Proteinoid Polymers and Nanocapsules for Cancer Diagnostics, Therapy and Theranostics: In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Synthesis and Characterization of Proteinoids and NCs
2.1. Preparation of Proteinoids
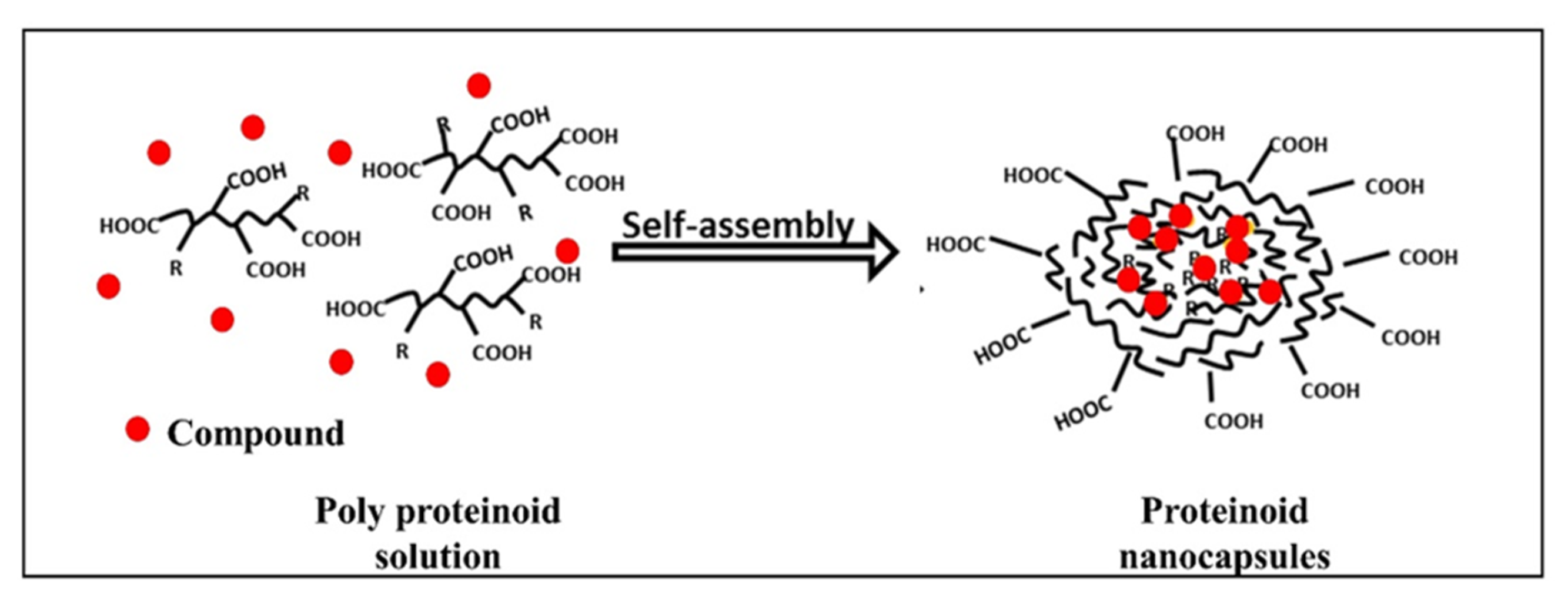
2.2. Preparation of Nanocapsules (NCs)
2.3. Characterization of Proteinoids and NCs
3. Cancer Diagnostics and Therapy, towards Theranostic Applications
3.1. Colorectal Cancer (CRC) Diagnostics
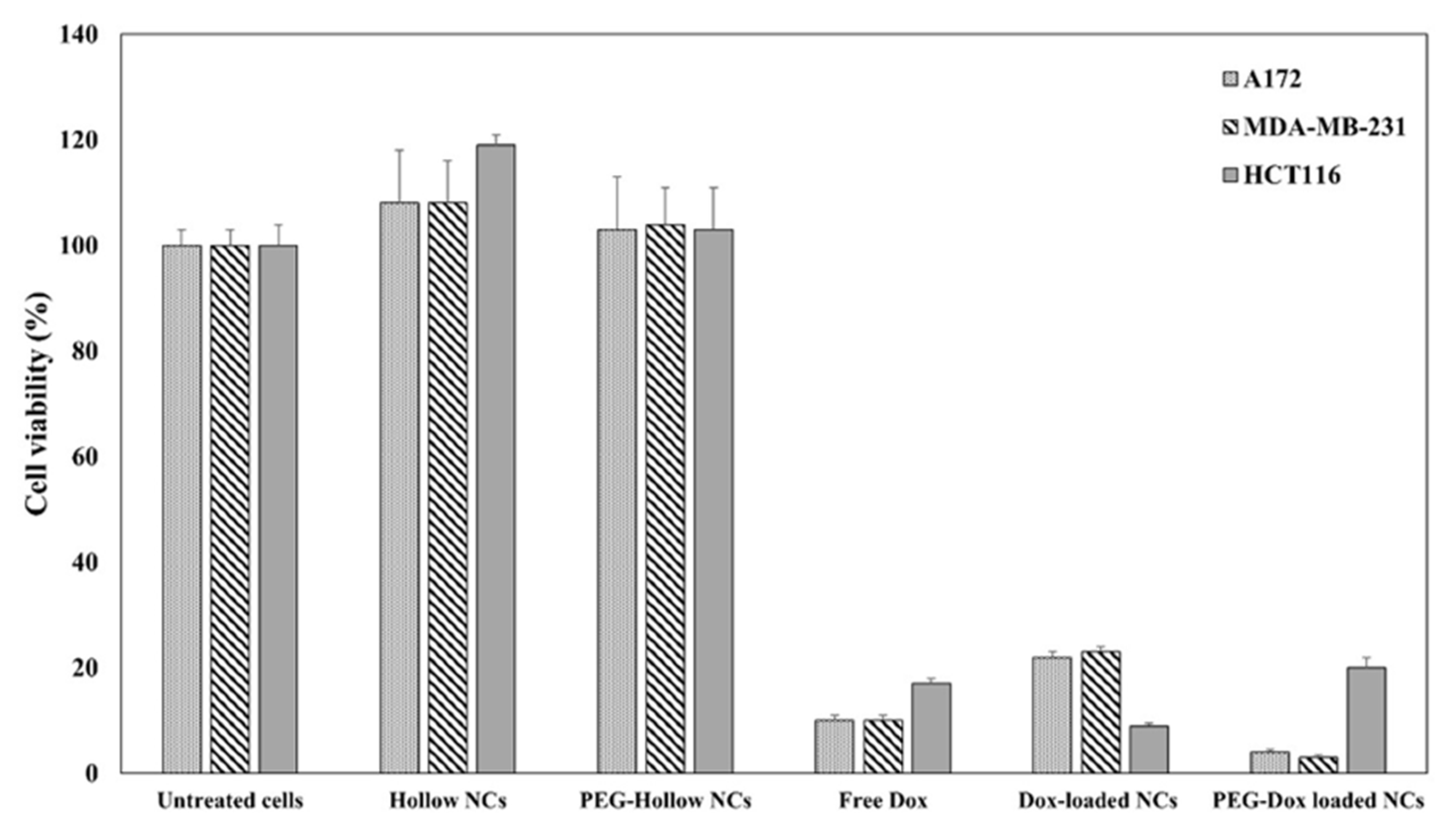
3.2. Cancer Therapy with Doxorubicin-Loaded NCs
4. Cancer Theranostics
4.1. RGD (ArgGlyAsp) for Specific Delivery to Tumors
4.2. Preparation and Characterization of RGD Proteinoids
4.3. Self-Assembly and Characterization of P(RGD) NCs
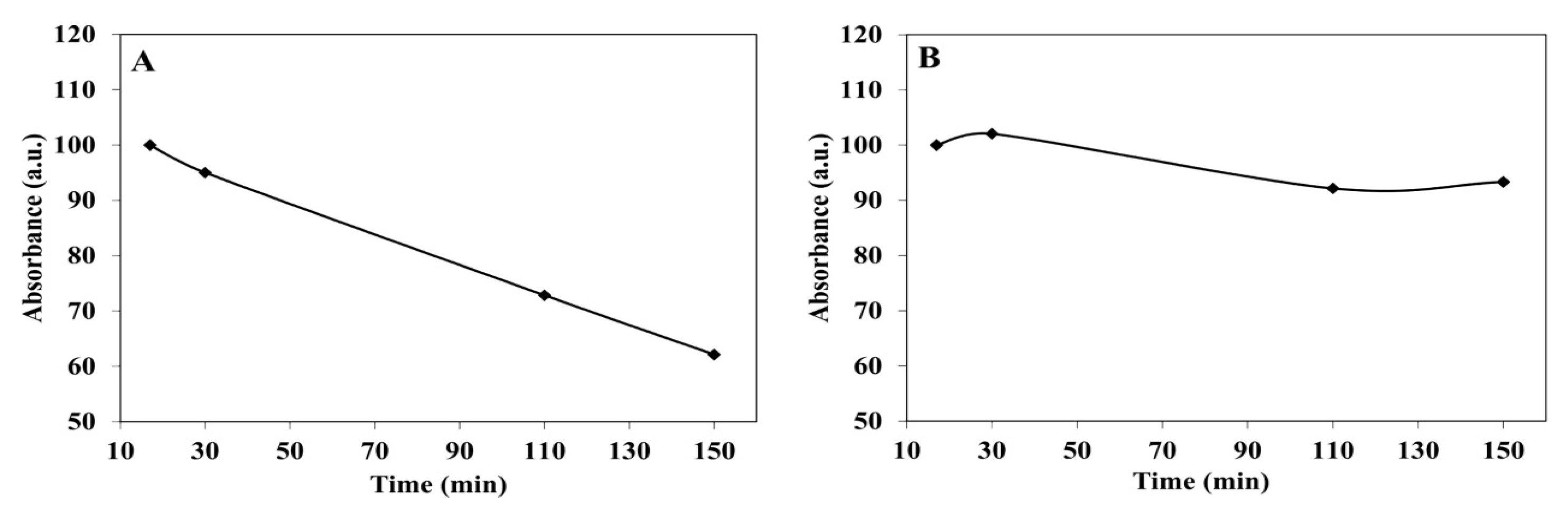
4.4. ICG Controlled Release
4.5. Optimization of RGD Configuration
4.6. Engineering of NIR Fluorescent PEGylated P(RDGD) NCs
5. Application of P(RGD) NCs for Cancer Tumors
5.1. mCherry-Labeled Tumor in CAM Model
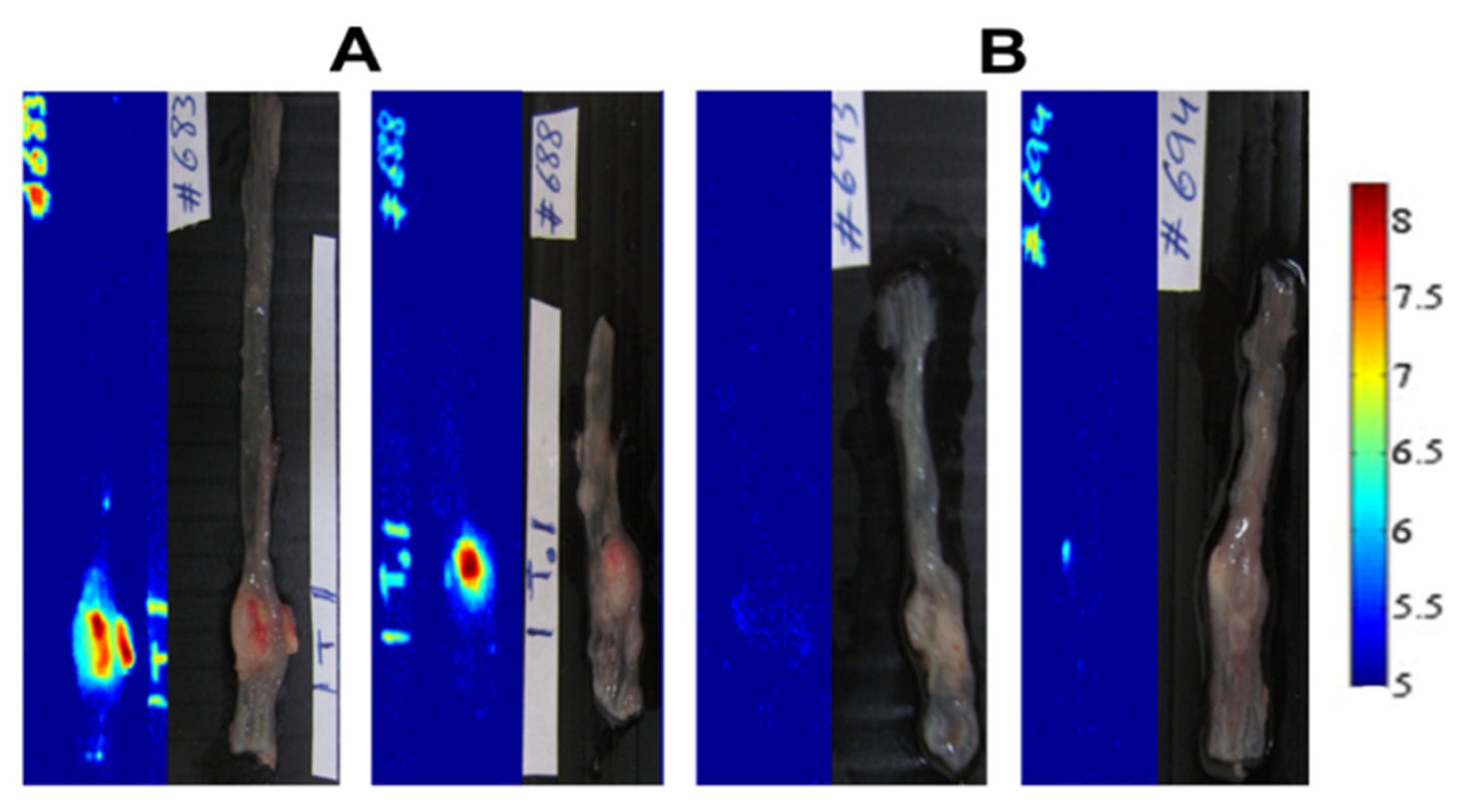
5.2. mCherry-Labeled Tumor in Mouse Model
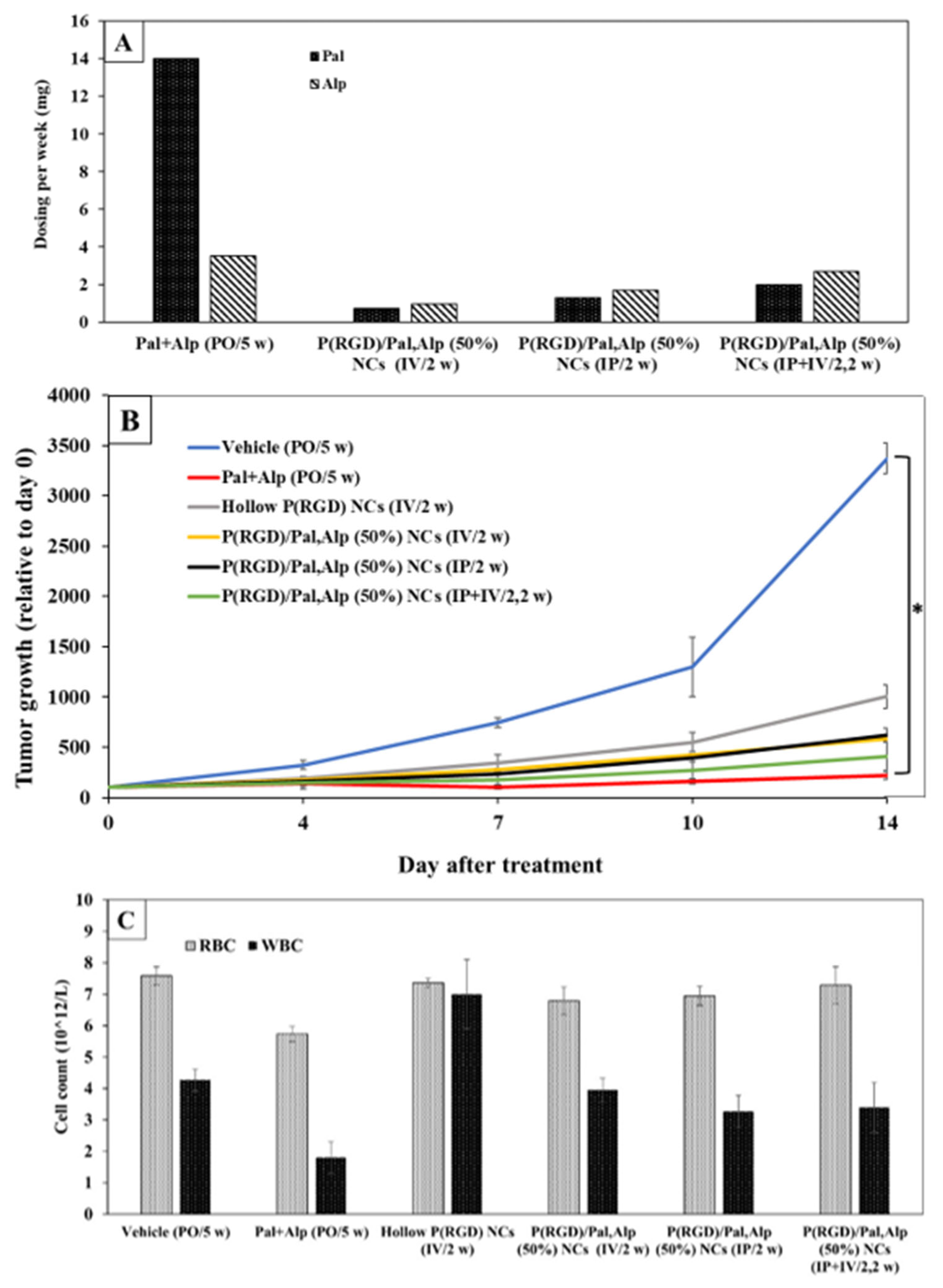
5.3. In Vivo Anti-Tumor Therapy with Cannabidiol (CBD)
5.4. Conjugation of TRAIL
5.5. Fluorescent NCs Containing Synergistic Drugs
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fox, S.W.; Jungck, J.R.; Nakashima, T. From proteinoid microsphere to contemporary cell: Formation of internucleotide and peptide bonds by proteinoid particles. Orig. Life 1974, 5, 227–237. [Google Scholar] [CrossRef]
- Fox, S.W.; Mccauley, R.J.; Fukushim, T.; Windsor, C.R.; Montgome, P.O. Selective action in boundaries of particles of thermal proteinoid. Fed. Proc. 1967, 26, 749. [Google Scholar]
- Fox, S.W. The proteinoid theory of the origin of life and competing ideas. Am. Biol. Teach 1974, 36, 161–172. [Google Scholar] [CrossRef]
- Harada, K.; Fox, S.W. The thermal condensation of glutamic acid and glycine to linear peptides. J. Am. Chem. Soc. 1958, 80, 2694–2697. [Google Scholar] [CrossRef]
