47.9%. MITV and MA of the SLA group were significantly lower than control (p = 0.034 and p = 0.039). TREV significantly higher in SLA than control (p = 0.046). No differences between SLA and control in TIEV, MRTV and RAMV. No differences between clockwise or counterclockwise rotation. | Ms treated by SLA have lower values of torque and angular momentum at insertion, but higher values of total energy absorbed than the control. The osseointegration created by the treatment, and influenced by the location and rotation, is sufficient to resist orthodontic forces. |
| Niwa, K.et al., 2009 [76] | White Japanese rabbit | 21 | Central hook in Ti that connect Ms to a 5 mm disc in pure perforated Ti; n = 6 × 21= 126 | 1. Plasma spray method—flame heat β-TCP powder, depositing α-TCP on the surface of the disc; 2. Hydrothermal processing of α-TCP | n = 42 T1: α-TCP; n = 42 T2: HA | n = 42, no treatment | 1, 2 and 3 s | Radiographic evaluation: Ms position and bone characteristics; tensile strength test. Histological analysis. | Stability time | Deposition of compounds. Surface morphology. Healing | Bone contact: notable radiopacity in α-TCP and HA (2 weeks). Bond strength: extensive bone formation in all groups: the α-TCP group showed a larger area than the AH group (p < 0.01) and both larger than control (p < 0.01). Average elastic strength in Mpa: 1 week—C 0.006, T1 0.003, T2 0.019; 2 weeks: C 0.365, T1 1.004, T2 1.063; 3 weeks: C 2.849, T1 3.686, T2 3.161. | Two weeks after placement: formation of new osteoid tissue around Ms treated with α-TCP and HA. At 2 weeks, greater bone volume was formed in the disc perforations of the α-TCP group than in the HA. |
| Mo, S.S. et al., 2010 [77] | White rabbit | 42 | 9.5 mm × 1.8 mm (C-implant, Seoul, Republic of Korea), n = 412 | SLA | N = 180, SLA | n = 160, no treatment | 2 days, 1, 6 and 10 s | Digital measurement of removal torques. Measuring success rate | Stability | - | Failure of 13 Ms. Success rate: 96.8%. RTVs: higher values for the SAE group (p < 0.0001); significant difference between load periods (p < 0.001); no differences between loaded or unloaded groups. | SLA treatment for Ms, subject to different loading protocols, allows similar success rates between them. RTVs were higher for SLA treated group than machined control during the first 6 weeks of healing. |
| Cho, I.C. et al., 2012 [78] | Beagle dog, M, 1 year old | 6 | Ti-6AL4V (BioMaterials Korea, Seoul, Republic of Korea), 8 mm × 1.45 mm, n = 54 = 18+ 36 | (n = 18) SLA: 100 µm aluminum particles, 2 min, 2 bar + 30% HCl, 60% H2SO4 and diluting solution. (n = 18) SLAO: SLA + oxidative anodization 3 min, 250 V | n = 6 + 12SLA; n = 6 + 12 SLAO | n = 6 + 12, no treatment | 8 s | Digital measurement of insertion and removal torque: analysis of maximum torque, total energy and peak energy | Stability | - | Three highest insertion values in control (p < 0.01); removal: no differences in VTE and NPE between groups, with SLAO having higher MRTV than SLA and control (p < 0.001). | SLAO treatment can be an effective way to reduce the damage due to the insertion of Ms, both in the tissue and in the device, and can also improve its stability. |
| Choi, S-H. et al., 2012 [79] | Beagle dog, 1 year old | 2 | 7 mm × 1.5 mm (BioMaterials Korea, Seoul, Republic of Korea), n = 8 | AO + immediate load | AO | No treatment | 12 s | Measurement of initial torque and mobility. SEM and AFM: surface and roughness analysis | Stability | Surface properties | No differences in torque and initial mobility between groups. SEM: used AO group tip turns become smooth. AFM: roughness of the turns of the AO group used significantly lower than in its initial state (p < 0.05); turns of group AO used rougher than those of group C used and not used (p < 0.05). | Ms subjected to AO obtained better surface characteristics than machined surfaces, despite differences in texture at insertion and in the initial loading period. |
| Karmarker, S. et al., 2012 [80] | White rabbit, 10 months | 3 | Absoanch (DENTOS Inc., Daegu, Republic of Korea), 6 mm × 1.3 mm, n = 36 | n = 18, AO | n = 18, anodized surface | n = 18, no treatment | 6 s | Digital tests, maximum insertion and removal torque | Stability | - | ITVs: no differences between groups. RTVs: higher in the anodized group than in the control (3.79 ± 1.39 Ncm vs. 2.05 ± 1.07 Ncm) (p < 0.01). The interface forces calculated from the RTV were 10.6 MPa and 5.74 MPa for the experimental and control groups, respectively. | Anodizing of Ms can enhance initial stability, through greater retention capacity, enhancing absolute anchorage. |
| Omasa, S. et al., 2012 [81] | Sprague-Dawley rat, M, 6 weeks; Fischer mouse, M, 6 weeks | 30; 9 | Ti (MogiShokai CO, Japan), 7.3 mm × 1.4 mm (Keisei Medical Industrial CO, Tokyo, Japan) 3.5 mm × 1.5 mm; n = 78 | LLLT (Ga-Al-Ar) at 830 nm, 200 mW, 195 J/cm2,135 s × 2 points, 54 J/session, 1×/day, 7 days | GLLLT, n = 39; LLLT, right tibia | n = 39, no treatment, left tibia | 7 and 35 days | Micro-CT: levels of new bone formation; PCR: BMP-2 expression analysis | Stability and osteogenesis | - | Osteogenesis: new bone formation observed at 5 days in both groups, but stronger in LLLT; BV significantly higher in the LLLT group than in the control on day 7 (1.53× higher); BMP-2 expression: 1.92× higher in LLLT than in the control on the 1st day, but similar in the rest. | LLLT therapy improved the stability of Ms and accelerated bone formation around them by increasing BMP-2 gene expression. |
| Uysal, T. et al., 2012 [82] | New Zealand White rabbit, M, adults | 15 | Ti Dual-Top 8 (Jeil Medical, Republic of Korea) mm × 1.4 mm, n = 60 | LED: Osseo Pulse LED (Biolux Research), 618 nm, 20 mW/cm2, 20 min, for 10 days | n = 10 × 3 LED (G1 0 cN, G3 150 cN and G5 300 cN) | n = 10 × 3 No treatment (G2 0 cN, G4 150 cN and G6 300 cN) | 1 and 21 days | RFA (OsstellMentor): stability assessment | Stability | - | Similar initial stability among all groups. Significant differences in the ISQ quotient between the experimental group and the control; significant increase between groups of forces in the LED group; in control, the greater the force, the lower the ISQ. | Photobiomodulation therapy with LED light achieved greater stability than control over the 21 days, and may have a favorable effect on healing and insertion of orthodontic Ms. |
| Cho, Y.C. et al., 2013 [83] | Beagle dog | 4 | Model 1016106 (Orlus™, Ortholution, Seoul, Republic of Korea) Original SLA 1.45 mm × 6 mm, n = 32 | Laser beam with plasma ion penetration at low temperatures | n = 16, treatment plasma ion implantation; source SLA | n = 16, without treatment; origin SLA | 0–12 s | Insertion torque sensor; periotest: mobility; histological analysis | Stability | Mobility, bone parameters, success rate | SR: 100% for control; 93.75% experimental group. Insertion torque: slightly higher experimental (p = 0.61). Contact: control 64.2% and experimental 72.1%, 3 weeks; 12 weeks, control 66.2% and experimental 63.4%. (p = 0.11 and p = 0.65). | Ion-modified Ms have similar biological characteristics to the control in terms of the outcomes evaluated. |
| Pinto, M. et al., 2013 [84] | New Zealand rabbit, M, 4 months | 16 | Ti6Al4V: INP and TF 9 mm × 1.5 mm, n = 32 × 2 | LLLT: 21 days, 2 days apart, 10 sessions, external and internal point of the right tibia, 90 J/cm2, 25 s, 2.5 J. | n = 32, LLLT in both brands, right tibia | n = 32, without treatment of both brands, left tibia | 21 and 36 days | Mechanical removal test: measurement of load, maximum force and displacement values | Stability | - | Control: (vs. TF) 108.58 ± 17.92 to (vs. IND) 124.63 ± 13.43 N/cm². LLLT: 137.37 ± 11.78 (IND) to 177.39 ± 24.9 (TF) N/cm². Greater removal force for treated groups. Significant difference between TF control and INP control (p < 0.05). Values increased in groups with LLLT, especially in the TF group. | Low-intensity laser therapy was able to increase the stability of Ms. All types of Ms observed are effective for clinical use, with or without LLLT. |
