Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery
Abstract
1. Introduction
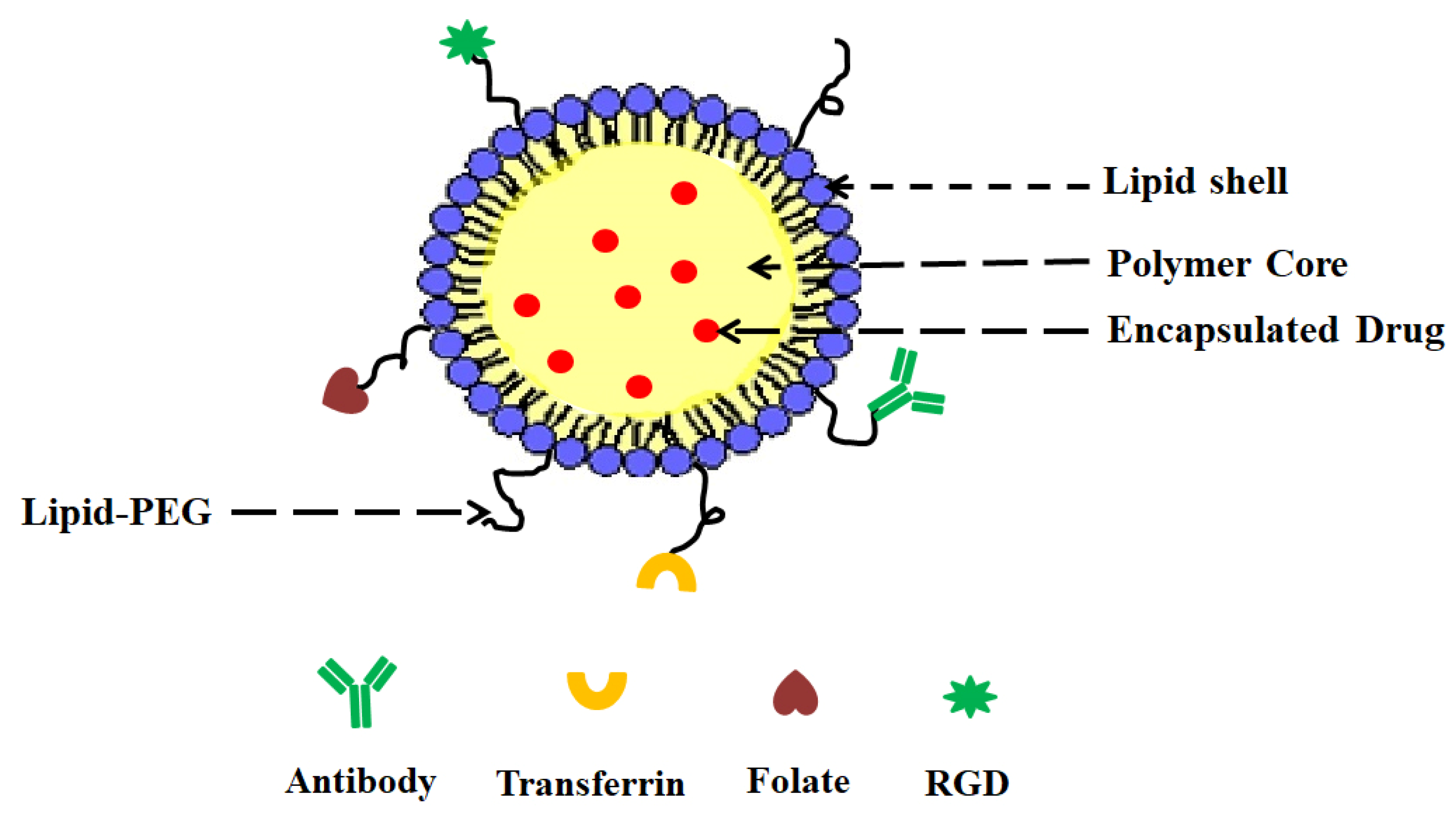
- (i)
- A drug encapsulating polymer core;
- (ii)
- A lipid layer surrounding the polymer core;
- (iii)
- An outer lipid–PEG layer.
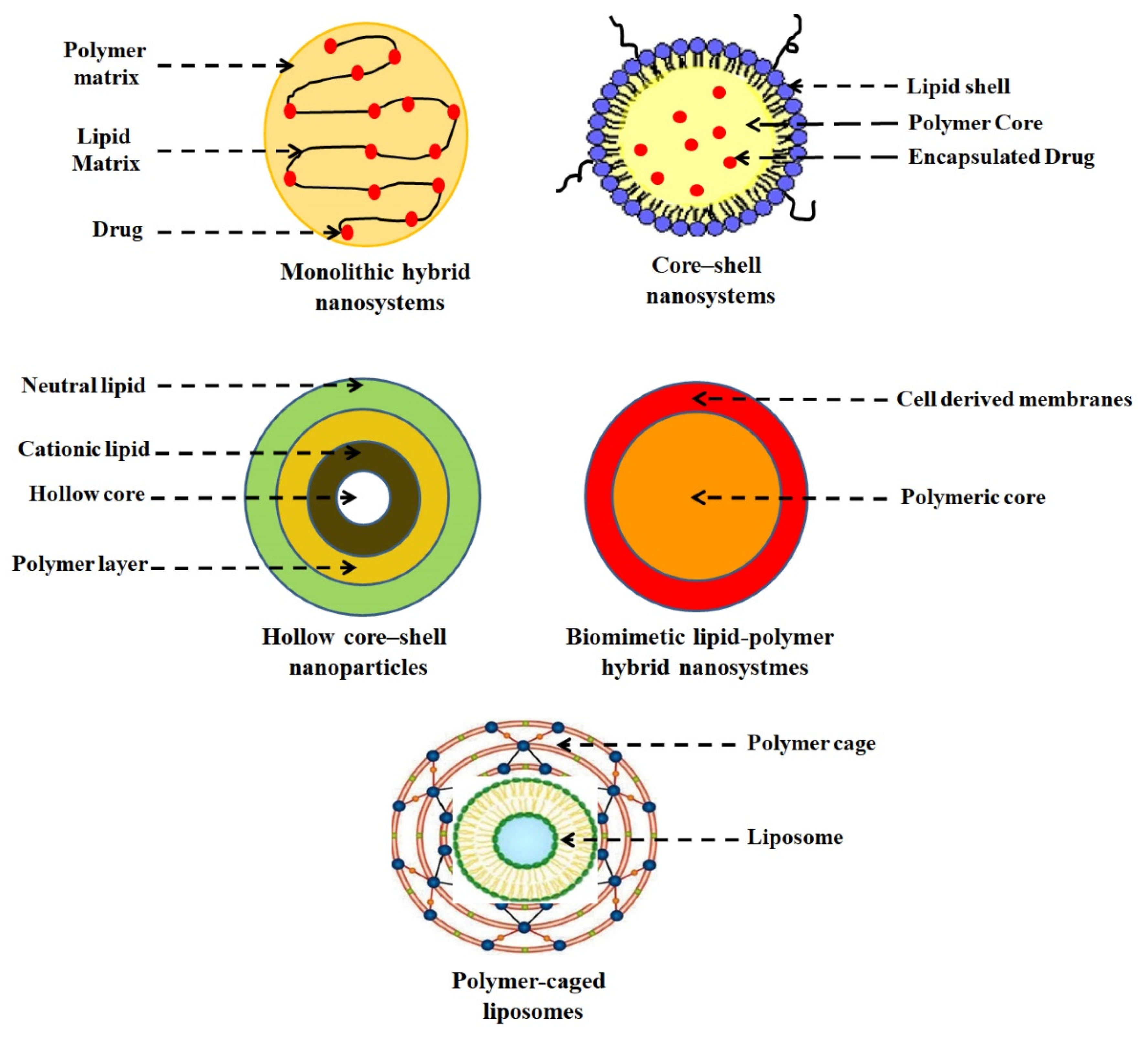
2. Advantages and Limitations of LPHNPs
3. LPHNPs Composition and Its Influence
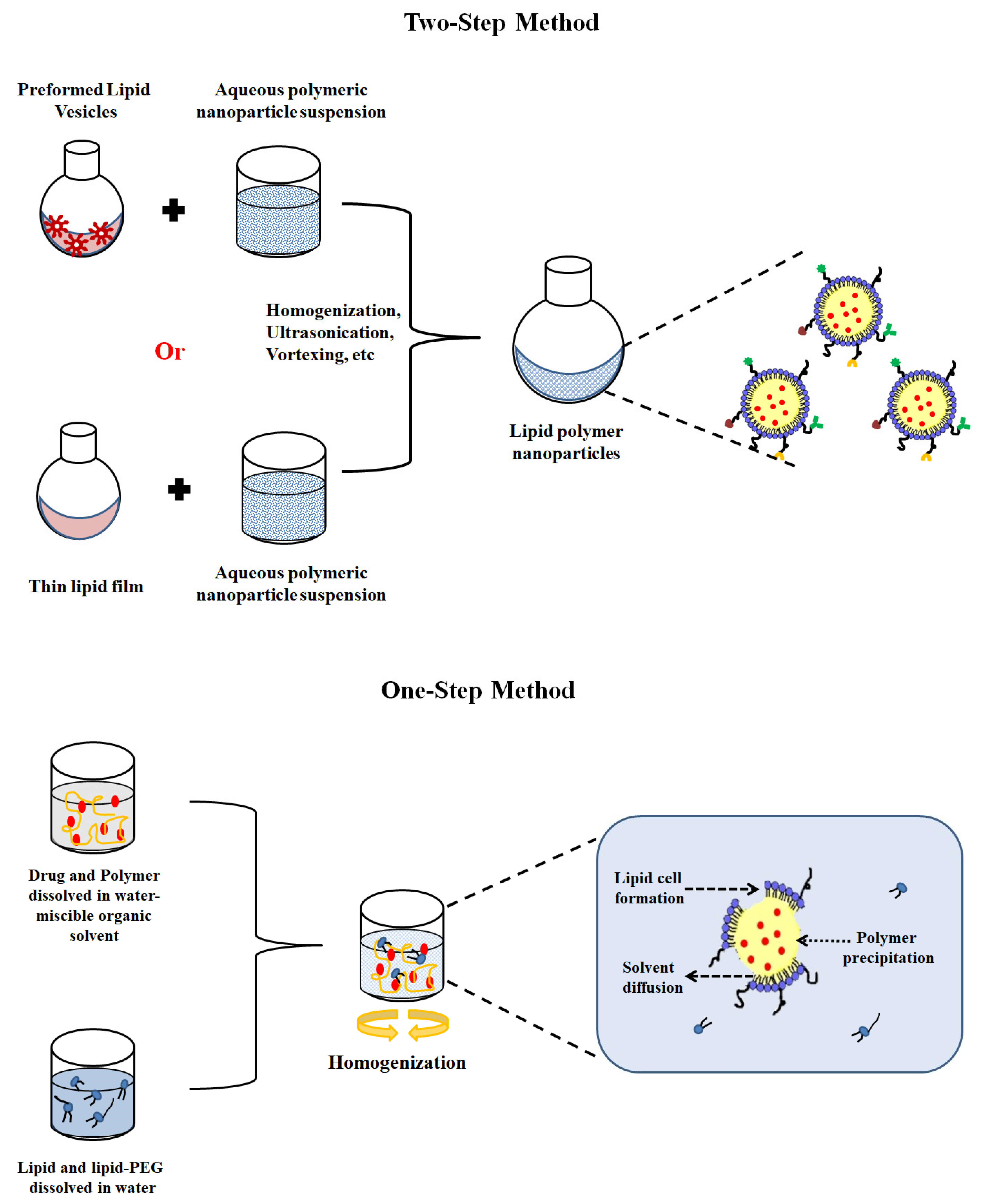
4. LPHNP Fabrication Methods
5. Surface Functionalization of LPHNPs
6. Stimuli-Responsive Drug Release from LPHNPs
7. Patents on LPHNPs
8. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Nakmode, D.; Bhavana, V.; Thakor, P.; Madan, J.; Singh, P.K.; Singh, S.B.; Rosenholm, J.M.; Bansal, K.K.; Mehra, N.K. Fundamental Aspects of Lipid-Based Excipients in Lipid-Based Product Development. Pharmaceutics 2022, 14, 831. [Google Scholar] [CrossRef]
- Marzi, M.; Rostami Chijan, M.; Zarenezhad, E. Hydrogels as Promising Therapeutic Strategy for the Treatment of Skin Cancer. J. Mol. Struct. 2022, 1262, 133014. [Google Scholar] [CrossRef]
- Bansal, K.K.; Rosenholm, J.M. Synthetic Polymers from Renewable Feedstocks: An Alternative to Fossil-Based Materials in Biomedical Applications. Ther. Deliv. 2020, 11, 297–300. [Google Scholar] [CrossRef]
- Bansal, K.K.; Kakde, D.; Purdie, L.; Irvine, D.J.; Howdle, S.M.; Mantovani, G.; Alexander, C. New Biomaterials from Renewable Resources—Amphiphilic Block Copolymers from δ-Decalactone. Polym. Chem. 2015, 6, 7196–7210. [Google Scholar] [CrossRef]
- Bansal, K.; Sasso, L.; Makwana, H.; Awwad, S.; Brocchini, S.; Alexander, C. Nanopharmacy: Exploratory Methods for Polymeric Materials; John Wiley & Sons: Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2017; Volume 1, ISBN 978-3-527-34054-5. [Google Scholar]
- Jain, P.; Vaidya, A.; Jain, R.; Shrivastava, S.; Khan, T.; Jain, A. Ethyl Cellulose Coated Chitosan Microspheres of Metronidazole as Potential Anti-Amoebic Agent. J. Bionanosci. 2017, 11, 599–607. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Agrawal, R.K.; Jain, S.K. Pectin–Metronidazole Prodrug Bearing Microspheres for Colon Targeting. J. Saudi Chem. Soc. 2015, 19, 257–264. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Attama, A.A.; Momoh, M.A.; Builders, P.F.; Attama, A.A.; Momoh, M.A.; Builders, P.F. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Recent Advances in Novel Drug Carrier Systems; IntechOpen: London, UK, 2012; ISBN 978-953-51-0810-8. [Google Scholar]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond Liposomes: Recent Advances on Lipid Based Nanostructures for Poorly Soluble/Poorly Permeable Drug Delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Chapter 8—Recent Advances in Lipid Nanocarriers Applicable in the Fight against Cancer**This Chapter Is Sincerely Dedicated to the Memory of Prof. Ervin Wolfram (1923–1985), the Long-Time Head of the Department of Colloid Chemistry and Colloid Technology, the Faculty of Science, Eötvös Loránd University, Budapest, Hungary. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 219–294. ISBN 978-0-12-816200-2. [Google Scholar]
- Mendozza, M.; Caselli, L.; Salvatore, A.; Montis, C.; Berti, D. Nanoparticles and Organized Lipid Assemblies: From Interaction to Design of Hybrid Soft Devices. Soft Matter 2019, 15, 8951–8970. [Google Scholar] [CrossRef]
- Lipid Polymer Hybrid Nanoparticles: A Novel Approach for Drug Delivery. IntechOpen. Available online: https://www.intechopen.com/chapters/69735 (accessed on 9 August 2023).
- Miri, V.; Jangde, R.K.; Singh, D.; Suresh, P.K. Lipid-Polymer Hybrid Nanoparticles for Topical Drug Delivery System. J. Drug Deliv. Ther. 2023, 13, 113–120. [Google Scholar] [CrossRef]
- Bangera, P.D.; Kara, D.D.; Tanvi, K.; Tippavajhala, V.K.; Rathnanand, M. Highlights on Cell-Penetrating Peptides and Polymer-Lipid Hybrid Nanoparticle: Overview and Therapeutic Applications for Targeted Anticancer Therapy. AAPS PharmSciTech 2023, 24, 124. [Google Scholar] [CrossRef]
- Baghel, Y.S.; Bhattacharya, S. Lipid Polymeric Hybrid Nanoparticles: Formulation Techniques and Effects on Glioblastoma. Pharm. Sci. 2021, 28, 174–193. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–Polymer Hybrid Nanoparticles as a next-Generation Drug Delivery Platform: State of the Art, Emerging Technologies, and Perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Sengel-Turk, C.T.; Hascicek, C. Design of Lipid-Polymer Hybrid Nanoparticles for Therapy of BPH: Part I. Formulation Optimization Using a Design of Experiment Approach. J. Drug Deliv. Sci. Technol. 2017, 39, 16–27. [Google Scholar] [CrossRef]
- Chouhan, M.D.; Mujariya, D.R. Design and Development of Lipid Polymer Hybrid Nanoparticles for Combinatorial Drug Delivery. Int. J. Creat. Res. Thoughts 2023, 11, e645. [Google Scholar]
- Rouco, H.; García-García, P.; Évora, C.; Díaz-Rodríguez, P.; Delgado, A. Screening Strategies for Surface Modification of Lipid-Polymer Hybrid Nanoparticles. Int. J. Pharm. 2022, 624, 121973. [Google Scholar] [CrossRef]
- Bokare, A.; Takami, A.; Kim, J.H.; Dong, A.; Chen, A.; Valerio, R.; Gunn, S.; Erogbogbo, F. Herringbone-Patterned 3D-Printed Devices as Alternatives to Microfluidics for Reproducible Production of Lipid Polymer Hybrid Nanoparticles. ACS Omega 2019, 4, 4650–4657. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Correia, A.; Rehman, M.; Balasubramanian, V.; Khan, M.M.; Santos, H.A. Lipid-Polymer Hybrid Nanoparticles for Controlled Delivery of Hydrophilic and Lipophilic Doxorubicin for Breast Cancer Therapy. Int. J. Nanomed. 2019, 14, 4961–4974. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Pan, J.; Liu, B.; Feng, S.-S. Folic Acid Conjugated Nanoparticles of Mixed Lipid Monolayer Shell and Biodegradable Polymer Core for Targeted Delivery of Docetaxel. Biomaterials 2010, 31, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Alshaker, H.; Böhler, T.; Srivats, S.; Chao, Y.; Cooper, C.; Pchejetski, D. Core Shell Lipid-Polymer Hybrid Nanoparticles with Combined Docetaxel and Molecular Targeted Therapy for the Treatment of Metastatic Prostate Cancer. Sci. Rep. 2017, 7, 5901. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Gianella, A.; Cormode, D.P.; Zhao, Y.; Meijerink, A.; Langer, R.; Farokhzad, O.C.; Fayad, Z.A.; Mulder, W.J.M. Engineering of Lipid-Coated PLGA Nanoparticles with a Tunable Payload of Diagnostically Active Nanocrystals for Medical Imaging. Chem. Commun. 2012, 48, 5835–5837. [Google Scholar] [CrossRef]
- Jain, A.K.; Bataille, C.J.R.; Milhas, S.; Miller, A.; Zhang, J.; Rabbitts, T.H. Immunopolymer Lipid Nanoparticles for Delivery of Macromolecules to Antigen-Expressing Cells. ACS Appl. Bio Mater. 2020, 3, 8481–8495. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Luo, W.; Guo, Q.; Li, X.; Zhang, Q.; Li, J. In Vitro and in Vivo Effect of Hyaluronic Acid Modified, Doxorubicin and Gallic Acid Co-Delivered Lipid-Polymeric Hybrid Nano-System for Leukemia Therapy. Drug Des. Dev. Ther. 2019, 13, 2043–2055. [Google Scholar] [CrossRef]
