Marginal Bone Level and Biomechanical Behavior of Titanium-Indexed Abutment Base of Conical Connection Used for Single Ceramic Crowns on Morse-Taper Implant: A Clinical Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
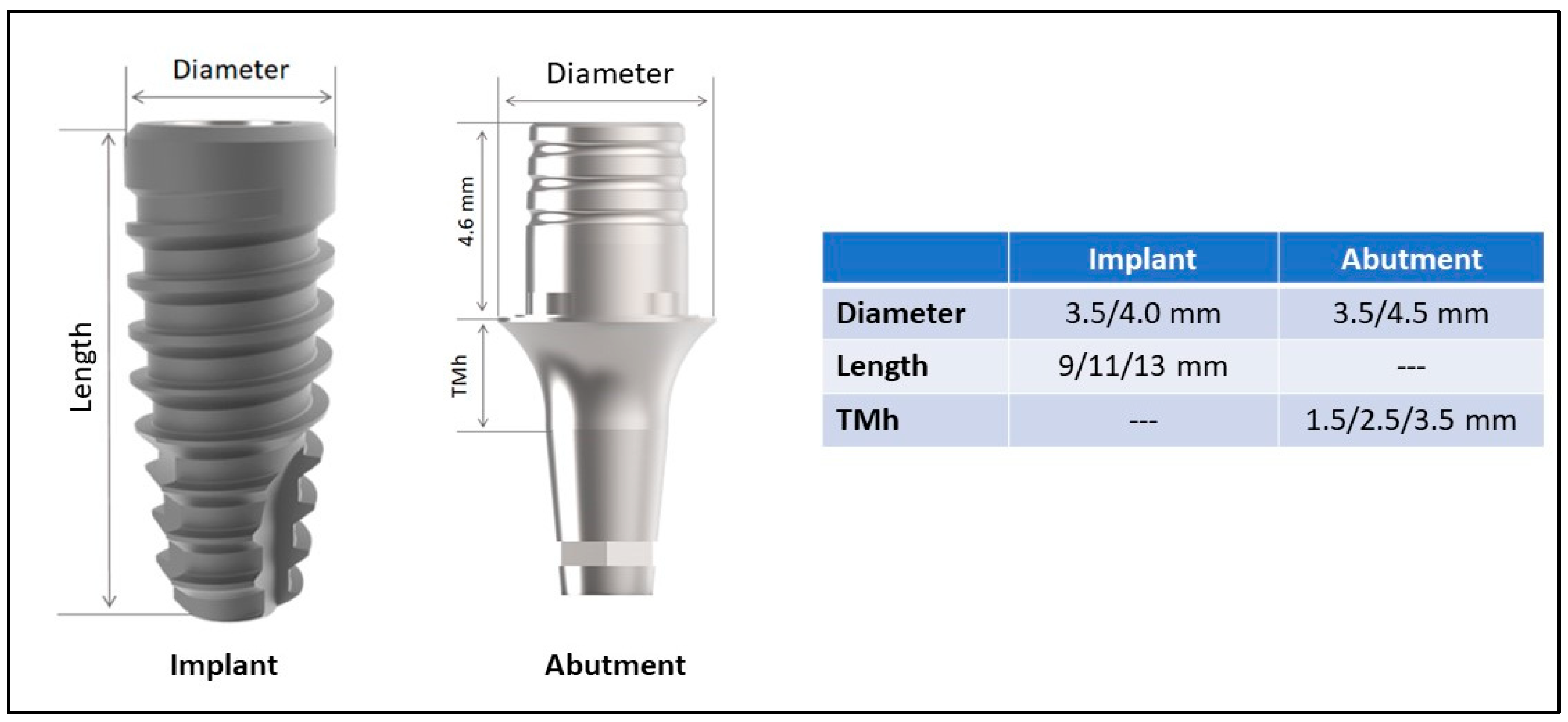
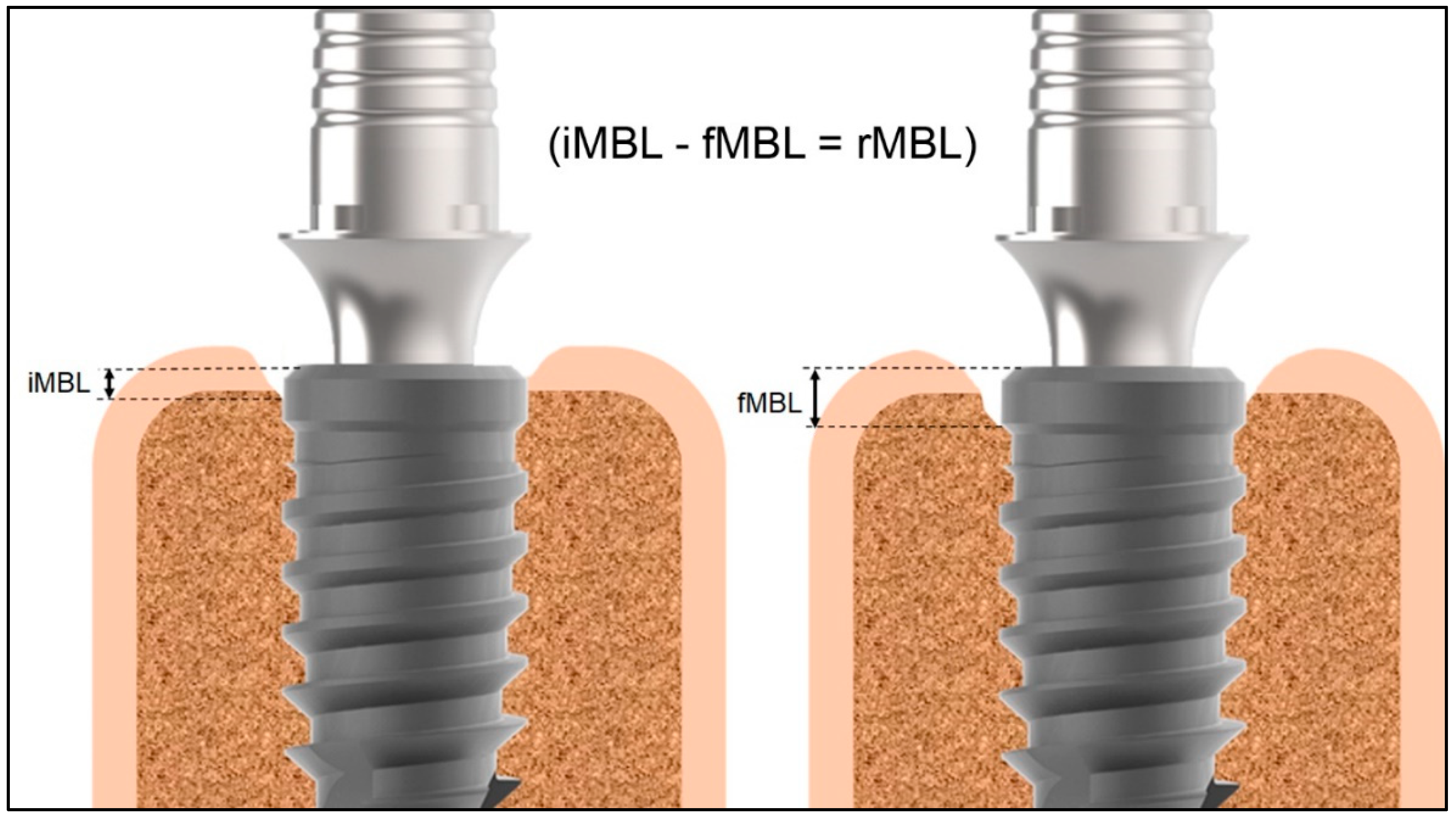
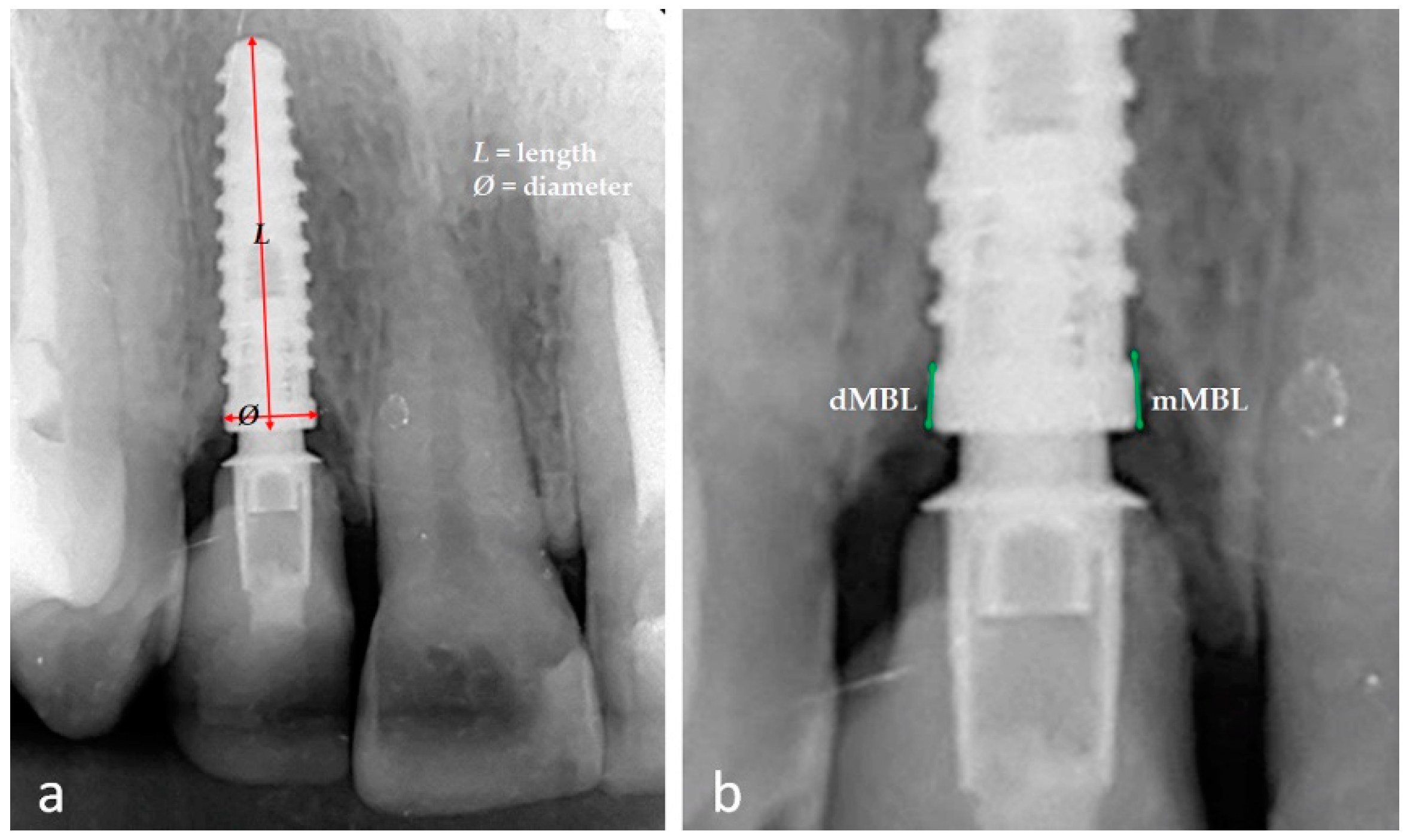
2.2. Data Collection and Variable Studied
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niedermaier, R.; Stelzle, F.; Riemann, M.; Bolz, W.; Schuh, P.; Wachtel, H. Im-plant-supported immediately loaded fixed full-arch dentures: Evaluation of implant survival rates in a case cohort of up to 7 years. Clin. Implant. Dent. Relat. Res. 2017, 19, 4–19. [Google Scholar] [CrossRef]
- French, D.; Ofec, R.; Levin, L. Long term clinical performance of 10 871 dental im-plants with up to 22 years of follow-up: A cohort study in 4247 patients. Clin. Implant. Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tis-sues in health and disease. Periodontol. 2000 2018, 76, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martín, I.; Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Sanz, M. Effects of modified abutment characteristics on peri-implant soft tissue health: A systematic re-view and meta-analysis. Clin. Implant. Dent. Relat. Res. 2018, 29, 118–129. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials 2020, 13, 1131. [Google Scholar] [CrossRef]
- Passos, S.P.; May, L.; Faria, R.; Özcan, M.; Bottino, M.A. Implant-abutment gap versus microbial colonization: Clinical significance based on a literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Gadalla, H.; Lundgren, T. Bacterial Colonization of the Implant-Abutment Interface (IAI) of Dental Implants with a Sloped Marginal Design: An in-vitro Study. Clin. Implant. Dent. Relat. Res. 2016, 18, 161–167. [Google Scholar] [CrossRef]
- Gois, D.M.; Fo Gois-Santos, V.T.; Silva, R.S.; Marqueti, A.C.; Cortes, A.R.G.; Trento, C.L. Evaluation of sealing between abutment and inner connection of cone morse dental implant: Microgaps between implant and abutment. Clin. Lab. Res. Dent. 2018, 2018, 1–6. [Google Scholar]
- Alves, D.C.C.; De Carvalho, P.S.P.; Martinez, E.F. In Vitro Microbiological Analysis of Bacterial Seal at the Implant-Abutment Interface Using Two Morse Taper Implant Models. Braz. Dent. J. 2014, 25, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.C.L.C.M.; Sperandio, M.; Napimoga, M.H. Association between implant-abutment microgap and implant circularity to bacterial leakage: An in vitro study using tapered connection implants. Int. J. Oral Maxillofac. Implant. 