| Cuairán, C. et al., 2014 [85] | Dog without breed, M, 1–2 years | 3 | Ti6Al4V(Neodent, Curitiba, Brazil), 5 mm × 1.6 mm, n = 60 | Treatment with Zoledronate: 16 µg in 50 µL of phosphate saline solution at the site of the burr cavity, before insertion | GZ: n = 30, Zoledronate Injection | n = 30, no treatment (injection of 50 µL of saline solution) | 0–8 s | RFA (Osstell Mentor): stability assessment; micro-CT: BIC 3D | Stability longitudinal | Scarring around the MI | Stability: significantly less stable controls (p < 0.05). BIC: superficial bone layers with less bone in both groups than the two deeper layers (p < 0.05); at 8 weeks, greater amount of cortical bone around the control and greater amount of bone trabecular in GZ. | A single, localized dose of Zoledronate was able to prevent significant loss of stability over time, with control demonstrating significant losses at 4 weeks and an increase in the 6th, losing again until the 8th week. |
| Miura, K.et al., 2014 [86] | Sprague-Dawley rat, M, 6 months | 7 | Ti 1.4 mm × 4 μm; n = 14 | LIPUS, 15 min/day, 2 weeks; I:30 mW/cm2, F:3.0 MHz at 20% cycle; | LIPUS + right tibia implant | No treatment + implant left tibia | 2 s | FE-SEM: O/M contact ratio | Stability | Mobility (periotest) | Experimental group: good O/M contact; O/M Ratio = 72.9 ± 10.2%. Control: gaps in the O/M interface; O/M Ratio = 52.3 ± 9.0% (p < 0.05) | LIPUS increased bone/microimplant contact and reduced microimplant mobility in growing rats. |
| Oh, EJ. et al., 2014 [44] | Wistar rat, M, 7 weeks | 16 | Ti6Al4V (Jeil Medical, Republic of Korea), 4 mm × 1.4 mm; n = 32 | n = 16 APH treatment: Glycerol/H2O/NH4F solution, 20 V, 1 h + cyclic calcification by immersion in NaH2PO4 (0.05 M), 80 °C and Ca(OH)2 (100 °C) 1 min/cycle for 30 cycles + heat treatment 500 °C, 2 h | (n = 16)APH; AH:anodizing + heat | (n = 16) UT: no treatment | 3 days, 3 and 6 s | FE-SEM; roughness tests (Surftest SV-3000); microscopy: hydrophilicity; digital torque sensor; EDS/XRD; histological analysis: %BIC | Stability | Surface properties | The smaller the contact angle, the greater the roughness; higher in APH. Biomechanical stability/strength: higher RTVs in APH at 3 and 6 weeks (p < 0.05); RTVs: higher at 3 and 6 weeks (p < 0.05). Osseointegration: little bone formation at 3 s in UT, unlike APH (%BIC: UT8.25 ± 6.67% vs. 84.00 ± 8.47%, p< 0.05); at 6 weeks formation occurred better and faster in APH (p < 0.05): better stability. | APH treatment accelerated the formation of hydroxyapatite, improved bone formation and presented surfaces with bioactivity and biocompatibility, which will allow an improvement in the initial stability of Ms. Indication for poor quality bone, where rapid healing and osseointegration is required. |
| Oh, NH. et al., 2014 [87] | New Zealand White rabbit | 12 (n = 6 diabetic and n = 6 healthy) | 8.5 mm × 1.8 mm (C-implant, Seoul, Republic of Korea); n = 48 | SLA. Experimental group in diabetic rabbits induced for 4 weeks | Diabetic rabbits, treated with SLA and without treatment | Healthy rabbits, treated with SLA and without treatment | 4 and 8 s | Body mass measurement; blood glucose test; insertion and removal torque and energy tests; %BIC Analysis | Stability | Body mass and blood glucose levels | ITVs: no significant differences between groups and treatments, but higher total insertion energy in the control. RTVs: no differences between diabetics and non-diabetics, but with higher total removal energy in the diabetic group, without significant difference (p > 0.05); in the diabetic group, slightly higher removal torque and energy in the SLA group, but control without differences in treatments. %BIC: higher in control than in diabetic patients, with no statistical difference between the two. | The use of Ms in diabetic patients will have similar results as in healthy patients. Surface treatments in these conditions also seem to be applicable to diabetic patients, with results consistent with those obtained for healthy patients. |
| Yadav, S. et al., 2015 [50] | White New Zealand rabbit, M, 4–5 months | 8 | 6 mm × 1.6 mm n = 128 | G2 (n = 32): AE: 0.11 mol/L and HCl for 20 min; G3 (n = 32): gritblasting with alumina; G4 (n = 32): gritblasting+ AE 0.11 mol/L HCl 65 °C for 20 min | G2: AE (n = 32); G3: gritblasting (n = 32); G4: gritblasting+ AE (n = 32) | n = 32, G1: no treatment | 8 s | Profilometer Goniometer. Removal torque test; histological analysis: BIC | Biological integration | Stability | RTVs: no significant interaction between bone type and surface. Medium roughness: Ra—G4: 3.69; G3: 4.88; G2: 1.78; G1 1.13 p = 0.001. Rq—G4 4.87; G3 7.06; G2 3.27; G1 2.56 p = 0.001. O/M Contact: significantly greater in G4 than G3, G2 and G1 and with no differences between them. Average %BIC: Tibias—G4 66.34%; G3 53.07%; G2 50.64%; G1 39.30%; Femur—G4 68.94%; G3 49.10%; G2 48.30%; G1 45.28%. | Contact with liquids was greater for the control and lower for gritblasted with conditioning. The removal torque in both the tibia and femur was greater in the gritblasted with conditioning group. |
| El-Wassefy, N. et al., 2015 [88] | White New Zealand rabbit, M, 6 months | 4 | Absoanch (DENTOS Inc., Daegu, Republic of Korea) 7 mm × 1.4 mm, n = 40 | G1: autoclave 30 min at 121 °C and 18 psi; G2: gamma ray sterilization for one night at 25 kGy; G3: UV sterilization, 90 min 254 nm | n = 30; G1: autoclave; G2: gamma rays; G3: UV light | n = 10, unused, untreated | 30 days | SEM; absorption spectrometry; healing analysis; analysis bone histology | Property surface and mechanics (potential reuse) | Ionic release; stability | All Ms had stability, good mechanical fixation and no signs of inflammation or reaction. | Autoclave sterilization treatment showed better results in terms of cellular activity than the other treatments. |
| Ganzorig, K. et al., 2015 [47] | Sprague-Dawley rat, 6 weeks | 40 | Ti6Al4V (Nishimura Metal, Japan) and 1.6 mm × 1.5 mm SS, n = 140 | LIPUS: Osteotron_D IB, Ito Co; 1.5 MHz, 30 mW/cm2; 24 h after insertion, 20 min/day in the right tibia (1×/15 min in cells) | GLIPUS, n = 80, LIPUS, right tibia | n = 80, without treatment and left tibia | 0–14 days | SEM and micro-CT: Bone morphology, CBT (mm and %) and BIC | Stability and osteogenesis and | Morphology surface and cellular | From day 3 to day 14, BIC gradually increased in all groups. LIPUS increased BD density, CBT and bone formation rate after insertion (p < 0.05). Increase in GLIPUS of ALP regulation in vitro, on the 3rd day (p < 0.05). | It is suggested that the application of LIPUS improves bone formation around titanium alloy and stainless steel Ms and may therefore improve their initial stability and success rate throughout the treatment. |
| Goymen, M. et al., 2015 [89] | New Zealand White rabbit, M, 6 months | 17 | Ti6Al4V (Jeil Medical, Republic of Korea), 8 mm × 1.4 mm, n = 68 | n = 48 LLLT: GaAlAs diode laser (Cheese dental laser, Wuhan Gigaa Optronics Technology Co. Ltd.), 810 nm, 0.3 W, area 5.85 cm2; 195–390 s/point for 10 days. | G2 (n = 12):LLLT 10 J/cm2 without load; G3 (n = 12): LLLT 20 J/cm2 without load; G5 (n = 12): LLLT 10 J/cm2 with 150 g load; G6 (n = 12): LLLT 20 J/cm2 with load 150 g | G1 (n = 8): without LLT and without load; G4 (n = 12): without LLT and 150 g load | 10 days and 4 s | Digital measurement of initial torque %BIC and BT analysis | Stability | - | BIC: G6 with the highest value of 83.11 ± 1.75 (p < 0.05), followed by groups 5 and 3, group 1 with the lowest value of 36.15 ± 2.45; presence of differences between all groups. BT: significant differences between group 1 and each of the others (p < 0.05); group 1 with the lowest value and group 3 with the highest (1.93 ± 0.31 compared to 2.16 ± 0.16), followed by 4 and 2; no significant differences between groups. No significant correlation between BIC and BT values. | Using Ms as orthodontic anchorage is easy, effective and a reliable method. The BIC values of the groups with 20 J/cm2 (3 and 6) were higher than the others. LLLT can be a method that allows increasing the stability of M. There was no relationship between BIC and BT values. |
