Specimen Shape and Elution Time Affect the Mineralization and Differentiation Potential of Dental Pulp Stem Cells to Biodentine
Abstract
1. Introduction
2. Materials and Methods
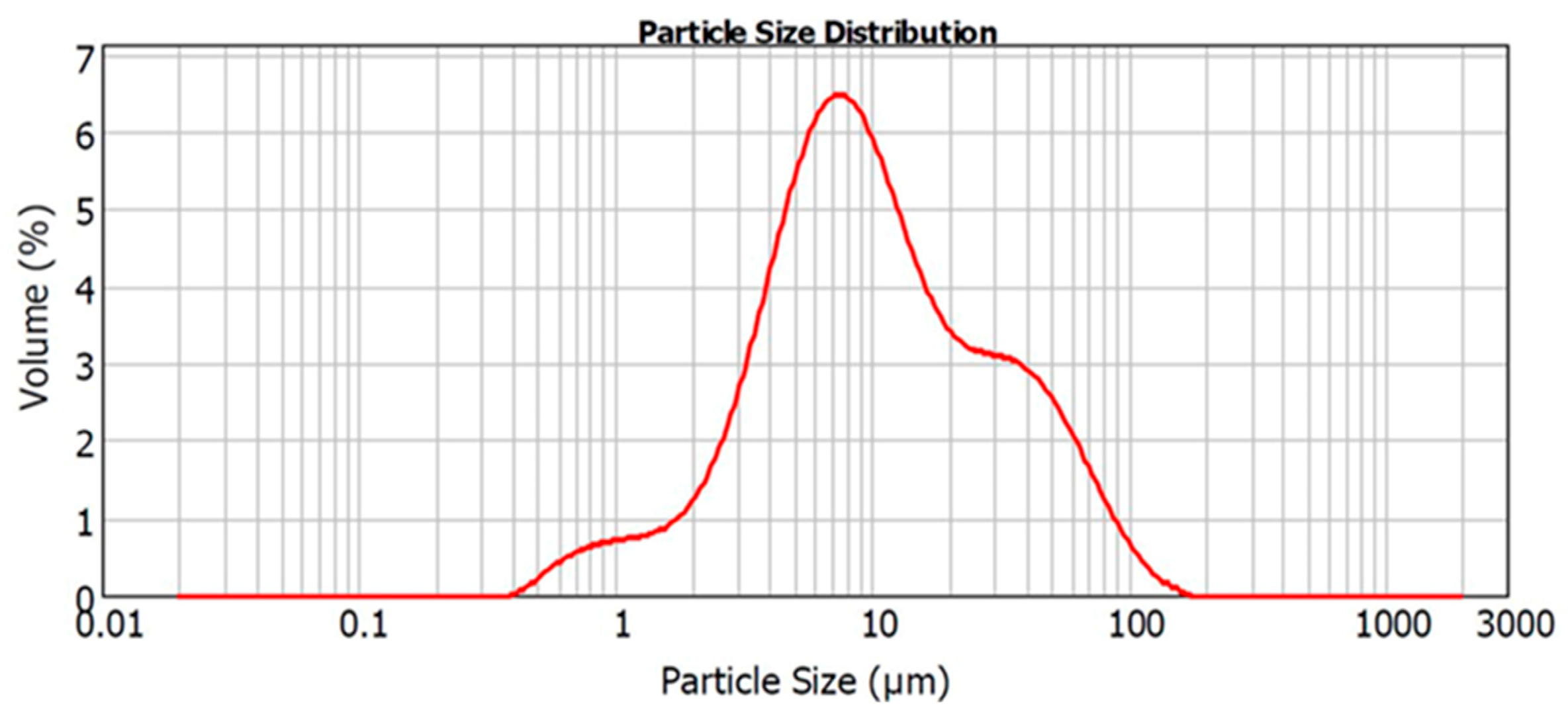
2.1. Material and Elution Preparation
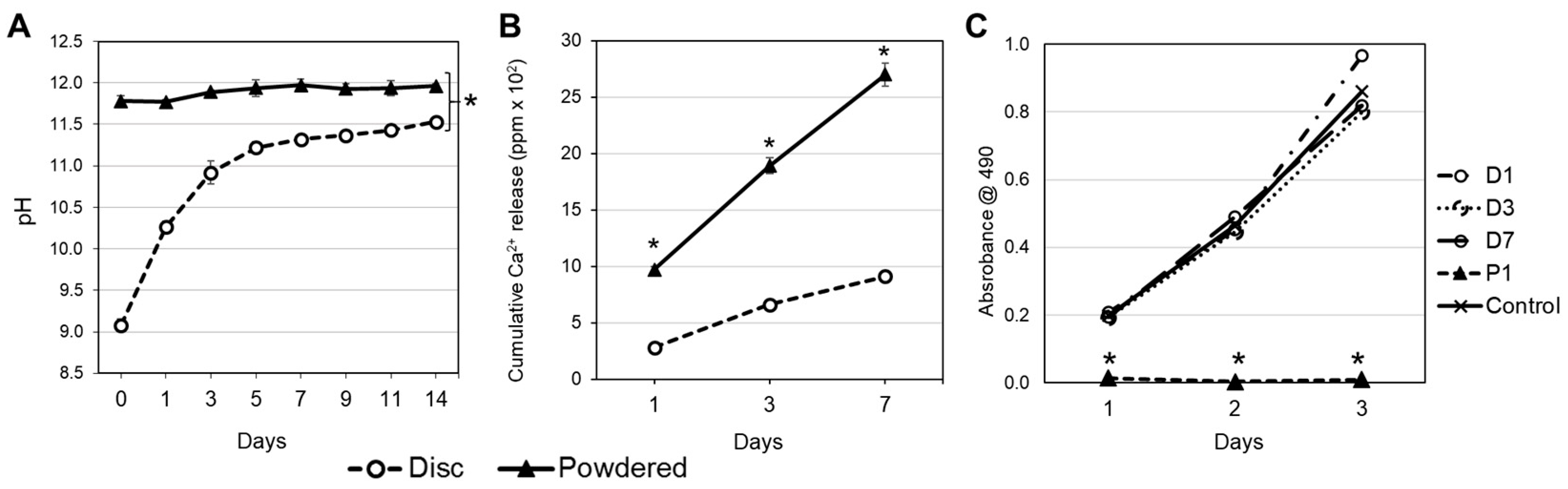
2.2. pH Measurement of Leachate
2.3. Calcium Release
2.4. Cell Culture and Characterization
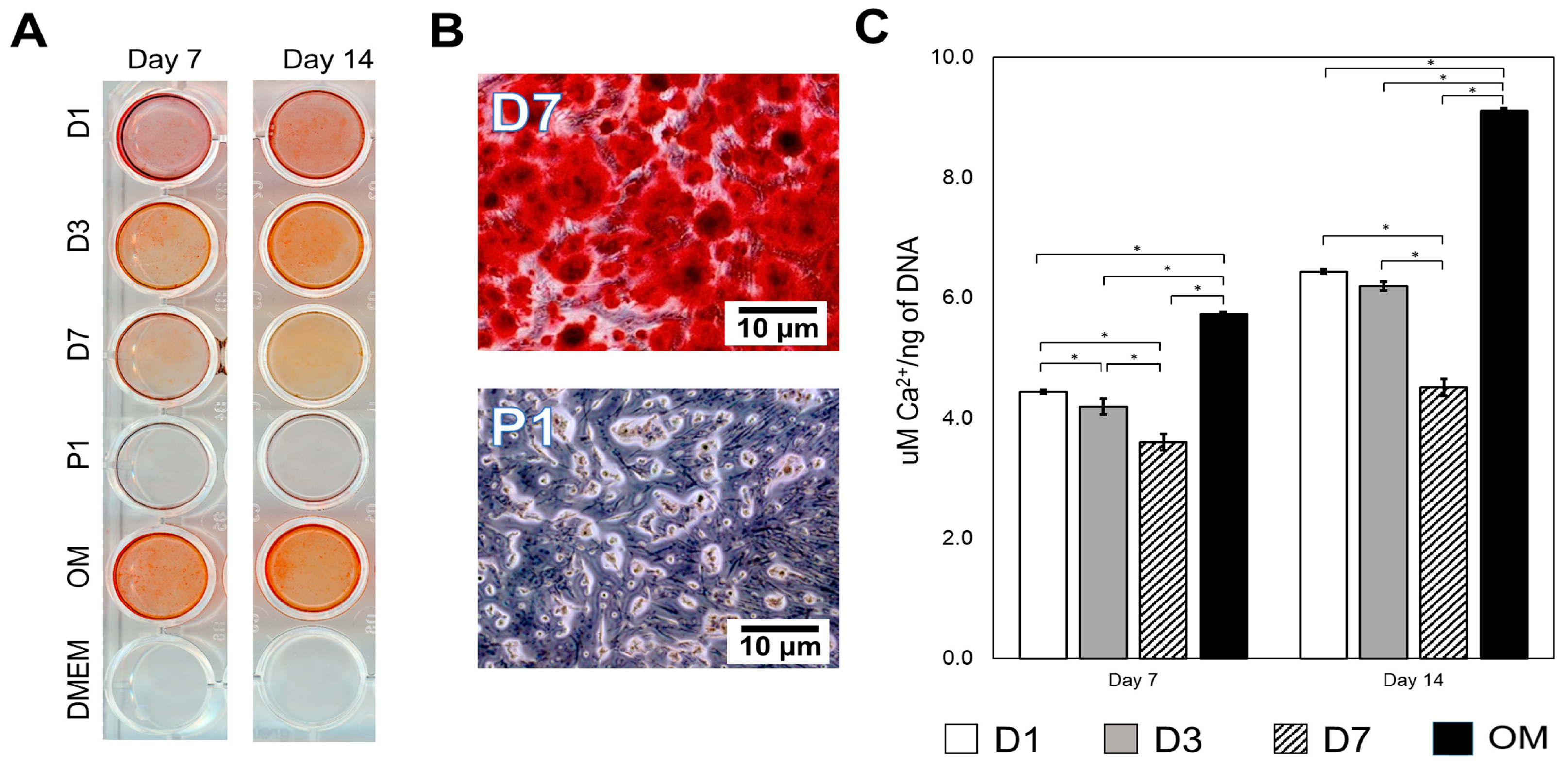
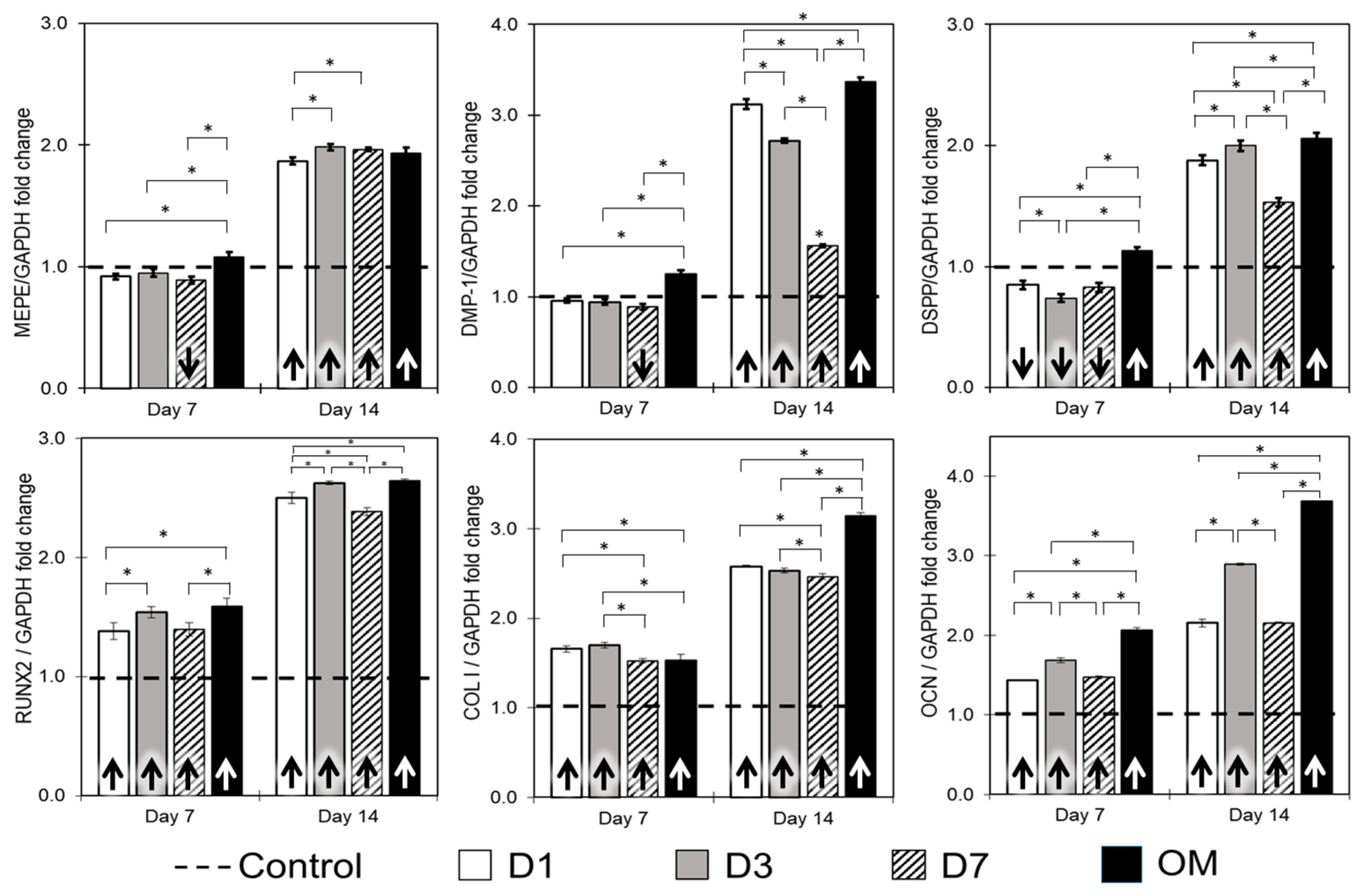
2.5. Cell Proliferation and Differentiation
2.6. Statistical Analysis
3. Results
3.1. pH Variation, Calcium Release and Cell Proliferation
3.2. Mineralization Potential and Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, C.; Sübay, R.; Ostro, E.; Suzuki, S.; Suzuki, S. Tunnel defects in dentin bridges: Their formation following direct pulp capping. Oper. Dent. 1995, 21, 4–11. [Google Scholar]
- Ferracane, J.L.; Cooper, P.R.; Smith, A.J. Can interaction of materials with the dentin-pulp complex contribute to dentin regeneration? Odontology 2010, 98, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Vallés, M.; Roig, M.; Duran-Sindreu, F.; Martínez, S.; Mercadé, M. Color stability of teeth restored with biodentine: A 6-month in vitro study. J. Endod. 2015, 41, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Abuarqoub, D.; Aslam, N.; Jafar, H.; Abu Harfil, Z.; Awidi, A. Biocompatibility of biodentine™® with periodontal ligament stem cells: In vitro study. Dent. J. 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. Biodentinetm induces tgf-β1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Cornélio, A.; Rodrigues, E.; Salles, L.; Mestieri, L.; Faria, G.; Guerreiro-Tanomaru, J.; Tanomaru-Filho, M. Bioactivity of mta plus, biodentine and an experimental calcium silicate-based cement on human osteoblast-like cells. Int. Endod. J. 2016, 50, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.B.; Pieroni, K.; Nelson-Filho, P.; Silva, R.A.B.; Hernandez-Gaton, P.; Lucisano, M.P.; Paula-Silva, F.W.G.; de Queiroz, A.M. Furcation perforation: Periradicular tissue response to biodentine as a repair material by histopathologic and indirect immunofluorescence analyses. J. Endod. 