Alveolar Bone Microstructure Surrounding Orthodontic Anchor Screws with Plasma Surface Treatment in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
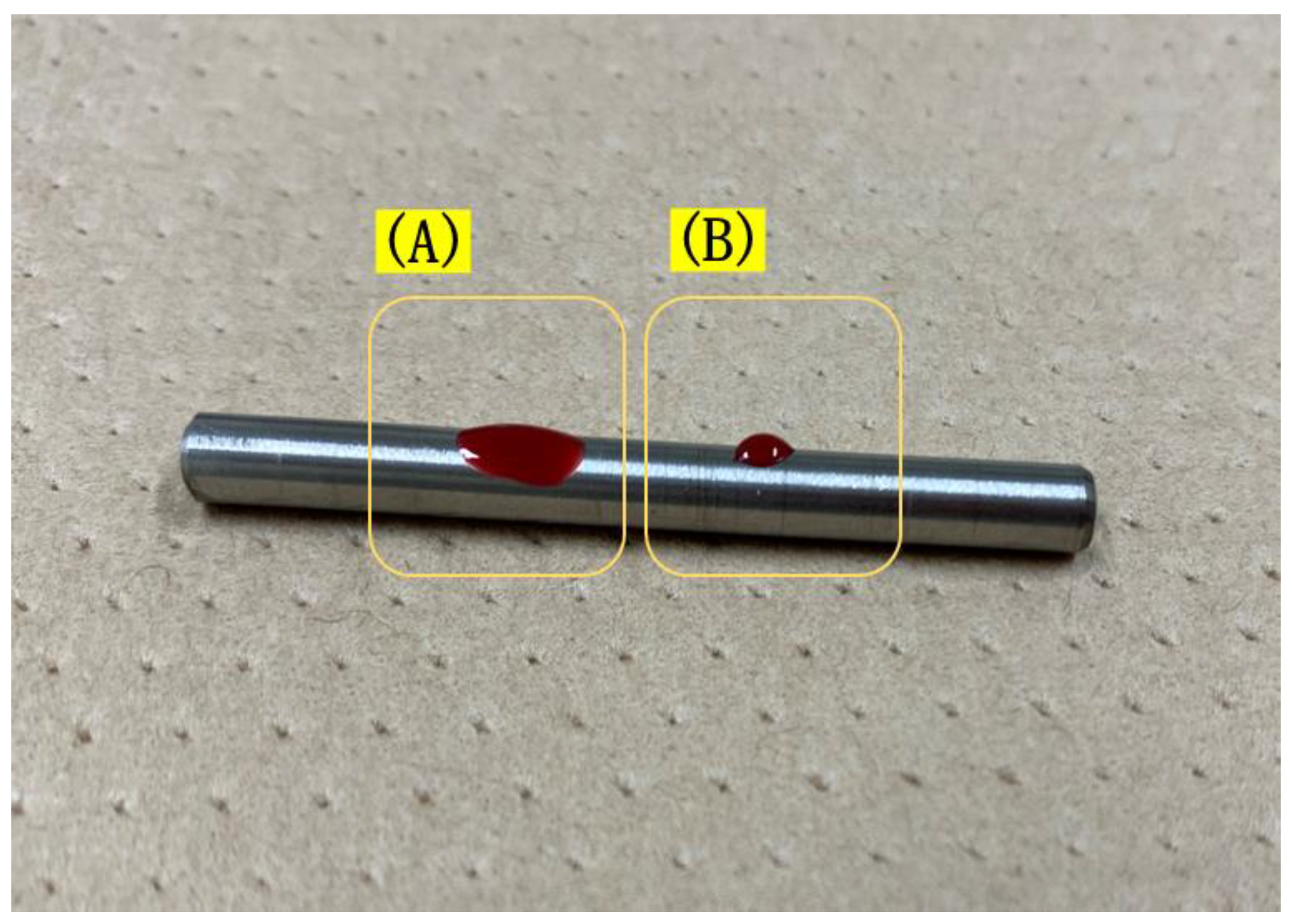
2.2. Anchor Screw Surface Treatment
2.3. Experimental Procedure
2.4. Micro-CT Scanning
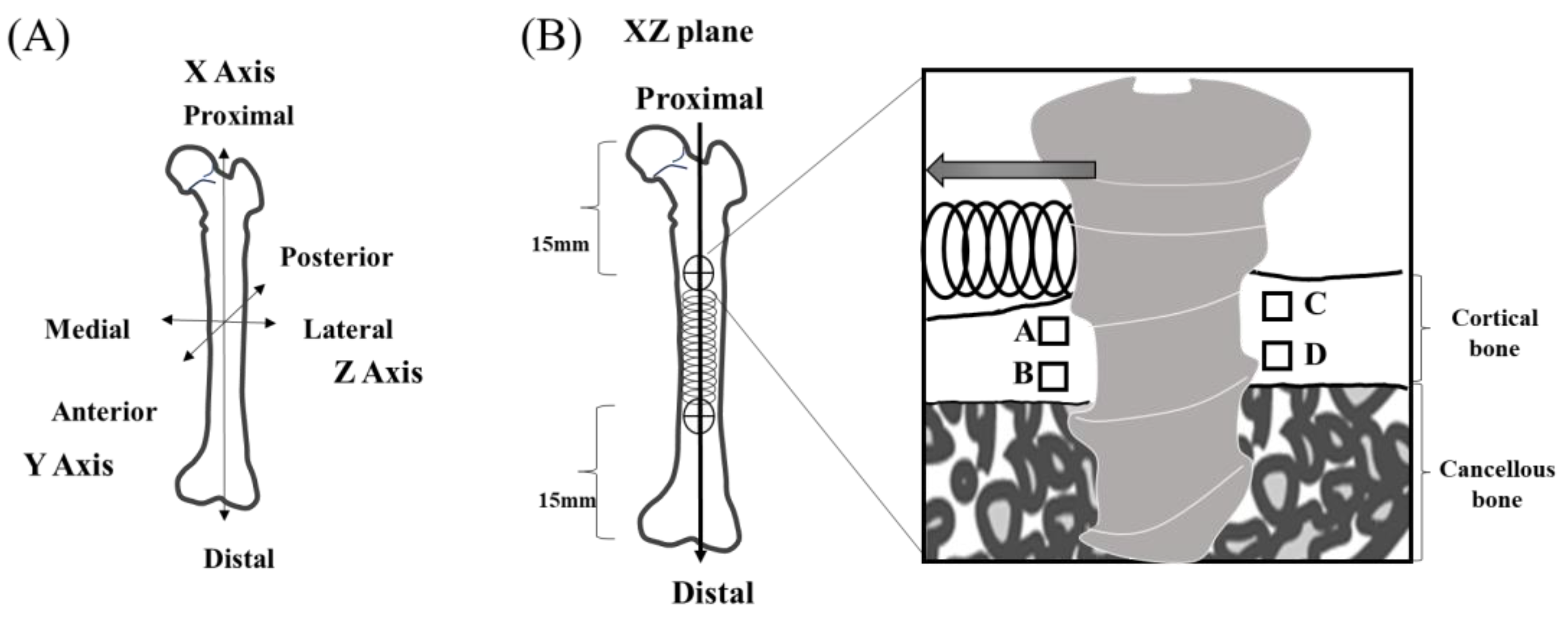
2.5. Regions of Interest
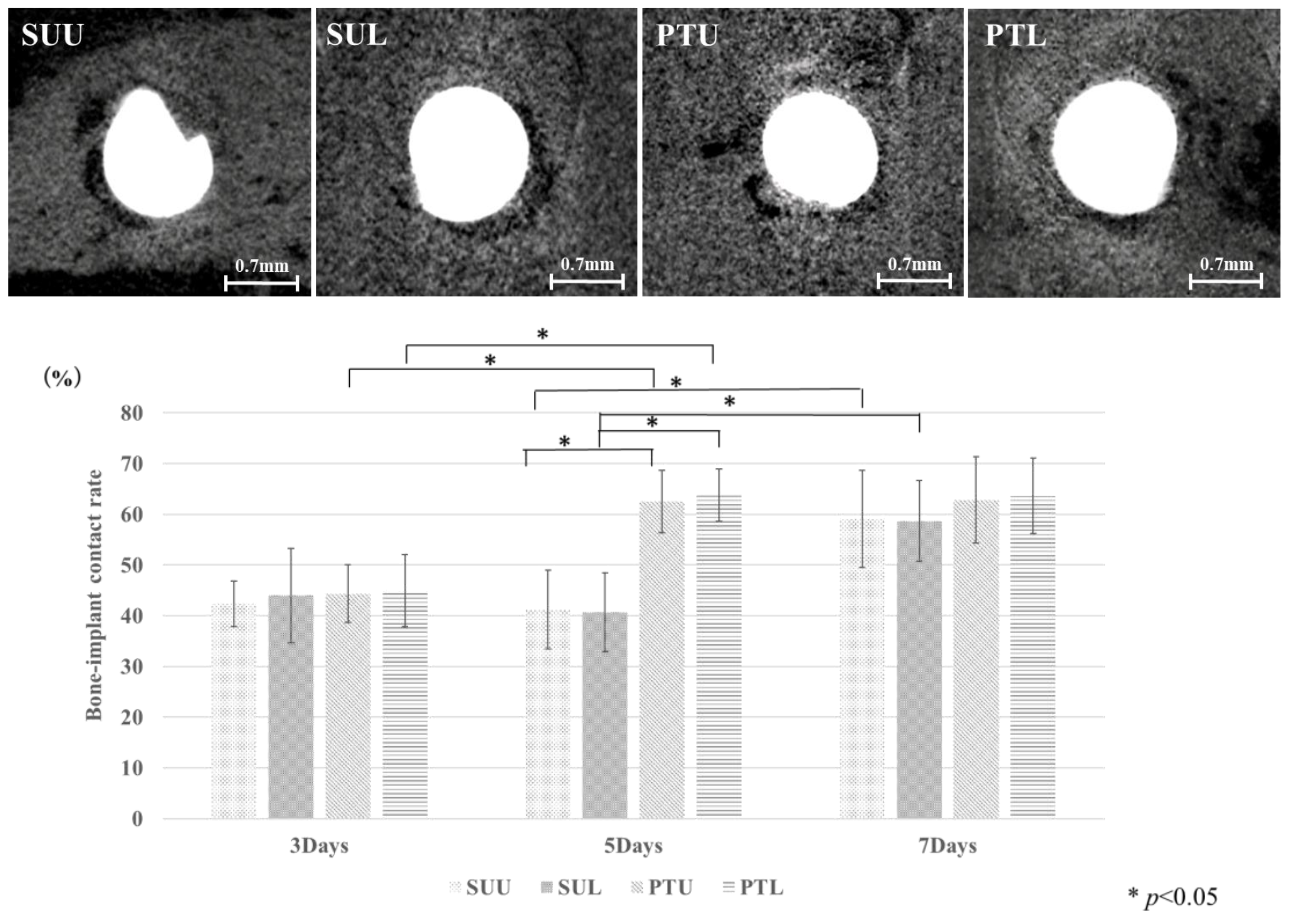
2.6. Bone–Implant Contact (BIC)
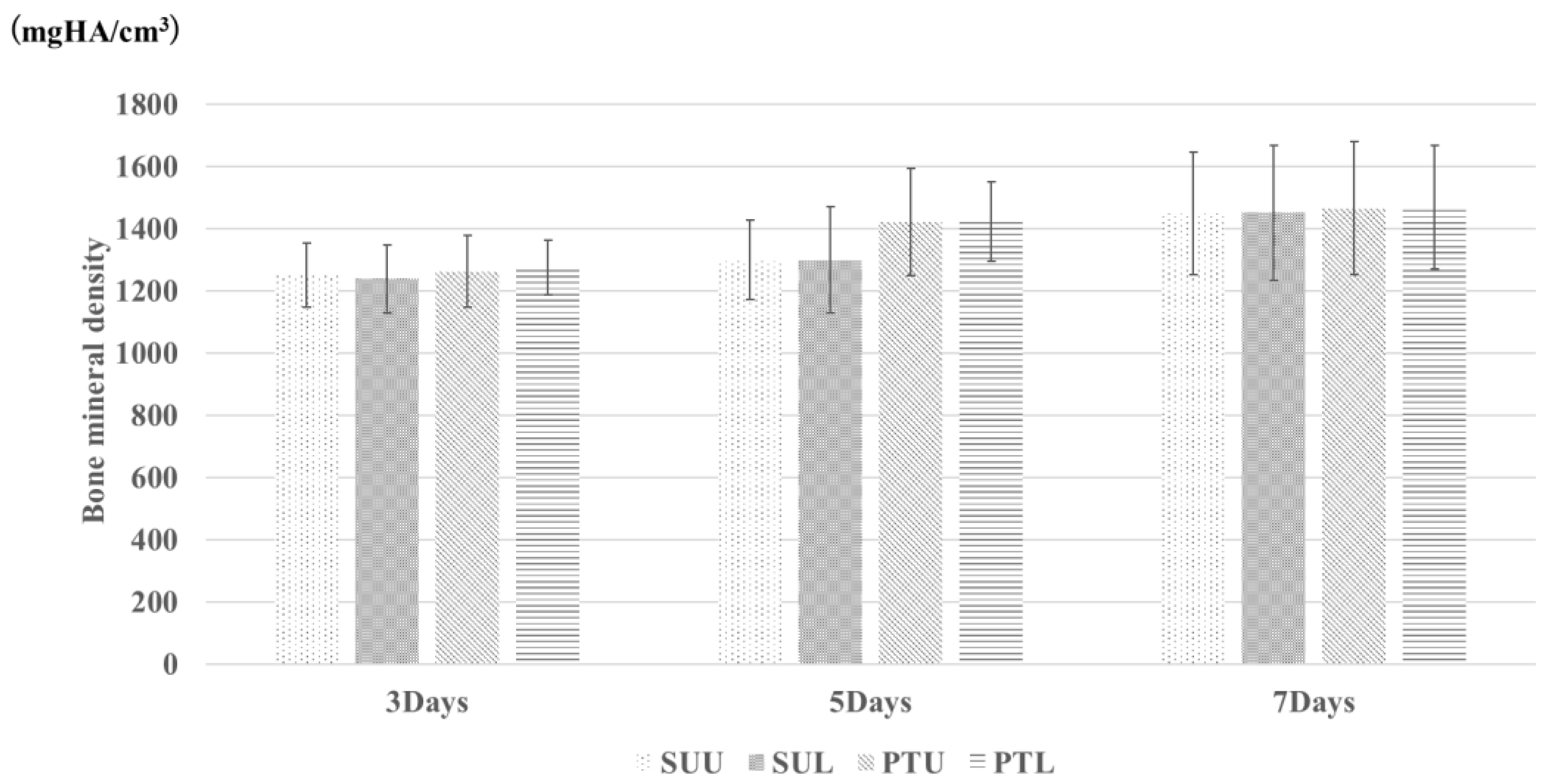
2.7. Bone Mineral Density (BMD)
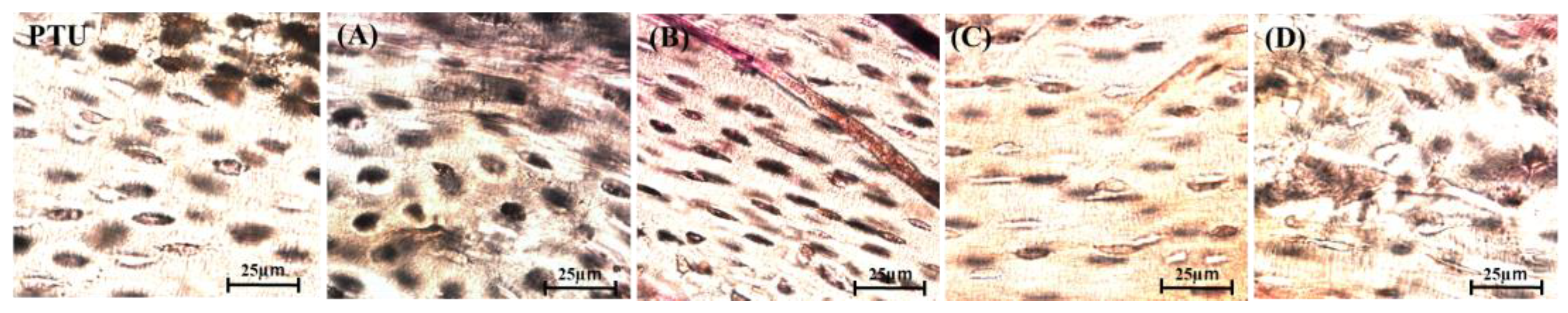
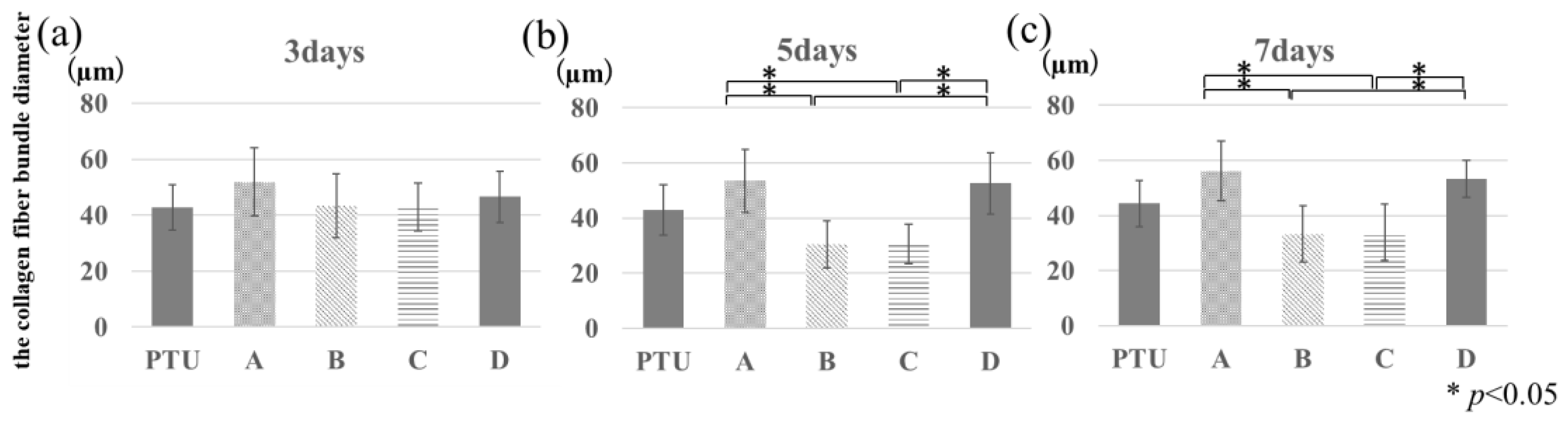
2.8. Histological Evaluation
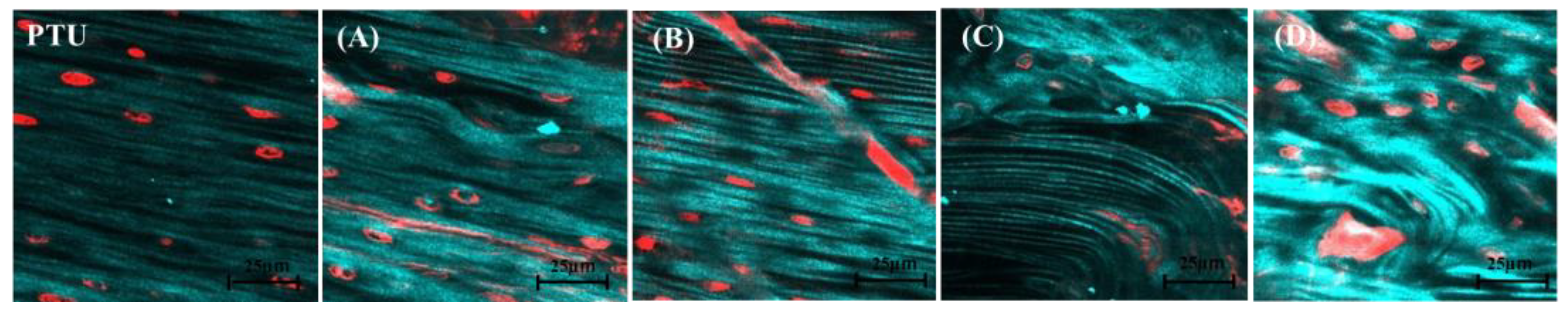
2.9. Second Harmonic Generation Imaging (SHG Imaging)
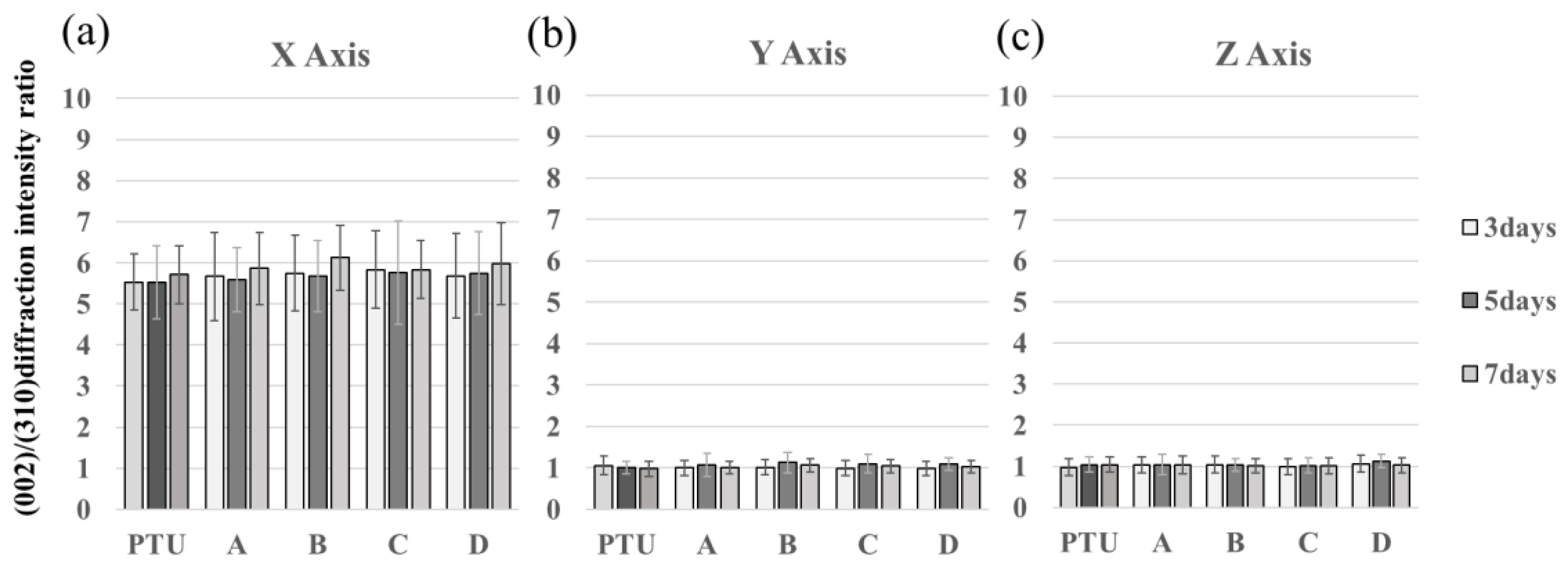
2.10. BAp Crystal Orientation
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, L.; Zhenjin, Y.; Jing, Z.; Ping, X.; Quan, W.; Yan, Y.; Yu, H. Comparison of anchorage efficiency of orthodontic mini-implant and conventional anchorage reinforcement in patients requiring maximum orthodontic anchorage: A systematic review and meta-analysis. J. Evid. Based. Dent. Pract. 2020, 20, 101401. [Google Scholar]