| Jang, I. et al. 2015 [90] | White New Zealand rabbit, F, 13–14 weeks | 4 | Dual Top 6 mm × 1.6 mm (Jeil Medical, Republic of Korea), n = 8 | n = 4 AO in 2 steps: Electrolyte bath. Drugs inside nanotubes. | n = 4 G2:TiO2 nanotube array | n = 4 G1 without treatment | 8 s | FE-SEM: morphological characteristics of Micro-CT microimplants: %BIC and %BV/TV | Stability | Tissue and bone volume | Experimental groups with higher average Ms/bone contact than control (52.8 ± 18.1% vs. 29.3 ± 15.6%, p = 0.016). Tissue and bone volumes were also higher in the experimental group (BV/TV%): AO 81.2 ± 4.2% and C 73.3 ± 16.4%, p = 0.386. | Nanotubes promoted a rough surface and greater contact with bone, allowing osseointegration. Physical properties were maintained after use in rabbits. |
| Liang, Y. et al., 2015 [48] | Sprague-Dawley rat, F, 3 months | 36 (excised number of ovaries n = 24) | 6.6 mm × 1.5 mm, n = 72 | Application of strontium (Sr): polishing + HCl + CaCl2 + ultrasonic cleaning in acetone, 10 min + H2O + drying + ECD (electrochemical deposition with platinum mesh) | n = 12 G1: Without ECD, ovariectomy; n = 12 G2: ECD with Sr, ovariectomy | n = 12, no treatment + without ovariectomy | 2 and 4 s | Histological analysis. Confocal microscopy: determination of DDL, MAR and MS/BS. Measurement maximum removal torque | Stability | Success rate. Surface properties | Histological analysis: G1—less bone formation and poor continuity; G2 new bone formation and increased lamellar layer thickness and discontinuity. %BIC, BV%TV and BT significantly higher in G2. RTVs: G2: 27.94 ± 1.43, control 22.04 ± 2.11 and G1 25.30 ± 1.38 N.cm, with significant differences between experimental and control (p < 0.001) and between experimental (p < 0.01). | Strontium treatment is easily performed by ECD. This has a promoting effect on the osteointegration of Ms in animals with osteopenia and may be a new treatment protocol for patients with osteoporosis. |
| Miyawaki, S. et al., 2015 [45] | White New Zealand rabbit, 1 M, 1 F, 14 weeks | 2 | Ti 6 mm × 1.6 mm, n = 4 | SpikeAnchor, Ti6Al4V, two portions: portion that receives forces and portion with spikes that contact the cortical bone | Implantation in the femur with SpikeAnchor | No Spike | 4 s | Lateral displacement test: mechanical retention forces; visual analysis of device insertion | Stability | Depth implementation | Retention force was significantly greater in the experimental group at different displacements (p < 0.05). The smaller the displacement, the greater differences in the forces applied in the experiment. The spikes were implanted 0.3 mm after application of compressive forces. | SpikeAnchor allowed automatic implantation over time of spikes into cortical bone. There was an increase in Ms stability of 3–5 times when compared to the experimental group and the control, with absolute anchorage. |
| Sirisa-Ard, A. et al., 2015 [91] | New Zealand rabbit, M, adults | 24 | Ti6Al4V (Russell Symes & Co, Australia), 6 mm × 1.5 mm, n = 47 | n = 23, SLA treatment | n = 23, treatment SLA | n = 24, without treatment (MA) | 8 and 16 s | Digital removal torque tests; %BIC analysis | Stability | - | RTVs: at 0 s, SLA group with higher values than MA (7.21 Ncm vs. 5.38 Ncm, p < 0.05); at 8 s, MA group with higher values than SLA (8 Ncm vs. 6.59 Ncm), but without significance; comparing the 0 s and the 8 s, there were no significant differences in the SLA group. %BIC: statistically significant decrease in values from 0–8 weeks for MA (p = 0.003); higher in the SLA group at 8 s. | SLA surface treatment does not increase the removal torque of Ms. |
| Tabuchi, M. et al., 2015 [49] | Sprague-Dawley rats, 8 weeks | 6 | 6 mm × 1.4 mm (Jeil Medical, Republic of Korea), (n = 12) | UV light for 12 min with TheraBeam Super Osseo device. | UV Light | No treatment | 3 s | SEM and EDX; ELISA; CLSM; mechanical Tests at 0.5, 10, 15, 20, 25, 30, 35 and 40 N | Stability | Surface characteristic; cell adhesion, behavior and proliferation | Lateral pressure test: smaller movement in the photofunctionalized group (p < 0.05, except at 40 N); the greater the force, the greater the displacement, but photofunctionalization reduced this effect, except at 40 N (p < 0.01); at 3 weeks experimental group: continuous bone, newly formed, without visible interface between the cortex and the space of the marrow. | The displacement of photofunctionalized Ms was 30–40% less than untreated ones. It is suggested that photofunctionalization increases the bioactivity of the Ms under study, as well as their anchoring capacity and stability, without changing the mechanical morphology itself. |
| Tabuchi, M. et al., 2015 [92] | Sprague-Dawley rat, M, 12 weeks | - | Ti6Al4V (Jeil Medical, Republic of Korea), 6 mm × 1.4 mm | Ultraviolet light: TheraBeam Super Osseo, 12 min | UV Light | No treatment | 3 s | SEM. Measurement of insertion and removal torque. Lateral force resistance analysis. EDX. | Stability | Surface properties | Initial SEM: control with hydrophobic surface; UV became superhydrophilic. RTVs: no differences at 0 weeks; at 3 weeks, higher in UV than control. Final SEM: UV group with regenerated bone more intact and with continuity than control. Faulty, non-cohesive O/M interface in both groups. Resistance to lateral force: lower in the control group, with a displacement 1.4× greater than UV. | The surface treated by UV converted from hydrophobic to superhydrophilic. At 3 weeks of healing, the removal torque in the UV group was greater than control. The interface between complexes was faulty, with non-cohesive fracture. Subject to lateral forces, the displacement was greater for the untreated group. |
| Villani, G. et al., 2015 [93] | Dog without breed, M, adult | 6 | Ti6Al4V (Conexão, São Paulo, Brazil), 6.0 mm × 2 mm, n = 36 = 6 × 6 | AE: HNO3 + HCl + H2SO4. | n = 18, AE (rough R), with and without load 1.0 N | n = 18, Untreated (smooth S), with and without load 1.0 N | 16 s | Digital measurement of insertion and removal torques. Periotest. Digital displacement measurement. | Stability | - | No significant performance differences between groups. ITVs high and low initial mobility in all groups. RTVs smaller than ITVs in both groups. AE allowed a rougher surface, which resulted in higher RTVs and lower mobility than control, and therefore, greater secondary stability, but p > 0.05. | Higher success rate for Ms treated with acid-etching. Primary stability greater than stability after 16 weeks. No differences in stability between groups, according to the parameters evaluated. |
| Bayani, S. et al., 2016 [94] | German Shepherd dog M, 6–8 months | 3 | 8 mm × 1.6 mm (Jeil Medical, Republic of Korea), n = 60 | n = 30 Treatment with growth factor obtained from plasma (PRGF) | n = 30, PRGF, sub-groups with (150 g) or without immediate load | n = 30; without treatment, sub-groups with or without immediate loading | 12 s | Maximum removal torque measurement test | Stability primary | - | RTVs: there were differences between the four groups—GPRGF without load > GPRGF with load > Gcontrol without load > Gcontrol with load. | Application of PRGF significantly increased the removal torque and, therefore, the stability of the Ms. |