- Dong, W.; Wang, X.; Liu, C.; Zhang, X.; Zhang, X.; Chen, X.; Kou, Y.; Mao, S. Chitosan Based Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Enoxaparin. Int. J. Pharm. 2018, 547, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Sailor, G.U.; Ramani, V.D.; Shah, N.; Parmar, G.R.; Gohil, D.; Balaraman, R.; Seth, A. Design of Experiment Approach Based Formulation Optimization of Berberine Loaded Solid Lipid Nanoparticle for Antihyperlipidemic Activity. Indian J. Pharm. Sci. 2021, 83, 204–218. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yang, H.; Yoon, I.-S.; Kim, S.-B.; Ko, S.-H.; Shim, J.-S.; Sung, S.H.; Cho, H.-J.; Kim, D.-D. Nanocomplexes Based on Amphiphilic Hyaluronic Acid Derivative and Polyethylene Glycol–Lipid for Ginsenoside Rg3 Delivery. J. Pharm. Sci. 2014, 103, 3254–3262. [Google Scholar] [CrossRef]
- Wei, W.; Sun, J.; Guo, X.-Y.; Chen, X.; Wang, R.; Qiu, C.; Zhang, H.-T.; Pang, W.-H.; Wang, J.-C.; Zhang, Q. Microfluidic-Based Holonomic Constraints of SiRNA in the Kernel of Lipid/Polymer Hybrid Nanoassemblies for Improving Stable and Safe In Vivo Delivery. ACS Appl. Mater. Interfaces 2020, 12, 14839–14854. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Mahesh, A.; Mahadevan, S.; Mandal, A.B. Synthesis and Characterization of Curcumin Loaded Polymer/Lipid Based Nanoparticles and Evaluation of Their Antitumor Effects on MCF-7 Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 1913–1922. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, H.; Kamboj, A. Development and Evaluation of Isradipine via Rutin-Loaded Coated Solid-Lipid Nanoparticles. Interv. Med. Appl. Sci. 2018, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-Y.; Liu, X.-Y.; Chen, C.-J.; Wang, J.-C.; Feng, Q.; Yu, M.-Z.; Ma, X.-F.; Pei, X.-W.; Niu, Y.-J.; Qiu, C.; et al. Core-Shell Type Lipid/RPAA-Chol Polymer Hybrid Nanoparticles for in Vivo SiRNA Delivery. Biomaterials 2014, 35, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Yuet, K.; Zhang, L.; Gu, F.X.; Huynh-Le, M.; Radovic-Moreno, A.F.; Kantoff, P.W.; Bander, N.H.; Langer, R.; Farokhzad, O.C. ChemoRad Nanoparticles: A Novel Multifunctional Nanoparticle Platform for Targeted Delivery of Concurrent Chemoradiation. Nanomedicine 2010, 5, 361–368. [Google Scholar] [CrossRef]
- Salvador-Morales, C.; Zhang, L.; Langer, R.; Farokhzad, O.C. Immunocompatibility Properties of Lipid–Polymer Hybrid Nanoparticles with Heterogeneous Surface Functional Groups. Biomaterials 2009, 30, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Yadav, R.B.; Kushwaha, K.; Yadav, S.; Sharma, S.; Agrawal, U. Lipid-Polymer Hybrid Nanoparticles: Development & Statistical Optimization of Norfloxacin for Topical Drug Delivery System. Bioact. Mater. 2017, 2, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Basto, P.A.; Zhang, L.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-Step Assembly of Homogenous Lipid-Polymeric and Lipid-Quantum Dot Nanoparticles Enabled by Microfluidic Rapid Mixing. ACS Nano 2010, 4, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Troutier, A.-L.; Delair, T.; Pichot, C.; Ladavière, C. Physicochemical and Interfacial Investigation of Lipid/Polymer Particle Assemblies. Langmuir 2005, 21, 1305–1313. [Google Scholar] [CrossRef]
- Thevenot, J.; Troutier, A.-L.; David, L.; Delair, T.; Ladavière, C. Steric Stabilization of Lipid/Polymer Particle Assemblies by Poly(Ethylene Glycol)-Lipids. Biomacromolecules 2007, 8, 3651–3660. [Google Scholar] [CrossRef]
- Yeh, M.-K.; Chang, W.-K.; Tai, Y.-J.; Chiang, C.-H.; Hu, C.; Hong, P.-D. The Comparison of Protein-Entrapped Liposomes and Lipoparticles: Preparation, Characterization, and Efficacy of Cellular Uptake. Int. J. Nanomed. 2011, 6, 2403–2417. [Google Scholar] [CrossRef][Green Version]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–Shell-Type Lipid–Polymer Hybrid Nanoparticles as a Drug Delivery Platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef]
- Wakaskar, R.R. General Overview of Lipid-Polymer Hybrid Nanoparticles, Dendrimers, Micelles, Liposomes, Spongosomes and Cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid Polymer Hybrid Nanocarriers: Insights into Synthesis Aspects, Characterization, Release Mechanisms, Surface Functionalization and Potential Implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-Assembled Lipid-Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Vaiyapuri, R.; Zhang, L.; Chan, J.M. Lipid-Coated Polymeric Nanoparticles for Cancer Drug Delivery. Biomater. Sci. 2015, 3, 923–936. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-Polymer Hybrid Nanoparticles: Synthesis Strategies and Biomedical Applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-Step Assembly of Cationic Lipid–Polymer Hybrid Nanoparticles for Systemic Delivery of SiRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Wu, B.; Zhou, W.; Wang, C.-X.; Wang, Q.; Yu, H.; Zhuo, R.-X.; Liu, Z.-L.; Huang, S.-W. Two-Component Reduction-Sensitive Lipid–Polymer Hybrid Nanoparticles for Triggered Drug Release and Enhanced in Vitro and in Vivo Anti-Tumor Efficacy. Biomater. Sci. 2016, 5, 98–110. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Antitumor Activity of Gemcitabine Hydrochloride Loaded Lipid Polymer Hybrid Nanoparticles (LPHNs): In Vitro and in Vivo. Int. J. Pharm. 2020, 580, 119246. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double Emulsion Solvent Evaporation Techniques Used for Drug Encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Montazam, S.H. Comparison of Microencapsulation by Emulsion-Solvent Extraction/Evaporation Technique Using Derivatives Cellulose and Acrylate-Methacrylate Copolymer as Carriers. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 144–152. [Google Scholar] [CrossRef]
- Jose, C.; Amra, K.; Bhavsar, C.; Momin, M.; Omri, A. Polymeric Lipid Hybrid Nanoparticles: Properties and Therapeutic Applications. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 555–588. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Han, L.; Qiang, Z.; Zhang, X.; Gao, W.; Zhao, K.; Song, Y. Synergistic Combination Therapy of Lung Cancer Using Paclitaxel- and Triptolide-Coloaded Lipid–Polymer Hybrid Nanoparticles. Drug Des. Dev. Ther. 2018, 12, 3199–3209. [Google Scholar] [CrossRef]
