Enhancing the Antifouling Properties of Alumina Nanoporous Membranes by GO/MOF Impregnated Polymer Coatings: In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation and Separation of Alumina NPMBs
2.1.2. Preparation of Graphene Oxide (GO)
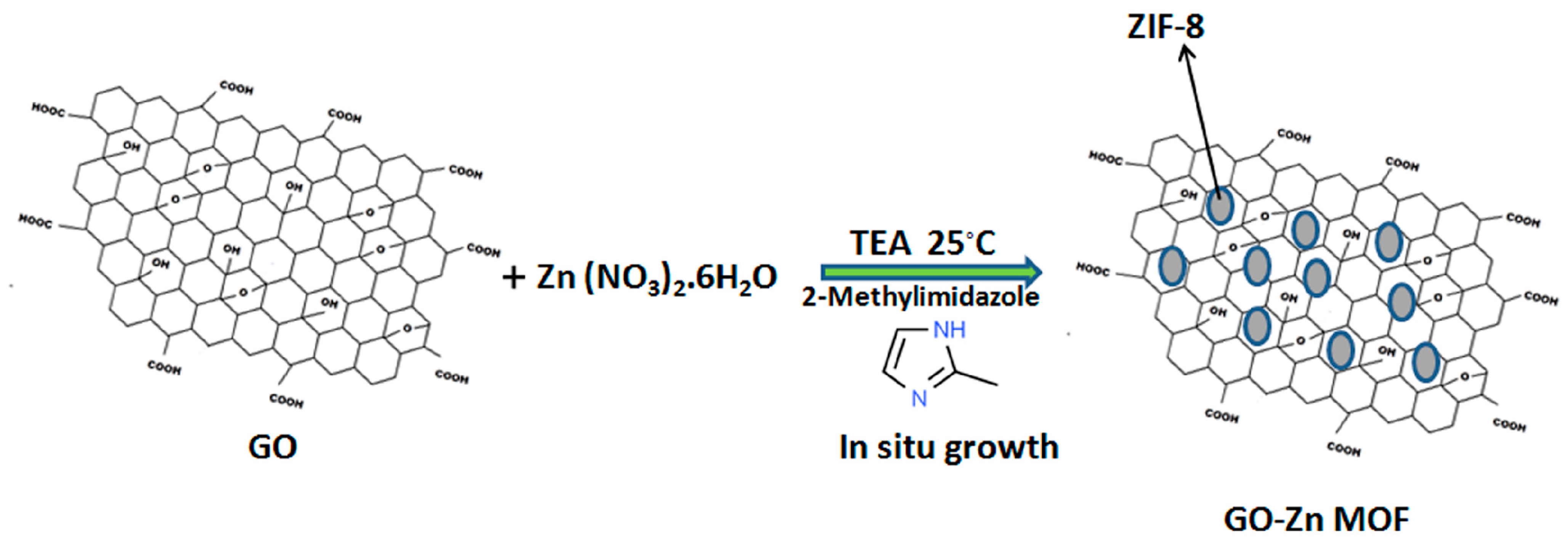
2.1.3. Preparation of Zinc—MOF/GO (ZIF-8 @ GO) Composites
2.2. Coating Materials Preparation
2.2.1. Scanning Electron Microscopy
2.2.2. Transmission Electron Microscopy
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. X-ray Diffraction Analysis (XRD)
2.3. Contact Angle Measurement
2.4. Atomic Force Microscopy (AFM)
2.5. In Vitro Studies
2.5.1. Preparation of HEPES-Buffered ACSF
2.5.2. Changes of ACSF Ions Concentrations
2.6. Antifouling Tests
2.6.1. Adsorption of Bovine Serum Albumin (BSA)
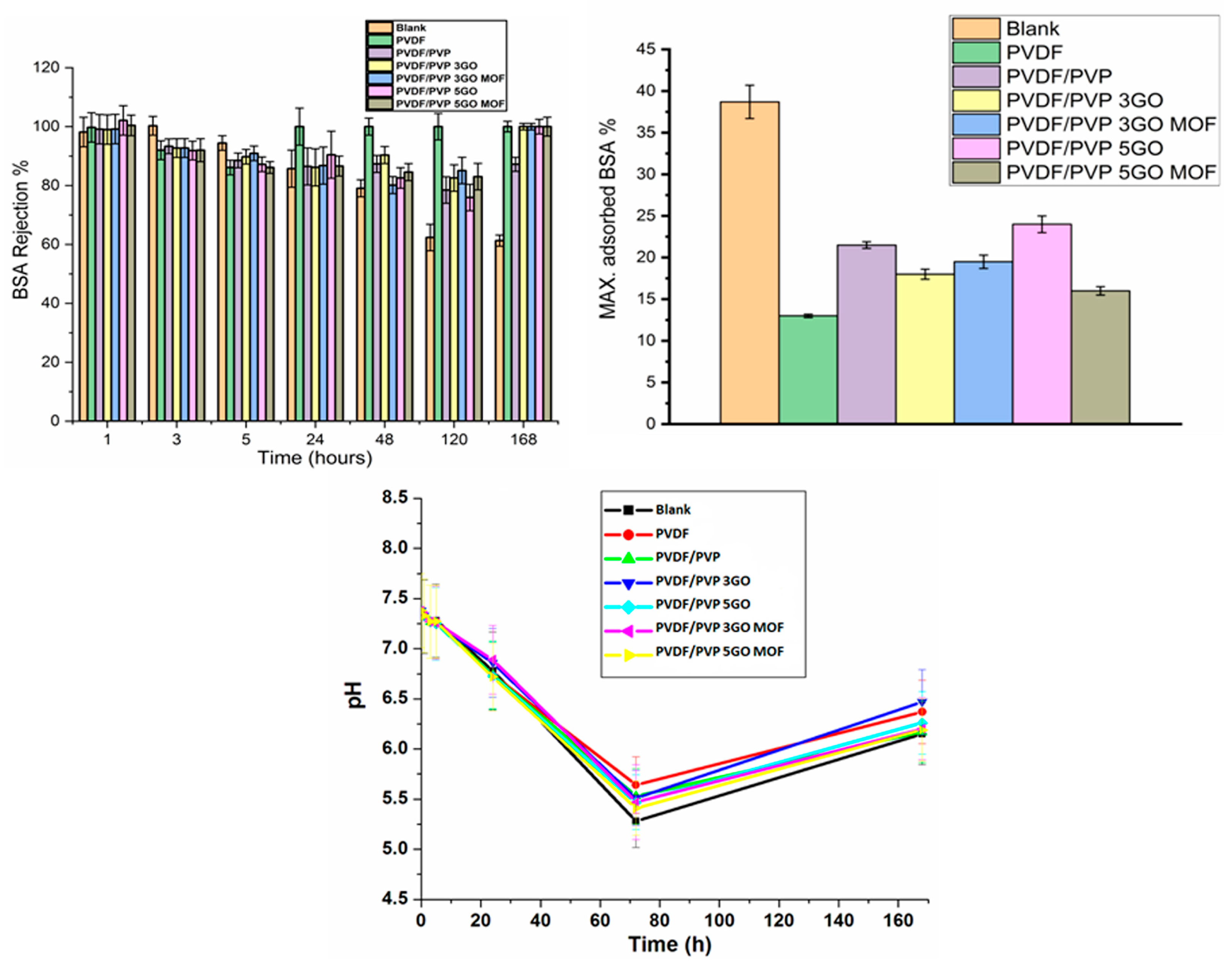
2.6.2. Changes in pH Values
2.7. Anti-Biofouling Activity against Bacteria
2.8. Drug Delivery through Different Membranes
2.8.1. Drug Loading
2.8.2. Drug Release
3. Results and Discussion
3.1. Scanning Electron Microscopy
3.2. Characterizations of the Coating Materials
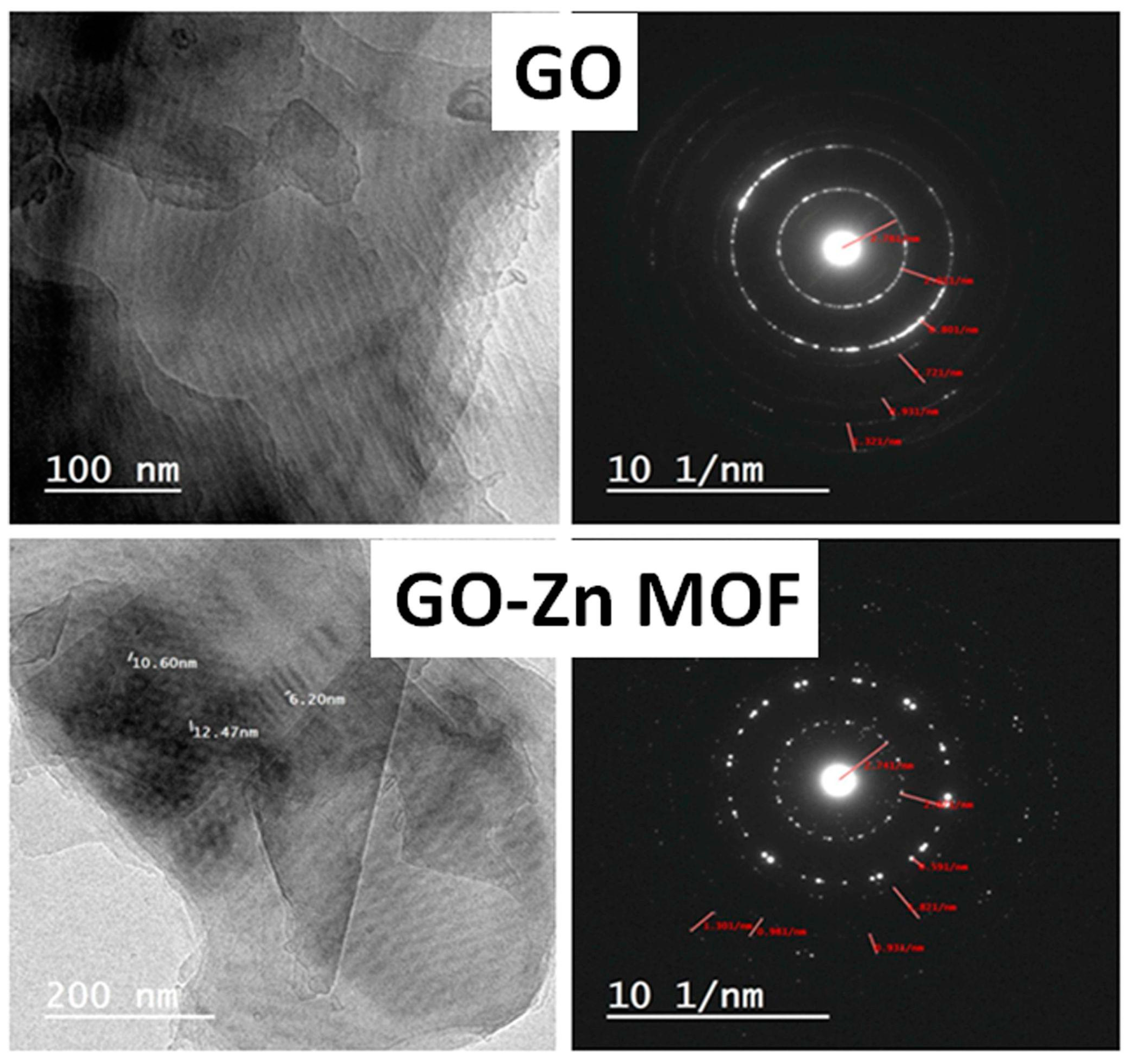
3.2.1. Transmittance Electron Microscopy (TEM)
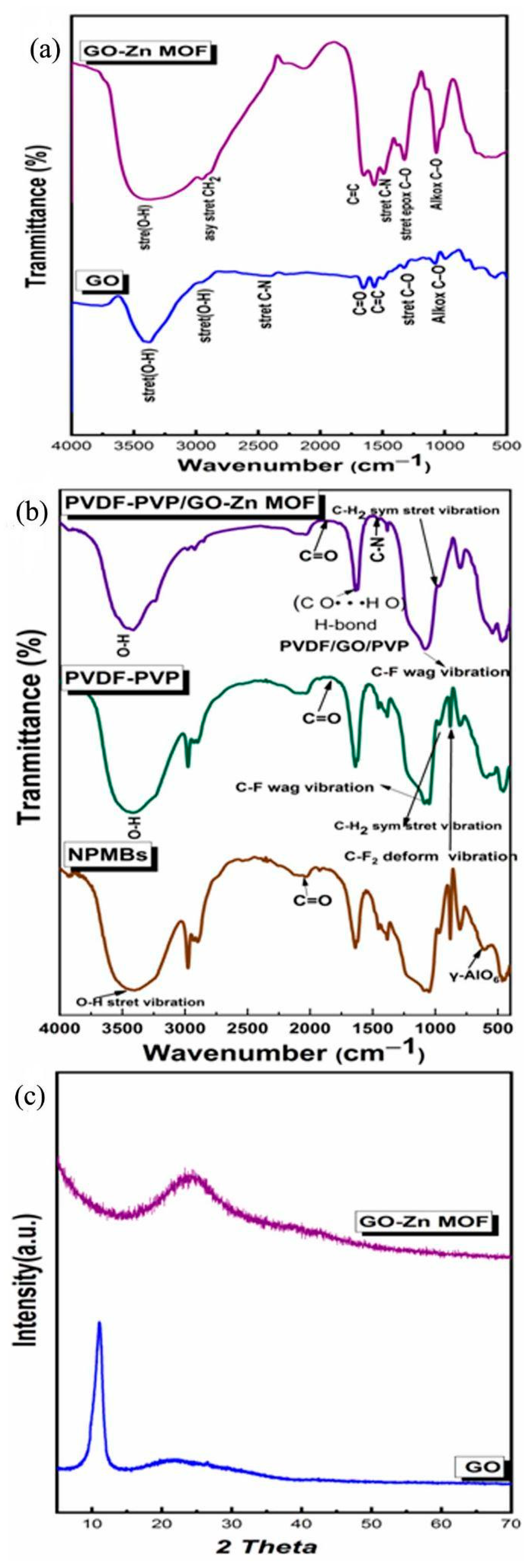
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR) of GO and GO-Zn MOF Composites
3.2.3. FTIR Analysis of NBMBs and Coated Membranes
3.2.4. X-ray Diffraction (XRD)
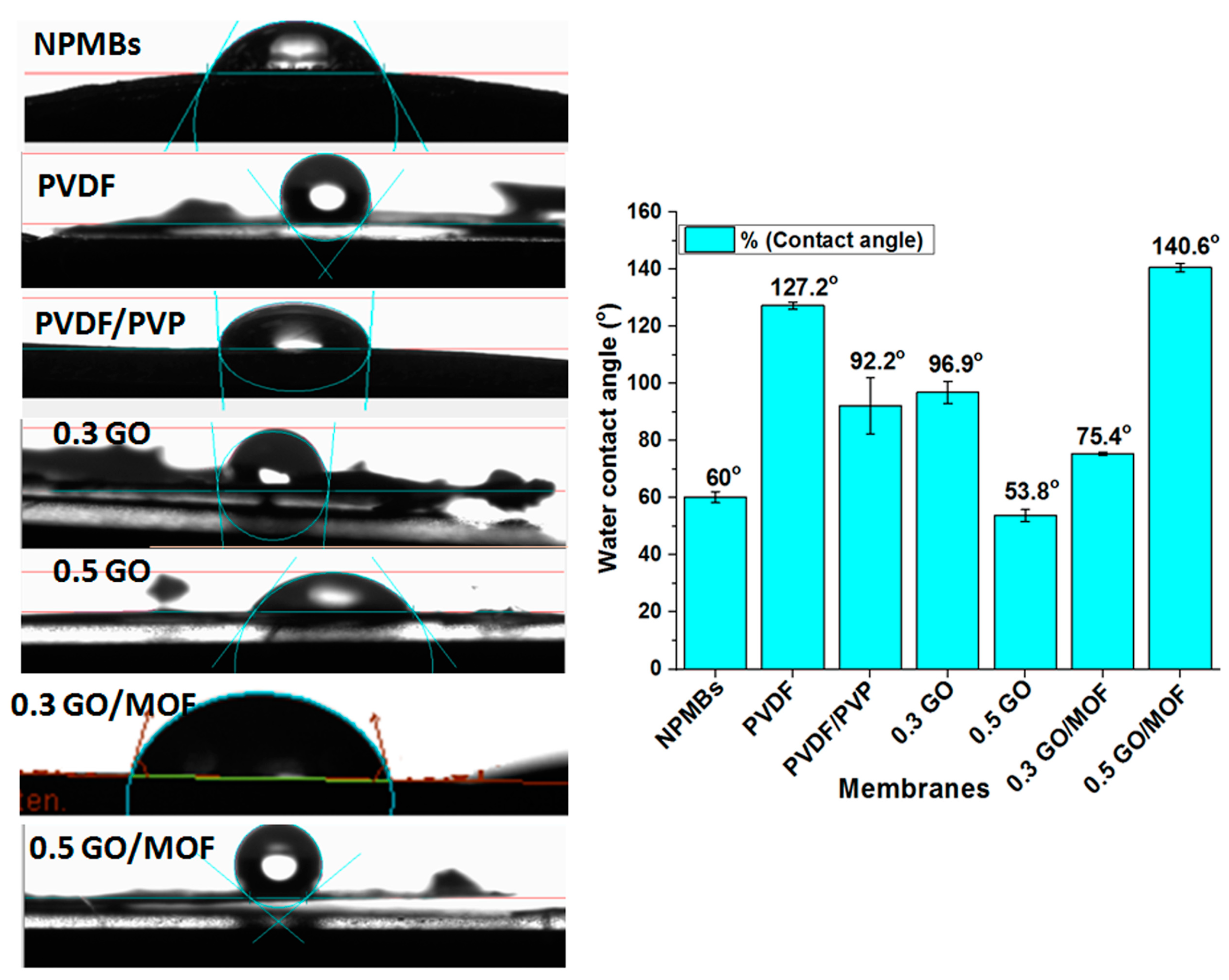
3.3. Contact Angle
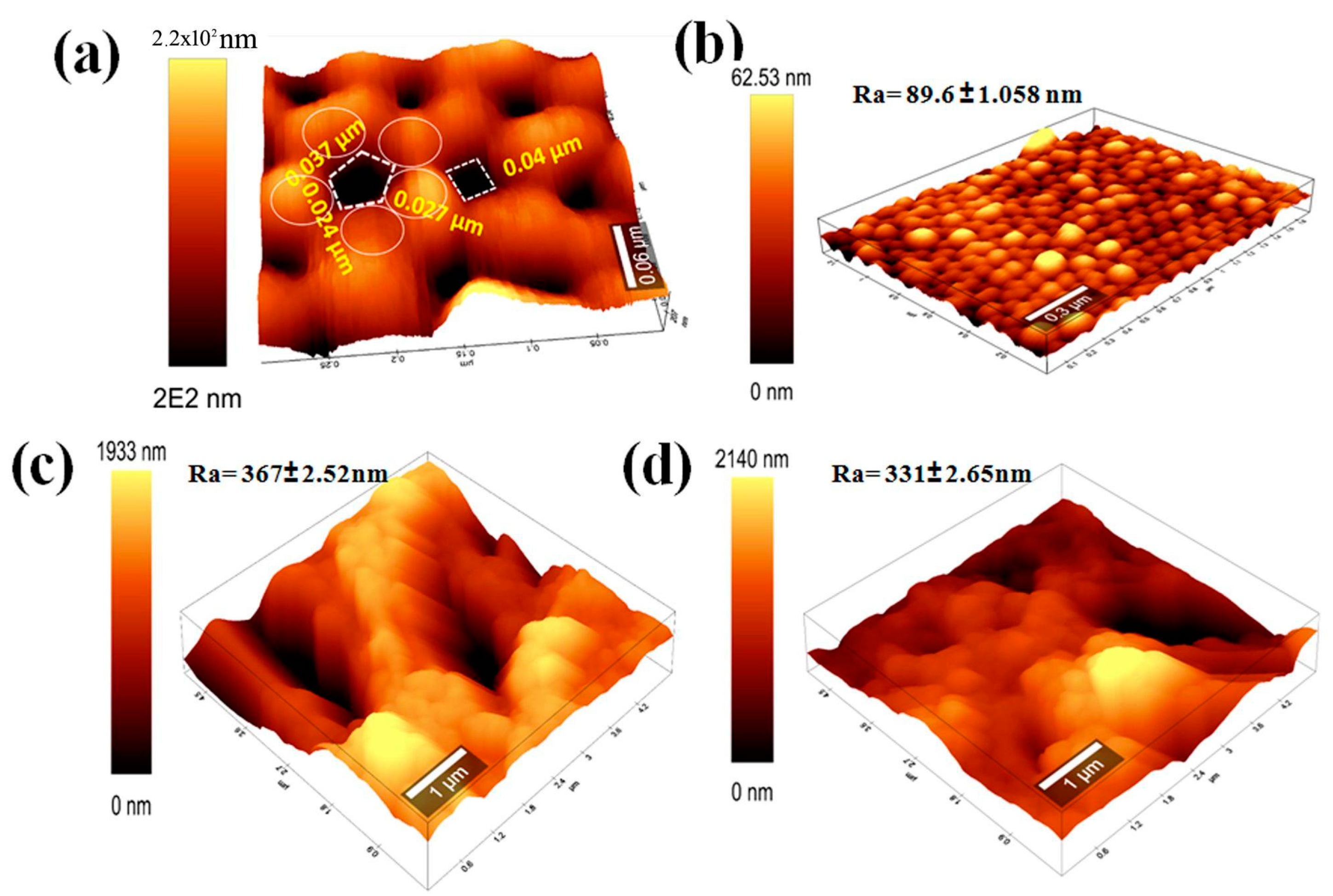
3.4. Atomic Force Microscopy
3.5. Stability of Composite under ACSF Conditions
3.5.1. ACSF Ions Concentrations
3.5.2. Antifouling Tests
Adsorption of Bovine Serum Albumin
SEM of the Membranes after BSA Static Immersion
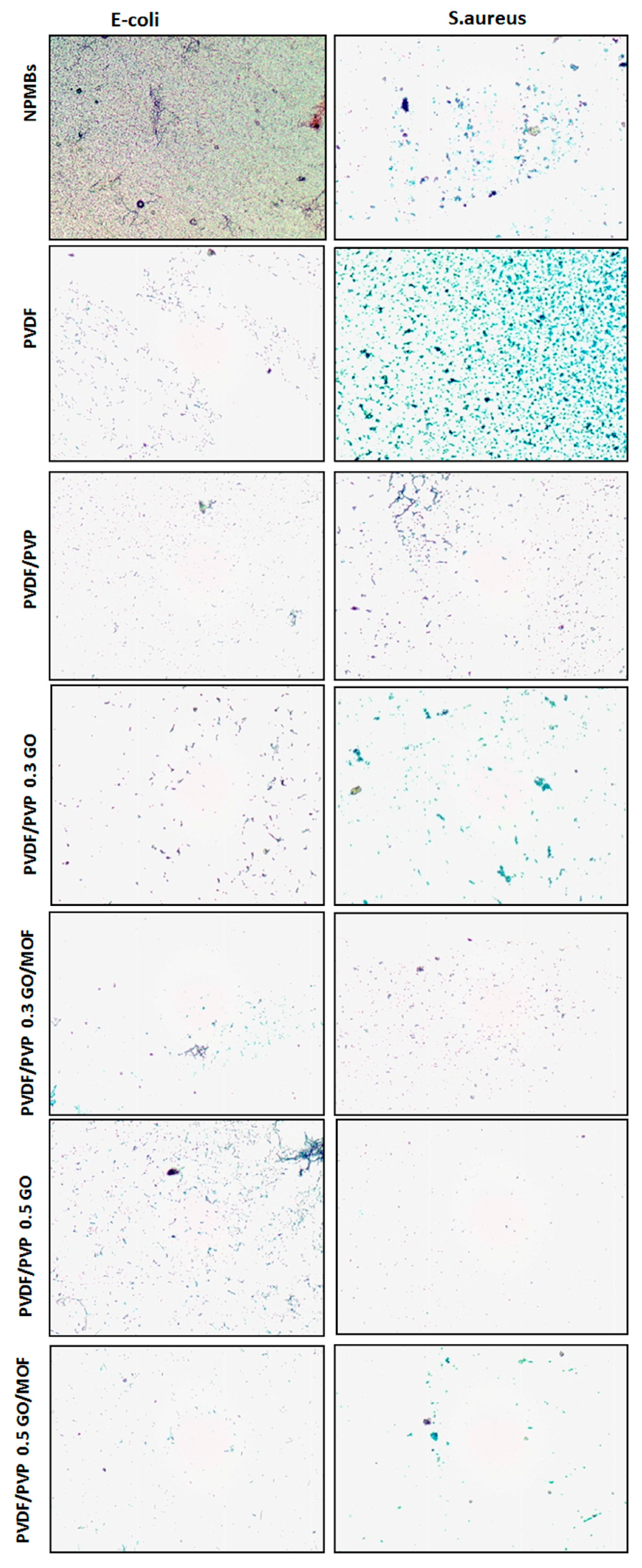
Anti-Biofouling Activity against Bacteria