- Matsuno, K. Electrical excitability of proteinoid microspheres composed of basic and acidic proteinoids. Biosystems 1984, 17, 11–14. [Google Scholar] [CrossRef]
- Przybylski, A.T. Excitable cell made of thermal proteinoids. Biosystems 1985, 17, 281–288. [Google Scholar] [CrossRef]
- Kumar, A.B.M.; Jayakumar, R.; Rao, P.K. Synthesis and aggregational behavior of acidic proteinoid. Sci. Polym. Chem. 1996, 34, 2915–2924. [Google Scholar] [CrossRef]
- Kumar, A.B.M.; Rao, P.K. Preparation and characterization of pH-sensitive proteinoid microspheres for the oral delivery of methotrexate. Biomaterials 1998, 19, 725–732. [Google Scholar] [CrossRef]
- Lugasi, L.; Grinberg, I.; Margel, S. Targeted delivery of CBD-loaded poly(RGD) proteinoid nanoparticles for antitumor therapy. J. Nanomed. Nanotech. 2020, 18, 552. [Google Scholar]
- Kiel, S.; Kolitz-Domb, M.; Corem-Salkmon, E.; Margel, S. Engineered doxorubicin delivery system using proteinoid-poly(L-lactic acid) polymeric nanoparticles of narrow size distribution and high molecular weight for cancer treatment. Int. J. Nanotechnol. Nanomed. 2017, 2, 1–11. [Google Scholar]
- Kolitz-Domb, M.; Margel, S. Recent advances of novel proteinoids and proteinoid nanoparticles and their applications in biomedicine and industrial uses. Isr. J. Chem. 2018, 58, 1277–1285. [Google Scholar] [CrossRef]
- Hadad, E.; Rudnick-Glick, S.; Ithaki, E.; Avivi, M.Y.; Grinberg, I.; Elias, Y.; Margel, S. Engineering of doxorubicin-encapsulating and TRAIL-conjugated poly(RGD) proteinoid nanocapsules for drug delivery applications. Polymers 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Hadad, E.; Rudnick-Glick, S.; Grinberg, I.; Kolitz-Domb, M.; Chill, J.H.; Margel, S. Synthesis and characterization of Poly(RGD) proteinoid polymers and NIR fluorescent nanoparticles of optimal D, L-configuration for drug-delivery applications— in vitro study. ACS Omega 2020, 5, 23568–23577. [Google Scholar] [CrossRef] [PubMed]