- Lee, J.-J.; Lee, S.Y.; Park, J.-H.; Kim, D.-D.; Cho, H.-J. Cholesterol-Modified Poly(Lactide-Co-Glycolide) Nanoparticles for Tumor-Targeted Drug Delivery. Int. J. Pharm. 2016, 509, 483–491. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Weecharangsan, W.; Piao, L.; Darby, M.; Mao, Y.; Koynova, R.; Yang, X.; Li, H.; Xu, S.; et al. Transferrin-Conjugated Lipid-Coated PLGA Nanoparticles for Targeted Delivery of Aromatase Inhibitor 7alpha-APTADD to Breast Cancer Cells. Int. J. Pharm. 2010, 390, 234–241. [Google Scholar] [CrossRef]
- Aryal, S.; Hu, C.-M.J.; Zhang, L. Combinatorial Drug Conjugation Enables Nanoparticle Dual-Drug Delivery. Small 2010, 6, 1442–1448. [Google Scholar] [CrossRef]
- Chan, J.M.; Rhee, J.-W.; Drum, C.L.; Bronson, R.T.; Golomb, G.; Langer, R.; Farokhzad, O.C. In Vivo Prevention of Arterial Restenosis with Paclitaxel-Encapsulated Targeted Lipid-Polymeric Nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 19347–19352. [Google Scholar] [CrossRef]
- Dehaini, D.; Fang, R.H.; Luk, B.T.; Pang, Z.; Hu, C.-M.J.; Kroll, A.V.; Yu, C.L.; Gao, W.; Zhang, L. Ultra-Small Lipid–Polymer Hybrid Nanoparticles for Tumor-Penetrating Drug Delivery. Nanoscale 2016, 8, 14411–14419. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Z.; Votruba, A.R.; Vilos, C.; Farokhzad, O.C. Differentially Charged Hollow Core/Shell Lipid-Polymer-Lipid Hybrid Nanoparticles for Small Interfering RNA Delivery. Angew. Chem. Int. Ed. Engl. 2011, 50, 7027–7031. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, H.; Yu, M.; Liao, Z.; Wang, X.; Zhang, F.; Ji, W.; Wu, B.; Han, J.; Zhang, H.; et al. Paclitaxel Loaded Folic Acid Targeted Nanoparticles of Mixed Lipid-Shell and Polymer-Core: In Vitro and in Vivo Evaluation. Eur. J. Pharm. Biopharm. 2012, 81, 248–256. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Fang, R.H.; Wang, K.-C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle Biointerfacing by Platelet Membrane Cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- JC Bose, R.; Lee, S.-H.; Park, H. Lipid Polymer Hybrid Nanospheres Encapsulating Antiproliferative Agents for Stent Applications. J. Ind. Eng. Chem. 2016, 36, 284–292. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Arai, Y.; Ahn, J.C.; Park, H.; Lee, S.-H. Influence of Cationic Lipid Concentration on Properties of Lipid-Polymer Hybrid Nanospheres for Gene Delivery. Int. J. Nanomed. 2015, 10, 5367–5382. [Google Scholar] [CrossRef]
- Aryal, S.; Hu, C.-M.J.; Fang, R.H.; Dehaini, D.; Carpenter, C.; Zhang, D.-E.; Zhang, L. Erythrocyte Membrane-Cloaked Polymeric Nanoparticles for Controlled Drug Loading and Release. Nanomedicine 2013, 8, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the Lipid Layer of Lipid–PLGA Hybrid Nanoparticles for Enhanced in Vitro Cellular Uptake and Improved Stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal Targeting of Tumour Cells and Neovasculature with a Nanoscale Delivery System. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef]
- Wu, B.; Yu, P.; Cui, C.; Wu, M.; Zhang, Y.; Liu, L.; Wang, C.-X.; Zhuo, R.-X.; Huang, S.-W. Folate-Containing Reduction-Sensitive Lipid-Polymer Hybrid Nanoparticles for Targeted Delivery of Doxorubicin. Biomater. Sci. 2015, 3, 655–664. [Google Scholar] [CrossRef]
- Gu, L.; Shi, T.; Sun, Y.; You, C.; Wang, S.; Wen, G.; Chen, L.; Zhang, X.; Zhu, J.; Sun, B. Folate-Modified, Indocyanine Green-Loaded Lipid-Polymer Hybrid Nanoparticles for Targeted Delivery of Cisplatin. J. Biomater. Sci. Polym. Ed. 2017, 28, 690–702. [Google Scholar] [CrossRef]
- Zheng, M.; Gong, P.; Zheng, C.; Zhao, P.; Luo, Z.; Ma, Y.; Cai, L. Lipid-Polymer Nanoparticles for Folate-Receptor Targeting Delivery of Doxorubicin. J. Nanosci. Nanotechnol. 2015, 15, 4792–4798. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, X.; Zhang, X.; Liu, J.; Jiang, Q. Targeted Delivery of 10-Hydroxycamptothecin to Human Breast Cancers by Cyclic RGD-Modified Lipid-Polymer Hybrid Nanoparticles. Biomed. Mater. 2013, 8, 025012. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Fu, C.; Xie, X.; Peng, F.; You, J.; Tang, H.; Wang, Z.; Li, P.; Chen, J. IRGD-Modified Lipid-Polymer Hybrid Nanoparticles Loaded with Isoliquiritigenin to Enhance Anti-Breast Cancer Effect and Tumor-Targeting Ability. Int. J. Nanomed. 2017, 12, 4147–4162. [Google Scholar] [CrossRef]
- Shi, K.; Zhou, J.; Zhang, Q.; Gao, H.; Liu, Y.; Zong, T.; He, Q. Arginine-Glycine-Aspartic Acid-Modified Lipid-Polymer Hybrid Nanoparticles for Docetaxel Delivery in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2015, 11, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.; Chu, K.; Gullapalli, A.; Dunn, S.S.; Enlow, E.M.; Luft, J.C.; Tian, S.; Napier, M.E.; Pohlhaus, P.D.; Rolland, J.P.; et al. Delivery of Multiple SiRNAs Using Lipid-Coated PLGA Nanoparticles for Treatment of Prostate Cancer. Nano Lett. 2012, 12, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Palange, A.L.; Di Mascolo, D.; Carallo, C.; Gnasso, A.; Decuzzi, P. Lipid-Polymer Nanoparticles Encapsulating Curcumin for Modulating the Vascular Deposition of Breast Cancer Cells. Nanomedicine 2014, 10, 991–1002. [Google Scholar] [CrossRef]
- Su, X.; Wang, Z.; Li, L.; Zheng, M.; Zheng, C.; Gong, P.; Zhao, P.; Ma, Y.; Tao, Q.; Cai, L. Lipid–Polymer Nanoparticles Encapsulating Doxorubicin and 2′-Deoxy-5-Azacytidine Enhance the Sensitivity of Cancer Cells to Chemical Therapeutics. Mol. Pharm. 2013, 10, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Z.; Li, C.; Duan, G.; Wang, K.; Li, Q.; Tao, T. RGD Peptide-Modified, Paclitaxel Prodrug-Based, Dual-Drugs Loaded, and Redox-Sensitive Lipid-Polymer Nanoparticles for the Enhanced Lung Cancer Therapy. Biomed. Pharmacother. 2018, 106, 275–284. [Google Scholar] [CrossRef]
- Guo, P.; Buttaro, B.A.; Xue, H.Y.; Tran, N.T.; Wong, H.L. Lipid-Polymer Hybrid Nanoparticles Carrying Linezolid Improve Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA) Harbored inside Bone Cells and Biofilms. Eur. J. Pharm. Biopharm 2020, 151, 189–198. [Google Scholar] [CrossRef]
- Bakar-Ates, F.; Ozkan, E.; Sengel-Turk, C.T. Encapsulation of Cucurbitacin B into Lipid Polymer Hybrid Nanocarriers Induced Apoptosis of MDAMB231 Cells through PARP Cleavage. Int. J. Pharm. 2020, 586, 119565. [Google Scholar] [CrossRef]
- Ai, X.; Duan, Y.; Zhang, Q.; Sun, D.; Fang, R.H.; Liu-Bryan, R.; Gao, W.; Zhang, L. Cartilage-Targeting Ultrasmall Lipid-Polymer Hybrid Nanoparticles for the Prevention of Cartilage Degradation. Bioeng. Transl. Med. 2021, 6, e10187. [Google Scholar] [CrossRef]
- Fraix, A.; Conte, C.; Gazzano, E.; Riganti, C.; Quaglia, F.; Sortino, S. Overcoming Doxorubicin Resistance with Lipid–Polymer Hybrid Nanoparticles Photoreleasing Nitric Oxide. Mol. Pharm. 2020, 17, 2135–2144. [Google Scholar] [CrossRef]