2018, 33, 505–511. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Aboalrejal, A.N. Microgap and Micromotion at the Implant Abutment Interface Cause Marginal Bone Loss Around Dental Implant but More Evidence is Needed. J. Evid. Based Dent. Pract. 2018, 18, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Shibli, J.A.; Aramburú, J.S., Jr.; Sánchez de Val, J.E.M.; Calvo-Girardo, J.L.; Dedavid, B.A. Effects of different torque levels on the implant-abutment interface in a conical internal connection. Braz. Oral Res. 2016, 30, e40. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Dedavid, B.A.; Marín, J.M.G.; Canullo, L. Behavior of implant and abutment sets of three different connections during the non-axial load application: An in vitro experimental study using a radiographic method. Bio-Med. Mater. Eng. 2022, 33, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Grobecker-Karl, T.; Karl, M. Correlation between Micromotion and Gap Formation at the Implant-Abutment Interface. Int. J. Prosthodont. 2017, 30, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.; Delgado-Ruiz, R.; Frutos, J.; Prados-Privado, M.; DeDavid, B.; Marín, J.; Guirado, J. Misfit of Three Different Implant-Abutment Connections Before and After Cyclic Load Application: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 822–829. [Google Scholar] [CrossRef]
- Vetromilla, B.M.; Brondani, L.P.; Pereira-Cenci, T.; Bergoli, C.D. Influence of different implant-abutment connection designs on the mechanical and biological behavior of single-tooth implants in the maxillary esthetic zone: A systematic review. J. Prosthet. Dent. 2019, 121, 398–403.e3. [Google Scholar] [CrossRef]
- Gehrke, S.A.; De Carvalho Serra, R. Load fatigue performance of conical implant-abutment connection: Effect of torque level and interface junction. Minerva Stomatol. 2015, 64, 1–7. [Google Scholar] [PubMed]
- Degidi, M.; Perrotti, V.; Shibli, J.A.; Novaes, A.B.; Piattelli, A.; Iezzi, G. Equicrestal and Subcrestal Dental Implants: A Histologic and Histomorphometric Evaluation of Nine Retrieved Human Implants. J. Periodontol. 2011, 82, 708–715. [Google Scholar] [CrossRef]
- Cerutti-Kopplin, D.; Neto, D.J.R.; Valle, A.L.D.; Pereira, J.R. Influence of reverse torque values in abutments with or without internal hexagon indexes. J. Prosthet. Dent. 2014, 112, 824–827. [Google Scholar] [CrossRef]
