Characteristics of Chitosan Films with the Bioactive Substances—Caffeine and Propolis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan-Based Films
2.2. Antiradical Effect of Chitosan-Based Solutions
2.3. Antimicrobial Activity of Chitosan-Based Films
2.4. Mechanical Properties of Chitosan-Based Films
2.5. Scanning Electron Microscopy
2.6. Fourier Transform Infrared Spectroscopy
2.7. Statistical Analysis
3. Results
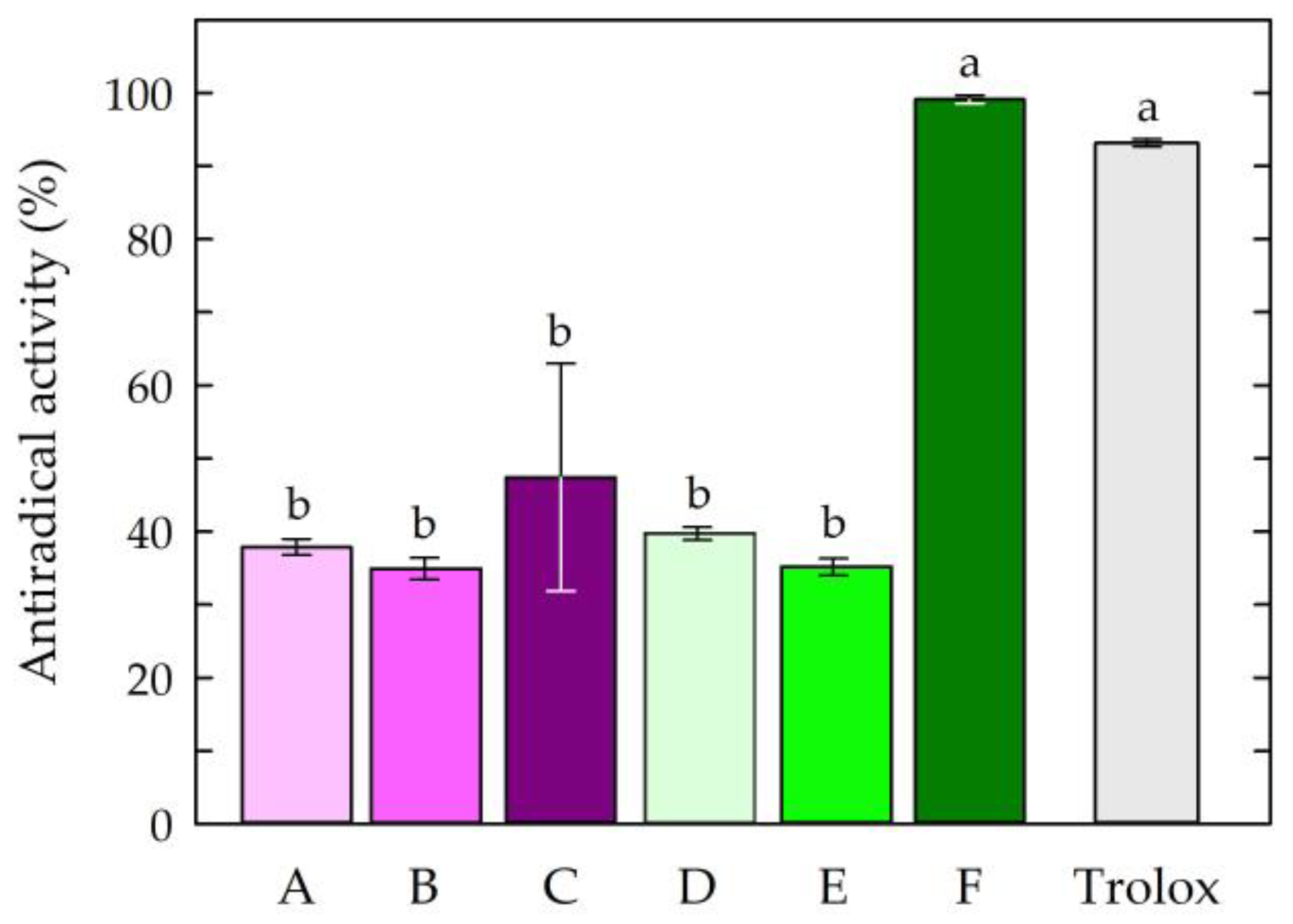
3.1. Antioxidant Activity of the Film-Forming Solutions
3.2. Antimicrobial Activity of the Chitosan-Based Films
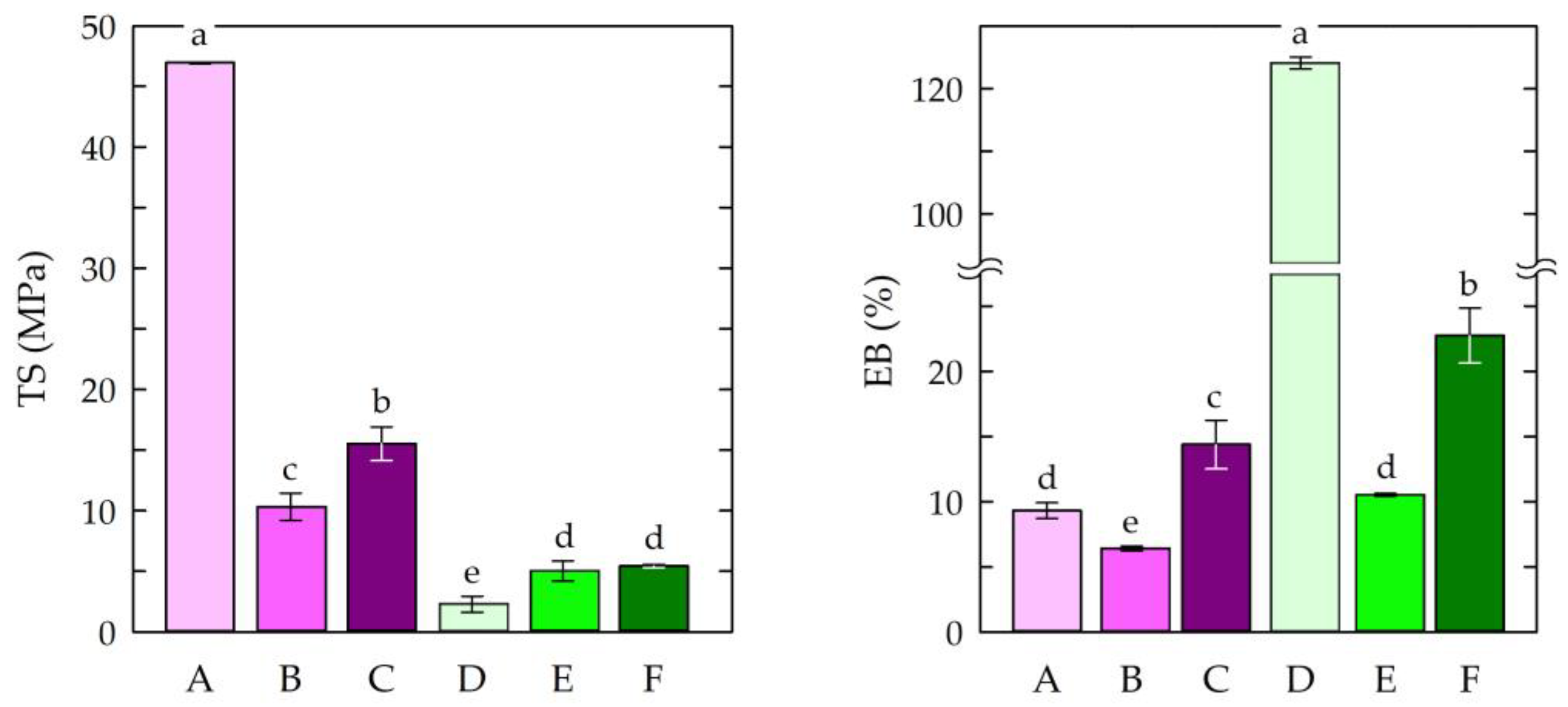
3.3. Mechanical Properties
3.4. Scanning Electron Microscopy (SEM)
3.5. Fourier Transform Infrared Spectroscopy (ATR-FTIR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials—A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Stefanowska, K.; Woźniak, M.; Dobrucka, R.; Ratajczak, I. Chitosan with Natural Additives as a Potential Food Packaging. Materials 2023, 16, 1579. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional Properties of Chitosan-Based Films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan Based Antimicrobial Films for Food Packaging Applications. E-Polym. 2008, 8, 093. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Tănase, E.; Popa, V.I.; Popa, M.E. Sustainable Alternative For Food Packaging: Chitosan Biopolymer—A Review. AgroLife Sci. J. 2015, 4, 52–62. [Google Scholar]
- Darder, M.; Karan, A.; del Real, G.; DeCoster, M.A. Cellulose-Based Biomaterials Integrated with Copper-Cystine Hybrid Structures as Catalysts for Nitric Oxide Generation. Mater. Sci. Enging. C 2020, 108, 110369. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Cellulose Nano-Films as Bio-Interfaces. Front. Chem. 2019, 7, 535. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Hu, B.; Guo, Y.; Li, H.; Liu, X.; Fu, Y.; Ding, F. Recent Advances in Chitosan-Based Layer-by-Layer Biomaterials and Their Biomedical Applications. Carbohydr. Polym. 2021, 271, 118427. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of Chitosan for Biomedical Applications. Chitosan Based Biomater. 2017, 1, 3–30. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, Q.; Long, Y.; Xu, C.; Fang, H.; Chen, Y.; Dai, H. A Chitosan Based Scaffold with Enhanced Mechanical and Biocompatible Performance for Biomedical Applications. Polym. Degrad. Stab. 2020, 181, 109322. [Google Scholar] [CrossRef]
- Sathya Seeli, D.; Das, A.; Prabaharan, M. Zinc Oxide–Incorporated Chitosan–Poly(Methacrylic Acid) Polyelectrolyte Complex as a Wound Healing Material. JFB 2023, 14, 228. [Google Scholar] [CrossRef]
- Zhou, J.; Li, N.; Liu, P.; Liu, Z.; Gao, L.; Jiao, T. Preparation of Fluorescently Labeled Chitosan-Quercetin Drug-Loaded Nanoparticles with Excellent Antibacterial Properties. JFB 2022, 13, 141. [Google Scholar] [CrossRef]