- Papadopoulos, M.A.; Papageorgiou, S.N.; Zogakis, I.P. Clinical effectiveness of orthodontic miniscrew implants: A meta-analysis. J. Dent. Res. 2011, 90, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Yamada, K.; Deguchi, T.; Kyung, H.M.; Takano-Yamamoto, T. Class II malocclusion treated with miniscrew anchorage: Comparison with traditional orthodontic mechanics outcomes. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.N.; Basha, S.; Al-Thomali, Y. Maxillary molar distalization with miniscrew-supported appliances in Class II malocclusion: A systematic review. Angle Orthod. 2018, 88, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sung, S.J. Nonsurgical correction of a Class III skeletal anterior open-bite malocclusion using multiple microscrew implants and digital profile prediction. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 283–293. [Google Scholar] [CrossRef]
- Reynders, R.; Ronchi, L.; Bipat, S. Mini-implants in orthodontics: A systematic review of the literature. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 564.e1–564.e19. [Google Scholar] [CrossRef]
- Nkenke, E.; Fenner, M. Indications for immediate loading of implants and implant success. Clin. Oral Implant. Res. 2006, 17, 19–34. [Google Scholar] [CrossRef]
- Alharbi, F.; Almuzian, M.; Bearn, D. Miniscrews failure rate in orthodontics: Systematic review and meta-analysis. Eur. J. Orthod. 2018, 40, 519–530. [Google Scholar] [CrossRef]
- Ramírez-Ossa, D.M.; Escobar-Correa, N.; Ramírez-Bustamante, M.A.; Agudelo-Suárez, A.A. An umbrella review of the effectiveness of temporary anchorage devices and the factors that contribute to their success or failure. J. Evid. Based. Dent. Pract. 2020, 20, 101402. [Google Scholar] [CrossRef]
- Mo, S.S.; Kim, S.H.; Kook, Y.A.; Jeong, D.M.; Chung, K.R.; Nelson, G. Resistance to immediate orthodontic loading of surface-treated mini-implants. Angle Orthod. 2010, 80, 123–129. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Razak, S.I.A.; Rehman, S.; Hasan, A.; Qureshi, S.; Stojanović, G.M. Bioactive scaffold (sodium alginate)-g-(nHAp@SiO2@GO) for bone tissue engineering. Int. J. Biol. Macromol. 