- de Oliveira Silva, T.S.; Mendes Alencar, S.M.; da Silva Valente, V.; de Moura, C.D.V.S. Effect of internal hexagonal index on removal torque and tensile removal force of different Morse taper connection abutments. J. Prosthet. Dent. 2017, 117, 621–627. [Google Scholar] [CrossRef]
- Caricasulo, R.; Malchiodi, L.; Ghensi, P.; Fantozzi, G.; Cucchi, A. The influence of implant-abutment connection to peri-implant bone loss: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Moergel, M.; Rocha, S.; Messias, A.; Nicolau, P.; Guerra, F.; Wagner, W. Clinical and radiographic performance of self-locking conical connection implants in the posterior mandible: Five-year results of a two-centre prospective study. Clin. Oral Implant. Res. 2021, 32, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Macedo, J.P.; Pereira, J.; Vahey, B.R.; Henriques, B.; Benfatti, C.A.M.; Magini, R.S.; López-López, J.; Souza, J.C.M. Morse taper dental implants and platform switching: The new paradigm in oral implantology. Eur. J. Dent. 2016, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Peruzetto, W.M.; Martinez, E.F.; Peruzzo, D.C.; Joly, J.C.; Napimoga, M.H. Microbiological Seal of Two Types of Tapered Implant Connections. Braz. Dent. J. 2016, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, I.H.; Kul, E. Investigation of the Type of Angled Abutment for Anterior Maxillary Implants: A Finite Element Analysis. J. Prosthodont. 2022, 31, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Guncu, M.; Aktas, G.; Guncu, G.; Anıl, D.; Turkyilmaz, I.; Antonoff, L. Clinical, Technical, and Radiologic Outcomes of 182 Implant-Supported Zirconia Single Crowns Using Titanium-Base Abutments: A Retrospective Study. Int. J. Prosthodont. 2022, 35, 553–559. [Google Scholar] [CrossRef]
- Ding, T.A.; Woody, R.D.; Higginbottom, F.L.; Miller, B.H. Evaluation of the ITI Morse taper implant/abutment design with an internal modification. Int. J. Oral Maxillofac. Implant. 2003, 18, 865–872. [Google Scholar]
- Villarinho, E.A.; Cervieri, A.; Shinkai, R.S.A.; Grossi, M.L.; Teixeira, E.R. The Effect of a Positioning Index on the Biomechanical Stability of Tapered Implant-Abutment Connections. J. Oral Implant. 2015, 41, 139–143. [Google Scholar] [CrossRef]
- Martins, C.M.; Ramos, E.V.; Kreve, S.; De Carvalho, G.A.P.; Franco, A.B.G.; De Macedo, L.G.S.; Silva, A.D.M.; Dias, S.C. Reverse torque evaluation in indexed and nonindexed abutments of Morse Taper implants in a mechanical fatigue test. Dent. Res. J. 2019, 16, 110–116. [Google Scholar] [CrossRef]
