Potential of Bacterial Cellulose in Reconstructive Surgery of Body Integumentary System: Preliminary Studies in Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Culture Media
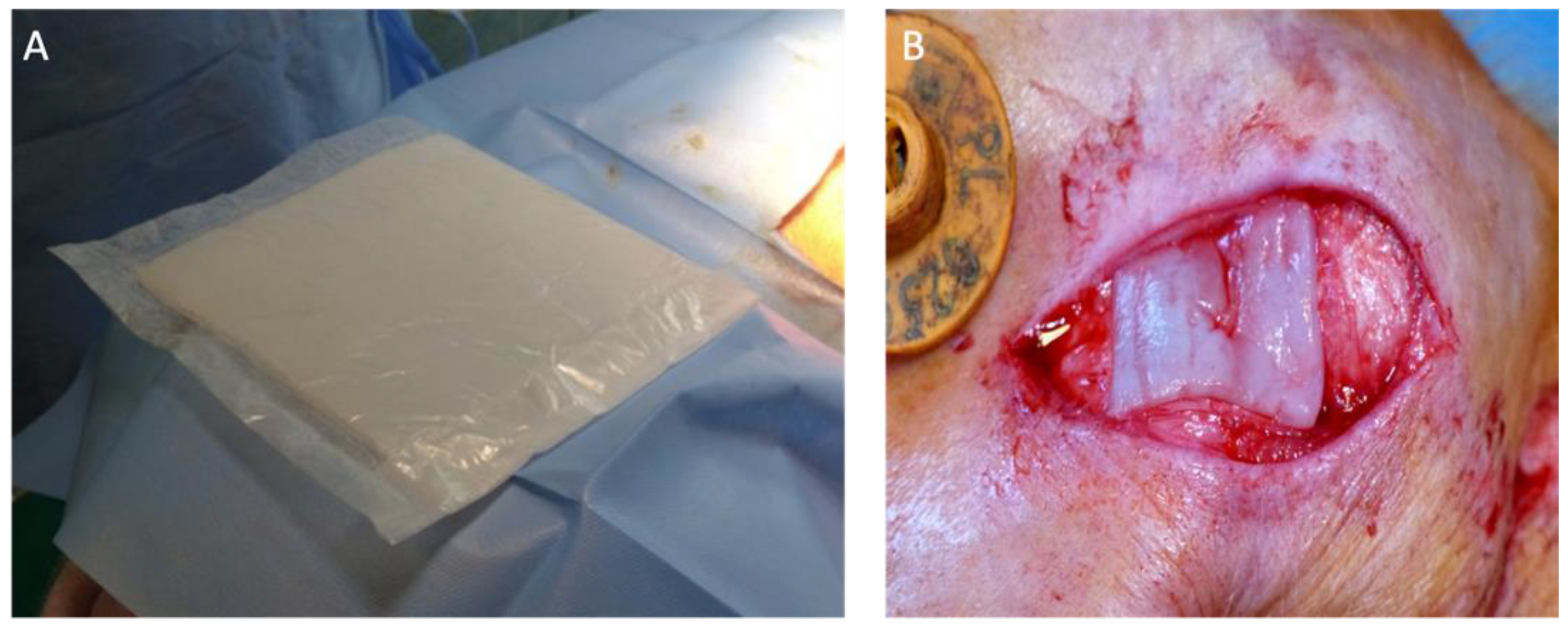
2.3. Surgical Procedures
2.4. Time of Observation
2.5. Clinical Evaluation
2.6. Histopathological Examination
3. Results
3.1. Clinical Evaluation
3.2. Histopathological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin Substitutes and Bioscaffolds: Temporary and Permanent Coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ludwicka, K.; Kolodziejczyk, M.; Gendaszewska-Darmach, E.; Chrzanowski, M.; Jędrzejczak-Krzepkowska, M.; Rytczak, P.; Bielecki, S. Stable composite of bacterial nanocellulose and perforated polypropylene mesh for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Jankau, J.; Błażyńska-Spychalska, A.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Pankiewicz, T.; Ludwicka, K.; Dettlaff, A.; Pęksa, R. Bacterial Cellulose Properties Fulfilling Requirements for a Biomaterial of Choice in Reconstructive Surgery and Wound Healing. Front. Bioeng. Biotechnol. 2022, 9, 805053. [Google Scholar] [CrossRef]
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound dressings as growth factor delivery platforms for chronic wound healing. Expert Opin. Drug Deliv. 2021, 18, 737–759. [Google Scholar] [CrossRef]
- Kołaczkowska, M.; Siondalski, P.; Kowalik, M.M.; Pęksa, R.; Długa, A.; Zając, W.; Dederko, P.; Kołodziejska, I.; Malinowska-Pańczyk, E.; Sinkiewicz, I.; et al. Assessment of the usefulness of bacterial cellulose produced by Gluconacetobacter xylinus E25 as a new biological implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 97, 302–312. [Google Scholar] [CrossRef]
- Martínez Ávila, H.; Feldmann, E.-M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Müller, R.; Hendriks, J.; Rotter, N.; et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 2015, 44, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Jaroennonthasit, W.; Lam, N.T.; Sukyai, P. Evaluation of carbon sources from sugar industry to bacterial nanocellulose produced by Komagataeibacter xylinus. Int. J. Biol. Macromol. 2021, 191, 299–304. [Google Scholar] [CrossRef]
- Tapking, C.; Popp, D.; Branski, L.K. Pig Model to Test Tissue-Engineered Skin. Methods Mol. Biol. 2019, 1993, 239–249. [Google Scholar] [CrossRef]
- Voipio, H.-M.; Baneux, P.; Gomez DeSegura, I.A.; Hau, J.; Wolfensohn, S. Guidelines for the veterinary care of laboratory animals: Report of the FELASA/ECLAM/ESLAV Joint Working Group on Veterinary Care. Lab. Anim. 2008, 42, 1–11. [Google Scholar] [CrossRef]
- Guillen, J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar]