- Kolitz-Domb, M.; Grinberg, I.; Corem-Salkmon, E.; Margel, S. Engineering of near infrared fluorescent proteinoid-poly(L-lactic acid) particles for in vivo colon cancer detection. J. Nanobiotechnol. 2014, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Park, D.; Kim, J.C. Disulfide proteinoid micelles responsive to reduction. J. Dispers. Sci. Technol. 2018, 40, 1413–1422. [Google Scholar] [CrossRef]
- Adamatzky, A. Towards proteinoid computers. hypothesis paper. Biosystems 2021, 208, 104480. [Google Scholar] [CrossRef]
- Sharma, S.; Mougoyannis, P.; Tarabella, G.; Adamatzky, A. A review on the protocols for the synthesis of proteinoids. arXiv 2022, arXiv:2212.02261. [Google Scholar]
- Fox, S.W.; Harada, K. Thermal copolymerization of amino acids in the presence of phosphoric acid. Arch. Biochem. Biophys. 1960, 86, 281–285. [Google Scholar] [CrossRef]
- Itzhaki, E.; Hadad, E.; Moskovits, N.; Stemmer, S.M.; Margel, S. Tumor-targeted fluorescent proteinoid nanocapsules encapsulating synergistic drugs for personalized cancer therapy. Pharmaceuticals 2021, 6, 648. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762. [Google Scholar] [CrossRef]
- Van den Bos, J.; Wieringa, F.P.; Bouvy, N.D.; Stassen, L.P.S. Optimizing the image of fluorescence cholangiography using ICG: A systematic review and ex vivo experiments. Surg. Endosc. 2018, 32, 4820–4832. [Google Scholar] [CrossRef] [PubMed]
- Bhavane, R.; Starosolski, Z.; Stupin, I.; Ghaghada, K.B.; Annapragada, A. NIR-II fluorescence imaging using indocyanine green nanoparticles. Sci. Rep. 2018, 81, 14455. [Google Scholar] [CrossRef] [PubMed]
- Lwin, T.M.; Murakami, T.; Miyake, K.; Yazaki, P.J.; Shivley, J.E.; Hoffman, R.M.; Bouvet, M. Tumor-specific labeling of pancreatic cancer using a humanized anti-CEA antibody conjugated to a near-infrared fluorophore. Ann. Surg. Oncol. 2018, 25, 1079–1085. [Google Scholar] [CrossRef]