- Bharate, V.; Kumar, Y.; Koli, D.; Pruthi, G.; Jain, V. Effect of different abutment materials (zirconia or titanium) on the crestal bone height in 1 year. J. Oral Biol. Craniofacial Res. 2020, 10, 372–374. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Pei, X.; Han, J.; Wang, J. Network meta-analysis of survival rate and complications in implant-supported single crowns with different abutment materials. J. Dent. 2019, 88, 103115. [Google Scholar] [CrossRef] [PubMed]
- Schepke, U.; Meijer, H.J.; Kerdijk, W.; Raghoebar, G.M.; Cune, M. Stock Versus CAD/CAM Customized Zirconia Implant Abutments—Clinical and Patient-Based Outcomes in a Randomized Controlled Clinical Trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 74–84. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Muñoz, A.; Pina, V.; Sola-Ruiz, M. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.M.S.; Elias, C.N.; Monteiro, E.S.; Coimbra, M.E.R.; Santana, A.I.C. Galvanic Corrosion of Ti Dental Implants Coupled to CoCrMo Prosthetic Component. Int. J. Biomater. 2021, 2021, 1313343. [Google Scholar] [CrossRef] [PubMed]
- Amine, M.; Merdma, W.; El Boussiri, K. Electrogalvanism in Oral Implantology: A Sys-tematic Review. Int. J. Dent. 2022, 2022, 4575416. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Cascos-Sanchez, R.; Yilmaz, B.; Lam, W.; Pow, E.; Highsmith, J.D.R.; Gómez-Polo, M. Effect of Fabrication Technique on the Microgap of CAD/CAM Cobalt–Chrome and Zirconia Abutments on a Conical Connection Implant: An In Vitro Study. Materials 2021, 14, 2348. [Google Scholar] [CrossRef] [PubMed]
- Molinero-Mourelle, P.; Roccuzzo, A.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Highsmith, J.D.R.; Gómez-Polo, M. Microleakage assessment of CAD-CAM Cobalt-Chrome and Zirconia abutments on a conical connection dental implant: A comparative in vitro study. Clin. Oral Implant. Res. 2022, 33, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Al-Thobity, A.M. Titanium Base Abutments in Implant Prosthodontics: A Literature Review. Eur. J. Dent. 2022, 16, 49–55. [Google Scholar] [CrossRef]
- Maghsoudi, P.; Slot, D.E.; van der Weijden, F.G.A. Bone remodeling around dental implants after 1–1.5 years of functional loading: A retrospective analysis of two-stage implants. Clin. Exp. Dent. Res. 2022, 8, 680–689. [Google Scholar] [CrossRef]
- Pan, Y.H.; Lin, H.K.; Lin, J.C.-Y.; Hsu, Y.-S.; Wu, Y.-F.; Salamanca, E.; Chang, W.-J. Evaluation of the Peri-Implant Bone Level around Platform-Switched Dental Implants: A Retrospective 3-Year Radiographic Study. Int. J. Environ. Res. Public Heal. 2019, 16, 2570. [Google Scholar] [CrossRef] [PubMed]