3.6. Drug Delivery
Drug Releasing Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Staples, M.; Daniel, K.; Cima, M.; Langer, R. Application of micro- and nanoelectromechanical devices to drug delivery. Pharm. Res. 2006, 23, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Long, D.M., Jr.; Rosenbaum, R. Silicone rubber: A new diffusion property useful for general anesthesia. Science 1966, 154, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Greatbatch, W.; Holmes, C.F. History of implantable devices. IEEE Eng. Med. Biol. Mag. 1991, 10, 38–41. [Google Scholar] [CrossRef]
- Tokuda, D.; Ng, T.; Shiosaka, S.; Tano, Y.; Ohta, J. Implantable microimagers. Sensors 2008, 8, 3183–3204. [Google Scholar]
- Berger, T.W.; Baudry, M.; Brinton, R.D.; Liaw, J.S.; Marmarelis, V.Z.; Park, A.Y.; Sheu, B.J.; Tanguay, A.R. Brain-implantable biomimetic electronics as the next era in neural prosthetics. Proc. IEEE 2001, 89, 993–1012. [Google Scholar] [CrossRef]
- Li, C.M.; Dong, H.; Cao, X.; Luong, J.H.; Zhang, X. Implantable electrochemical sensors for biomedical and clinical applications: Progress, problems, and future possibilities. Curr. Med. Chem. 2007, 14, 937–951. [Google Scholar]
- Regar, E.; Sianos, G.; Serruys, P.W. Stent development and local drug delivery. Br. Med. Bull. 2001, 59, 227–248. [Google Scholar] [CrossRef]
- Hulse, G.K.; Tait, R.J.; Comer, S.D.; Sullivan, M.A.; Jacobs, I.G.; Arnold-Reed, D. Reducing hospital presentations for opioid overdose in patients treated with sustained release naltrexone implants. Drug Alcohol. Depend. 2005, 79, 351–357. [Google Scholar] [CrossRef]
