The Effect of Titanium Surface Topography on Adherent Macrophage Integrin and Cytokine Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium
2.2. Cell Culture
2.3. Macrophage Viability and Morphology
2.4. Cytokine Secretion
2.5. Integrin Gene Expression
2.6. Statistical Analysis
3. Results
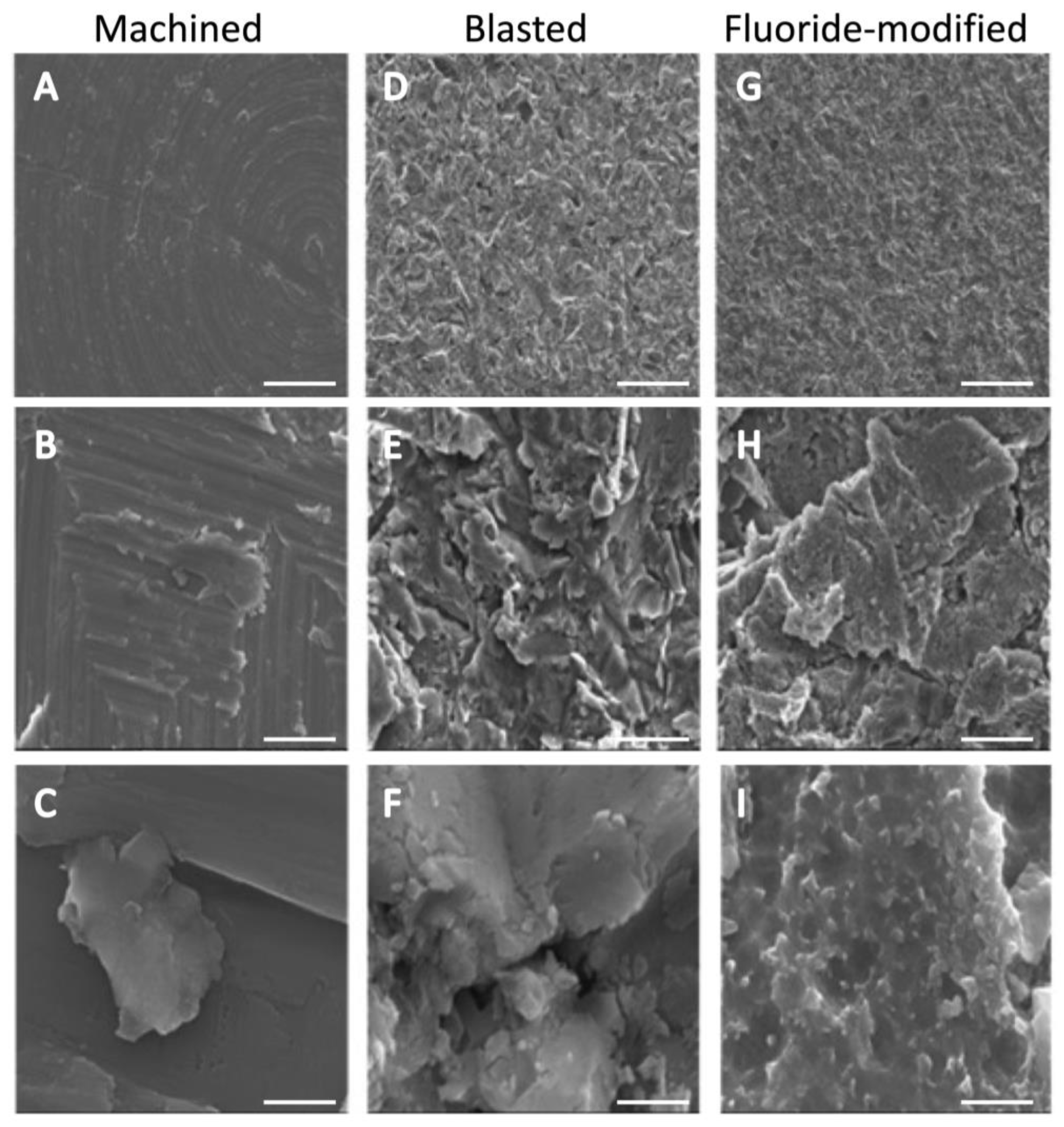
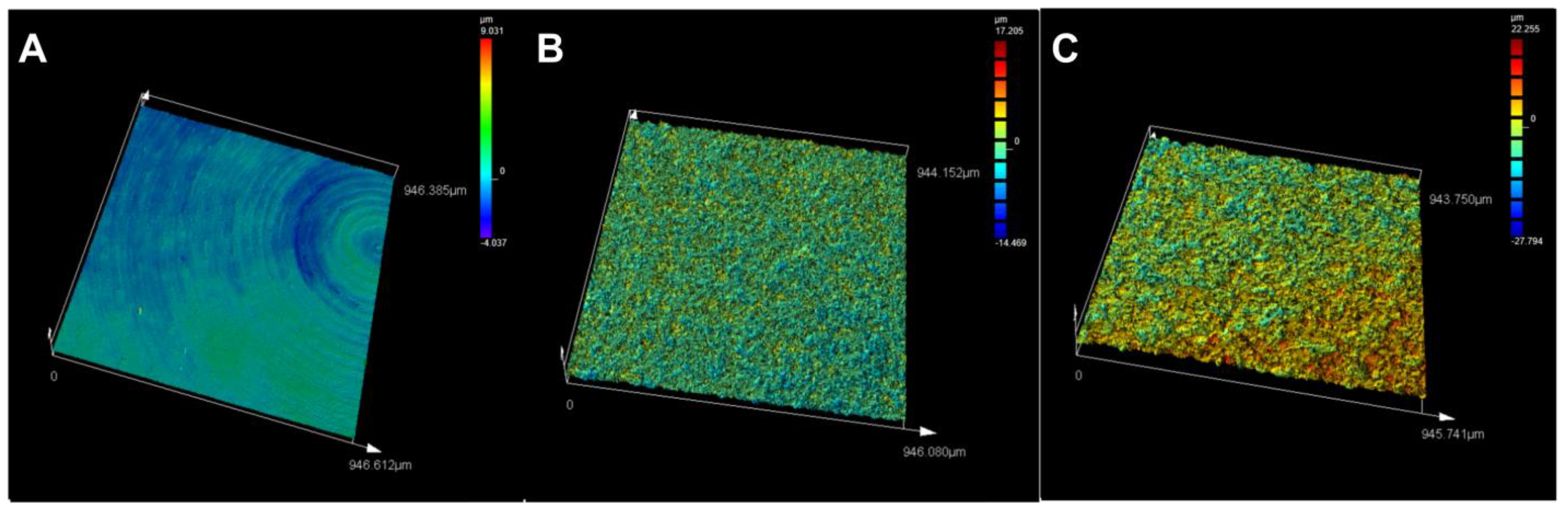
3.1. Titanium Surface Characterisation
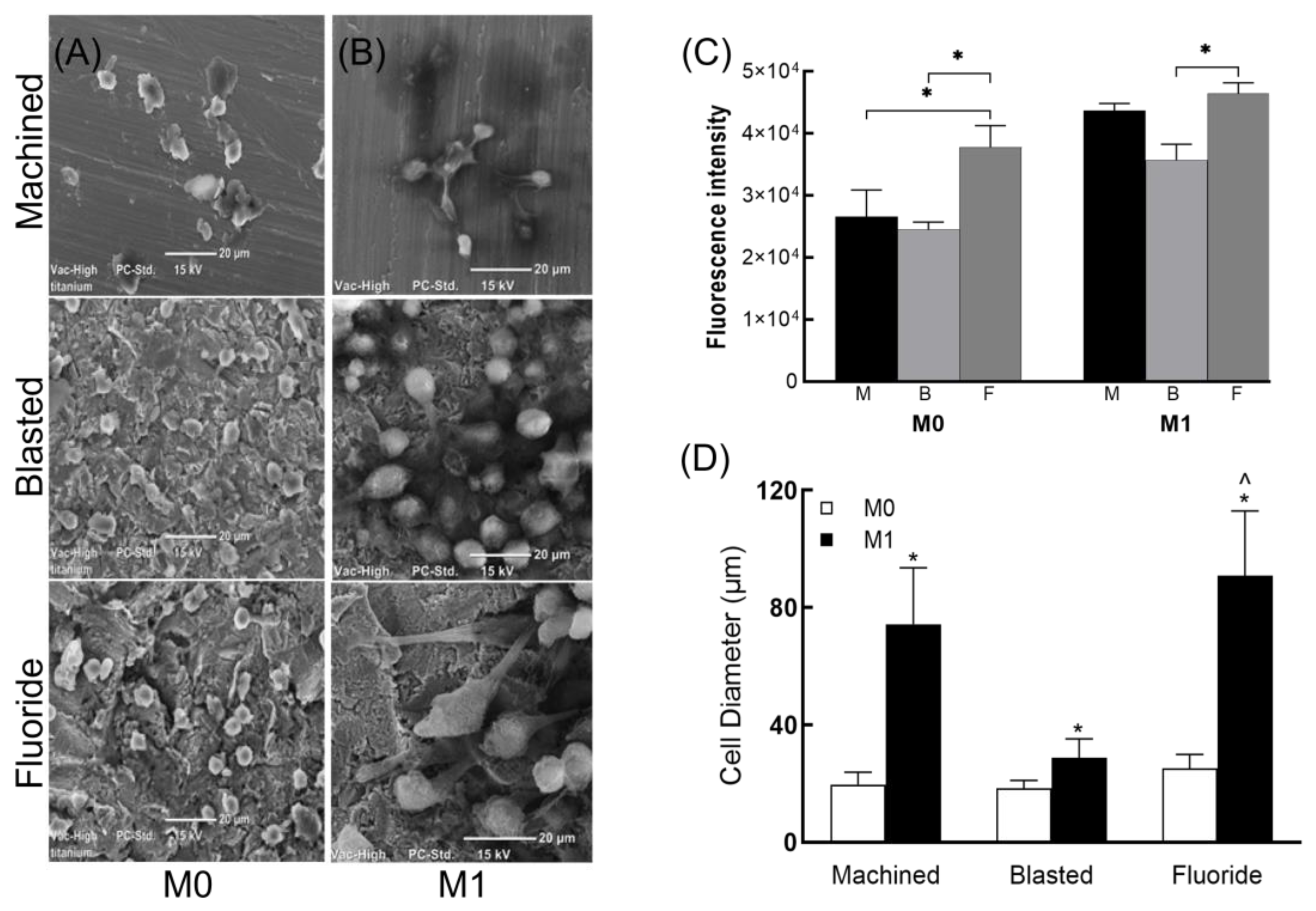
3.2. Macrophage Viability and Morphology
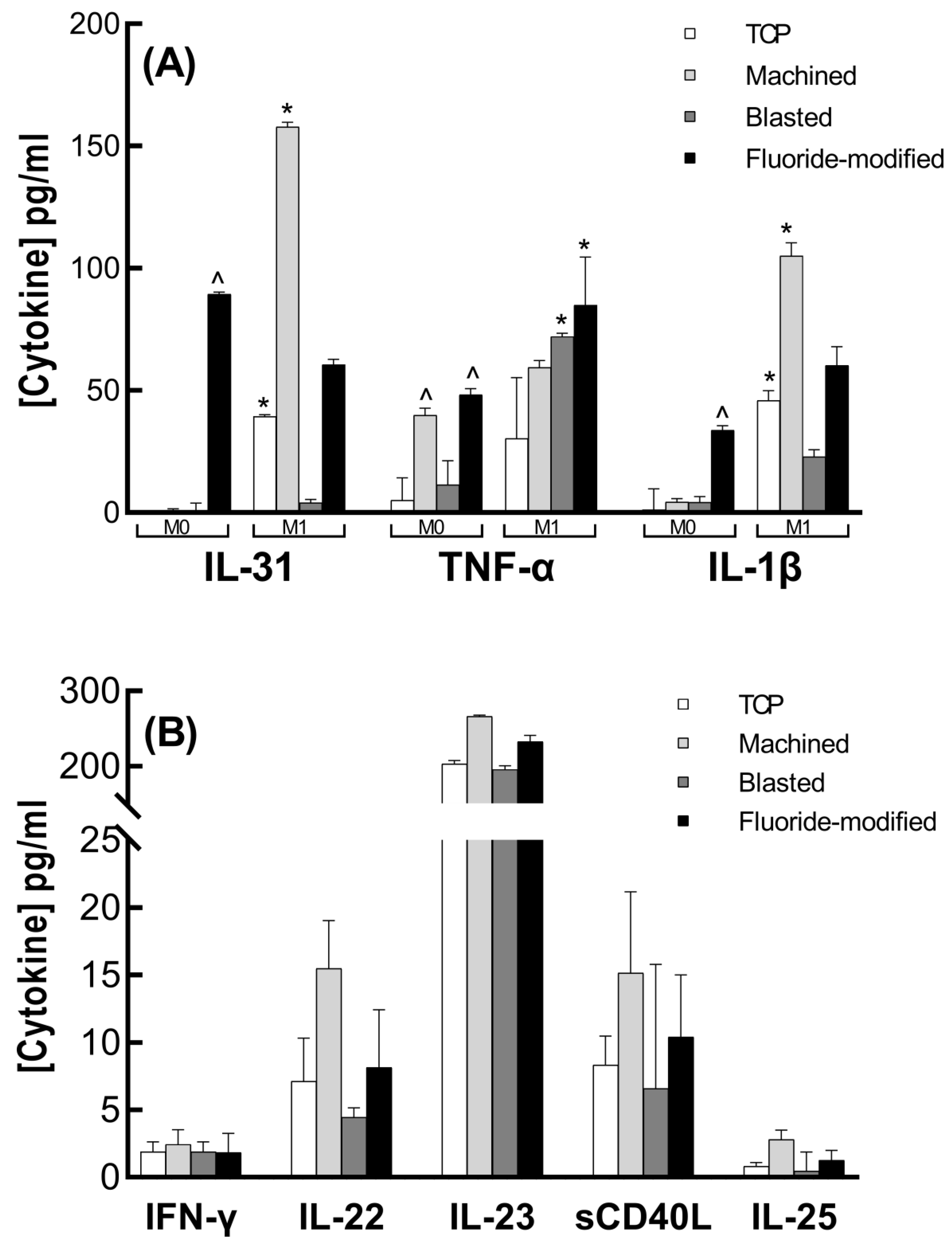
3.3. Cytokine Analysis
3.4. Integrin Gene Expression
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shirazi, S.; Ravindran, S.; Cooper, L.F. Topography-mediated immunomodulation in osseointegration; Ally or Enemy. Biomaterials 2022, 291, 121903. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Shanley, L.C.; Mahon, O.R.; Kelly, D.J.; Dunne, A. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater. 2021, 133, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.; Gadegaard, N.; Oreffo, R. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Shimaoka, M.; Springer, T.A. Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2003, 2, 703–716. [Google Scholar] [CrossRef]
- Podolnikova, N.P.; Kushchayeva, Y.S.; Wu, Y.; Faust, J.; Ugarova, T.P. The role of integrins αMβ2 (Mac-1, CD11b/CD18) and αDβ2 (CD11d/CD18) in macrophage fusion. Am. J. Pathol. 2016, 186, 2105–2116. [Google Scholar] [CrossRef] [Green Version]