- Paker, E.S.; Senel, M. Polyelectrolyte Multilayers Composed of Polyethyleneimine-Grafted Chitosan and Polyacrylic Acid for Controlled-Drug-Delivery Applications. JFB 2022, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Aalipour, H.; Shirazi, M.J.S.; Jebali, A.; Chi, H.; Hamedi, S.; Wang, N.; Li, T.; Moravvej, H. Physically Crosslinked Chitosan/PVA Hydrogels Containing Honey and Allantoin with Long-Term Biocompatibility for Skin Wound Repair: An In Vitro and In Vivo Study. JFB 2021, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The Versatility of Collagen and Chitosan: From Food to Biomedical Applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-Based Hydrogels: From Preparation to Biomedical Applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; Des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and Chitosan Derivatives in Drug Delivery and Tissue Engineering. Adv. Polym. Sci. 2011, 244, 19–44. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Hao, J.Y.; Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Schoung, J.Y.; Tsai, Y.H.; Huang, Y. Bin Control of Wound Infections Using a Bilayer Chitosan Wound Dressing with Sustainable Antibiotic Delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound Healing and Antimicrobial Effect of Active Secondary Metabolites in Chitosan-Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent Progress of Collagen, Chitosan, Alginate and Other Hydrogels in Skin Repair and Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Marine Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules 2022, 12, 155. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, S.; Jain, P. Leveraging Advances in Chemistry to Design Biodegradable Polymeric Implants Using Chitosan and Other Biomaterials. Int. J. Biol. Macromol. 2021, 169, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef]
- Rasul, R.M.; Tamilarasi Muniandy, M.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A Review on Chitosan and Its Development as Pulmonary Particulate Anti-Infective and Anti-Cancer Drug Carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef]
- Rostami, E. Progresses in Targeted Drug Delivery Systems Using Chitosan Nanoparticles in Cancer Therapy: A Mini-Review. J. Drug Deliv. Sci. Technol. 2020, 58, 101813. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Ashassi-Sorkhabi, H.; Afkari, R.; Kazempour, A. Cross-Linked Chitosan in Nano and Bead Scales as Drug Carriers for Betamethasone and Tetracycline. Int. J. Biol. Macromol. 2019, 131, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y.; Liu, J. Chitosan-Based Nanoscale and Non-Nanoscale Delivery Systems for Anticancer Drugs: A Review. Eur. Polym. J. 2021, 154, 110533. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Omura, Y.; Shigemoto, M.; Akiyama, T.; Saimoto, H.; Shigemasa, Y.; Nakamura, I.; Tsuchido, T. Antimicrobial Activity of Chitosan with Different Degrees of Acetylation and Molecular Weights. Biocontrol. Sci. 2003, 8, 25–30. [Google Scholar] [CrossRef]