- Gultepe, E.; Nagesha, D.; Sridhar, S.; Amiji, M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Deliv. Rev. 2010, 62, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal lymphatics affect microglia responses and anti-Abeta immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rusinek, H.; Butler, T.; Glodzik, L.; Pirraglia, E.; Babich, J.; Mozley, P.D.; Nehmeh, S.; Pahlajani, S.; Wang, X.; et al. Decreased CSF clearance and increased brain amyloid in Alzheimer’s disease. Fluids Barriers CNS 2022, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C.; Sullivan, J.P.O.; Vaszko, B. The direct observation of barrier layers in porous anodic oxide films. J. Electrochem. Soc. 1968, 115, 618–620. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Mor, G.; Grimes, C.A.; Desai, T.A. Surface modification of nanoporous alumina surfaces with poly(ethylene glycol). Langmuir 2004, 20, 8035–8041. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gosele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Gultepe, E.; Nagesha, D.; Menon, L.; Busnaina, A.; Sridhar, S. High-throughput assembly of nanoelements in nanoporous alumina templates. Appl. Phys. Lett. 2007, 90, 163119. [Google Scholar] [CrossRef]
- Sedel, L. Evolution of alumina-on-alumina implants: A review. Clin. Orthop. Relat. Res. 2000, 379, 48–54. [Google Scholar] [CrossRef]
- La Flamme, K.E.; Popat, K.C.; Leoni, L.; Markiewicz, E.; La Tempa, T.J.; Roman, B.B.; Grimes, C.A.; Desai, T.A. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials 2007, 28, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Dinh, T.T.L.; Ahn, Y.-H. Enhanced antifouling performance of PVDF ultrafiltration membrane by blending zinc oxide with support of graphene oxide nanoparticle. Chemosphere 2022, 241, 125068. [Google Scholar] [CrossRef] [PubMed]