- Spinato, S.; Stacchi, C.; Lombardi, T.; Bernardello, F.; Messina, M.; Zaffe, D. Biological width establishment around dental implants is influenced by abutment height irrespective of vertical mucosal thickness: A cluster randomized controlled trial. Clin. Oral Implant. Res. 2019, 30, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Galli, C.; Smerieri, A.; Macaluso, G.M.; Manfredi, E.; Ghiacci, G.; Toffoli, A.; Bonanini, M.; Lumetti, S. The Effect of Age, Gender, and Insertion Site on Marginal Bone Loss around Endosseous Implants: Results from a 3-Year Trial with Premium Implant System. BioMed Res. Int. 2014, 2014, 369051. [Google Scholar] [CrossRef]
- Yoo, S.-Y.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y. Clinical and radiographic evaluations of implants as surveyed crowns for Class I removable partial dentures: A retrospective study. J. Adv. Prosthodont. 2022, 14, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Tironi, F.; Orlando, F.; Azzola, F.; Corbella, S.; Francetti, L.A. A Retrospective Analysis on Marginal Bone Loss around Tilted and Axial Implants in Immediate-Loaded All-On-4 with a Long-Term Follow-Up Evaluation. Prosthesis 2022, 4, 15–23. [Google Scholar] [CrossRef]
- Aldahlawi, S.; Demeter, A.; Irinakis, T. The effect of implant placement torque on crestal bone remodeling after 1 year of loading. Clin. Cosmet. Investig. Dent. 2018, 10, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Júnior, J.A.; Treichel, T.L.E.; Prado, T.D.D.; Dedavid, B.A.; de Aza, P.N. Effects of insertion torque values on the marginal bone loss of dental implants installed in sheep mandibles. Sci. Rep. 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Berberi, A.N.; Sabbagh, J.M.; Aboushelib, M.N.; Noujeim, Z.F.; Salameh, Z.A. A 5-year comparison of marginal bone level following immediate loading of single-tooth implants placed in healed alveolar ridges and extraction sockets in the maxilla. Front. Physiol. 2014, 5, 29. [Google Scholar] [CrossRef]
- Vignoletti, F.; Sanz, M. Immediate implants at fresh extraction sockets: From myth to reality. Periodontol. 2000 2014, 66, 132–152. [Google Scholar] [CrossRef]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef]
- Ibrahim, A.; Chrcanovic, B.R. Dental Implants Inserted in Fresh Extraction Sockets versus Healed Sites: A Systematic Review and Meta-Analysis. Materials 2021, 14, 7903. [Google Scholar] [CrossRef]