- Makwana, V.; Karanjia, J.; Haselhorst, T.; Anoopkumar-Dukie, S.; Rudrawar, S. Liposomal doxorubicin as targeted delivery platform: Current trends in surface functionalization. Int. J. Pharm. 2021, 593, 120117. [Google Scholar] [CrossRef]
- Neun, B.W.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Understanding the role of anti-PEG antibodies in the complement activation by Doxil in vitro. Molecules 2018, 23, 1700. [Google Scholar] [CrossRef] [PubMed]
- Bavli, Y.; Winkler, I.; Chen, B.M.; Roffler, S.; Cohen, R.; Szebeni, J.; Barenholz, Y. Doxebo (doxorubicin-free Doxil-like liposomes) is safe to use as a pre-treatment to prevent infusion reactions to PEGylated nanodrugs. J. Control. Release 2019, 306, 138–148. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the Molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Temming, K.; Schiffelers, R.M.; Molema, G.; Kok, R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updat. 2005, 8, 381–402. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef]
- Asati, S.; Pandey, V.; Soni, V. RGD Peptide as a targeting moiety for theranostic purpose: An update study. Int. J. Pept. Res. Ther. 2018, 25, 49–65. [Google Scholar] [CrossRef]
- Kieffer, B.; Mer, G.; Mann, A.; Lefèvre, J.F. Structural studies of two antiaggregant RGDW peptides by 1H and 13C NMR. Int. J. Pept. Protein Res. 1994, 44, 70–79. [Google Scholar] [CrossRef]
- Zeng, C.; Shang, W.; Wang, K.; Chi, C.; Jia, X.; Feng, C.; Yang, D.; Ye, J.; Fang, C.; Tian, J. Intraoperative identification of liver cancer microfoci using a targeted near-infrared fluorescent probe for imaging-guided surgery. Sci. Rep. 2016, 6, 21959. [Google Scholar] [CrossRef]
- Oddo, L.; Paradossi, G.; Cerroni, B.; Ben-Harush, C.; Ariel, E.; Di Meco, F.; Ram, Z.; Grossman, R. In vivo biodistribution of engineered lipid microbubbles in rodents. ACS Omega 2019, 4, 13371–13381. [Google Scholar] [CrossRef]