- Javad, M.P.; Mahreen, A.; Ahmed, M. Biomedical antifouling polymer nanocomposites. In Advances in Biomedical Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Sharma, P.; Agrawal, S.; Rathore, M.S.; Shahi, V.K. Cross-linked anion-exchange membrane with side-chain grafted multi-cationic spacer for electrodialysis: Imparting dual anti-fouling anti-bacterial characteristics. J. Membr. Sci. 2022, 660, 120871. [Google Scholar] [CrossRef]
- Makhetha, T.A.; Moutloali, R.M. Stable zeolitic imidazolate framework-8 supported onto graphene oxide hybrid ultrafiltration membranes with improved fouling resistance and water flux. Chem. Eng. J. Adv. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Hydrophilic ZIF-8 decorated GO nanosheets improve biocompatibility and separation performance of polyethersulfone hollow fiber membranes: A potential membrane material for bioartificial liver application. Mater. Sci. Eng. 2018, 9, 524–540. [Google Scholar] [CrossRef]
- Shiraishi, T.; Kikuchi, T.; Fukushi, K.; Shinotoh, H.; Nagatsuka, S.I.; Tanaka, N.; Irie, T. Estimation of plasma IC50 of donepezil hydrochloride for brain acetylcholinesterase inhibition in monkey using N-[11C] methylpiperidin-4-yl acetate ([11C], M.P.4.A.). PET Neuropsychopharmacol. 2005, 30, 2154–2161. [Google Scholar] [CrossRef]
- Kapruwan, P.; Ferré-Borrull, J.; Marsal, L.F. Nanoporous anodic alumina platforms for drug delivery applications: Recent advances and perspective. Adv. Mater. Interfaces 2020, 7, 2001133. [Google Scholar] [CrossRef]
- Salim, N.; Siddiqa, A.; Shahida, S.; Qaisar, S. PVDF based Nanocomposite Membranes: Application towards Wastewater treatment. Madridge J. Nanotechnol. Nanosci. 2019, 4, 139–147. [Google Scholar] [CrossRef]
- ASTM-D 7334-08; Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. ASTM: West Conshohocken, PA, USA, 2013.
- Kamen, Y.; Káradóttir, R.T. Combining whole-cell patch clamp and dye loading in acute brain slices with bulk RNA sequencing in embryonic to aged mice. STAR Protocols. 2021, 2, 100439. [Google Scholar] [CrossRef]