- Amor, I.B.; Hemmami, H.; Laouini, S.E.; Abdelaziz, A.G.; Barhoum, A. Influence of Chitosan Source and Degree of Deacetylation on Antibacterial Activity and Adsorption of AZO Dye from Water. Biomass Convers. Biorefin. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Stanicka, K.; Dobrucka, R.; Woźniak, M.; Sip, A.; Majka, J.; Kozak, W.; Ratajczak, I. The Effect of Chitosan Type on Biological and Physicochemical Properties of Films with Propolis Extract. Polymers 2021, 13, 3888. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Butt, J. Chitosan Films Are NOT Antimicrobial. Biotechnol. Lett. 2011, 33, 417–421. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K.; Pathak, P. Static Intermittent Fed-Batch Production of Bacterial Nanocellulose from Black Tea and Its Modification Using Chitosan to Develop Antibacterial Green Packaging Material. J. Clean. Prod. 2021, 279, 123608. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Eng. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and Neuroprotective Effects of Caffeine against Alzheimer’s and Parkinson’s Disease: Insight into the Role of Nrf-2 and A2AR Signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.P.; Naghetini, C.C.; Santos, V.R.; Antonio, A.G.; Farah, A.; Glória, M.B.A. Influence of Natural Coffee Compounds, Coffee Extracts and Increased Levels of Caffeine on the Inhibition of Streptococcus Mutans. Food Res. Int. 2012, 49, 459–461. [Google Scholar] [CrossRef]
- Monente, C.; Bravo, J.; Vitas, A.I.; Arbillaga, L.; De Peña, M.P.; Cid, C. Coffee and Spent Coffee Extracts Protect against Cell Mutagens and Inhibit Growth of Food-Borne Pathogen Microorganisms. J. Funct. Foods 2015, 12, 365–374. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The Use of Propolis as a Functional Food Ingredient: A Review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Sip, A.; Babicka, M.; Rogoziński, T.; Ratajczak, I. Aktywność Przeciwutleniająca i Przeciwbakteryjna Miodu, Propolisu Oraz Pyłku Kwiatowego. Postępy Fitoter. 2020, 21, 123. [Google Scholar] [CrossRef]
- Farag, M.R.; Abdelnour, S.A.; Patra, A.K.; Dhama, K.; Dawood, M.A.O.; Elnesr, S.S.; Alagawany, M. Propolis: Properties and Composition, Health Benefits and Applications in Fish Nutrition. Fish Shellfish Immunol. 2021, 115, 179–188. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. 2013, 2013, 11. [Google Scholar] [CrossRef]
- da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S. Propolis as a Novel Antibacterial Agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Corbo, M.R.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth Biocontrol of Foodborne Pathogens and Spoilage Microorganisms of Food by Polish Propolis Extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M.; Alam, R.; Łochyńska, M. Antifungal Properties of Chemically Defined Propolis from Various Geographical Regions. Microorganisms 2022, 10, 364. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.I.; Roslan, A.; Saari, N.S.; Hashim, K.H.; Kalamullah, M.R. Ethanolic Extract of Propolis for Biodegradable Films Packaging Enhanced with Chitosan. AIP Conf. Proc. 2017, 1885, 20231. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antifungal Properties of Hydroxypropylmethylcellulose Based Films Containing Propolis as Affected by Moisture Content. Carbohydr. Polym. 2010, 82, 1174–1183. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Antibacterial Effectiveness of Chitosan–Propolis Coated Polypropylene Films against Foodborne Pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef]
- Stefanowska, K.; Woźniak, M.; Majka, J.; Sip, A.; Mrówczyńska, L.; Waśkiewicz, A.; Kozak, W.; Dobrucka, R.; Ratajczak, I. A New Approach to Obtain Chitosan Films—Characteristics of Films Prepared with Tea and Coffee Kombucha as Natural Chitosan Solvents. Ind. Crops Prod. 2023, 197, 116634. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. Phenolic Profile and Antioxidant Activity of Propolis Extracts from Poland. Nat. Prod. Commun. 2019, 14, 1934578X19849777. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of Bioactive Compounds Potential and Antioxidant Activity of Brown, Green and Red Propolis from Brazilian Northeast Region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Miłek, M.; Ciszkowicz, E.; Tomczyk, M.; Sidor, E.; Zaguła, G.; Lecka-Szlachta, K.; Pasternakiewicz, A.; Dżugan, M. The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells. Molecules 2022, 27, 725. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidant 2021, 10(1), 71. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dastidar, D.G.; Paul, P.; Dutta, S.; Basu, D.; Sharma, S.R.; Basu, S.; Sarker, R.K.; Sen, A.; Sarkar, A.; et al. Inhibition of Biofilm Formation of Pseudomonas Aeruginosa by Caffeine: A Potential Approach for Sustainable Management of Biofilm. Arch. Microbiol. 2020, 202, 623–635. [Google Scholar] [CrossRef]
- Ramanavièienë, A.; Mostovojus, V.; Bachmatova, I.; Ramanavièius, A. Anti-Bacterial Effect of Caffeine on Escherichia Coli and Pseudomonas Fluorescens. Acta Med. Litu. 2003, 10, 185–188. [Google Scholar]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Ramos-García, M.d.L.; Martínez-González, M.d.C.; Hernández-Romano, J. Physicochemical Characterization and Antimicrobial Activity of Edible Propolis-Chitosan Nanoparticle Films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.J.E.; Chorianopoulos, N. Probiotic Incorporation in Edible Films and Coatings: Bioactive Solution for Functional Foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Humelnicu, A.C.; Samoilă, P.; Cojocaru, C.; Dumitriu, R.; Bostănaru, A.C.; Mareș, M.; Harabagiu, V.; Simionescu, B.C. Chitosan-Based Therapeutic Systems for Superficial Candidiasis Treatment. Synergetic Activity of Nystatin and Propolis. Polymers 2022, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.B.; Svetaz, L.