| Cha, BK. et al., 2016 [95] | New Zealand White rabbit, 13–14 weeks | 5 | Dual-Top (Jeil Medical, Republic of Korea)), Ti6Al4V, 6 mm × 1.6 mm, n = 12 | TiO2 nanotubes by AO + machined tunnel (1.5 mg/mL rhBMP-2 transport system): anodization with glycol-ethylene and 0.5 wt% NH4F at 60 V 60 min+ ultrasonic cleaning; anodizing again after opening the window on the nanotubes 15 V, 15 min + tunnel | GTM: n = 4 machined tunnel; GTNM: n = 4 TiO2 nanotubes + tunnel | GCM n = 4, no treatment | 8 s | micro-CT: bone assessment of %BV and %BS. Microscopy %BIC, %BA | Stability | Surface properties | %BIC and %BS: similar GTM and GTNM values, but higher than the control, but without significant differences. %BA: new bone formation at 3 and 6 weeks in tunnel groups, and this is greater than control. No beneficial effect of nanotubes added to the tunnel was found. | Ms with machined tunnels can be coupled to TiO2 nanotube arrays. Pharmacological transport of rhBMP-2 to bone was successful. Bone surface values are higher in the experimental groups (nanotubes > tunnel only), as well as the amount of new bone formation. It would improve osteogenesis performance, but it has not been proven that nanotubes have additional effects to those of tunnel itself. |
| Choi, S-H. et al., 2016 [96] | Beagle dog, M, 12–15 months | 12 | Ti6Al4V (BioMaterials Korea, Seoul, Republic of Korea), 7 mm × 1.45 mm, n = 96 = 8 × 1 2 | n = 48, AO: immersion in solution with electrolytes for 3 min, at 250 V | n = 48 AO | n = 48, no treatment | 3, 9 and 12 s | SEM, AFM, insertion and removal torques, analysis of %BIC and %BV/TV | Stability | Surface properties; success rate | ITVs (N.cm): similar in both the 3-week and 12-week load groups. RTVs (N.cm): no significant differences between groups at 12 weeks. BIC and BV/TV: decrease in values, but without significant differences. | The maximum insertion and removal torque values, as well as BIC and BV/TV ratios after 3 and 12 weeks, of the anodized group, are not significantly different. The treatment has not proven to be clinically superior. |
| Espinar-Escalona, E. et al., 2016 [28] | New Zealand White rabbit | 10 | MI Ti pure (HDC Company, Sarcedo, Italy), 9 mm × 2 mmn = 20 | AE: immersion in 0.35 M HF, 15 s, 25 °C. Gritblasting: alumina particles 600 µm, 0.25 MPa until roughness saturation. GBA: acetone, 15 min + H20 + drying in N2 | n = 5 AE: condition then acidic; n = 5 GB—alumina beam; n = 5 GBA—AE + GB | n = 5 No treatment | 10 s | FE-SEM: O/M interface analysis. Manual measurement of removal torque. %BIC analysis. | Stability | Surface properties | Roughness of GB and GBA significantly greater than control and AE, with AE greater than control. %BIC:GBA 79 ± 12% and GB 75 ± 15% (p < 0.05), AE 26 ± 6%, C 19 ± 7%. No differences in removal torque between AE and control groups, but higher values for GB and GBA. Greater roughness and wettability in treatments than in control, leading to higher %BIC and higher RTV. | Surface treatments on Ms such as AE, GB and GBA are effective in modifying the surface and improving osseointegration and stability of the device. These create removal torques that do not compromise stability and do not promote fractures. Wettability was the parameter that showed the most influence on torque, based on the values obtained in AE. |
| Gansukh, O. et al., 2016 [97] | New Zealand rabbit, 3 months | 24 | Dual-Top ((Jeil Medical, Republic of Korea)), Ti6Al4V, 6 mm × 1.6 mm, n = 96 | RBM (resorbable blasting media) treatment: sandblasting with RBM, CaP and HNO3 | n = 48, RBM | n = 48, no treatment | 0, 2 and 4 s | SEM; measurement of maximum insertion and removal torques; RAM; analysis of %BIC and %BA | Stability | surface properties | SEM: rough and reticulated RBM surfaces; smooth control; higher roughness values in RBM. Mechanical analysis: RBM group with lower MIT values and higher MRT and RAM than the control (p < 0.05). No difference in BIC at 4 weeks, but higher BA in the RBM group. Control presents new bone formation after resorption. | RBM surface treatment can maintain the initial stability of Ms. |
| Kang, HK et al., 2016 [51] | Beagle dog, F, 10–15 months | 6 | 316 stainless steel, 6 mm × 1.2–1.3 mm, n = 48 | Nd-YAG 1064 nm Q-switched | n = 12,Nd-YAG Q-Laser switched | n = 12, without treatment | 0 and 8 s | Survival rate; insertion torque; micro-CT; histological analysis; mobility | Stability | Surface properties: roughness, topography | No failures. Surface roughness: higher values in the treated group (p < 0.05), triple increase; no differences in RTVs and 2D and 3D BIC between groups. There was no increase in fracture resistance, despite an increase in surface roughness. | Surface roughness increased by more than three times due to surface reactions created by the laser. No differences in secondary stability between treated and control groups. |
| Kim, H.Y. et al., 2016 [52] | White New Zealand rabbit, M, | 25 | OSSH1606 (Osstem Implant, Seul, Republic of Korea), 1.6 mm × 6 mm Ti6Al4V, n = 150 | G1: hydrochloric and nitric AE; G2: RBM, Ca3(PO4)2 beam and acid wash; G3: Ca3(PO4)2 and acid wash | G1, G2 and G3 | G0, no treatment | 1, 2, 4 and 8 s | Torque test, SR; histological analysis; SEM; EDS | Stability | Sharpness | Osseointegration: at 4 weeks, control RTV significantly decreased, but was increased in G1 (p < 0.05 in both); G4 with increased values at 2 weeks, and with higher values at 8 weeks (p < 0.05). Calcium and phosphorus infiltration on the bone-like surface was detected in the hybrid/G4 group. | Partial/hybrid RBM group with greater stability compared to the other groups, without a reduction in cutting capacity. |
| Takahashi, M. et al., 2016 [98] | Sprague-Dawley rat, M, 6 weeks | 12 | Pure Ti, 4 mm × 1.4 mm; n = 24 | n = 12, UV Light, 15 min, on the TheraBeam Affiny device, Ushio. Load and no load immediately | n = 12 ultraviolet light. Insertion into the right tibia | n = 12; no treatment. Insertion into the left tibia | 2 s | Micro-CT: bone assessment of BT and BD; FAITH-SEM:O/M contact analysis (BSC) | Stability | Density and bone thickness | FE-SEM: controls showed some gaps at the O/M interface; UV groups with uniform contact; with loading or without immediate loading, BSCs were higher in the UV groups than in the controls (UV 72.7 ± 9.9% and 71.5 ± 16.5% vs. 38.1 ± 26.8% and 40.4 ± 23.5%, p < 0. 05). | In this way, UV light on Ms subject, or not, to immediate loading, improved their stability by increasing their contact with the underlying bone. |
| Jang, I. et al., 2017 [99] | White New Zealand rabbit, X1 3–14 weeks | 6 | Dual Top 6 mm × 1.6 mm (Jeil Medical, Republic of Korea), n = 24 | n = 18 TO 2 steps, in an electrolyte bath. Drugs inside nanotubes | n = 18 = 3 × 6 G2: OA without drug; G3: OA with RhBMP-2; G4: AO with ibuprofen | n = 6 G1 without treatment | 8 s and 5 days | Method for detecting drug transport. Histological analysis: % BIC | Stability | Pharmacological transport capacity | Ibuprofen group with significantly higher BIC than control (71.6% vs. 44.3%). Successful use of nanotubes as a carrier. %BIC: Control: 44.3 ± 23.7% G2: 60.1 ± 13.7% G3: 24.6 ± 20.7% G4: 71.6 ± 17.8% p < 0.05. | Ibuprofen acted as an anti-inflammatory, allowing better osteointegration than in other groups. |
| Fernandes, DJ et al., 2017 [20] | New Zealand White rabbit, F, 6 months | 12 | Ti6Al4V grade V (ASTM, West Conshohocken, PA, USA) 6 mm × 1.5 mm, n = 48 | AE: (HNO3 + H2O + H2SO4) under magnetic stirring + HNO3 again | n = 24 AE (1, 4 and 8 s) | n = 24, no treatment (1, 4 and 8 s) | 1, 4 and 8 s | AAS: analysis of blood concentration of Al and V ions. Measurement of insertion and removal torque. Histological analysis | Stability | Surface properties. Blood concentrations and histological analysis | Surface: intercommunicating micropores in the experimental group; larger TiO2 layer in treated Ms, and with lower % of Al and V. Torque: higher values for treated group and ITVs > RTVs. Histological analysis: dense Ca/P particles with proliferating osteoblasTS at the O/M interface of the treated group at 4 s, new bone formed at 8 s. | Acid-etching treatment of the surface of Ms improves surface morphology and mechanical stability, with early signs of osseointegration. It also allowed a reduction in the release of Al and V ions. |