- Ni, X.X.; Li, J.H.; Yu, L.P. Correction: A novel PVDF hybrid membrane with excellent active–passive integrated antifouling and antibacterial properties based on a PDA guiding effect. Mater. Adv. 2022, 3, 2945. [Google Scholar] [CrossRef]
- Xu, W.; Zhuang, H.; Xu, Z.; Huang, M.; Gao, S.; Li, Q.; Zhang, G. Design and Construction of Ag@MOFs Immobilized PVDF Ultrafiltration Membranes with Anti-bacterial and Antifouling Properties. Adv. Polym. Technol. 2020, 2020, 5456707. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation and performance of PVDF/ZnO hybrid membranes and their application in removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Akhshabi, S.; Biazar, E.; Singh, V.; Keshel, S.H.; Geetha, N. The effect of the carbodiimide cross-linker on the structural and biocompatibility properties of collagen–chondroitin sulfate electrospun mat. Int. J. Nanomed. 2018, 13, 4405–4416. [Google Scholar] [CrossRef]
- Jarosz, M.; Pawlik, A.; Szuwarzyński, M.; Jaskuła, M.; Sulka, G.D. Nanoporous anodic titanium dioxide layers as potential drug delivery systems: Drug release kinetics and mechanism. Colloids Surf. B 2016, 143, 447–454. [Google Scholar] [CrossRef]
- Sreedharan, S.M.; Singh, R. Ciprofloxacin functionalized biogenic gold nanoflowers as nanoantibiotics against pathogenic bacterial strains. Int. J. Nanomed. 2019, 14, 9905–9916. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Geng, C.; Zhao, F.; Wang, Q.; Zheng, S.; Liu, Y.; Niu, H.; Zhang, J.; Dong, H. Anti-biofouling property anti-leaching investigation of modifier for PVDF ultrafiltration membrane by incorporating antibacterial grapheme oxide derivatives. J. Environ. Chem. Eng. 2022, 10, 108558. [Google Scholar] [CrossRef]