- Sengel-Turk, C.T.; Alcigir, M.E.; Ekim, O.; Bakar-Ates, F.; Hascicek, C. Clinicopathological and Immunohistochemical Evaluation of Lonidamine-Entrapped Lipid–Polymer Hybrid Nanoparticles in Treatment of Benign Prostatic Hyperplasia: An Experimental Rat Model. Eur. J. Pharm. Biopharm. 2020, 157, 211–220. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, L.; Wang, T.; Liu, Q.; Wang, J.; Wang, Y.; Tu, Z.; Lin, F. Doxorubicin and Edelfosine Combo-Loaded Lipid-Polymer Hybrid Nanoparticles for Synergistic Anticancer Effect Against Drug-Resistant Osteosarcoma. Onco Targets Ther. 2020, 13, 8055–8067. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Abdel-Bar, H.M.; Elmowafy, E.; Al-Jamal, K.T.; Awad, G.A.S. An Integrated Vitamin E-Coated Polymer Hybrid Nanoplatform: A Lucrative Option for an Enhanced in Vitro Macrophage Retention for an Anti-Hepatitis B Therapeutic Prospect. PLoS ONE 2020, 15, e0227231. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Mandal, N.; Patel, G.; Kirar, S.; Reddy, Y.N.; Kushwah, V.; Jain, S.; Kalia, Y.N.; Bhaumik, J.; Banerjee, U.C. Co-Administration of Zinc Phthalocyanine and Quercetin via Hybrid Nanoparticles for Augmented Photodynamic Therapy. Nanomedicine 2021, 33, 102368. [Google Scholar] [CrossRef] [PubMed]
- Jadon, R.S.; Sharma, M. Docetaxel-Loaded Lipid-Polymer Hybrid Nanoparticles for Breast Cancer Therapeutics. J. Drug Deliv. Sci. Technol. 2019, 51, 475–484. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.; Meng, F.; Zhang, X.; Ma, Q.; Zhuang, Y.; Liu, H.; Pang, M.; Cai, T.; Cai, Y. Polymer-Lipid Hybrid Nanoparticles: A Novel Drug Delivery System for Enhancing the Activity of Psoralen against Breast Cancer. Int. J. Pharm. 2019, 561, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Development and Characterization of Gemcitabine Hydrochloride Loaded Lipid Polymer Hybrid Nanoparticles (LPHNs) Using Central Composite Design. Int. J. Pharm. 2018, 548, 255–262. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Yang, X.; Jia, M.; Li, Y.; Huang, Y.; Lin, J.; Wu, S.; Hou, Z. Mitomycin C-Soybean Phosphatidylcholine Complex-Loaded Self-Assembled PEG-Lipid-PLA Hybrid Nanoparticles for Targeted Drug Delivery and Dual-Controlled Drug Release. Mol. Pharm. 2014, 11, 2915–2927. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Kushwah, V.; Garg, N.K.; Kesharwani, P.; Ghoshal, G.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Methotrexate and Beta-Carotene Loaded-Lipid Polymer Hybrid Nanoparticles: A Preclinical Study for Breast Cancer. Nanomedicine 2017, 12, 1851–1872. [Google Scholar] [CrossRef]
- Ayad, C.; Libeau, P.; Lacroix-Gimon, C.; Ladavière, C.; Verrier, B. LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro MRNA Transfection Compared to Liposomes. Pharmaceutics 2021, 13, 377. [Google Scholar] [CrossRef]
- Garg, N.K.; Tyagi, R.K.; Sharma, G.; Jain, A.; Singh, B.; Jain, S.; Katare, O.P. Functionalized Lipid–Polymer Hybrid Nanoparticles Mediated Codelivery of Methotrexate and Aceclofenac: A Synergistic Effect in Breast Cancer with Improved Pharmacokinetics Attributes. Mol. Pharm. 2017, 14, 1883–1897. [Google Scholar] [CrossRef]
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-Small Cell Lung Cancer Combination Therapy: Hyaluronic Acid Modified, Epidermal Growth Factor Receptor Targeted, PH Sensitive Lipid-Polymer Hybrid Nanoparticles for the Delivery of Erlotinib plus Bevacizumab. Biomed. Pharmacother. 2020, 125, 109861. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wang, J.; Liu, H. Chemo-Immune Synergetic Therapy of Esophageal Carcinoma: Trastuzumab Modified, Cisplatin and Fluorouracil Co-Delivered Lipid-Polymer Hybrid Nanoparticles. Drug Deliv. 2020, 27, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yang, F.; Xin, J.; Bai, X. An Efficient and Long-Acting Local Anesthetic: Ropivacaine-Loaded Lipid-Polymer Hybrid Nanoparticles for the Control of Pain. Int. J. Nanomed. 2019, 14, 913–920. [Google Scholar] [CrossRef]
- Shafique, M.; Ur Rehman, M.; Kamal, Z.; Alzhrani, R.M.; Alshehri, S.; Alamri, A.H.; Bakkari, M.A.; Sabei, F.Y.; Safhi, A.Y.; Mohammed, A.M.; et al. Formulation Development of Lipid Polymer Hybrid Nanoparticles of Doxorubicin and Its In-Vitro, in-Vivo and Computational Evaluation. Front. Pharmacol. 2023, 14, 1025013. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Li, Y. Sorafenib-Loaded Ligand-Functionalized Polymer-Lipid Hybrid Nanoparticles for Enhanced Therapeutic Effect Against Liver Cancer. J. Nanosci. Nanotechnol. 2019, 19, 6866–6871. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-Chitosan Hybrid Nanoparticles for Controlled Delivery of Cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef]
- Nair, R.; Kumar, A.C.; Priya, V.K.; Yadav, C.M.; Raju, P.Y. Formulation and Evaluation of Chitosan Solid Lipid Nanoparticles of Carbamazepine. Lipids Health Dis. 2012, 11, 72. [Google Scholar] [CrossRef]
- Tezgel, Ö.; Szarpak-Jankowska, A.; Arnould, A.; Auzély-Velty, R.; Texier, I. Chitosan-Lipid Nanoparticles (CS-LNPs): Application to SiRNA Delivery. J. Colloid Interface Sci. 2018, 510, 45–56. [Google Scholar] [CrossRef]
- Saeed, R.M.; Abdullah, M.; Ahram, M.; Taha, M.O. Novel Ellipsoid Chitosan-Phthalate Lecithin Nanoparticles for SiRNA Delivery. Front. Bioeng. Biotechnol. 2021, 9, 695371. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Wu, X.Y. Simultaneous Delivery of Doxorubicin and GG918 (Elacridar) by New Polymer-Lipid Hybrid Nanoparticles (PLN) for Enhanced Treatment of Multidrug-Resistant Breast Cancer. J. Control. Release 2006, 116, 275–284. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-Modified Lipid-Polymer Hybrid Nanoparticles for Targeted Paclitaxel Delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef]
- Monirinasab, H.; Asadi, H.; Rostamizadeh, K.; Esmaeilzadeh, A.; Khodaei, M.; Fathi, M. Novel Lipid-Polymer Hybrid Nanoparticles for SiRNA Delivery and IGF-1R Gene Silencing in Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2018, 48, 96–105. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Hou, X.; Xie, X.; Shi, J.; Shen, J.; He, Y.; Wang, Z.; Feng, N. Functional Lipid Polymeric Nanoparticles for Oral Drug Delivery: Rapid Mucus Penetration and Improved Cell Entry and Cellular Transport. Nanomedicine 2019, 21, 102075. [Google Scholar] [CrossRef]
- Parvez, S.; Yadagiri, G.; Gedda, M.R.; Singh, A.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Modified Solid Lipid Nanoparticles Encapsulated with Amphotericin B and Paromomycin: An Effective Oral Combination against Experimental Murine Visceral Leishmaniasis. Sci. Rep. 2020, 10, 12243. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, B.A.; Tenne, R. Polymer-Assisted Fabrication of Nanoparticles and Nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Nguyen, S.T. Smart Nanoscale Drug Delivery Platforms from Stimuli-Responsive Polymers and Liposomes. Macromolecules 2013, 46, 9169–9180. [Google Scholar] [CrossRef]