2017, 43, 1137–1142. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, S.-Y.; Ann, H.-J.; Kum, K.-Y.; Kim, E.-C. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J. Endod. 2014, 40, 1194–1200. [Google Scholar] [CrossRef]
- Birant, S.; Gokalp, M.; Duran, Y.; Koruyucu, M.; Akkoc, T.; Seymen, F. Cytotoxicity of neomta plus, proroot mta and biodentine on human dental pulp stem cells. J. Dent. Sci. 2021, 16, 971–979. [Google Scholar] [CrossRef]
- Xie, H.; Dubey, N.; Shim, W.; Ramachandra, C.J.A.; Min, K.S.; Cao, T.; Rosa, V. Functional odontoblastic-like cells derived from human ipscs. J. Dent. Res. 2018, 97, 77–83. [Google Scholar] [CrossRef]
- Daltoé, M.O.; Paula-Silva, F.W.G.; Faccioli, L.H.; Gatón-Hernández, P.M.; De Rossi, A.; Silva, L.A.B. Expression of mineralization markers during pulp response to biodentine and mineral trioxide aggregate. J. Endod. 2016, 42, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Natu, V.P.; Dubey, N.; Loke, G.C.; Tan, T.S.; Ng, W.H.; Yong, C.W.; Cao, T.; Rosa, V. Bioactivity, physical and chemical properties of mta mixed with propylene glycol. J. Appl. Oral Sci. 2015, 23, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.-R.; Emara, R.; Taher, M.M.; Al-Allaf, F.A.; Almalki, M.; Almasri, M.A.; Siddiqui, S.S. Effects of mineral trioxide aggregate, calcium hydroxide, biodentine and emdogain on osteogenesis, odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral Health 2019, 19, 133. [Google Scholar] [CrossRef]
- Thangaraju, P.; Varthya, S.B. ISO 10993: Biological evaluation of medical devices. In Medical Device Guidelines and Regulations Handbook; Springer: Berlin/Heidelberg, Germany, 2022; pp. 163–187. [Google Scholar]