- Jansen, L.A.; De Caigny, P.; Guay, N.A.; Lineaweaver, W.C.; Shokrollahi, K. The evidence base for the acellular dermal matrix AlloDerm: A systematic review. Ann. Plast. Surg. 2013, 70, 587–594. [Google Scholar] [CrossRef]
- Jansen, L.A.; Macadam, S.A. The Use of AlloDerm in Postmastectomy Alloplastic Breast Reconstruction: Part II. A Cost Analysis. Plast. Reconstr. Surg. 2011, 127, 2245–2254. [Google Scholar] [CrossRef]
- Thoma, D.S.; Benić, G.I.; Zwahlen, M.; Hämmerle, C.H.F.; Jung, R.E. A systematic review assessing soft tissue augmentation techniques. Clin. Oral Implant. Res. 2009, 20, 146–165. [Google Scholar] [CrossRef]
- Amorim, W.L.; Costa, H.O.; De Souza, F.C.; De Castro, M.G.; Da Silva, L. Experimental study of the tissue reaction caused by the presence of cellulose produced by Acetobacter xylinum in the nasal dorsum of rabbits. Braz. J. Otorhinolaryngol. 2009, 75, 200–207. [Google Scholar] [CrossRef]
- Wippermann, J.; Schumann, D.; Klemm, D.; Kosmehl, H.; Salehi-Gelani, S.; Wahlers, T. Preliminary Results of Small Arterial Substitute Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 592–596. [Google Scholar] [CrossRef]
- Ai, F.-F.; Mao, M.; Zhang, Y.; Kang, J.; Zhu, L. Experimental study of a new original mesh developed for pelvic floor reconstructive surgery. Int. Urogynecology J. 2019, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Högset, O.; Bredberg, G. Plaster of Paris: Thermal Properties and Biocompatibility A Study on an Alternative Implant Material for Ear Surgery. Acta Oto-Laryngol. 2009, 101, 445–452. [Google Scholar] [CrossRef]
- Sevastjanova, N.A.; Mansurova, L.A.; Dombrovska, L.E.; Slutskii, L.I. Biochemical characterization of connective tissue reaction to synthetic polymer implants. Biomaterials 1987, 8, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Vince, D.G.; Hunt, J.A.; Williams, D.F. Quantitative assessment of the tissue response to implanted biomaterials. Biomaterials 1991, 12, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Schadel, A.; Thun, G.; Stork, L.; Metzler, R. Immunodiffusion and Immuno-histochemical Investigations on the Reactivity of Oxide Ceramic Middle-Ear Implants. ORL 1993, 55, 216–221. [Google Scholar] [CrossRef]
- Ribot, E.J.; Tournier, C.; Aid-Launais, R.; Koonjoo, N.; Oliveira, H.; Trotier, A.J.; Rey, S.; Wecker, D.; Letourneur, D.; Vilamitjana, J.A.; et al. 3D anatomical and perfusion MRI for longitudinal evaluation of biomaterials for bone regeneration of femoral bone defect in rats. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pogorelova, N.; Rogachev, E.; Digel, I.; Chernigova, S.; Nardin, D. Bacterial Cellulose Nanocomposites: Morphology and Mechanical Properties. Materials 2020, 13, 2849. [Google Scholar] [CrossRef]