- Geng, C.; Fan, L.A.; Niu, H.; Liu, L.; Zhao, F.; Zhang, J.; Dong, H.; Yu, S. Improved anti-organic fouling and antibacterial properties of PVDF ultrafiltration membrane by one-step grafting imidazole-functionalized graphene oxide. Mater. Sci. Eng. C 2021, 131, 112517. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, Y.; Uliana, A.A.; Zhu, J. Zeolitic Imidazolate Framework/Graphene Oxide Hybrid Nanosheets Functionalized Thin Film Nanocomposite Membrane for Enhanced Antimicrobial Performance. ACS Appl. Mater. Interfaces 2016, 8, 25508–25519. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Buldakov, D.A.; Tishkin, A.A.; Lukashin, A.V.; Eliseev, A.A. Liquid permeation and chemical stability of anodic alumina membranes. Beilstein J. Nanotechnol. 2017, 8, 561–570. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinyl pyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Sun, W.; Shen, F.; Wang, Z.; Zhang, Y.; Wan, Y. An ultrathin, porous and in-air hydrophilic/underwater oleophobic coating simultaneously increasing the flux and antifouling property of membrane for membrane distillation. Desalin 2018, 445, 40–50. [Google Scholar] [CrossRef]
- Wang, Z.; Zuilhof, H. Antifouling Properties of Fluoropolymer Brushes toward Organic Polymers: The Influence of Composition, Thickness, Brush Architecture, and Annealing. Langmuir 2016, 32, 6571–6581. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Ahn, Y.H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity permeability antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Nackaerts, R.; Thwala, J.M.; Mamba, B.B.; Verliefde, A.R.D. Hydrophilic fouling-resistant GO-ZnO/PES membranes for wastewater reclamation. J. Membr. Sci. 2017, 524, 43–55. [Google Scholar] [CrossRef]