- Schmalz, G. Use of cell cultures for toxicity testing of dental materials—Advantages and limitations. J. Dent. 1994, 22, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- ISO E. 10993-5; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Li, X.; Pedano, M.S.; Li, S.; Sun, Z.; Jeanneau, C.; About, I.; Hauben, E.; Chen, Z.; Van Landuyt, K.; Van Meerbeek, B. Preclinical effectiveness of an experimental tricalcium silicate cement on pulpal repair. Mater. Sci. Eng. C 2020, 116, 111167. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Guillén, A.; López-García, S.; Rodríguez-Lozano, F.J.; Sanz, J.L.; Lozano, A.; Llena, C.; Forner, L. Comparative cytocompatibility of the new calcium silicate-based cement neoputty versus neomta plus and mta on human dental pulp cells: An in vitro study. Clin. Oral Investig. 2022, 26, 7219–7228. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, S.; Alcántara-Dufeu, R.; Luengo Machuca, L.; Ferrada, L.; Sánchez-Sanhueza, G.A. Real-time evaluation of the biocompatibility of calcium silicate-based endodontic cements: An in vitro study. Clin. Exp. Dent. Res. 2023, 9, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Zanini, M.; Sautier, J.M.; Berdal, A.; Simon, S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J. Endod. 2012, 38, 1220–1226. [Google Scholar] [CrossRef]
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W.-X. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase ii pathways. J. Endod. 2014, 40, 937–942. [Google Scholar] [CrossRef]
- Luo, Z.; Li, D.; Kohli, M.R.; Yu, Q.; Kim, S.; He, W.-X. Effect of biodentine™ on the proliferation, migration and adhesion of human dental pulp stem cells. J. Dent. 2014, 42, 490–497. [Google Scholar] [CrossRef]
- Pérard, M.; Le Clerc, J.; Meary, F.; Pérez, F.; Tricot-Doleux, S.; Pellen-Mussi, P. Spheroid model study comparing the biocompatibility of biodentine and mta. J. Mater. Sci. Mater. Med. 2013, 24, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Ciołek, L.; Jaegermann, Z.; Zaczyńska, E.; Czarny, A.; Biernat, M.; Gąsiński, A.; Jastrzębska, A.; Gloc, M.; Olszyna, A. Cytotoxicity and antibacterial activity of mineral trioxide aggregate cement with radiopacity introduced by ZrO2. Bioinorg. Chem. Appl. 2022, 2022, 9574245. [Google Scholar] [CrossRef] [PubMed]