- Nosova, A.S.; Koloskova, O.O.; Nikonova, A.A.; Simonova, V.A.; Smirnov, V.V.; Kudlay, D.; Khaitov, M.R. Diversity of PEGylation Methods of Liposomes and Their Influence on RNA Delivery. Med. Chem. Commun. 2019, 10, 369–377. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Clawson, C.; Ton, L.; Aryal, S.; Fu, V.; Esener, S.; Zhang, L. Synthesis and Characterization of Lipid–Polymer Hybrid Nanoparticles with PH-Triggered Poly(Ethylene Glycol) Shedding. Langmuir 2011, 27, 10556–10561. [Google Scholar] [CrossRef]
- Wang, J. Combination Treatment of Cervical Cancer Using Folate-Decorated, PH-Sensitive, Carboplatin and Paclitaxel Co-Loaded Lipid-Polymer Hybrid Nanoparticles. Drug Des. Dev. Ther. 2020, 14, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Singh, B.; Kushwah, V.; Tyagi, R.K.; Sharma, R.; Jain, S.; Katare, O.P. The Ligand (s) Anchored Lipobrid Nanoconstruct Mediated Delivery of Methotrexate: An Effective Approach in Breast Cancer Therapeutics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2043–2060. [Google Scholar] [CrossRef]
- Omar, S.H.; Osman, R.; Mamdouh, W.; Abdel-Bar, H.M.; Awad, G.A.S. Bioinspired Lipid-Polysaccharide Modified Hybrid Nanoparticles as a Brain-Targeted Highly Loaded Carrier for a Hydrophilic Drug. Int. J. Biol. Macromol. 2020, 165, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Yougan, C.; Yuanyuan, D.; Chenyao, Z.; Congming, X. Anti Prostate Cancer Therapy: Aptamer-Functionalized, Curcumin and Cabazitaxel Co-Delivered, Tumor Targeted Lipid-Polymer Hybrid Nanoparticles. Biomed. Pharmacother. 2020, 127, 110181. [Google Scholar]
- Zhang, H.; Dong, S.; Zhang, S.; Li, Y.; Li, J.; Dai, Y.; Wang, D. PH-Responsive Lipid Polymer Hybrid Nanoparticles (LPHNs) Based on Poly (β-Amino Ester) as a Promising Candidate to Resist Breast Cancers. J. Drug Deliv. Sci. Technol. 2021, 61, 102102. [Google Scholar] [CrossRef]
- Yao, C.; Wu, M.; Zhang, C.; Lin, X.; Wei, Z.; Zheng, Y.; Zhang, D.; Zhang, Z.; Liu, X. Photoresponsive Lipid-Polymer Hybrid Nanoparticles for Controlled Doxorubicin Release. Nanotechnology 2017, 28, 255101. [Google Scholar] [CrossRef]
- Wang, J.; Su, G.; Yin, X.; Luo, J.; Gu, R.; Wang, S.; Feng, J.; Chen, B. Non-Small Cell Lung Cancer-Targeted, Redox-Sensitive Lipid-Polymer Hybrid Nanoparticles for the Delivery of a Second-Generation Irreversible Epidermal Growth Factor Inhibitor—Afatinib: In Vitro and in Vivo Evaluation. Biomed. Pharmacother. 2019, 120, 109493. [Google Scholar] [CrossRef]
- Joshy, K.S.; Augustine, R.; Mayeen, A.; Alex, S.M.; Hasan, A.; Thomas, S.; Chi, H. NiFe2O4/Poly(Ethylene Glycol)/Lipid–Polymer Hybrid Nanoparticles for Anti-Cancer Drug Delivery. New J. Chem. 2020, 44, 18162–18172. [Google Scholar] [CrossRef]



| Polymer Used | Lipid Used | Reference |
|---|---|---|
 Polylactic-co-glycolic acid (PLGA) | 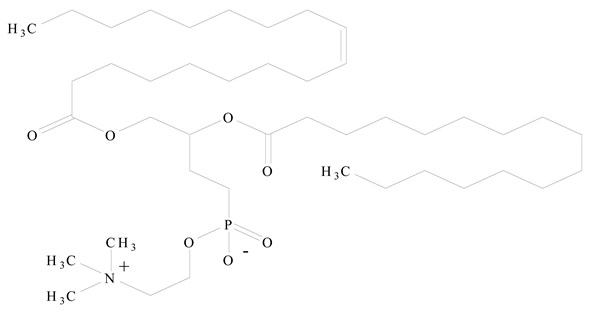 Phosphatidyl choline (lecithin) | [27] |
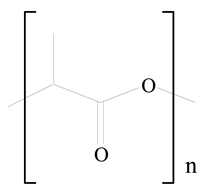 Polylactic acid (PLA) | 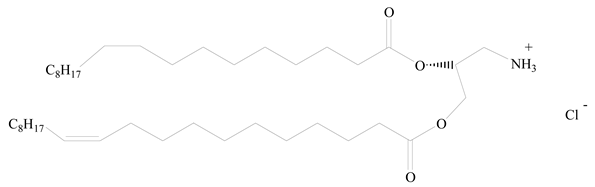 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) | [28] |
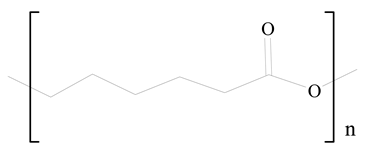 Polycaprolactone (PCL) |  1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) | [29] |
 D-Glucosamine and N-acetyl-D-glucosamine (chitosan) | 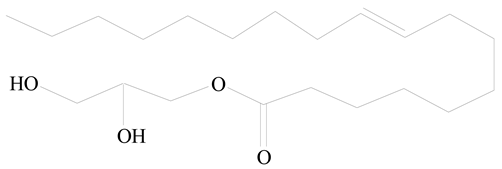 Glyceryl monooleate (GMO) | [30] |
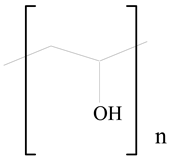 Poly(vinyl alcohol) (PVA) |  Stearic acid | [31] |
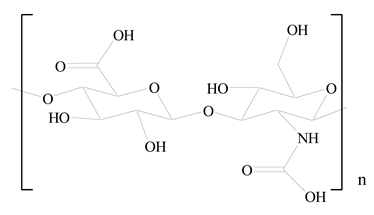 Hyaluronic acid (HA) | 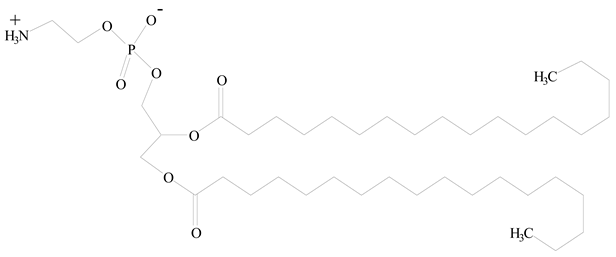 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) | [32] |
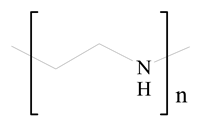 Polyethylenimine (PEI) |  1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) | [33] |
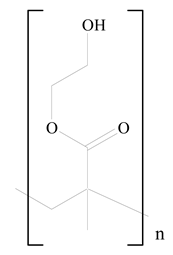 Poly(2-hydroxyethyl methacrylate) (PHEMA) | 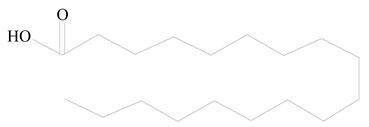 Stearic acid | [34] |
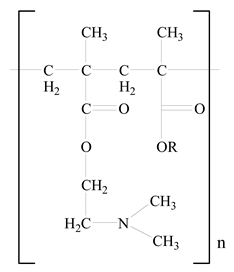 Eudragit |  Glycerol monostearate | [35] |
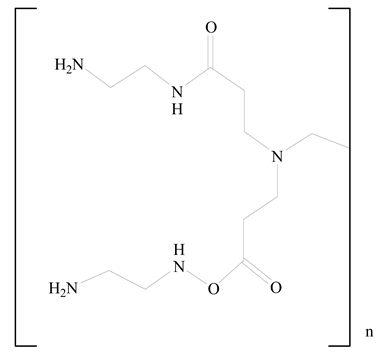 Polyamidoamine | 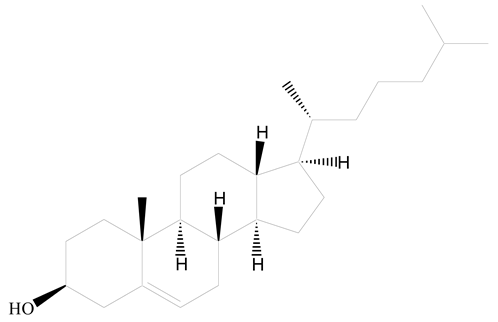 (3β)-Cholest-5-en-3-ol (cholesterol) | [36] |
 Polylactic-co-glycolic acid (PLGA) | 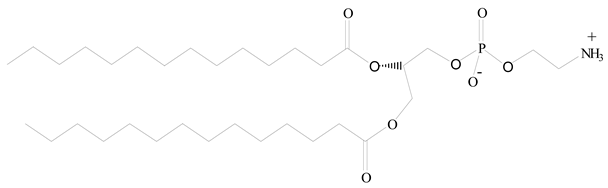 1,2-Dimyristoyl-sn-glycero-3-phosphoethanolaminediethylene (DMPE) | [37] |
 Polylactic-co-glycolic acid (PLGA) |  Diethylenetriaminepentaacetate (DTPA) | [37] |
 Polylactic-co-glycolic acid (PLGA) | 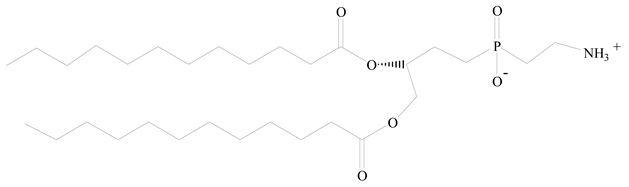 1,2-Dilauroyl-sn-glycero-3-phosphocholine structure (DLPC) | [25] |
| Polymer | Lipid | Polymer-to-Lipid Ratio (w/w) | Method of Preparation | Drugs | Targeting Ligand | Size (nm) | PdI | Zeta Potential (mV) | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| PLGA | Lecithin/DSPE-PEG | NR | One step | AuNC/QD | NA | 50–100 | 0.11 | NA | Diagnostic/optical imaging | [27] |
| PLGA | Cholesterol | NR | ESE | CUR | NA | 202 and 223 | ~0.10 | −8.0 and −0.6 | Cancer therapy | [57] |
| PLGA | EPC, DOPE | 3.5/15 | Solvent injection method | 7-APTADD | Transferrin | 170 ± 7 | NR | −18.9 ± 1.5 | Cancer therapy (SKBR-3 breast cancer cells) | [58] |
| PLGA | Lecithin, DMPE, DTPA/ DSPE-PEG | 1/0.15 | Single-step nanoprecipitation | DTX, 90Y, 111In | A10 Apt | ~65 | NR | ~35 | Prostate cancer therapy | [37] |
| PLGA | Lecithin–DSPE-PEG | 1/0.38 | Nanoprecipitation process | PTXL and GEM | NA | 70 ± 1 | NR | −53 ± 2 | Cancer therapy (XPA3 pancreatic cancer cells) | [59] |
| PLGA | Soybean lecithin-DSPE-PEG | 9/2.25 | - | PTX | NA | ~60 | NR | NR | Coronary artery disease | [60] |
| PLGA | DSPE-PEG | 1/0.4 | Modified nanoprecipitation process | DTX | FA | <25 | NR | −10 | Cytotoxicity in KB cells (CCL17) | [61] |
| PLGA | EPC-DSPE-PEG | 14/1 | Modified double emulsion method | Cy3-siRNA | NA | 225 ± 8 | NR | −10 | Gene delivery for cancer cells (GFP-HeLa cells and of HeLa cells) | [62] |
| PLGA | PEG-OQLCS-cholesterol | 1/1 | o/w emulsification solvent evaporation | PTX | FA | 184 ± 8 | NR | −24 ± 4 | Cancer therapy (Hela cervical and A549 lung cancer cells) | [63] |
| PLGA | DOTAP, DSPE-PEG, cholesterol | NR | Double ESE method | BSA | NA | ~144 | NR | +4.5–5.6 | Vaccine delivery | [64] |
| PLGA | DOPC, DOTAP, DSPE-PEG | 2/15 | Spontaneous emulsification and solvent diffusion (SESD) method | Sirolimus or propolis | Fluorescent (NBD-PC lipid and NIR dye–DiD (40 mg)) | 150–250 | 0.14–0.26 | + 8 to −33 | Cytotoxic studies against human aortic endothelial primary cells (HAECs) | [65] |
| PLGA | DOTAP | 1/0.24 | Double ESE method with self-assembly | Plasmid DNA | NA | 150–250 | 0.08–0.17 | +36 to +64 | Cancer therapy (HEK293T embryonic kidney, HeLa cervical, HaCaT-immortalized human keratinocytes, and HepG2 immortal cancer cells) | [66] |