- Adair, B.; Xiong, J.; Maddock, C.; Goodman, S.; Arnaout, M.; Yeager, M. Three-dimensional EM structure of the ectodomain of integrin αVβ3 in a complex with fibronectin. J. Cell Biol. 2005, 168, 1109–1118. [Google Scholar] [CrossRef]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β2 integrins as regulators of dendritic cell, monocyte, and macrophage function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Leal, R.A.; Healey, G.D.; Corradetti, B. Biomimetic immunomodulation strategies for effective tissue repair and restoration. Adv. Drug Deliv. Rev. 2021, 179, 113913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.R.; Fernandes, N.A.R.; Gonzalez Maldonado, L.A.; Rossa Junior, C. Comparison of monocytic cell lines U937 and THP-1 as macrophage models for in vitro studies. Biochem. Biophys. Rep. 2022, 32, 101383. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Aldo, P.B.; Craveiro, V.; Guller, S.; Mor, G. Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am. J. Reprod. Immunol. 2013, 70, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying in Vitro Polarization? Front. Pharmacol. 2018, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; So, E.C.; Strome, S.E.; Zhang, X. Impact of Detachment Methods on M2 Macrophage Phenotype and Function. J. Immunol. Methods 2015, 26, 56–61. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Y.; Zhou, M.; Xu, Y.; Zhang, Q.; Wu, L.; Liu, S.; Zhang, M.; Zhang, L.; Wu, Z.; et al. The Culture Dish Surface Influences the Phenotype and Dissociation Strategy in Distinct Mouse Macrophage Populations. Front. Immunol. 2022, 13, 920232. [Google Scholar] [CrossRef]
- Masaki, C.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implant. Res. 2005, 16, 650–656. [Google Scholar] [CrossRef]
- Monjo, M.; Petzold, C.; Ramis, J.M.; Lyngstadaas, S.P.; Ellingsen, J.E. In vitro osteogenic properties of two dental implant surfaces. Int. J. Biomater. 2012, 2012, 181024. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.S.; Su, Y.L.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef]
- Wu, S.; Xia, B.; Mai, S.; Feng, Z.; Wang, X.; Liu, Y.; Liu, R.; Li, Z.; Xiao, Y.; Chen, Z.; et al. Sodium Fluoride under Dose Range of 2.4-24 μM, a Promising Osteoimmunomodulatory Agent for Vascularized Bone Formation. ACS Biomater. Sci. Eng. 2019, 5, 817–830. [Google Scholar] [CrossRef]
- De la Fuente, B.; Vázquez, M.; Rocha, R.A.; Devesa, V.; Vélez, D. Effects of sodium fluoride on immune response in murine macrophages. Toxicol. Vitr. 2016, 34, 81–87. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Goschorska, M.; Kolasa, A.; Łukomska, A.; Jakubczyk, K.; Dec, K.; Chlubek, D. Fluoride as a factor initiating and potentiating inflammation in THP1 differentiated monocytes/macrophages. Toxicol. Vitr. 2015, 29, 1661–1668. [Google Scholar] [CrossRef]