| Maino, B. et al., 2017 [100] | New Zealand White rabbit, M, 6 months | 8 | Ti6Al4V Spider Screw (HDC Company, Sarcedo, Italy), 6.5 mm × 1.5 mm, n = 64 | SLA | n = 32, SLA, with and without load 100 g | n = 32, without treatment with and without load 100 g | 12 s | X-ray. Digital measurement of removal torque SEM. Histological analysis | Stability | Surface properties | SEM: visible bone adhesion to SLA Ms. RTVs: significantly higher for SLA than control, with and without load. Histological analysis: loaded group, presence of irregular fibers, with modified cortical bone around, indicating remodeling and bone loss. %BIC: no significant differences. Decreased cortical bone area significantly in SLA under load. | Grade V titanium in SLA Ms has greater bone retention than machined surfaces, both at 0 and 3 months. This is due to better interconnection with the tissues. The use of this treatment is recommended for clinical use in situations of difficult retention and stability. |
| Yun, SD. et al., 2017 [101] | Beagle dog, M, 12 months | 4 | OSSH140 6 (MS) and OSSH1406HE(ES) (Osstem Implant, Seul, Republic of Korea), 6 mm × 1.4 mm; n = 56 (14 × 4) + 40 = 96 | Machined and original AE, placed in the jaw of beagle dogs, removed at 4 weeks and treated to be reused and implanted in the jaw | n = 56; GA: jetair-water; GB: mechanical cleaning; GC: mechanical + chemical cleaning | n = 40 (20 MS + 20 ES); GD: unused, autoclave | 8 and 12 s | SEM: differences on the surface; EDS: elemental composition; insertion torque tester; light microscopy: %BIC and %BV | Stability | Surface properties | Remaining compounds reduced in each cycle, without deformation. Biological response: %BIC and %BV higher at 4 s than at 8 s (p < 0.01), higher in ES than MS, but without significance; %BV of controls higher than experimental groups (p < 0.01), but without differences in %BIC. | Reused Ms can have a surface composition equivalent to new devices. The use of protocols with a mechanical and chemical component seemed to produce better results. The biological response already produces other variant results, which may imply that these treatments are not the most suitable from a biomechanical perspective. |
| Jang, T- H. et al., 2018 [102] | White New Zealand rabbit, M, 5 months | 21 (two tibias) | Ti6Al4V (Osstem Implant, Seul, Republic of Korea), 6 mm × 1.4 mm, n = 126 | n = 42 AE with nitric or hydrochloric acid. ECG: immediate immersion after EA in calcium chloride in a sealed location | n = 42, ECG: AE with calcium chloride immersion; n = 42, EG: AE without immersion | n = 42, CG no treatment | 1, 4 and 7 s | Mechanical testing of cutting capacity and removal torque; SEM: analysis of changes in surface morphology | Stability | Capacity sharp | Surface: porous structures present in both experimental groups. RTVs: at 1 week were significantly higher in the experimental groups (p < 0.05), with no differences between EG and ECG; at 4 and 7 weeks, higher in the ECG group, and lower in the control group (p < 0.05); no changes in control over time. | We can increase the stability of Ms, increasing surface roughness by acid, and prevent contamination by calcium chloride. |
| Bakopoulor, A. et al., 2019 [103] | New Zealand rabbit, M, 4 months | 6 | The Sydney Mini Screw (Ti grade 5) + Aarhus (Aarhus system, American Orthodontics, Sheboygan, WI, USA), n = 24 | iBGS; 8 weeks; injection through 1 cc cavity | T1: n = 8 SMS + IBGs; T2: n = 8 SMS | n = 8, no treatment and Aarhus | 8 s | EA: stress point analysis; Micro-CT: stability; histological analysis | Stability primary | Dispersal from iBGs | All groups with cortical integration. iBGS group filled gaps, but with a greater inflammatory response, derived from a greater number of white blood cells (T0); at T8 complete healing—iBGS disperses with empty SMS. Control: uniform integration across the Ms in T0 and T8. | Biocompatibility and uniform integration with and without iBGS. Good delivery of iBGS to the bed, almost completely replacing the missing bone. Good results with the concentrations used. |
| Oga, Y. et al., 2019 [61] | Japanese white rabbit, F, 14 weeks | 11 | Dual-Top (Jeil Medical, Republic of Korea), 6 mm × 1.6 mm + auxiliary built-in device Ti6Al4V, F136-96(ASTM, West Conshohocken, PA, USA), PCT + Durometer silicone rings; n = 42 | n = 22 Ti6Al4V auxiliary built-in device with two parts: capture of compressive forces + three peaks inserted into the bone cortex, X | n = 11 4 s; n = 11 8 s; Placing with auxiliary device | n = 9 4 s; n = 11 8 s; no treatment | 4 and 8 s | Micro-CT: assessment of bone thickness, M/O interface and insertion depth | Stability | Bone thickness and insertion depth | Lateral displacement 0.01, 0.02 or 0.03 mm: device had significant effects on lateral displacement (p < 0.01), not taking time into account. Retention force: experimental at 4 s and 8 s greater than controls at all displacements. | The automatic anchoring auxiliary device coupled to the Ms increased its stability, on average, by 1.6 to 2.8×. It may be possible to allow the use of Ms that are smaller in length and diameter, fundamental characteristics in substrates that are more difficult to insert. |
| Yucesoy, T. et al., 2019 [104] | New Zealand rabbit, M, 9 months | 18 | Original SLA pure Ti, 8 mm × 1.8 mm, n = 72 | Osseo Pulse LED, 618 nm, 20 mW/cm2, 5 min, for 21 days. Ozone therapy: (Ozonytron XL, Mymed); concentration 10–100 µg/mL, 90%, 30 s every 3 days | LED; Ozone. three sub-groups: immediate load G1, at 4 s G2 and at 8 s G3 | Without treatment three sub-groups: immediate load G1, at 4 s G2 and at 8 s G3 | 0, 3, 4, 7, 8 and 11 s | SEM and IFM: surface characteristics biomechanical analysis. RR FA: stability assessment. %BIC analysis | Stability | Surface properties | Ozone G2 with significantly higher values of bone volume than the remaining G2, and the same was true for G3 photo and ozone. RFA: no statistical differences between groups. Histomorphometric analysis: ozone G2 and photo G2 with significantly higher % of osteointegrated areas than the G2 control, and in G3, photobiomodulation obtained greater values | Both photobiomodulation and ozone therapy are safe and effective methods for increasing bone volume, and therefore Ms stability, with implications not only in orthodontics, but in all areas that use implants. |
| Cho, Y.C. et al., 2021 [105] | White New Zealand rabbit, | 12 | Discs 316L 15 mm, 1.2 mm; n = 6 + Micro316L machined 2 mm × 10 mm, n = 6 | AO:C6H8O7 and HF, 25 °C for 30 s + H cleaning. The anodizing (0.5, 1, 3.5 V) 5 min in H2SO4 solution 1 M at 98% and C6H8O7 85% at 25 °C | n = 6 Anodizing | n = 6, no treatment | 8 s | FE-SEM; XRD: crystallization phase; XPS: chemical analysis; histological analysis | Stability | Deposition of compounds; surface morphology | Group 5 V: deposition of double porous chromium oxide. Fibroblasts proliferate in all groups after 24 h, but in a well-distributed manner on anodized surfaces, adhering to flat and rough areas; anodized group shows larger osseointegration than I control. | Anodizing at 5 V on 316L BSS Ms can generate porous structures on their surface. This surface shows potential in improving cell adhesion and recovery in order to promote a higher survival rate of the Ms. |