- Uo, M.; Watari, F.; Yokoyama, A.; Matsuno, H.; Kawasaki, T. Tissue reaction around metal implants observed by X-ray scanning analytical microscopy. Biomaterials 2001, 22, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, J.; Liu, S.; Chen, L.; Chen, M.; Yu, X.; Ma, N.; Zhang, J.; Chen, X.; Zhong, L.; et al. Magnetic resonance imaging for non-invasive clinical evaluation of normal and regenerated cartilage. Regen. Biomater. 2021, 8, rbab038. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.P.; Witte, F.; Tozzi, G. Applications of X-ray computed tomography for the evaluation of biomaterial-mediated bone regeneration in critical-sized defects. J. Microsc. 2019, 277, 179–196. [Google Scholar] [CrossRef] [PubMed]


| Congestion | Chronic Inflammation | Purulent Infiltration | Fibrosis | Presence of Giant Cells | Presence of Cellulose | |
|---|---|---|---|---|---|---|
| Ear—Pig no. 1 | 1 | 2 | 0 | 2 | 1 | 0 |
| Ear—Pig no. 2 | 1 | 2 | 0 | 2 | 0 | 0 |
| Ear—Pig no. 3 | 1 | 3 | 0 | 2 | 1 | 1 |
| Muscle—Pig no. 1 | 2 | 3 | 1 | 2 | 1 | 1 |
| Muscle—Pig no. 2 | Missed excision site | |||||
| Muscle—Pig no. 3 | 0 | 1 | 0 | 2 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błażyńska-Spychalska, A.; Kur, M.; Brzeski, T.; Zając, W.; Pankiewicz, T.; Bielecki, S.; Woliński, J.; Jankau, J. Potential of Bacterial Cellulose in Reconstructive Surgery of Body Integumentary System: Preliminary Studies in Animals. J. Funct. Biomater. 2023, 14, 397. https://doi.org/10.3390/jfb14080397
Błażyńska-Spychalska A, Kur M, Brzeski T, Zając W, Pankiewicz T, Bielecki S, Woliński J, Jankau J. Potential of Bacterial Cellulose in Reconstructive Surgery of Body Integumentary System: Preliminary Studies in Animals. Journal of Functional Biomaterials. 2023; 14(8):397. https://doi.org/10.3390/jfb14080397
Chicago/Turabian StyleBłażyńska-Spychalska, Agata, Martyna Kur, Tomasz Brzeski, Wacław Zając, Teresa Pankiewicz, Stanisław Bielecki, Jarosław Woliński, and Jerzy Jankau. 2023. "Potential of Bacterial Cellulose in Reconstructive Surgery of Body Integumentary System: Preliminary Studies in Animals" Journal of Functional Biomaterials 14, no. 8: 397. https://doi.org/10.3390/jfb14080397
APA StyleBłażyńska-Spychalska, A., Kur, M., Brzeski, T., Zając, W., Pankiewicz, T., Bielecki, S., Woliński, J., & Jankau, J. (2023). Potential of Bacterial Cellulose in Reconstructive Surgery of Body Integumentary System: Preliminary Studies in Animals. Journal of Functional Biomaterials, 14(8), 397. https://doi.org/10.3390/jfb14080397