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Saavedra, P.; Wunderlin, D.; Feresin, G.E.; Tapia, A. Urban Propolis from San Juan Province (Argentina): Ethnopharmacological Uses and Antifungal Activity against Candida and Dermatophytes. Ind. Crops Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Liaudanskas, M.; Ivanauskas, L.; Ivaskiene, M.; Ramanauskiene, K. Extracts of Poplar Buds (Populus balsamifera L., Populus nigra L.) and Lithuanian Propolis: Comparison of Their Composition and Biological Activities. Plants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Bouchelaghem, S. Propolis Characterization and Antimicrobial Activities against Staphylococcus aureus and Candida albicans: A Review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef]
- Gucwa, K.; Kusznierewicz, B.; Milewski, S.; van Dijck, P.; Szweda, P. Antifungal Activity and Synergism with Azoles of Polish Propolis. Pathogens 2018, 7, 56. [Google Scholar] [CrossRef]
- Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In Vitro Activity of Propolis on Oral Microorganisms and Biofilms. Antibiotics 2021, 10, 1045. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Nowak, P.; Ratajczak, I. Effect of the Solvent on Propolis Phenolic Profile and Its Antifungal, Antioxidant, and In Vitro Cytoprotective Activity in Human Erythrocytes under Oxidative Stress. Molecules 2020, 25, 4266. [Google Scholar] [CrossRef]
- Haščík, P.; Čuboň, J. The Antimicrobial Activity of Honey, Bee Pollen Loads and Beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and Calorimetric Investigations on the Antimicrobial Actions of Different Propolis Extracts: An in Vitro Approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Homez-Jara, A.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of Chitosan Edible Films Obtained with Various Polymer Concentrations and Drying Temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Qiao, C.; Liu, R.; Liu, Q.; Xu, J.; Yao, J. Structure and Properties of Citric Acid Cross-Linked Chitosan/Poly(Vinyl Alcohol) Composite Films for Food Packaging Applications. Carbohydr. Polym. 2023, 312, 120842. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Zhi, X.; Du, B.; Yuan, S. Preparation of Elastic and Antibacterial Chitosan-Citric Membranes with High Oxygen Barrier Ability by in Situ Cross-Linking. ACS Omega 2020, 5, 1086–1097. [Google Scholar] [CrossRef]
- Chen, J.; Han, S.; Huang, M.; Li, J.; Zhou, M.; He, J. Green Crosslinked Nanofibers Membrane Based on CS/PVA Combined with Polybasic Organic Acid for Tympanic Membrane Repair. Int. J. Polym. Mater. 2022, 71, 291–301. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sankari, G.; Ponnusamy, S. Vibrational Spectral Investigation on Xanthine and Its Derivatives—Theophylline, Caffeine and Theobromine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 117–127. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarić, I.; Mršić, G.; Ježek, D. Improving the Controlled Delivery Formulations of Caffeine in Alginate Hydrogel Beads Combined with Pectin, Carrageenan, Chitosan and Psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef]