- Karkehabadi, H.; Ahmadyani, E.; Najafi, R.; Khoshbin, E. Effect of biodentine coated with emdogain on proliferation and differentiation of human stem cells from the apical papilla. Mol. Biol. Rep. 2022, 49, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Woo, S.M.; Lee, B.N.; Koh, J.T.; Nör, J.; Hwang, Y.C. Effect of biodentine and bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int. Endod. J. 2014, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-M.; Shen, Y.; Wang, Z.-J.; Li, L.; Zheng, Y.-F.; Häkkinen, L.; Haapasalo, M. In vitro cytotoxicity evaluation of a novel root repair material. J. Endod. 2013, 39, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Küden, C.; Karakaş, S.N.; Batmaz, S.G. Comparative chemical properties, bioactivity, and cytotoxicity of resin-modified calcium silicate–based pulp capping materials on human dental pulp stem cells. Clin. Oral Investig. 2022, 26, 6839–6853. [Google Scholar] [CrossRef]
- Agrafioti, A.; Taraslia, V.; Chrepa, V.; Lymperi, S.; Panopoulos, P.; Anastasiadou, E.; Kontakiotis, E.G. Interaction of dental pulp stem cells with biodentine and mta after exposure to different environments. J. Appl. Oral Sci. 2016, 24, 481–486. [Google Scholar] [CrossRef]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.P.; Islam, I.; Neto, A.H.C. Graphene oxide-based substrate: Physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent. Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef]
- Main, C.; Mirzayan, N.; Shabahang, S.; Torabinejad, M. Repair of root perforations using mineral trioxide aggregate: A long-term study. J. Endod. 2004, 30, 80–83. [Google Scholar] [CrossRef]