- Xie, J.; Yan, C.; Yan, Y.; Chen, L.; Song, L.; Zang, F.; An, Y.; Teng, G.; Gu, N.; Zhang, Y. Multi-modal Mn–Zn ferrite nanocrystals for magnetically-induced cancer targeted hyperthermia: A comparison of passive and active targeting effects. Nanoscale 2016, 8, 16902–16915. [Google Scholar] [CrossRef] [PubMed]
- Hadad, E.; Rudnick-Glick, S.; Grinberg, I.; Yehuda, R.; Margel, S. Engineering of NIR fluorescent PEGylated poly(RGD) proteinoid polymers and nanoparticles for drug delivery applications in chicken embryo and mouse models. RSC Adv. 2020, 10, 34364–34372. [Google Scholar] [CrossRef]
- Bossion, A.; Heifferon, K.V.; Meabe, L.; Zivic, N.; Taton, D.; Hedrick, J.L.; Long, T.E.; Sardon, H. Opportunities for organocatalysis in polymer synthesis via step-growth methods. Prog. Polym. Sci. 2019, 90, 164–210. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, P.K.; Chung, B.H. Self-assembled levan nanoparticles for targeted breast cancer imaging. Chem. Commun. 2015, 51, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Chen, Y.; Mount, C.W.; Gombotz, W.R.; Li, X.; Pun, S.H. Evaluation of temperature-sensitive, indocyanine green-encapsulating micelles for noninvasive near-infrared tumor imaging. Pharm. Res. 2010, 27, 1900–1913. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenes 2018, 213, 425–532. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2020, 897, 1619–1626. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials 2014, 35, 4835–4847. [Google Scholar] [CrossRef]
- Yin, H.Q.; Mai, D.S.; Gan, F.; Chen, X.J. One-step synthesis of linear and cyclic RGD conjugated gold nanoparticles for tumour targeting and imaging. RSC Adv. 2014, 4, 9078–9085. [Google Scholar] [CrossRef]
- Baek, A.; Kim, Y.; Lee, J.W.; Lee, S.C.; Cho, S.R. Effect of polydeoxyribonucleotide on angiogenesis and wound healing in an in vitro model of osteoarthritis. Cell Transplant. 2018, 27, 1623–1633. [Google Scholar] [CrossRef]