- Araújo, M.G.; Wennström, J.L.; Lindhe, J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin. Oral Implant. Res. 2006, 17, 606–614. [Google Scholar] [CrossRef]
- Araújo, M.G.; Silva, C.; Souza, A.B.; Sukekava, F. Socket healing with and without immediate implant placement. Periodontology 2000 2019, 79, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Raes, F.; Lenzi, C.; Eccellente, T.; Ortolani, M.; Luongo, G.; Mangano, F. Immediate Loading of Single Implants: A 2-Year Prospective Multicenter Study. Int. J. Periodontics Restor. Dent. 2017, 37, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Reside, G.J.; Raes, F.; Garriga, J.S.; Tarrida, L.G.; Wiltfang, J.; Kern, M.; De Bruyn, H. Immediate Provisionalization of Dental Implants Placed in Healed Alveolar Ridges and Extraction Sockets: A 5-year Prospective Evaluation. Int. J. Oral Maxillofac. Implant. 2014, 29, 709–717. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Catena, A.; Pérez-Sayáns, M.; Fernández-Barbero, J.E.; O’Valle, F.; Padial-Molina, M. Early marginal bone loss around dental implants to define success in implant dentistry: A retrospective study. Clin. Implant. Dent. Relat. Res. 2022, 24, 630–642. [Google Scholar] [CrossRef]
- Bordin, D.; Cury, A.A.D.B.; Faot, F. Influence of Abutment Collar Height and Implant Length on Stress Distribution in Single Crowns. Braz Dent. J. 2019, 30, 238–243. [Google Scholar] [CrossRef]
- Pico, A.; Martín-Lancharro, P.; Caneiro, L.; Nóvoa, L.; Batalla, P.; Blanco, J. Influence of abutment height and implant depth position on interproximal peri-implant bone in sites with thin mucosa: A 1-year randomized clinical trial. Clin. Oral Implant. Res. 2019, 30, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ao, X.; Xie, P.; Jiang, F.; Chen, W. The biological width around implant. J. Prosthodont. Res. 2021, 65, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; Suárez, F.; Óvalle, F.; Spinato, S.; Catena, A. Prosthetic Abutment Height is a Key Factor in Peri-implant Marginal Bone Loss. J. Dent. Res. 2014, 93 (Suppl. S7), 80S–85S. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, Y.; Qiu, L.; Yu, H. Influence of buccal emergence profile designs on pe-ri-implant tissues: A randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2022, 24, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Soulami, S.; Slot, D.E.; van der Weijden, F. Implant-abutment emergence angle and profile in relation to peri-implantitis: A systematic review. Clin. Exp. Dent. Res. 2022, 8, 795–806. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Bercianos, M.; Aguerrondo, J.G.; Calvo-Guirado, J.L.; Prados-Frutos, J.C. Influence of Mucosal Thickness, Implant Dimensions and Stability in Cone Morse Implant Installed at Subcrestal Bone Level on the Peri-Implant Bone: A Prospective Clinical and Radiographic Study. Symmetry 2019, 11, 1138. [Google Scholar] [CrossRef]