- Ingerman, L.; Jones, D.G.; Keith, S.; Rosemond, Z.A. Toxicological Profile for Aluminum. 2008, Reported by Agency for Toxic Substances and Disease Registry. 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333. Available online: https://stacks.cdc.gov/view/cdc/5289 (accessed on 15 February 2024).
- Tofighy, M.A.; Mohammadi, T.; Sadeghi, M.H. High-flux PVDF/PVP nanocomposite ultrafiltration membrane incorporated with graphene oxide nanoribbones with improved antifouling properties. J. Appl. Polym. Sci. 2020, 138, e49718. [Google Scholar] [CrossRef]
- Moaness, M.; Mabrouk, M.; Ahmed, M.M.; Das, D.B.; Beherei, H.H. Novel zinc-silver nanocages for drug delivery and wound healing: Preparation, characterization and antimicrobial activities. Int. J. Pharm. 2022, 616, 121559. [Google Scholar] [CrossRef]
- Aminudin, N.N.; Basri, H.; Yunos, M.Z.; Harun, Z.; Sean, G.P. Effect of pH on Bovine Serum Albumin (BSA) Solution to Polysulfone-based Membrane. J. Teknol. 2014, 70, 77–79. [Google Scholar] [CrossRef][Green Version]
- Zolghadr, E.; Firouzjaei, M.D.; Aktij, S.A.; Aghaei, A.; Wujcik, E.K.; Sadrzadeh, M.; Rahimpour, A.; Afkhami, F.A.; LeClair, P.; Elliott, M.; et al. An ultrasonic-assisted rapid approach for sustainable fabrication of antibacterial and anti-biofouling membranes via metal-organic frameworks. Mater. Today Chem. 2022, 26, 101044. [Google Scholar] [CrossRef]



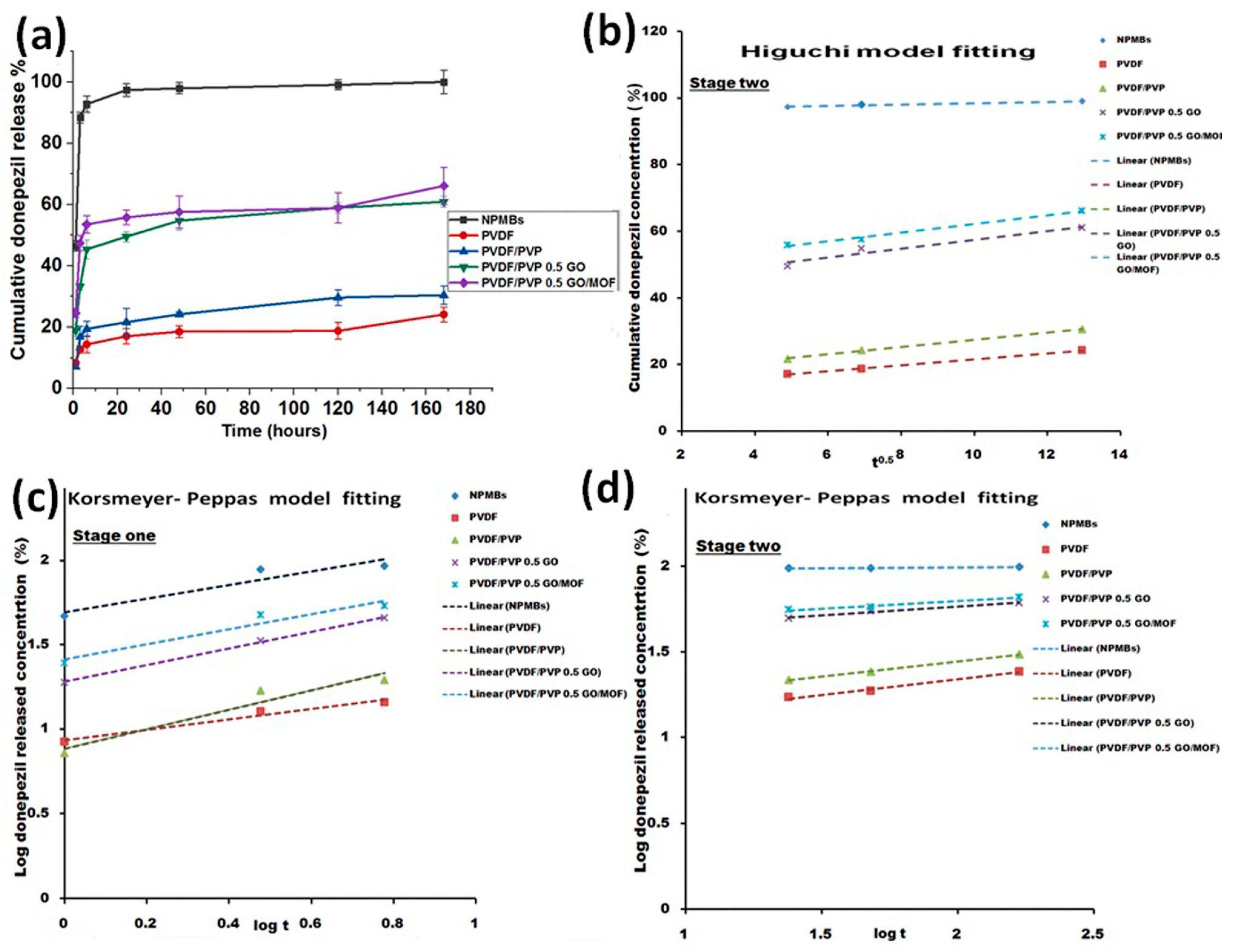
| Formula Code | R2-Value † | Korsmeyer–Peppas Model | n | RE0–6 h ‡ (%) | |||
| Zero-Order | Fickian Diffusion | Korsmeyer–Peppas Model | t50** (Hours) | t90*** (Hours) | |||
| NPMBs | 0.724292 | 0.82468 | 0.894398 | 1.17 | 3.19 | 0.403 | 92.7 |
| PVDF | 0.867891 | 0.9383 | 0.968293 | 16.7 | 31.9 | 0.307 | 14.4 |
| PVDF/PVP | 0.825071 | 0.906514 | 0.935472 | 10.7 | 19.8 | 0.575 | 19.44 |
| PVDF/PVP 0.5 GO | 0.973343 | 0.998133 | 0.998647 | 4.3 | 8.6 | 0.5 | 45.3 |