| Choi, S- H. et al., 2021 [106] | Beagle dog, M, 12–15 months | 8 | Ti-6Al-4V (Orlus™, Ortholution, Seoul, Republic of Korea), 4/7 mm × 1.8 mm; n = 64 = 8 × 8 | n = 32 UV light, 12 min, by TheraBeam Super Osseo device, | n = 32, UV light; G4mm and G7mm | n = 32, no treatment; G4mm and G7mm | 7 and 28 days; 8 s | Measurement of success rate: mobility ≤ 1 mm (Periotest); Maximum insertion torque and micro-CT tests: %BA and %BV/TV analysis; histological analysis: %BIC | Stability | Success rate | Success rate: 100% in experimental 7 mm and 87.5% in control 7 mm. ITVs and RTVs: values increase with increasing length and surface treatment (p < 0.05). %BV/TV: trend equivalent to %BA, but without significant interaction. %BIC: values increase with the use of surface treatment and with length (p = 0.021 and p = 0.014, respectively). | Photofunctionalization using UV light can significantly increase biomechanical stability compared to untreated Ms. |
| Auciello, O. et al., 2022 [107] | Wistar rat M, X | 10 | Ti-6Al-4V AbsoAnchor (DENTOS Inc., Daegu, Republic of Korea); n = 20 | Tungsten + UNCD 30 days; argon gas + argon gas/CH4 0.8 sccm at 90 mbar + MO 1200 W | n = 10 Treatment UNCD | n = 10, no treatment | 30 days | SEM and AFM; EDS: chemical composition; analysis with optical microscope: BIC | Tissue response Biocompatibility | Stability surface roughness | Larger W interface with greater roughness with UNCD ( + 0.3 nm rms); UNCD 3–5 nm film; UNCD granules increase surface roughness; osteointegration in both, but with control with more bone tissue. Average BIC% values: C—53.40 ± 13%; UNCD—58.82 ± 9%. | UNCD treatment with excellent biocompatibility. No differences in osteointegration. It can protect against titanium corrosion. |
| Im, C. et al., 2022 [30] | New Zealand White rabbit, M, 8 weeks | 3 | Ti–6Al–4V Ms ELI (Fort Wayne Metals Research Products Co., Fort Wayne, IN, USA) 3.3 mm × 1.4 mm | AO (HNO3 + HF+ H2O) 10 s + H2O + 1.4 wt% NH4F) 20 V, 60 min + P: 0.5 vol% silicate, 5 min + drying 1 h + 20× (0.05 M NaH2PO4 and Ca(OH)2, 90 °C, 1 min interval). Heating 500 °C, 2 h | AH: anodizing and heat treatment; APH: anodizing, pre-calcification and heat | UT: no treatment | 4 s | Digital measurement of removal torque; FE-SEM: analysis of surface morphology; EDS: analysis of differences in elemental composition | Stability | Surface properties. Bioactivity. Cytotoxicity | Surface: AH—dense and aligned formation of nanotubes, with protrusions; APH—presence of granular Ca3(PO4)2 precipitate. Bioactivity: presence of Ca in the AH and APH groups, absence in the UT; FE-SEM: removal causes fractures not only at the interface (UT), but also in places of attached bone tissue, mainly in APH. | The APH surface obtained well-aligned nanotubes with a dense structure. Precipitates of calcium phosphate and hydroxyapatite were obtained in clusters, which allow greater binding to proteins that form new endogenous bone. Compared to the control, the experimental groups showed significantly higher removal torque. |
| Li, M. et al., 2022 [67] | Wistar rat, F, 8 months | 144 | Mini-pin (original SLA) 5.0 mm × 1.1 mm, n = 144 | Immersion in simulated body fluid 24 h, 37 °C for BioCaP formation + immersion in SBF 5× 24 h, 37 °C for deposition of amorphous layer + 20 mL calcium phosphate solution | G2—No treatment, bath in PBS + BSA; G3 -amorphous treatment; G4—amorphous treatment + BSA; G5—TC; G6—TC+ BSA | G1: no treatment or BSA | 3 days, 1, 2 and 4 s | SEM; FTIR; CLSM; spectrometry; activity n phosphatase alkaline and %BIC | Stability | Bioactivity by transport of biological agents | The thickness of crystalline layers is seven times greater than that of amorphous ones. Treatment by crystalline BioCaP allowed pharmacological transport. There was an increase in bone/Ms contact in the 1st week in G4, but in other groups this increase occurred at 2 and 4 weeks. | The pharmacological transport capacity was 10 times greater in the BioCaP treatment than in the amorphous ones. The contact between bone and Ms increased after the 1st week in the crystalline group, unlike the others, suggesting that this treatment is a technique that can increase stability and increase the success rate of Ms. |
| Seker, ED et al., 2022 [108] | White New Zealand rabbit, F, 12 months | 14 | Pure Ti grade IV, 8 mm × 1.8 mm, n = 56 | n = 56, SLA: Al2O3 + AE hydrochloric and sulfuric, 90 °C, 15 min (G1) and 18 min (G2) + ultrasonic cleaning with acetone, 70% ethanol and H2O +sterilization by gamma rays | n = 28, G2: SLA of roughness 1.5 µm (no load and immediate load at 4 and at 8 weeks) | n = 28, G1: roughness SLA 1 µm (no load and immediate load at 4 and 8 weeks) | 0, 4, 8 s | SEM, EDS, IFM, RFA, ISQ measurement and histological analysis | Stability | Morphology surface | Surface after use, without damage or deformation, and composition identical to mineralized bone. Stability: differences between initial ISQ values and differences between beginning and end without significance; there are intragroup differences (loading protocol), p < 0.05. BIC: in G2, higher values at 8 weeks and in immediate loading. | No significant differences in bone volume between groups. Greater stability in Ms subjected to loading after the healing period, instead of immediate loading: higher osseointegration rate in G2. |
| Byeon, S. et al., 2023 [31] | Sprague-Dawley rats, M, 8 weeks old | 18 | Ti-6Al-4V ELI alloy rods used to make Mis 3.3 mm × 1.4 mm, n = 36 | APH: HNO3 + HF (H2O ratio of 12:7:81 for 10 s)+ ultrasonic cleaning in distilled water for 5 min + dryer at 50 °C for 24 h. SBF group: 10 mL of SBF for 5 days and 10 days, at 37 °C. Ibandronate groups: 10 min or 60 min in ibandronate ar −0.05 MpPa e and lyophilized with N2 gas + H2O for 7 days | 2 Groups: n = 9 AN—immersion in SBF. N = 9 IB—immersion in ibandronate | n = 18 UN: untreated, machined | 4 w | FE-SEM. RTV test | Stability | Surface properties | The removal torque values were higher in the AN and IB groups compared to the UN group, and the IB group showed the highest values. IB (8.48 ± 2.34 N/cm): statistically significant differences among all test groups. FE-SEM: UN—no adhesion to bone material and it showed the interfacial fracture; AN and IB—locally attached bone materials, with a mixture of cohesive and interfacial fractures, better observed in IB. All groups had new bone formation, but only IB had thick expansion of bone along the threads of Ms. | In the UN and AN groups, new bone was not formed or partially formed along the threads of the Ms. In the IB group, new bone was formed thickly along the threads. It was concluded that this method must be surface treatment to enhance the bioactivity and osseointegration, but it is necessary to study the biostability and biofunctionality on the surface of Ms according to the concentration of the drug using the sustained release method of the drug. |
| Nishioka-Sakamoto, K. et al., 2023 [69] | Wistar rats, M, 11 weeks old, 250–300 g | 20 | 4.5 mm in length and 1.2 mm in diameter (ACE, Brockton, Mass) | Bone holes with different diameters (1.6, 2.1, or 2.5 mm) were drilled in the rat tibias. After a healing period of 2 or 4 weeks, orthodontic force was applied. The holes were filled with e β-TCP beta-tricalcium phosphate | n = 18 β-TCP | n = 20 untreated | 0, 2 and 4 weeks | In vivo micro-CT, ex-vivo micro-CT, histological analysis, bone morphometry | Stability due to bone healing | Bone formation and percentage | The bone hole of 1.6 mm in diameter was employed as the OAS-loosening model. When b-TCP was inserted into the bone hole, the linear distance and mesial tipping angle of the OAS movement decreased markedly. The values of bone morphometry significantly increased with b-TCP filling. | An OAS-loosening model was established in rats and demonstrated that the loosening OAS was stabilized by b-TCP filling through bone formation. b-TCP may be useful for fixation of a loosening OAS. |