2022, 222, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M. Future prospects for bio-functionalized implants. Dep. Bull. Paper 2015, 3, 185–197. [Google Scholar]
- Choi, S.H.; Shin, J.; Cha, J.K.; Kwon, J.S.; Cha, J.Y.; Hwang, C.J. Evaluation of success rate and biomechanical stability of ultraviolet-photofunctionalized miniscrews with short lengths. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 158–166. [Google Scholar] [CrossRef]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. NIH Consens. Statement 2000, 17, 1–45. [Google Scholar]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Nakano, T.; Kaibara, K.; Tabata, Y.; Nagata, N.; Enomoto, S.; Marukawa, E.; Umakoshi, Y. Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone 2002, 31, 479–487. [Google Scholar] [CrossRef]
- Isidor, F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin. Oral Implant. Res. 1996, 7, 143–152. [Google Scholar] [CrossRef]
- Park, H.S.; Jeong, S.H.; Kwon, O.W. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 18–25. [Google Scholar] [CrossRef]
- Matsumoto, T.; Matsunaga, S.; Kasahara, M.; Kasahara, N.; Nakano, T.; Ishimoto, T.; Nishii, Y. Evaluation of the microstructural characteristics of bone surrounding anchor screws placed under a horizontal load by exploring the orientation of biological apatite crystals and collagen fiber anisotropy. J. Hard Tissue Biol. 2022, 31, 79–86. [Google Scholar] [CrossRef]
- Motoyoshi, M.; Inaba, M.; Ono, A.; Ueno, S.; Shimizu, N. The effect of cortical bone thickness on the stability of orthodontic mini-implants and on the stress distribution in surrounding bone. Int. J. Oral Maxillofac. Surg. 2009, 38, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Krishnaraj, R.; Duraisamy, S.; Ravi, K.; Dilip, S.; Charles, A.; Sushil, N.C. Comparison of rate of canine retraction and anchorage potential between mini-implant and conventional molar anchorage: An in vivo study. Contemp. Clin. Dent. 2018, 9, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.K.; Dharmadeep, G.; Reddy, Y.M.; Cherukuri, S.; Raj, K.P.; Reddy, V. Comparison