- Nandal, S.; Shekhawat, H.; Ghalaut, P. A radiological evaluation of marginal bone around dental implants: An in-vivo study. Natl. J. Maxillofac. Surg. 2014, 5, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Orsi, I.A.; de Araujo, C.P.R. The influence of the connection, length and diameter of an implant on bone biomechanics. Acta Odontol. Scand. 2015, 73, 321–329. [Google Scholar] [CrossRef] [PubMed]
- lmayah, H.; Hassan, T.A.; Al-Jumaily, H.A.; Al-Ghurabi, Z.H. Evaluation of marginal bone loss around SLActive implants by CBCT using different implant dimensions and surgical approaches: A clinical and radiological prospective study. J. Osseointegr. 2021, 14, 21–25. [Google Scholar]
- Mumcu, E.; Bilhan, H.; Cekici, A. Marginal Bone Loss Around Implants Supporting Fixed Restorations. J. Oral Implant. 2011, 37, 549–558. [Google Scholar] [CrossRef]
- Desai, S.R.; Koulgikar, K.D.; Alqhtani, N.R.; Alqahtani, A.R.; Alqahtani, A.S.; Alenazi, A.; Heboyan, A.; Fernandes, G.V.O.; Mustafa, M. Three-Dimensional FEA Analysis of the Stress Distribution on Titanium and Graphene Frameworks Supported by 3 or 6-Implant Models. Biomimetics 2023, 8, 15. [Google Scholar] [CrossRef]


| Independent Variables (Respectively) | Mean (±SD) | Mean (±SD) | p-Value |
|---|---|---|---|
| Mesial vs. distal rMBL (in mm) | (n = 109) −0.67 (0.65) | (n = 109) −0.70 (0.63) | 0.5072 |
| Mesial rMBL: maxilla vs. mandible sites (in mm) | (n = 58) −0.53 (0.48) | (n = 51) −0.83 (0.77) | 0.0292 * |
| Distal rMBL: maxilla vs. mandible sites (in mm) | (n = 58) −0.57 (0.47) | (n = 51) −0.84 (0.75) | 0.0192 * |
| Mesial rMBL: healed vs. socket sites (in mm) | (n = 74) −0.71 (0.68) | (n = 35) −0.84 (0.46) | 0.1319 |
| Distal rMBL: healed vs. socket sites (in mm) | (n = 74) −0.70 (0.69) | (n = 35) −0.69 (0.48) | 0.7526 |
| Mesial rMBL: immediate provisionalization vs. no provisionalization (in mm) | (n = 83) −0.71 (0.67) | (n = 26) −0.80 (0.45) | 0.2224 |
| Distal rMBL: immediate provisionalization vs. no provisionalization (in mm) | (n = 83) −0.62 (0.51) | (n = 26) −0.73 (0.49) | 0.6650 |
| Mesial rMBL: women vs. men (in mm) | (n = 70) −0.65 (0.62) | (n = 39) −0.68 (0.64) | 0.5320 |