- Chen, M.; Hu, J.; Zhang, E.; Hu, J.; Wang, X.; Qin, G. The osteoimmunomodulatory effect of nanostructured TiFx/TiOx coating on osteogenesis induction. Biomed. Mater. 2021, 16, 045041. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Lv, L.; Shao, D.; Xie, Y.; Cao, Y.; Zheng, X. Engineering nanopatterned structures to orchestrate macrophage phenotype by cell shape. J. Funct. Biomater. 2022, 13, 31. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.S.B.; Hamlet, S.M.; Moon, H.J.; Ivanovski, S. Re-establishment of macrophage homeostasis by titanium surface modification in type II diabetes promotes osseous healing. Biomaterials 2021, 267, 120464. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Eichler, M.; Geis-Gerstorfer, J. Wetting behavior of dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 1256–1266. [Google Scholar] [PubMed]
- Cha, B.H.; Shin, S.R.; Leijten, J.; Li, Y.C.; Singh, S.; Liu, J.C.; Annabi, N.; Abdi, R.; Dokmeci, M.R.; Vrana, N.E.; et al. Integrin-mediated interactions control macrophage polarization in 3d hydrogels. Adv. Healthc. Mater. 2017, 6, 1700289. [Google Scholar] [CrossRef] [Green Version]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ITGA1 | GGTGCCCGAAGAGGAGTTAAAA | TCCTCGGTTATAGCTGCCAAGA |
| ITGA2 | TCAGGGCACTATCCGCACAAAGTA | CCAAAGGCACCAATAGACACATCG |
| ITGAM | TGATGCTGTTCTCTACGGGGAGCA | AACAGGTAAACAGCACCCCGGTTG |