of the anchorage value of the first molars supported with implant and first molars supported with second molar during en masse retraction. J. Int. Soc. Prev. Community Dent. 2020, 10, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, S.; Iijima, M.; Yasuda, Y.; Handa, K.; Koike, T.; Saito, T.; Mizoguchi, I. Effectiveness of methods for detaching orthodontic implants likely to fracture upon rotational torque—An animal study. Clin. Exp. Dent. Res. 2016, 2, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Suzuki, B. Placement and removal torque values of orthodontic miniscrew implants. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 669–678. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, J.H.; Chung, K.R.; Kook, Y.A.; Nelson, G. Removal torque values of surface-treated mini-implants after loading. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 36–43. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Sims, J.R.; Karp, S.; Ingber, D.E. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J. Cell Sci. 1992, 103, 1215–1222. [Google Scholar] [CrossRef]
- Liu, T.C.; Chang, C.H.; Wong, T.Y.; Liu, J.K. Finite element analysis of miniscrew implants used for orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 468–476. [Google Scholar] [CrossRef]
- Martin, R.B.; Ishida, J. The relative effects of collagen fiber orientation, porosity, density, and mineralization on bone strength. J. Biomech. 1989, 22, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kaibara, K.; Ishimoto, T.; Tabata, Y.; Umakoshi, Y. Biological apatite (BAp) crystallographic orientation and texture as a new index for assessing the microstructure and function of bone regenerated by tissue engineering. Bone 2012, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Alrbata, R.H.; Momani, M.Q.; Al-Tarawneh, A.M.; Ihyasat, A. Optimal force magnitude loaded to orthodontic microimplants: A finite element analysis. Angle Orthod. 2016, 86, 221–226. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okawa, K.; Matsunaga, S.; Kasahara, N.; Kasahara, M.; Tachiki, C.; Nakano, T.; Abe, S.; Nishii, Y. Alveolar Bone Microstructure Surrounding Orthodontic Anchor Screws with Plasma Surface Treatment in Rats. J. Funct. Biomater. 2023, 14, 356. https://doi.org/10.3390/jfb14070356
Okawa K, Matsunaga S, Kasahara N, Kasahara M, Tachiki C, Nakano T, Abe S, Nishii Y. Alveolar Bone Microstructure Surrounding Orthodontic Anchor Screws with Plasma Surface Treatment in Rats. Journal of Functional Biomaterials. 2023; 14(7):356. https://doi.org/10.3390/jfb14070356
Chicago/Turabian StyleOkawa, Keisuke, Satoru Matsunaga, Norio Kasahara, Masaaki Kasahara, Chie Tachiki, Takayoshi Nakano, Shinichi Abe, and Yasushi Nishii. 2023. "Alveolar Bone Microstructure Surrounding Orthodontic Anchor Screws with Plasma Surface Treatment in Rats" Journal of Functional Biomaterials 14, no. 7: 356. https://doi.org/10.3390/jfb14070356
APA StyleOkawa, K., Matsunaga, S., Kasahara, N., Kasahara, M., Tachiki, C., Nakano, T., Abe, S., & Nishii, Y. (2023). Alveolar Bone Microstructure Surrounding Orthodontic Anchor Screws with Plasma Surface Treatment in Rats. Journal of Functional Biomaterials, 14(7), 356. https://doi.org/10.3390/jfb14070356