| PVDF/PVP 0.5 GO/MOF | 0.820906 | 0.903313 | 0.939609 | 3.19 | 6.65 | 0.45 | 53.53 |
| Formula Code | R2-Value † | Korsmeyer–Peppas Model | n | RE6–168 h ‡ (%) | |||
| Zero-Order | Fickian Diffusion | Korsmeyer–Peppas Model | t50** (Hours) | t90*** (Hours) | |||
| NPMBs | 0.962 | 0.988 | 1 | --- | --- | 0.008 | 99.9 |
| PVDF | 0.998 | 0.998 | 0.984 | 30.1 | 43.3 | 0.18 | 24.2 |
| PVDF/PVP | 0.982 | 0.9981 | 0.9991 | 24 | 32.5 | 0.177 | 30.5 |
| PVDF/PVP 0.5 GO | 0.9022 | 0.948 | 0.979 | 14.4 | 11.8 | 0.104 | 60.95 |
| PVDF/PVP 0.5 GO/MOF | 0.99998 | 0.993 | 0.9660 | 14.4 | 10.2 | 0.0898 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moaness, M.; El-Sayed, S.A.M.; Beherei, H.H.; Mabrouk, M. Enhancing the Antifouling Properties of Alumina Nanoporous Membranes by GO/MOF Impregnated Polymer Coatings: In Vitro Studies. J. Funct. Biomater. 2024, 15, 50. https://doi.org/10.3390/jfb15030050
Moaness M, El-Sayed SAM, Beherei HH, Mabrouk M. Enhancing the Antifouling Properties of Alumina Nanoporous Membranes by GO/MOF Impregnated Polymer Coatings: In Vitro Studies. Journal of Functional Biomaterials. 2024; 15(3):50. https://doi.org/10.3390/jfb15030050
Chicago/Turabian StyleMoaness, Mona, Sara A. M. El-Sayed, Hanan H. Beherei, and Mostafa Mabrouk. 2024. "Enhancing the Antifouling Properties of Alumina Nanoporous Membranes by GO/MOF Impregnated Polymer Coatings: In Vitro Studies" Journal of Functional Biomaterials 15, no. 3: 50. https://doi.org/10.3390/jfb15030050
APA StyleMoaness, M., El-Sayed, S. A. M., Beherei, H. H., & Mabrouk, M. (2024). Enhancing the Antifouling Properties of Alumina Nanoporous Membranes by GO/MOF Impregnated Polymer Coatings: In Vitro Studies. Journal of Functional Biomaterials, 15(3), 50. https://doi.org/10.3390/jfb15030050