- Kazunori, K.; Glenn, S.; Masayuki, Y.; Teruo, O.; Yasuhisa, S. Block copolymer micelles as vehicles for drug delivery. J. Control. Release 1993, 24, 119–132. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428. [Google Scholar] [CrossRef]
- Peng, C.; Zheng, L.; Chen, Q.; Shen, M.; Guo, R.; Wang, H.; Cao, X.; Zhang, G.; Shi, X. PEGylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomography. Biomaterials 2012, 33, 1107–1119. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Liu, F.; Collot, M.; Anton, N. Dye-loaded nanoemulsions: Biomimetic fluorescent nanocarriers for bioimaging and nanomedicine. Adv. Healthc. Mater. 2021, 10, 2001289. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar]
- Proulx, S.T.; Luciani, P.; Derzsi, S.; Rinderknecht, M.; Mumprecht, V.; Leroux, J.C.; Detmar, M. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010, 70, 7053–7062. [Google Scholar] [CrossRef]
- Kue, C.S.; Tan, K.Y.; Lam, M.L.; Lee, H.B. Chick embryo chorioallantoic membrane (CAM): An alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp. Anim. 2015, 64, 129. [Google Scholar] [CrossRef]
- Ge, Z.; Chen, Q.; Osada, K.; Liu, X.; Tockary, T.A.; Uchida, S.; Dirisala, A.; Ishii, T.; Nomoto, T.; Toh, K.; et al. Targeted gene delivery by polyplex micelles with crowded PEG palisade and cRGD moiety for systemic treatment of pancreatic tumors. Biomaterials 2014, 35, 3416–3426. [Google Scholar] [CrossRef]
- Vu, M.N.; Kelly, H.G.; Wheatley, A.K.; Peng, S.; Pilkington, E.H.; Veldhuis, N.A.; Davis, T.P.; Kent, S.J.; Truong, N.P. Cellular interactions of liposomes and PISA nanoparticles during human blood flow in a microvascular network. Small 2020, 16, 2002861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, H.; Wong, S.; Lu, M.; Xiao, P.; Stenzel, M.H. Influence of nanoparticle shapes on cellular uptake of paclitaxel loaded nanoparticles in 2D and 3D cancer models. Polym. Chem. 2017, 8, 3317–3326. [Google Scholar] [CrossRef]