| PLGA | RBC membranes | 1/1 | - | DOX | NA | 70–90 | 0.10–0.25 | −10.0 ± 2.7 | Leukemia cell line, Kasumi-1 | [67] |
| PLGA | Platelet membranes | 5/1 | Nanoprecipitation process | DOX, Vancomycin | NA | ~115 | 0.11–0.24 | −30.5 ± 0.5 | Coronary restenosis | [68] |
| PLGA | PC/cholesterol/ PEG-DSPE | 50/27.5 | ESE technique | Combretastatin, DOX | NA | 80–120 | NR | NR | Cancer therapy (B16/F10 lung cancer cells) | [69] |
| PLGA | Soybean lecithin-PEG2000, DSPE–PEG2000 | 40/12 | Single-step assembly method | DOX | FA | 118 ± 3 | 0.12 ± 0.01 | −8.5 ± 2.4 | Cancer therapy (KB oral cavity squamous and COS-7 African green monkey SV40-transformed kidney fibroblast cancer cells) | [70] |
| PLGA | DLPC/DSPE–PEG | NR | ESE technique | DTX | FA | 263 ± 8 | 0.16–0.03 | −20.7 ± 1.2 | Cancer therapy (MCF7 breast and NIH/3T3 murine fibroblast cancer cells) | [25] |
| PLGA | Lecithin, DSPE-PEG | 0.3/1 | Single-step sonication method | Cisplatin | FA | 94 ± 2 | 0.18 ± 0.06 | −19.8 ± 2.4 | Cancer therapy (MCF7 breast and A549 lung cancer cells) | [71] |
| PLGA | DSPE, Lecithin-PEG | 1/0.15 | Emulsification/solvent diffusion method | DOX | FA | 118 ± 0.7 | 0.10 ± 0.03 | 15.1 ± 3.8 | Cancer therapy (MCF7 breast cancer cells) | [72] |
| PLGA | EPC/DSPE–PEG | 15/1 | Modified emulsification technique | 10-Hydroxycamptothecin | RGD | 249 | 0.29 | −25.6 | Cancer therapy (MCF7 and MDA-MB- 435s breast cancer cells) | [73] |
| PLGA | Lecithin/DSPE–PEG | 1/1 | Modified single-step nanoprecipitation | Isoliquiritigenin | RGD | 137 ± 2 | NR | −34.2 ± 1.2 | Cancer therapy (MCF7 and MDA-MB- 231 and 4T1 breast cancer cells) | [74] |
| PLGA | Lecithin/DSPE–PEG | 1/0.15 | W/O/W ESE process | DTX | RGD | 110 ± 13 | 0.13 | −25.6 ± 1.4 | Cancer therapy (C6 glioma and GBM brain cancer cell-bearing rats) | [75] |
| PLGA | DOTAP, DOPE | 0.4/0.17 | Combination of nanoprecipitation and self-assembly | siRNA | NA | 207 ± 4 | 0.09 ± 0.005 | 5.2 ± 1.5 | Cancer therapy (LNCaP, PC3, and DU145 prostate cancer cells) | [76] |
| PLGA | DPPC, DSPE-PEG | 1/0.2 | Single ESE technique | CUR | NA | 171 ± 8 | 0.174 ± 0.02 | NR | Cancer therapy (MDA-MB-231 breast and HUVECs human umbilical vein endothelial cancer cells) | [77] |
| PLGA | Soybean lecithin, DSPE-PEG | 1/0.2 | Nanoprecipitation method | DOX and 2′-deoxy-5- Azacytidine | NA | 80 ± 20 | NR | −34.0 | Cancer therapy (MDA-MB-231 breast (MB231) and HONE1cancer cells) | [78] |
| PLGA | Soybean lecithin | 2/1 | Emulsification-sonication method | PTX, CIS | Arg-Gly-Asp peptide sequence (RGD peptide) | 191 ± 5 | 0.16 ± 0.03 | −37.2 ± 3.9 | Cancer therapy (A549 lung cancer cells) | [79] |
| PLGA | Soybean lecithin, DSPE-PEG | 2/1 | Nanoprecipitation method | PTX, TL | NA | 160 ± 5 | 0.17 ± 0.03 | −30.4 ± 4.4 | Cancer therapy (A549 and A549/PTX (PTX-resistant) lung cancer cells) | [56] |
| PLGA | DSPE-mPEG, cholesterol | 4/1 | Nanoprecipitation technique | Linezolid | NA | 50–150 | 0.15 ± 0.05 | −44.0 ± 5.0 | Antibacterial activity (osteomyelitis; methicillin-resistant staphylococcus aureus (MRSA)) | [80] |
| PLGA | Lecithin, DSPE-PEG | 1/0.2 | One-step self-assembly method | Cucurbitacin B | NA | 94–112 | 0.09–0.12 | −30.0 ± 5.5 | Cancer therapy (MDAMB231 triple-negative human breast cancer cells) | [81] |
| PLGA | DSPE-PEG | 1/0.4 | Modified nanoprecipitation process | MK-8722 | WYRGRLC peptide | ~25 | NR | ~15 | Cartilage targeting for osteoarthritis | [82] |
| PLGA | DSPE-PEG, HSPC | 10/0.1 | Modified two-step method | DOX | NA | 142 ± 8 | 0.1 | −22.5 ± 4.0 | Cancer therapy (M14 DOX-resistant breast cancer cells) | [83] |
| PLGA | Lecithin, DSPE-PEG | 2/0.425 | One-step self-assembly method | Lonidamine | NA | 110 ± 3 | 0.17 ± 0.01 | −41.2 ± 0.5 | Benign prostatic hyperplasia (BPH) treatment | [84] |
| PLGA | DSPE-PEG | 1/0.2 | Nanoprecipitation method | DOX and edelfosine | Folic acid | 122 ± 2 | 0.14 | −18.4 ± 1.2 | Cancer therapy (MG63 bone cancer cells) | [85] |
| PLGA | Lecithin, cholesterol | 15/1 | Modified single-step nanoprecipitation self-assembly method | Entecavir (E) | Vitamin E | 188 ± 4 | 0.14 ± 0.02 | −21.6 ± 1.0 | Antiviral therapy (J774 macrophages cells) | [86] |
| PLGA | Soy lecithin, DSPE-PEG | NR | Modified nanoprecipitation method | Zinc phthalocyanine and quercetin | NA | 170 ± 20 | 0.30 ± 0.05 | −30 ± 10 | Cancer therapy (MCF7 breast cancer therapy) | [87] |
| PLGA | Lecithin, DSPE-PEG | 1/0.2 | Modified single-step nanoprecipitation process | DOX.HCl | NA | 173–208 | <0.3 | −31.7 to −28.0 | Cancer therapy (MDA-MB231 breast and PC3 prostate cancer cells) | [24] |
| PLGA | DSPE-PEG | 1/0.15 | Self-assembled nanoprecipitation technique | DTX | NA | 143 ± 5 | <0.26 | −12.2 ± 0.4 | Cancer therapy (MDA-MB231 breast cancer cells) | [88] |
| PLGA | Soy lecithin | 1/4 | Nanoprecipitation method | PSO | NA | 93 ± 2 | 0.25 ± 0.02 | −27.6 ± 0.3 | Cancer therapy (MCF7 breast cancer cells) | [89] |
| PLGA | Soy phosphatidylcholine (SPC), DSPE-PEG | 35/65 | Modified double ESE method | GEM.HCl | NA | 237 | <0.3 | −16.7 to −24.5 | Improve drug encapsulation efficiency and release properties | [90] |
| PLA | SPC/DPPE/DSPE | 1/0.2 | Reverse micelle− solvent evaporation technique combined with a self-assembly method | Mitomycin C | FA | 215 ± 5 | 0.14 ± 0.02 | −25.8 ± 2.3 | Cancer therapy (HeLa cervical and A549 lung cancer cells) | [91] |
| PLA | DSPE-PEG, SA | 1/0.4 | Self-assembled nanoprecipitation technique | MTX and BC | Fructose | 117 ± 4 | 0.29 ± 0.11 | −6.8 ± 0.2 | Cancer therapy (MCF-7 breast cancer cells) | [92] |
| PLA | DSPC, DOTAP | NR | Surfactant-free solvent diffusion method | LAH4-L1 peptides | NA | 151 ± 3 | 0.09 ± 0.01 | −64 ± 3 | Cytotoxicity (antigenic presenting cells, namely DC2.4, and epithelial HeLa cells) | [93] |
| PLA | DOTAP | 1/0.1 | Double emulsion solvent evaporation method. | EYFP mRNA | Monoclonal anti-CD7 | 157 ± 9 | 0.13 ± 0.02 | −16.2 ± 0.6 | Cancer therapy (A549-CD7 lung cancer cells) | [28] |
| PCL | DSPE-PEG | 2/1 | Single-step nanoprecipitation method | MTX and ACL | Fucose | 151 ± 5 | 0.18 ± 0.03 | +2.9 ± 0.8 | Cancer therapy (MCF7, MDA-MB231 breast cancer cells) | [94] |
| PCL | DSPE-PEG | 2/3 | One-step nanoprecipitation | GA and DOX | HA | 165 ± 4 | 0.16 ± 0.02 | −41.3 ± 2.8 | Cancer therapy (HL-60/ADR leukemia cells and K562/ADR bone cells) | [29] |
| PCL | SPC | 2/5 | Sonication method | ERL and BEV | HA | 100–120 | 0.12–0.15 | −21.2 ± 2.9 | Cancer therapy (A549 and H1975 lung cancer cells) | [95] |
| PCL | DSPE-PEG, SPC | 1/1 | Solvent displacement method | CIS and 5-FU | TAB | 105 ± 5 | 0.18 ± 0.20 | 28.5 ± 1.9 | Cancer therapy BE-3 esophageal cancer cells) | [96] |
| PCL | PEG-DSPE, lecithin | 5/1 | W/O/W double emulsification method | RPV | NA | 112 ± 2 | 0.16 ± 0.02 | −33.2 ± 3.2 | Cytotoxicity (BALB/c-3T3 fibroblast viability); In vivo analgesic and anesthesia effect in rats | [97] |