| Distal rMBL: women vs. men (in mm) | (n = 70) −0.69 (0.65) | (n = 39) −0.71 (0.66) | 0.3257 |
| TMh Abutment | Quantity | Mesial rMBL | Distal rMBL | t-Test p-Value |
|---|---|---|---|---|
| 1.5 mm | 31 (28.4%) | −1.13 ± 0.39 mm | −1.15 ± 0.43 mm | 0.9096 |
| 2.5 mm | 50 (45.9%) | −0.62 ± 0.61 mm | −0.66 ± 0.60 mm | 0.6649 |
| 3.5 mm | 28 (25.7%) | −0.25 ± 0.64 mm | −0.26 ± 0.65 mm | 0.3240 |
| ANOVA p-value | --- | <0.0001 * | <0.0001 * | --- |
| Implant Dimensions | Quantity | Mesial rMBL | Distal rMBL | t-Test p-Value |
|---|---|---|---|---|
| Ø 3.5 mm | 24 | −0.55 ± 0.46 mm | −0.64 ± 0.55 mm | 0.5619 |
| Ø 4.0 mm | 85 | −0.74 ± 0.53 mm | −0.58 ± 0.60 mm | 0.6420 |
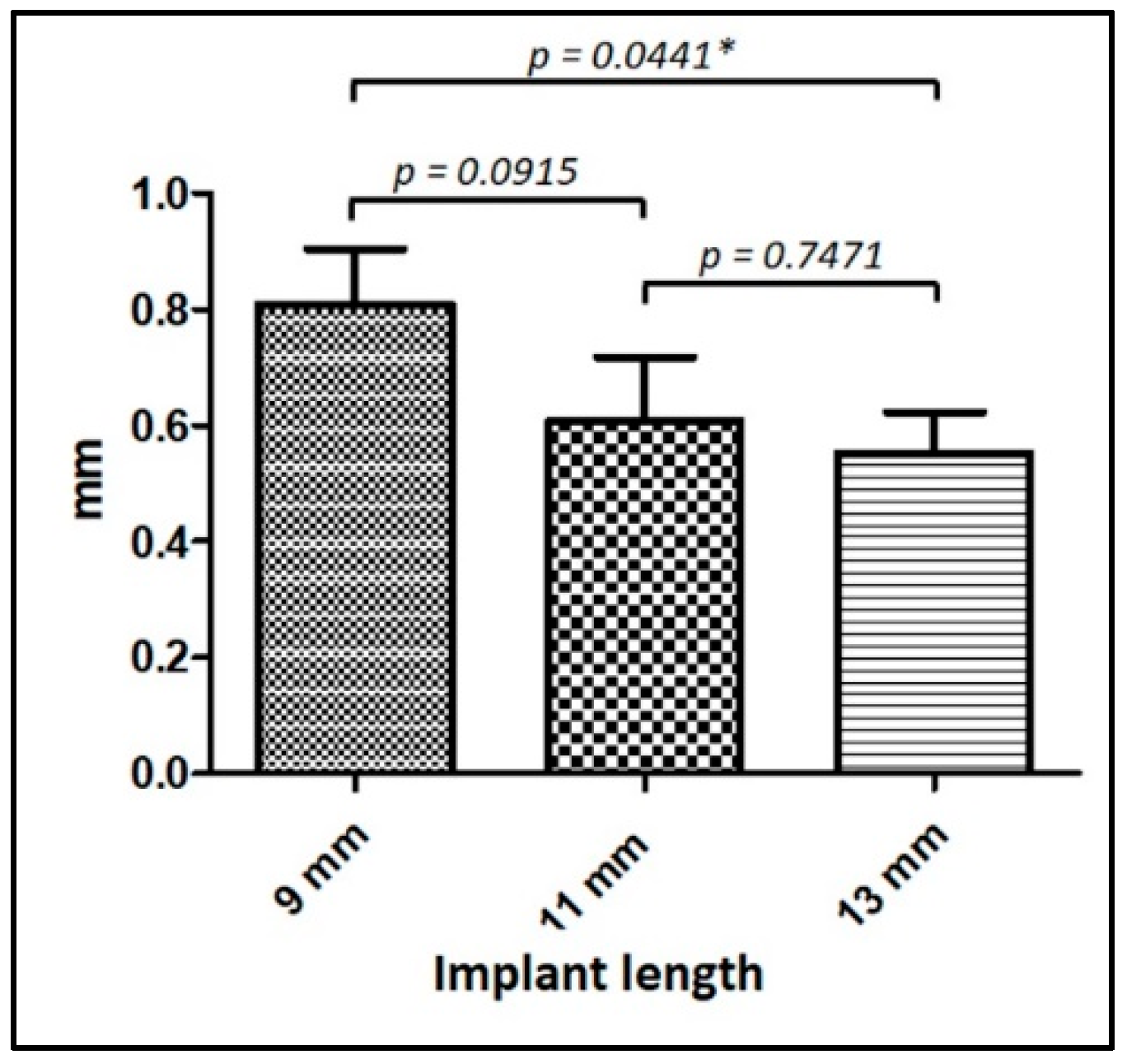
| L 9 mm | 51 | −0.79 ± 0.70 mm | −0.83 ± 0.74 mm | 0.6796 |
| L 11 mm | 25 | −0.66 ± 0.70 mm | −0.55 ± 0.50 mm | 0.8226 |
| L 13 mm | 33 | −0.48 ± 0.47 mm | −0.61 ± 0.49 mm | 0.3487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehrke, S.A.; Scarano, A.; Cortellari, G.C.; Fernandes, G.V.O.; Mesquita, A.M.M.; Bianchini, M.A. Marginal Bone Level and Biomechanical Behavior of Titanium-Indexed Abutment Base of Conical Connection Used for Single Ceramic Crowns on Morse-Taper Implant: A Clinical Retrospective Study. J. Funct. Biomater. 2023, 14, 128. https://doi.org/10.3390/jfb14030128
Gehrke SA, Scarano A, Cortellari GC, Fernandes GVO, Mesquita AMM, Bianchini MA. Marginal Bone Level and Biomechanical Behavior of Titanium-Indexed Abutment Base of Conical Connection Used for Single Ceramic Crowns on Morse-Taper Implant: A Clinical Retrospective Study. Journal of Functional Biomaterials. 2023; 14(3):128. https://doi.org/10.3390/jfb14030128
Chicago/Turabian StyleGehrke, Sergio Alexandre, Antonio Scarano, Guillermo Castro Cortellari, Gustavo Vicentis Oliveira Fernandes, Alfredo Mikail Melo Mesquita, and Marco Aurélio Bianchini. 2023. "Marginal Bone Level and Biomechanical Behavior of Titanium-Indexed Abutment Base of Conical Connection Used for Single Ceramic Crowns on Morse-Taper Implant: A Clinical Retrospective Study" Journal of Functional Biomaterials 14, no. 3: 128. https://doi.org/10.3390/jfb14030128
APA StyleGehrke, S. A., Scarano, A., Cortellari, G. C., Fernandes, G. V. O., Mesquita, A. M. M., & Bianchini, M. A. (2023). Marginal Bone Level and Biomechanical Behavior of Titanium-Indexed Abutment Base of Conical Connection Used for Single Ceramic Crowns on Morse-Taper Implant: A Clinical Retrospective Study. Journal of Functional Biomaterials, 14(3), 128. https://doi.org/10.3390/jfb14030128