- Tolibah, Y.A.; Kouchaji, C.; Lazkani, T.; Ahmad, I.A.; Baghdadi, Z.D. Comparison of mta versus biodentine in apexification procedure for nonvital immature first permanent molars: A randomized clinical trial. Children 2022, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Awawdeh, L.; Al-Qudah, A.; Hamouri, H.; Chakra, R.J. Outcomes of vital pulp therapy using mineral trioxide aggregate or biodentine: A prospective randomized clinical trial. J. Endod. 2018, 44, 1603–1609. [Google Scholar] [CrossRef]
- Karamichos, D.; Nikfarjam, F.; Beyer, K.; König, A.; Hofmann, M.; Butting, M.; Valesky, E.; Kippenberger, S.; Kaufmann, R.; Heidemann, D.; et al. Influence of biodentine®—A dentine substitute—On collagen type i synthesis in pulp fibroblasts in vitro. PLoS ONE 2016, 11, e0167633. [Google Scholar]
- Halkai, R.S.; Ishaq, S.S.; Halkai, K.R.; Zakaullah, S.; Diwanji, P.R.; Mahveen, S.U.; Algahtani, F.N.; Almohareb, R. Evaluation of ph and dimensional stability of mineral trioxide aggregate and biodentine with and without triple antibiotic medicament: An in vitro study. Saudi Endod. J. 2023, 13, 183–188. [Google Scholar] [CrossRef]
- Atmeh, A.R. Investigating the effect of bicarbonate ion on the structure and strength of calcium silicate-based dental restorative material—Biodentine. Clin. Oral Investig. 2020, 24, 4597–4606. [Google Scholar] [CrossRef] [PubMed]
- Abusrewil, S.; Brown, J.L.; Delaney, C.; Butcher, M.C.; Tiba, M.; Scott, J.A.; Ramage, G.; McLean, W. Chitosan enhances the anti-biofilm activity of biodentine against an interkingdom biofilm model. Antibiotics 2021, 10, 1317. [Google Scholar] [CrossRef]
- Kot, K.; Kucharski, Ł.; Marek, E.; Safranow, K.; Lipski, M. Alkalizing properties of six calcium-silicate endodontic biomaterials. Materials 2022, 15, 6482. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.-B.; Song, D.; Kim, H.-M.; Kim, S.-Y. Cytotoxicity and mineralization potential of four calcium silicate-based cements on human gingiva-derived stem cells. Coatings 2020, 10, 279. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Vercruysse, C.; Martens, L.; Verbeeck, R. Effect of exposed surface area, volume and environmental ph on the calcium ion release of three commercially available tricalcium silicate based dental cements. Materials 2018, 11, 123. [Google Scholar] [CrossRef]
- Santilli, F.; Fabrizi, J.; Pulcini, F.; Santacroce, C.; Sorice, M.; Delle Monache, S.; Mattei, V. Gangliosides and their role in multilineage differentiation of mesenchymal stem cells. Biomedicines 2022, 10, 3112. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Y.; Zhang, M.; Xu, J.; Zhang, C.; Li, J. Effect of biodentine on odonto/osteogenic differentiation of human dental pulp stem cells. Bioengineering 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, P.; Bellomo, G.; Orrenius, S. Calcium-mediated mechanisms in chemically induced cell death. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 449–470. [Google Scholar] [CrossRef] [PubMed]
- Haglund, R.; He, J.; Jarvis, J.; Safavi, K.E.; Spångberg, L.S.; Zhu, Q. Effects of root-end filling materials on fibroblasts and macrophages in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 95, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.S.; Rosa, V. Graphene nanosheets to improve physico-mechanical properties of bioactive calcium silicate cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Sakamoto, M.; Takeuchi, H.; Matsushima, K. Effects of pH on mineralization ability of human dental pulp cells. J. Endod. 2006, 32, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Finan, J.D.; Guilak, F. The effects of osmotic stress on the structure and function of the cell nucleus. J. Cell. Biochem. 2010, 109, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Torneck, C.; Moe, H.; Howley, T. The effect of calcium hydroxide on porcine pulp fibroblasts in vitro. J. Endod. 1983, 9, 131–136. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Fan, M. Bioaggregate and iroot bp plus optimize the proliferation and mineralization ability of human dental pulp cells. Int. Endod. J. 2013, 46, 923–929. [Google Scholar] [CrossRef]