| ITGB1 | TGAGCTGGACAGAGGAGGAGGAAG | GCCTCCTGCTGCTCAATGATGC |
| ITGB2 | GAAGGAAGCTGCCGGAAGGACAAC | GCGCTCACAGTTGATGGTGTCACA |
| ACTB | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
| Ra (µm) | Rz (µm) | Sa (µm) | Height Profile (µm) | |
|---|---|---|---|---|
| Machined | 0.214 | 1.803 | 0.361 | +9.031 to −4.037 |
| Blasted | 1.196 | 10.146 | 1.351 | +17.205 to −14.469 |
| Fluoride-modified | 2.31 | 20.226 | 2.449 | +22.255 to −27.794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitchai, M.S.; Ipe, D.S.; Hamlet, S. The Effect of Titanium Surface Topography on Adherent Macrophage Integrin and Cytokine Expression. J. Funct. Biomater. 2023, 14, 211. https://doi.org/10.3390/jfb14040211
Pitchai MS, Ipe DS, Hamlet S. The Effect of Titanium Surface Topography on Adherent Macrophage Integrin and Cytokine Expression. Journal of Functional Biomaterials. 2023; 14(4):211. https://doi.org/10.3390/jfb14040211
Chicago/Turabian StylePitchai, Manju Sofia, Deepak Samuel Ipe, and Stephen Hamlet. 2023. "The Effect of Titanium Surface Topography on Adherent Macrophage Integrin and Cytokine Expression" Journal of Functional Biomaterials 14, no. 4: 211. https://doi.org/10.3390/jfb14040211
APA StylePitchai, M. S., Ipe, D. S., & Hamlet, S. (2023). The Effect of Titanium Surface Topography on Adherent Macrophage Integrin and Cytokine Expression. Journal of Functional Biomaterials, 14(4), 211. https://doi.org/10.3390/jfb14040211