| Eudragit | SA | 2/1 | Combined process, using both probe sonication and magnetic stirring processes | DOX | NA | 121 ± 3 | 0.25 ± 0.003 | −33.9 ± 3.5 | Pharmacokinetic study on healthy rabbits | [98] |
| Eudragit | Glycerol monostearate, soy lecithin | NR | Modification of the homogenization followed by ultrasonication method | Isradipine | NA | 120–124 | NR | −28.6 | Hypertensive activity in Wistar rats | [35] |
| Polyamidoamine-grafted cholesterol | DOTAP, DOPE, cholesterol, DSPE-PEG | 5/1 | Thin-film hydration method | Anti-EGFR siRNA | Peptide (HAIYPRH, named as T7) modified | 99 ± 0.6 | NR | 42.5 ± 1.0 | Cancer therapy (MCF7 breast cancer cells) | [36] |
| Chitosan | DSPE-PEG | NR | ESE method | Sorafenib | Folic acid | 178.4 ± 2.6 | NR | −21.4 ± 1.5 | Cancer therapy (SMMC-7721 liver cancer cells) | [99] |
| Chitosan | GMO | 1/0.2 | Self-assembly method | Enoxaparin | NA | ~300 | <0.3 | ~20.0 | Develop orally applicable delivery system for hydrophilic macromolecules | [30] |
| Chitosan | Lipoid S75 | 1/20 | Single-step ionic gelation method | CIS | Fluorescent dyes, rhodamine 123, and rhodamine-PE | 181–245 | 0.3–0.4 | 20–30 | Cytotoxicity studies (doxorubicin-resistant A2780 ovarian carcinoma cell line) | [100] |
| Chitosan | Phospholipon R 80 H | NR | Solvent injection method | Carbamazepine | NA | 168 ± 1 | NR | −28.9 ± 2.0 | Anti epileptic treatment | [101] |
| Chitosan | Lecithin, DOTAP, DOPE | 1/2 | Ultrasonication process | AllStar negative-control fluorescent AF488-siRNA | siERK1(mouse) and FITC labeled anti-ERK1 (K-23) mouse | ~200 | 0.18–0.14 | 41–54 | Cytotoxicity studies (NIH3T3 mouse fibroblasts) | [102] |
| Chitosan | Soy bean lecithin | 1.25/1 | Syringe method | SLUG mRNA | NA | 180 | 0.2–0.5 | 20–40 | Cancer therapy (MDA-MB453 breast cancer cells) | [103] |
| PVA | SA | 1/0.279 | Solvent injection method | Berberine | NA | 395 ± 17 | 0.08 ± 0.01 | −18.3 ± 0.1 | Antihyperlipidemic activity | [31] |
| HA | Egg PC, DSPE-PEG | 7/2 | Solvent evaporation method | Ginsenoside Rg3 (S)-Rg3 | NA | 134 ± 5 | 0.24 ± 0.03 | −29.5 ± 1.3 | Cancer therapy (A549 lung cancer cells) | [32] |
| PEI- PCL | DOPE, DSPE-PEG | NR | Microfluidic technology | Anti-EGFR siRNA | NA | 200 ± 3 | 0.26 ± 0.04 | − 1.2 ± 1.8 | Cancer therapy (PC-3 prostate cancer cells) | [33] |
| PHEMA | SA | 4/1 | ESE method | CUR | NA | 184 | NR | −29.3 | Cancer therapy (MCF7 breast cancer cells) | [34] |
| PESO + Pluronic F-68 | SA | NR | Ultrasonication method | DOX and GG918 | NA | 272 ± 48 | NR | −19.4 ± 0.3 | Cancer therapy (MDA435/ LCC6/MDR1 breast cancer cells) | [104] |
| PESO | SA, tristearin | NR | ESE method | DOX | NA | 290 | NR | NR | Cancer therapy (EMT6/WT murine breast cancer cells) | [104] |
| PCL-PEG-PCL | Soybean, DSPE-PEG | 10/1 | Thin-film hydration and ultrasonic dispersion method | PTX | FA | 279 ± 8 | 0.17 ± 0.02 | −17.5 ± 1.1 | Cancer therapy (EMT6 breast cancer cells) | [105] |
| PLA-PEG-PLA | DDAB | 10.8/1.4 | Sonication method | FAM-siRNA | NA | 48 ± 2 | 0.25 ± 0.03 | 12 ± 4 | Cancer therapy (MCF7 breast cancer cells) | [106] |
| pHPMA-chitosan | DSPE-PEG2000; LIPOID S100 | NR | Nanoprecipitation | Vitamin B12 | NA | 135 | NR | ~18 | Mucus penetration and improved cell entry in Caco-2 colon cells | [107] |
| HPCD | Soy lecithin | NR | ESE method | Amphotericin B and paromomycin | Fmoc-Cl and FITC | 164 ± 17 | 0.39 ± 0.18 | −14.7 ± 3.4 | Anti-leishmaniasis activity | [108] |
| S. No. | Author/Owner | Patent No./Countries Covered | Priority Date | The Title of Invention | Polymer/Lipid Used | Applications |
|---|---|---|---|---|---|---|
| 1 | Zhang Xueqing, Teng Yilong, Chen Qijing/Rongcan Biomedical Technology (Shanghai) Co., Ltd., China | CN115784920A | 9 February 2023 | A kind of ionizable lipid compound with high transfection efficiency and its application | PEG/cholesterol, DOPE, and DSPC | Encapsulation of nucleic acids, targeting them to target cells and delivery of nucleic acids of specific genes into cells |
| 2 | Song Gengshen, Zhang Honglei, Chen Xichao, Wang Huanyu, Huang Dawei, Yu Xiaowen; Liu Yangjian, Li Yuqing, Yan Rucan, Qiao Lianyong, Li Xiaojuan, Chen Xiaoling, Sun Zhenlong, Wang Shuai, Dong Kai, Zhang Jinyu/Beijing Yuekang Kechuang Pharmaceutical Technology Co., Ltd., China | CN115784921A | 8 February 2023 | Extrahepatic-targeted cationic lipid compound with high efficiency and low toxicity and its composition | PEG/cholesterol, DOPE | The effective delivery of biologically active substances, including small drug molecules, proteins, peptides, and nucleic acids |
| 3 | Yang Hyun-Joo, Wang Jun, Chen Chaoran, Su Miao, Zhang Yuxi, Lin Song, Yu Boya, Du Xiaojiao/Univ South China Tech, China | CN115671045A | 30 December 2022 | Non-hepatic targeting nucleic acid nano-preparation as well as preparation method and application thereof | PEG-PLA or PLGA/cationic lipids-DOTMA, DOTAP, DORI, DSRIE, DOGS, DOSC. | To regulate genes, proteins, other targets, and the tumor microenvironment |
| 4 | Min Peng, Huang Dan, Li Zheng/Keyan Bioengineering Research (Tianjin) Co., Ltd., China | CN115778860A | 26 December 2022 | Composite of annatto seed oil and hydrogel and its preparation method and application | PGA/annatto seed oil | Cosmetic applications |
| 5 | Liu Xuhan, Zhang Jiancheng, Han Wei, Li Qin/Univ Shenzhen General Hospital, China | CN115645523A | 22 December 2022 | Application of polymer–lipid hybrid nanoparticles as immunologic adjuvant and immune preparation | PEG-PCL, PEG-PLA, PEG-PLGA, PEG-PDL/DOTAP, DOTMA, DDAB, ethyl PC, etc. | As immune adjuvants to improve humoral immunity and cellular immunity, they have advantages in resisting viral infections |
| 6 | Liu Qingwei, Tan Bibo, Li Yong, Fan Liqiao, Zhao Qun, Zhang Zhidong, Li Zhaoxing/Fourth Hospital of Hebei Medical University, China | CN115737823A | 19 December 2022 | A nanodrug delivery system targeting immune cells | DSPC, DMG-PEG/cholesterol, SA | In targeted therapy of tumor immune cells |
| 7 | Xu Congfei, Zhang Yue, Wang Yue, Zhao Gui, Wang Jun, Yang Xianzhu/South China University of Technology, China | CN115737841A | 7 December 2022 | Gene nanomedicine for enhancing T cell anti-tumor immune effect and its preparation method and application | PEG, PLGA/cholesterol derivatives | In chemotherapy and immunotherapy by enhancing the anti-tumor immune effect of T cells for gene nanomedicine |
| 8 | Guan Yixin, Zhang Yipeng, Wei Mengying, Liu Xiangrui/Zhejiang University, China | CN115721734A | 6 December 2022 | Solid lipid nanoparticle complex loaded with budesonide and preparation method thereof | Cellulose, chitosan/SA, monoglyceride stearate, and other fatty acids | The treatment of moderate to severe Crohn’s disease, ulcerative colitis, and other local inflammatory colorectal diseases |