- Araujo, L.B.; Cosme-Silva, L.; Fernandes, A.P.; Oliveira, T.M.; Cavalcanti, B.D.N.; Gomes Filho, J.E.; Sakai, V.T. Effects of mineral trioxide aggregate, biodentinetm and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. J. Appl. Oral Sci. 2018, 26, e20160629. [Google Scholar] [CrossRef]
- Tran, X.V.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Opsahl Vital, S.; Chaussain, C.; Boukpessi, T. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef]
- Bronckers, A.L.; Lyaruu, D.M.; Woltgens, J.H. Immunohistochemistry of extracellular matrix proteins during various stages of dentinogenesis. Connect. Tissue Res. 1989, 22, 65–70. [Google Scholar] [CrossRef]
- Balic, A.; Mina, M. Identification of secretory odontoblasts using dmp1-gfp transgenic mice. Bone 2011, 48, 927–937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balic, A.; Aguila, H.L.; Mina, M. Identification of cells at early and late stages of polarization during odontoblast differentiation using pobcol3.6gfp and pobcol2.3gfp transgenic mice. Bone 2010, 47, 948–958. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nekoofar, M.H.; Namazikhah, M.; Sheykhrezae, M.; Mohammadi, M.; Kazemi, A.; Aseeley, Z.; Dummer, P.M.H. pH of pus collected from periapical abscesses. Int. Endod. J. 2009, 42, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.; Collado-González, M.; López-García, S.; García-Bernal, D.; Moraleda, J.; Lozano, A.; Forner, L.; Murcia, L.; Oñate-Sánchez, R. Evaluation of changes in ion release and biological properties of neomta-plus and endocem-mta exposed to an acidic environment. Int. Endod. J. 2019, 52, 1196–1209. [Google Scholar] [CrossRef]
- Peng, W.; Liu, W.; Zhai, W.; Jiang, L.; Li, L.; Chang, J.; Zhu, Y. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J. Endod. 2011, 37, 1240–1246. [Google Scholar] [CrossRef]