- Morrish, C.; Whitehead, F.; Istivan, T.; Kasapis, S. The Effect of Trisodium Phosphate Crosslinking on the Diffusion Kinetics of Caffeine from Chitosan Networks. Food Chem. 2022, 381, 132272. [Google Scholar] [CrossRef]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and Effective Evaluation of the Antioxidant Capacity of Propolis Extracts Using DPPH Bleaching Kinetic Profiles, FT-IR and UV-Vis Spectroscopic Data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Wu, Y.W.; Sun, S.Q.; Zhao, J.; Li, Y.; Zhou, Q. Rapid Discrimination of Extracts of Chinese Propolis and Poplar Buds by FT-IR and 2D IR Correlation Spectroscopy. J. Mol. Struct. 2008, 883–884, 48–54. [Google Scholar] [CrossRef]


| Symbol | Solvent | Additives | |
|---|---|---|---|
| Caffeine | Propolis Extract | ||
| A | Acetic acid | -- | -- |
| B | Acetic acid | ✓ | -- |
| C | Acetic acid | ✓ | ✓ |
| D | Citric acid | -- | -- |
| E | Citric acid | ✓ | -- |
| F | Citric acid | ✓ | ✓ |
| Bacterial Strain | Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| B. subtilis | -- | -- | -- | 20 | 24 | 26 |
| E. faecalis | -- | -- | -- | 28 | 28 | 28 |
| E. coli | -- | 18 | -- | 22 | 28 | 28 |
| P. aeruginosa | -- | 18 | 18 | 20 | 25 | 27 |
| P. fluorescens | -- | -- | -- | 24 | 24 | 26 |
| S. enterica | -- | -- | 16 | 16 | 19 | 25 |
| S. aureus | -- | -- | -- | -- | -- | -- |
| Bacterial Strain | Activity | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| L. paracasei | -- | -- | -- | -- | -- | -- |
| L. rhamnosus | -- | -- | -- | S | S | S |
| L. plantarum | S | S | S | S | S | S |
| Fungal Strain | Activity | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| A. flavus | -- | -- ^ | -- ^ | -- | -- ^ | -- ^ |
| A. niger | -- | -- | -- | -- | -- | -- |
| C. albicans | -- | -- | -- | -- | -- | 22 * st |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanowska, K.; Woźniak, M.; Sip, A.; Mrówczyńska, L.; Majka, J.; Kozak, W.; Dobrucka, R.; Ratajczak, I. Characteristics of Chitosan Films with the Bioactive Substances—Caffeine and Propolis. J. Funct. Biomater. 2023, 14, 358. https://doi.org/10.3390/jfb14070358
Stefanowska K, Woźniak M, Sip A, Mrówczyńska L, Majka J, Kozak W, Dobrucka R, Ratajczak I. Characteristics of Chitosan Films with the Bioactive Substances—Caffeine and Propolis. Journal of Functional Biomaterials. 2023; 14(7):358. https://doi.org/10.3390/jfb14070358
Chicago/Turabian StyleStefanowska, Karolina, Magdalena Woźniak, Anna Sip, Lucyna Mrówczyńska, Jerzy Majka, Wojciech Kozak, Renata Dobrucka, and Izabela Ratajczak. 2023. "Characteristics of Chitosan Films with the Bioactive Substances—Caffeine and Propolis" Journal of Functional Biomaterials 14, no. 7: 358. https://doi.org/10.3390/jfb14070358
APA StyleStefanowska, K., Woźniak, M., Sip, A., Mrówczyńska, L., Majka, J., Kozak, W., Dobrucka, R., & Ratajczak, I. (2023). Characteristics of Chitosan Films with the Bioactive Substances—Caffeine and Propolis. Journal of Functional Biomaterials, 14(7), 358. https://doi.org/10.3390/jfb14070358