- Martin-Banderas, L.; Muñoz-Rubio, I.; Prados, J.; Álvarez-Fuentes, J.; Calderón-Montaño, J.M.; López-Lázaro, M.; Arias, J.L.; Leiva, M.C.; Holgado, M.A.; Fernández-Arévalo, M. In vitro and in vivo evaluation of Δ9-tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int. J. Pharm. 2015, 487, 205–212. [Google Scholar] [CrossRef]
- Kruyt, F.A.E. TRAIL and cancer therapy. Cancer Lett. 2008, 263, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, E.; Chausky-Barzakh, E.; Atkins, A.; Bareket-Samish, A.; Stemmer, S.M.; Moskovits, N.; Margel, S. Tumor-targeted poly(ArgGlyAsp) nanocapsules for personalized cancer therapy-In vivo study. Adv. Ther. 2023, 1–34. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600. [Google Scholar] [PubMed]
- Nur Husna, S.M.; Tan, H.-T.T.; Mohamud, R.; Dyhl-Polk, A.; Wong, K.K. Inhibitors targeting CDK4/6, PARP and PI3K in breast cancer: A review. Ther. Adv. Med. Oncol. 2018, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, S.; Markosian, C.; Ali, Z.; Rizvi, M. Palbociclib-induced pneumonitis: A case report and review of the literature. Cureus 2020, 12, e8929. [Google Scholar] [CrossRef]
- Anonymous. Alpelisib Palbociclib HPLC Report; Faculty of Life Sciences, Bar-Ilan University: Ramat Gan, Israel, 2019; pp. 6–7, 18–19. [Google Scholar]
- Bardia, A.; Chandarlapaty, S.; Linden, H.M.; Ulaner, G.A.; Gosselin, A.; Cartot-Cotton, S.; Cohen, P.; Doroumian, S.; Paux, G.; Celanovic, M.; et al. AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nat. Commun. 2022, 13, 4116. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, X.; Li, J.; Xie, X.; Huang, M.; Jiang, J.; Liao, Y.P.; Donahue, T.; Meng, H. Use of ratiometrically designed nanocarrier targeting CDK4/6 and autophagy pathways for effective pancreatic cancer treatment. Nat. Commun. 2020, 11, 4249. [Google Scholar] [CrossRef] [PubMed]