| 9 | Ying Bo/Suzhou Abogen Biosciences Co., Ltd., China | WO 2022/152141 A2 CN US | 14 January 2021 | Polymer conjugated lipid compounds and lipid nanoparticle compositions | PEG (DMG-PEG)/DSPC, cholesterol | The delivery of nucleic acid for therapeutic or prophylactic purposes, including vaccination |
| 10 | Kim Yong Hee, Yong Seok Beom, Chung Jee Young, Kim Seong Su, Kim Jae Hyun, Ra Se HeeIucf Hyu, South Korea | US2021379197A1 US, KR | 3 June 2020 | Dual targeting lipid–polymer hybrid NPs | PLLA, PGA, PLA, PLGA, PCL and PHBV/DSPE, DMPC, DLPC | Applied either alone or in various combination therapies with patient compliance |
| 11 | Shah Sunil, Ngu Sean/Max Biology Co., Ltd., USA | WO 2021/234548 A1 IL, CA, AU, US, KR | 18 May 2020 | Lipid–polymer compositions and methods of use | Poloxamer/PC, phosphatidylserine, phosphatidylglycerol, phosphatidylethanolamine, or phosphatidylinositol | Encapsulation of bioactive agent |
| 12 | Cai Yu, Zhuang Yong, Liu Hui, Ma Qianqian, Zhang Ronghua, Yang Li, Wang Panpan, Du Manling, Pang Mujuan/Univ Jinan, China | CN110960509A | 30 December 2019 | Baicalin polymer–lipid NPs as well as preparation method and application thereof | PEG- 2000, PLGA/distearoylphosphatidylethanolamine | Preparation of breast cancer treatment drugs |
| 13 | Chitkara Deepak, Pukale Sudeep Sudesh, Singh Arihant Kumar, Mittal Anupama, Sharma Saurabh/Incisive Element Llc, Nanobrid Innovations Private Limited, India | US 2021/0369631 A1 US, EP, JP | 2 February 2019 | A lipid–polymer hybrid nanoparticle | mPEG-PLA/Solid lipids (SA, GMS, compritol, precirol, cholesterol or cholic acid), liquid lipid (oleic acid, linoleic acid, miglyol, capmul MCM C8 or captex 355) | The delivery of antibiotics, proteins, peptides, and vaccines |
| 14 | Kaczmarek James, Anderson Daniel, Rhym Luke, Kauffman Kevin, Patel Asha/Massachusetts Inst Technology, USA | WO 2020/086965 A9 EP, CA, US, AU | 26 October 2018 | Polymer–lipids and compositions | PEG/cholesterol | PBAE polymers and formulations |
| 15 | Loo Say Chye Joachim, Baek Jongsuep, Tan Chuan Hao/Univ Nanyang Tech, Nat Univ Singapore, Singapore | WO 2019/135715A1 SG | 5 January 2018 | Lipid–polymer hybrid NPs | PLGA/DOTAP | Lipid–polymer NPs that contain active pharmaceutical ingredient for treating diseases |
| 16 | Uehara Keiji, Hatanaka Kentaro, Iwai Hiroto, Naoi Tomoyuki, Destito Giuseppe, Nugent Rachel Soloff/Kyowa Hakko Kirin Co., Ltd., Japan | WO2018225873A1 US, JP | 6 June 2017 | Nucleic acid-containing NPs | PEG, polyaminoacrylate/polyethylene-glycolated lipids, specifically polyethylene glycol-phosphatidylethanolamine and polyethylene glycol-diacylglycerol, etc. | Nucleic acid-containing NPs that can be delivered to immune system cells |
| 17 | Benjamin Frank Geldho/Modernatx, Inc., USA | WO2017223135A1 US | 24 June 2016 | Lipid NPs | PEG or PEG-DMG, PEG-DSG or PEG-DPG, disteroylphosphatidylcholine, cholesterol | Useful in enhancing the delivery of agents such as nucleic acids |
| 18 | Camilla Foged, Henrik Franzyk, Xianghui Zeng, Hanne Mørck Nielsen, Kaushik Thanki/Københavns Universitet, Copenhagen | WO2017158093A1 | 17 March 2016 | Nanoparticle compositions comprising PLGA derivatives and a lipid | PLGA/DOTAP | The delivery of drugs, with enhanced efficacy and reduced adverse effects |
| 19 | Pieter Jaap Gaillard, Jacob Rip/Eyesiu Medicines B.V., Netherlands | WO2017025588A1 TW KR RU CN EP US JP | 11 August 2015 | Pegylated lipid nanoparticle with bioactive lipophilic compound | PEA, PEG/neutral phospholipids comprise at least one of HSPC and DSPE | Systemic or topical delivery of lipophilic diagnostic or therapeutic agents |
| 20 | Jacob Klein, Ronit Goldberg, Jasmine Seror, Weifeng Lin, Reut Mashiach/Yeda Research and Development Co Ltd., Israel | US20170128365A1 US CA CN US ES EP ES WO EP | 15 June 2015 | Surface treatment by water-soluble polymers and lipids/liposomes | PEG/PC (DMPC) | Treating a synovial joint disorder associated with increased articular friction |
| 21 | Hatanaka Kentaro, Yagi Nobuhiro, Kuboyama Takeshi, Yagi Kaori, Hosoe Shintaro/Kyowa Kirin Co., Ltd., Japan | US11298326B2 | 24 March 2015 | Nucleic acid-containing lipid NPs | PEG/DSPE, DMPE, cholesterol | As a pharmaceutical and are more stable and smaller than conventional particles |
| 22 | Clive Allan Prestidge, Paul Matthew Joyce/University of South Australia, Australia | WO2016141413A1 US CN AU WO | 11 March 2015 | Drug delivery composition comprising polymer–lipid hybrid microparticles | PLGA/medium-chain triglyceride (MCT; Miglylol®812) | The oral delivery of poorly water-soluble drugs, anticancer formulations, and vaccinations |
| 23 | Nian Wu/Nian Wu, USA | WO2015085173A1 WO CA US CN JP CN EP | 5 December 2014 | Polymer–carbohydrate conjugates for drug delivery technology | PEG–carbohydrate conjugates with sterols or “fat-soluble” vitamins (“lipo-vitamin”) | Pharmaceuticals, cosmetics and nutraceuticals |
| 24 | Saavedra Steven Scott, Aspinwall Craig A, Ratnayaka Saliya N, Bright Leonard/The Arizona Board of Regents on behalf of the University of Arizona, USA | US 10576456 B2 US | 30 June 2014 | Systems and methods of preparing stabilized lipid assemblies | Methacrylate, tridecafluoro 1, 1, 2, 2-tetrahydrodimethylchlorosilane (PFDCS)/DPhPC | In ion selectivity, chemical or mechanical gating, inherent signal amplification, well-defined open and closed states and simple electrical readout |
| 25 | Geoffrey Gnana Jeba Jesudian, Vijay Kalyansundaram Shastri/Murli Krishna Pharma Pvt. Ltd., India | WO2015136477A1 | 12 March 2014 | NPs of polymer and lipid mixture core for targeted drug delivery | PLGA/GMS, glyceryl behenate | Pharmaceutical drug delivery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. J. Funct. Biomater. 2023, 14, 437. https://doi.org/10.3390/jfb14090437
Jain S, Kumar M, Kumar P, Verma J, Rosenholm JM, Bansal KK, Vaidya A. Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. Journal of Functional Biomaterials. 2023; 14(9):437. https://doi.org/10.3390/jfb14090437
Chicago/Turabian StyleJain, Shweta, Mudit Kumar, Pushpendra Kumar, Jyoti Verma, Jessica M. Rosenholm, Kuldeep K. Bansal, and Ankur Vaidya. 2023. "Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery" Journal of Functional Biomaterials 14, no. 9: 437. https://doi.org/10.3390/jfb14090437
APA StyleJain, S., Kumar, M., Kumar, P., Verma, J., Rosenholm, J. M., Bansal, K. K., & Vaidya, A. (2023). Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. Journal of Functional Biomaterials, 14(9), 437. https://doi.org/10.3390/jfb14090437