| Primer | Sequence |
|---|---|
| MEPE Forward | GGCCAGTGACTGCGATTAAAC |
| MEPE Reverse | CCTTCGAGTGTGCTTTAGCAT |
| DMP-1 Forward | CTCCGAGTTGGACGATGAGG |
| DMP-1 Reverse | TCATGCCTGCACTGTTCATTC |
| DSPP Forward | TGGCGATGCAGGTCACAAT |
| DSPP Reverse | CCATTCCCACTAGGACTCCCA |
| RUNX2 Forward | CACTGGCGCTGCAACAAGA |
| RUNX2 Reverse | CATTCCGGAGCTCAGCAGAATAA |
| COL I Forward | CTGACCTTCCTGCGCCTGATGTCC |
| COL I Reverse | GTCTGGGGCACCAACGTCCAAGGG |
| OCN Forward | ATGAGAGCCCTCAGACTCCTC |
| OCN Reverse | CGGGCCGTAGAAGCGCCGATA |
| D10 | D50 | D90 |
|---|---|---|
| 2.79 ± 0.04 | 9.73 ± 0.95 | 46.82 ± 6.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phang, V.; Malhotra, R.; Chen, N.N.; Min, K.-S.; Yu, V.S.H.; Rosa, V.; Dubey, N. Specimen Shape and Elution Time Affect the Mineralization and Differentiation Potential of Dental Pulp Stem Cells to Biodentine. J. Funct. Biomater. 2024, 15, 1. https://doi.org/10.3390/jfb15010001
Phang V, Malhotra R, Chen NN, Min K-S, Yu VSH, Rosa V, Dubey N. Specimen Shape and Elution Time Affect the Mineralization and Differentiation Potential of Dental Pulp Stem Cells to Biodentine. Journal of Functional Biomaterials. 2024; 15(1):1. https://doi.org/10.3390/jfb15010001
Chicago/Turabian StylePhang, Valene, Ritika Malhotra, Nah Nah Chen, Kyung-San Min, Victoria Soo Hoon Yu, Vinicius Rosa, and Nileshkumar Dubey. 2024. "Specimen Shape and Elution Time Affect the Mineralization and Differentiation Potential of Dental Pulp Stem Cells to Biodentine" Journal of Functional Biomaterials 15, no. 1: 1. https://doi.org/10.3390/jfb15010001
APA StylePhang, V., Malhotra, R., Chen, N. N., Min, K.-S., Yu, V. S. H., Rosa, V., & Dubey, N. (2024). Specimen Shape and Elution Time Affect the Mineralization and Differentiation Potential of Dental Pulp Stem Cells to Biodentine. Journal of Functional Biomaterials, 15(1), 1. https://doi.org/10.3390/jfb15010001