| Okawa, K. et al., 2023 [70] | Wistar rats, M, 25 weeks old, 400 g | 24 | Ti-6Al-4V titanium alloy, (Le Fort System, Pro-Seed, Japan) 3 mm × 1.2 mm, n = 48 | Plasma surface treatment with a PZ2 piezo brush® (Relyon Plasma, Regensburg, Germany) for 30 s, with or without load | n = 24 plasma treated with loading (PTL) or without loading (PTU) | n = 24, no treatment | 3, 5, 7 days and 2 weeks | Micro-CT: %BIC, bone density, histological analysis. Second harmonic generation imaging and XRD | Stability | Bone properties | Bone–implant contact rate increased more rapidly at an early stage in the treated surface group than in the untreated surface group. Collagen fiber bundle diameter showed that the measured values adjacent to regions A and D were significantly higher than those at regions B and C. | The results of bone contact rate and bone mineral content indicated that there was no significant difference between the surface-treated and untreated groups in the long term. Surface treatment of screws may accelerate initial bone remodeling. |
| Yamagata, K. et al., 2023 [71] | Japanese white rabbits, M, 3.5–4.0 kg | 8 | Dual-top (Jeil Medical, Republic of Korea), 5 mm × 1.3 mm. Kono Seisakusyo Corp, Chiba, Japan; raw material, Ti6Al4V, n = 32 | Novel auxiliary device with spikes | n = 16, auxiliary device | n = 16, no treatment | 28 and 56 days | Stability measurement Periotest. Histomorphometic analysis: BIC% and implantation spike depth | Stability | Implantation depth | The highest median PTV was 2.22 in the nonauxiliary group at 0 days and the lowest was 3.41 in the auxiliary group at 56 days. The interaction between the effects of the auxiliary device and days was not significant. There were no significant differences in BICs between the auxiliary and nonauxiliary groups at both 28 and 56 days | The results suggest that the use of Ms in conjunction with auxiliary devices provides stable skeletal anchorage, which may be useful in orthopedic treatments. |
| Lee, Y.T. et al., 2024 [109] | White New Zealand rabbit, M, 12 months | 12 | Ti (Mondeal Medical Systems, Germany) 9 mm × 2 mm, n = 48 | Formation of nanotubes on the surface by AO in 1 M H2SO4 and NaF at 3 or 7 V for 5 h + heating at 300 °C for 3 h | Set 2, n = 6 Microporous group; sets 3–5, 3 n = 6 groups of nanopores on the right tibias. 7 V | Set 1, n = 24 Placebo control: non-porous on left tibias | 12 s | Removal torque test with sensor; FE-SEM and SEM: composition surface and nanotubes | Stability | Surface morphology. Survival | Nanotube diameter increases with higher voltage and concentration; RTV: gradual increase from set1 to set 5, (p < 0.001). For 100% survival rate, minimum torque of 6.6 ± 0.8 N-cm and minimum thickness of 22.5 ± 4.8 nm. | Mts with nanoporous surfaces promoted higher survival rates and greater stability due to removal torques than microporous ones, the results being thought to be due to the greater thicknesses of TiO2 deposited on their surface by anodizing. |
| Author, Year | Study Design | Sex and Age | n | MI Type and Number | Surface Treatment | Experimental Group | Control Group | Period of Follow-Up | Test Used to Evaluate Outcomes | Primary Outcome | Secondary Outcomes | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chaddad, K. et al., 2008 [16] | NRCT | 13–65 years | 10 | DualTop™, Ti (C-implant, Seoul, Republic of Korea), n = 32 = 17 + 15 | Source SLA surface | n = 15, C-implant (SLA surface) | n = 17, Dual-top (machined surface) | 7, 14, 30, 60 and 150 days | Measurement of insertion torque | Success rate and stability | Surgical difficulty and post-surgical pain | SR: control 82.4%, SLA 93.4%. Devices always fail with torque < 15 Ncm. ITVs: influences SR (p < 0.05). Surface treatment did not create significant differences in SR. | The MI surface did not influence the SR of the same ITVs greater than 15 Ncm, which appear to be vital to the success of Ms under immediate loading. |
| Schaetzle, MA et al., 2009 [116] | RCT | 21 M/19 F; 27.9 years | 40 | Orthosystem (Straumann, Basel, Switzerland) pure Ti (palatine), original SLA, 4.2 mm × 4.1 mm n = 40 | modSLA (source SLA—0.25 grains- 0.5 mm and AE withHCl + H2SO4) + washing in N2 protection+ isotonic NaCl. | n = 20, modSLA | n = 20, SLA | 0–84 days | RFA | Stability | - | All Ms with high insertion stability (average 39.25 N/cm). ISQ: Both groups showed a tendency to decrease, which reversed at 42 and 63 days, respectively, for the modSLA and control groups; at 12 weeks, modSLA greater than control. | The chemical modification of the SLA treatment positively influenced initial osseointegration through greater effectiveness of the healing process. |
| Calderon, J.H. et al., 2011 [113] | NRCT | 6 M/7 F | 13 | IMTEK Ortho (3M, St. Paul, MN, USA), 6 mm mandible, 8 and 10 mm maxilla; n = 24 | Source SLA | SLA of origin, maxillary group and mandibular group. | - | 6 months | Overlay of occlusal radiographs and CBCT: displacement analysis | Stability | - | Angular displacement at 5 months: 65% ≤ 1°; 35% ≥ 2°. Less displacement in the mandibular group. Best results with 8 mm MI, without statistical differences. Length influences displacement variation. | Surface modification can improve osseointegration. All Ms were stable. No need for greater force to be applied when removing treated Ms. |
| Kim, SH. et al., 2012 [112] | Retro | 1 M/7 F, 17–46 years | 8 | Source SLA (C-implant, Seoul, Republic of Korea), 8.5 mm × 1.8 mm, n = 16 | Source SLA | SLA position after mass shrinkage | SLA starting position after placement (CBCT) | 9 months | Overlay of CBCT DICOM data: 3D change analysis | Stability | - | Average RTV of 23.69 Ncm. Relative values of position changes of the tail and head on the x-axis (intrusion and extrusion) were −0.04 ± 0.19 mm and 0.01 ± 0.18 mm. No significant difference between before and after retraction. | The C Ms with SLA surface remained stable during the application of forces. |
| Noorollahian, S. et al., 2012 [39] | RCT | - | 40 | 2 mm × 10 mm; n = 40 used on patients for 7 months | H20 and drying +H3PO4 37% 1 mL+ in NaOCl 5.25 mL (10 mL) for 30 min | n = 20 NP2: irrigation + drying; P2: irrigation + drying + phosphoric acid + NaOCl | n = 20, C2: autoclave | - | Torque measurements | Stability | - | ITVs: NP2 significantly smaller than P2 and C2, with no differences between them; RTVs and FTVs had no differences between all groups. | Absence of deleterious effects on the three torque values due to the use of AE, NaOCl and autoclave. Reused microimplants can be used. |
| Bratu, DC et al., 2014 [15] | NRCT | 20–38 years | 20 | 10 mm × 1.6 mm (MIS Implants Technologies, HaZafon, Israel), n = 40 | SLA | n = 20, SLA | n = 20, untreated, machined | 6 months | Measurement of insertion and removal torques | Stability | - | ITVs: control 18.55 ± 8.52 Ncm/SLA 20.45 ± 9.21 Ncm. RTVs: control 17.40 ± 8.18 Ncm/SLA 23.55 ± 9.68 Ncm. | SLA improved the secondary stability of Ms. 1.6 mm diameter suitable for good stability. |
| Ekizer, A. et al., 2016 [18] | RCT | 7 M/13 F; 16.77 ± 1.41 years | 20 | Titanium, 8 mm × 1.6 mm, n = 40 | LED: OsseoPulse LED (Biolux), 618 nm, 20 mW/cm2, 20 min/day, 21 days | n = 20, LED | n = 20, without treatment | 0, 1, 7 days, 1, 2 and 3 months | RFA | Canine distalization rate | Stability + inflammation | ISQ: similar values between groups in the 1st month; different stability between groups in the 2nd and 3rd months (LED > control, p < 0.01). | LED can accelerate orthodontic tooth movement. Has a positive effect on stability No effects on IL-1β. |
| Flieger, R. et al., 2019 [115] | RCT | 7 M/13 F; 32.5 ± 6.1 years | 20 | Grade V titanium, 10 mm × 1.4 mm; n = 40 | LLLT 635 nm (Lasotronix SmartM), 100 mW, 10 J, 100 s per point, 2 times/session, 199.04 mW/cm2 | n = 20 LLLT irradiation | n = 20, without treatment | 0, 3, 6, 9, 12 15, 30 and 60 days | Periotest: stability analysis; NRS-11 Pain Rating Scale | Stability | Pain perception | Lower Periotest values in the experimental group indicate greater stability at 3, 30 and 60 days (p < 0.05 in both). No MI failed over the 60 days. | LLLT 635 nm promotes secondary Ms stability. There are, however, no effects on pain appreciation. |