- Moskovits, N.; Peretz, I.; Chausky, E.; Itzhaki, E.; Shmuel, N.; Meerson, R.; Tarasenko, N.; Kaufman, A.; Stemmer, A.; Yaffe, R.; et al. Palbociclib in combination with sunitinib exerts a synergistic anti-cancer effect in patient-derived xenograft models of various human cancers types. Cancer Lett. 2022, 536, 215665. [Google Scholar] [CrossRef] [PubMed]









| Proteinoid Polymer | Amino Acid Content | Main Component of Amino Acids a | Segment |
|---|---|---|---|
| P(EF-PLLA) | L-glutamic acid L-phenylalanine | L-glutamic acid | Poly-L-lactic acid (PLLA) |
| P(KRHF) | L-lysine L-arginine L-histidine L-phenylalanine | L-lysine | PLLA |
| P(RGD) | D-arginine L-glycine L-aspartic acid | L-aspartic acid | ------ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itzhaki, E.; Elias, Y.; Moskovits, N.; Stemmer, S.M.; Margel, S. Proteinoid Polymers and Nanocapsules for Cancer Diagnostics, Therapy and Theranostics: In Vitro and In Vivo Studies. J. Funct. Biomater. 2023, 14, 215. https://doi.org/10.3390/jfb14040215
Itzhaki E, Elias Y, Moskovits N, Stemmer SM, Margel S. Proteinoid Polymers and Nanocapsules for Cancer Diagnostics, Therapy and Theranostics: In Vitro and In Vivo Studies. Journal of Functional Biomaterials. 2023; 14(4):215. https://doi.org/10.3390/jfb14040215
Chicago/Turabian StyleItzhaki, Ella, Yuval Elias, Neta Moskovits, Salomon M. Stemmer, and Shlomo Margel. 2023. "Proteinoid Polymers and Nanocapsules for Cancer Diagnostics, Therapy and Theranostics: In Vitro and In Vivo Studies" Journal of Functional Biomaterials 14, no. 4: 215. https://doi.org/10.3390/jfb14040215
APA StyleItzhaki, E., Elias, Y., Moskovits, N., Stemmer, S. M., & Margel, S. (2023). Proteinoid Polymers and Nanocapsules for Cancer Diagnostics, Therapy and Theranostics: In Vitro and In Vivo Studies. Journal of Functional Biomaterials, 14(4), 215. https://doi.org/10.3390/jfb14040215