| Park, H.J. et al., 2019 [117] | RCT | 13 M/27 F; 22.16 years | 40 | 1.6 mm × 6 mm; n = 98 | AE | n = 49 AE | n = 49, without treatment, machined | - | Success rate. SEM. Torque measurement (Sensor Mark-10) | Stability + success rate | Mobility and topography | Topography of different roughness. SR: experimental group greater than control (91.8% vs. 85.7%), p = 0.323. Average ITVs: higher in the experimental group, but without differences in surface and jaw treatment. | Higher SR in the experimental group, without statistically significant difference. The same occurred in primary stability. |
| Kim, SH. et al., 2012 [112] | Retro | 1 M/7 F, 17–46 years | 8 | Source SLA (C-implant, Seoul, Republic of Korea), 8.5 mm × 1.8 mm, n = 16 | Source SLA | SLA position after mass shrinkage | SLA starting position after placement (CBCT) | 9 months | Overlay of CBCT DICOM data: 3D change analysis | Stability | - | Average RTV of 23.69 Ncm. Relative values of position changes of the tail and head on the x-axis (intrusion and extrusion) were −0.04 ± 0.19 mm and 0.01 ± 0.18 mm. No significant difference between before and after retraction. | The C Ms with SLA surface remained stable during the application of forces. |
| Matys, J. et al., 2020 [114] | RCT | 8 M/14 F; 31.7 ± 9.7 years | 22 | Grade V titanium, 10 mm × 1.4 mm; n = 44 | LLLT 808 nm (SmartM Pro by Lasotronix), 100 mW, 4 J, 40 s/point, 2 times/session, 200 mW/cm2 | n = 22 LLLT irradiation | n = 22, without treatment (loss of 1) | 0, 3, 6, 9, 12 15, 30 and 60 days | Periotest: stability analysis; NRS-11 Pain Rating Scale | Stability | Pain perception | Stability: PTVs with significantly higher values in controls than in irradiated groups (p = 0.009). At 30 days, controls had a greater decrease in stability (p = 0.004). | LLLT increased secondary stability after 1 and 2 months of implantation. There were no differences in pain scores. |
| Rampurawala, A. et al., 2020 [14] | RCT | 18–45 years | 17 | AbsoAnchor (DENTOS Inc., Daegu, Republic of Korea), Teeth; 7 mm × 1.4 mm; n = 34 (24 after deletion) | UV-A rays (15 W, 350 ± 20 nm, 0.1 mW/cm2) and UV-C (15 W, 250 ± 20 nm, 2.0 mW/cm2), 15 min (before insertion) | n = 12, UV light | n = 12, no treatment | 6–8 months | SEM: contact O/M; EDX: Deposition of elements on the surface | Stability | Hydrophilicity and deposition of calcium and phosphorus | Increased hydrophilicity of the titanium surface. O/M Contact: higher score in the experimental group, with no statistical difference. Calcium and phosphorus deposition: no significant differences between groups. | UV light converted Ms surface: hydrophilic to superhydrophilic. Contact with the bone was greater in the lower region of the Ms in both groups. |
| Moghaddam, S. et al., 2021 [21] | RCT | 8 M/23; 18.5 years | 31 | Dual-Top Anchor System (Jeil Medical, Republic of Korea),10 mm × 2 mm, n = 62 | SLA: alumina particles 250 µm, 4 MPa + ultrasonic cleaning with acetone, 75% ethanol and H2O, 15 min + immersion in 0.11 HF mol/L and 0.09 mol/L HNO3, 25 °C, 10 min +drying at 50 °C, 24 h | n = 31, SLA | n = 31, no treatment | 3, 6, 10, 14, 18 weeks | Measurement clinical performance and mobility (<1 mm). Digital measurement of insertion and removal torques | Success rate | Stability | SR: SLA 90.32% vs. 83.87% control (p > 0.05); Success rate lower the lower the age in both groups (p = 0.025): <15 years 66.66% and >15 years 95.45%. Mean RTVs (N.cm) higher in the SLA group (p < 0.05): SLA 15.71 ± 5.563 and C 8.08 ± 2.481. No significant differences in mean ITVs (N.cm): 12.10 ± 6.295 vs. C 12.42 ± 5.755. | Surface roughness of orthodontic IMs by SLA treatment had no influence on the success rate, but increased removal torques significantly. |
| Manni, A. et al., 2022 [17] | RCT | 23 F/16 M; 15.55 ± 7.91 | 39 | Machined and original AE (Osstem Implant, Seul, Republic of Korea), 1.2–1.4 mm × 8 mm, n = 78 | AE of origin | n = 39, AE of origin | n = 39, untreated, machined | 9.3 months ± 1.31 | Measuring success rate: clinical performance analysis | Success rate | Stability | Failure rate: 25.6% control and 28.2% experimental. Cumulative SR of 26.9%. Average survival of failures: 52 days ± 58. No influence of surface treatment, Ms location, gender and diameter on stability. | The success rate did not vary statistically significantly between groups in cases of anchorage reinforcement during treatment with the Herbst device. |
| Durrani, O. et al., 2023 [110] | RCT | 32 M/60 F, 18 ± 1.8 years | 92 | Ti-6Al-4V alloy, 8 mm × 1.6 mm, self-drilling and self-tapping, n = 184 | Precipitation of HA: ultrassonic acetone cleaning 10 min + H2O + 20 mL CaCl2 0.1 mol/L + 20 mL K2HPO4 solution 0.1 mol/L with stirring for 60 min + H2O cleaning + autoclaving | n = 92, HA (46 on first quadrant, 46 on left quadrant) | n = 92, no treatment (46 on first quadrant, 46 on left quadrant) | Monthly over 18 months | Number of failing miniscrews | Failure rate | Stability | Non-significant statistical difference. Better survivability of the HA-coated Ms from the second to the fifth month, but p = 0.67. Left side failed more (19.6%vs4.3%, p = 0.002). | Ms coated with HA do not have any statistical difference in the failure when placed on the buccal shelf of the maxilla. The same method of using biomimetic precipitation of HA incorporated with other drugs on surfaces of Ms. |
| Ravi, J. et al., 2023 [111] | RCT | 9 F, 23–29 years | 9 | Self-drilling, tapered Ti Ms of 8-mm × 2 mm (A1 orthodontic Ms (Bioray Enterprises, Taipei, Taiwan) | SLA: sandblasted with large-grit alumina, followed by acid-etching with hydrochloric acid and sulfuric acid | n = 18, SLA | n = 18, no treatment | 4 weeks | Torque test with digital torque meter TQ-8800 | Stability | Failure rate | The mean maximum insertion torque was 17.9 ± 5.6 Ncm for surface-treated Ms and 16.4 ± 9.0 Ncm for non-surface-treated Ms. The mean maximum removal torque was 8.1 ± 2.9 Ncm for surface-treated Ms and 3.3 ± 1.9 Ncm for non-surface-treated. Among the failed Ms, 71.4% were non-surface-treated Ms and 28.6% were surface-treated Ms. | The insertion torque and failure rate did not differ significantly between the groups, whereas the removal torque was significantly higher in the surface-treated group. Thus, surface treatment using sandblasting and acid-etching may improve the secondary stability of self-drilling Ms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, A.L.; Travassos, R.; Nunes, C.; Ribeiro, M.P.; Santos, M.; Iaculli, F.; Paula, A.B.; Marto, C.M.; Caramelo, F.; Francisco, I.; et al. Surface Treatment of Dental Mini-Sized Implants and Screws: A Systematic Review with Meta-Analysis. J. Funct. Biomater. 2024, 15, 68. https://doi.org/10.3390/jfb15030068
Figueiredo AL, Travassos R, Nunes C, Ribeiro MP, Santos M, Iaculli F, Paula AB, Marto CM, Caramelo F, Francisco I, et al. Surface Treatment of Dental Mini-Sized Implants and Screws: A Systematic Review with Meta-Analysis. Journal of Functional Biomaterials. 2024; 15(3):68. https://doi.org/10.3390/jfb15030068
Chicago/Turabian StyleFigueiredo, Ana Luísa, Raquel Travassos, Catarina Nunes, Madalena Prata Ribeiro, Mariana Santos, Flavia Iaculli, Anabela Baptista Paula, Carlos Miguel Marto, Francisco Caramelo, Inês Francisco, and et al. 2024. "Surface Treatment of Dental Mini-Sized Implants and Screws: A Systematic Review with Meta-Analysis" Journal of Functional Biomaterials 15, no. 3: 68. https://doi.org/10.3390/jfb15030068
APA StyleFigueiredo, A. L., Travassos, R., Nunes, C., Ribeiro, M. P., Santos, M., Iaculli, F., Paula, A. B., Marto, C. M., Caramelo, F., Francisco, I., & Vale, F. (2024). Surface Treatment of Dental Mini-Sized Implants and Screws: A Systematic Review with Meta-Analysis. Journal of Functional Biomaterials, 15(3), 68. https://doi.org/10.3390/jfb15030068









