Combination of Enzymes with Materials to Give Them Antimicrobial Features: Modern Trends and Perspectives
Abstract
1. Introduction
2. Enzymes in Combinations with Other Enzymes, Antibiotics, Nanoparticles and Antimicrobial Peptides in the Content of Various Materials with Antimicrobial Properties
| Enzymes | Material | Antimicrobial Properties | Effect of Enzyme Presence on Microorganisms |
|---|---|---|---|
| Enzymes | |||
| Glucose oxidase (GOx) [16] | Cellulose beads | Growth inhibition of Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus | Oxidative cell damage by H2O2 |
| GOx [17] | Electrospun chitosan mats | Growth inhibition of E. coli and S. aureus | |
| GOx [18] | Polyester | Inhibition of S. epidermidis and E. coli growth | |
| GOx [19] | Poly(vinyl alcohol)/polycaprolactone multilayer system membrane | Inhibition of the E. coli growth | |
| GOx [20] | Chitosan with magnetic nanoparticle | Inhibition of proliferation of S. aureus suspended cells and biofilms | |
| α-Chymotrypsin [21] | Low-density polyethylene | Significant decrease in E. coli biofilm formation, reducing the number of adhered cells (up to 70.7%) and the matrix polysaccharide bio-volume (up to 80%) | Degradation of bacterial biofilms |
| Glycoside hydrolase [22] | Silica glass, polydimethylsiloxane and polystyrene | Significant reduce of surface attachment and P. aeruginosa biofilm formation (3-log reduction in surface associated bacteria) | Preventing of biofilm formation due to hydrolysis of poly-β-1,6-N-acetyl glucosamine |
| Glycoside hydrolase (Dispersin B) [23] | Fe3O4@SiO2 | 60% and 40% removal of S. aureus and Bacillus cereus biofilms; insignificant degradation of P. putida biofilms | Degradation of polysaccharides in the biofilms |
| Lysozyme [24] | Wool | Reduce the concentration of cells E. coli up to 95% | Destruction of bacterial cell wall |
| Lysozyme and tyrosinase [25] | Polyamide | Growth inhibition of Micrococcus lysodeikticus | |
| α-Amylase and alkaline pectinase [26] | Cotton | * MIC is 1156.3 μg/mL for S. aureus, 1156.3 μg/mL for S. epidermidis, 1156.3 μg/mL for E. coli, 18,500 μg/mL for P. aeruginosa and 4625 μg/mL for Candida albicans | |
| α-Amylase and lysozyme [27] | Polyethersulfone membrane | Decrease in formation of S. aureus and S. epidermidis biofilms | Prevention of attachment of microorganisms to the surface |
| Cellobiose dehydrogenase and deoxyribonuclease I [28] | Chitosan | Penetration through the matrix of polymicrobial biofilms of C. albicans and S. aureus and affect the embedded microbial cells | Disruption of the biofilm formation through degradation of extracellular DNA as a structural component of the formed biofilms |
| Dispersin B and endolysin SAL-1 [29] | Recombinant spider silk | Bacteriolytic effect and inhibition of S. aureus biofilm formation | Lysis of bacterial cells |
| Enzymes with antibiotics | |||
| Lysozyme in combination with nisin [30] | Nanocrystalline cellulose | Growth inhibition of Bacillus subtilis and S. aureus | Destruction of bacterial cell wall; reduction in inhibitory concentration compared to lysozyme and nisin in free forms |
| Alcalase in combination with ciprofloxacin [31] | Carbopol Aqua SF1 nanogel | 6-fold decrease in the biofilm mass and 3-log reduction in bacterial cells: S. aureus, Pseudomonas aeruginosa, S. epidermidis, Klebsiella pneumoniae, E. coli, Enterococcus faecalis | Degradation of bacterial biofilms and boosting of antibiotic action |
| Alginate lyase in combination with ciprofloxacin [32] | Chitosan | Significant reduction in P. aeruginosa biofilm aggregation; MIC is 0.125 μg/mL | |
| Alginate lyase in combination with ceftazidime or amikacin [33] | Inhibition of P. aeruginosa biofilm formation; MIC is 64 mg/mL | ||
| Ficin with gentamicin, ciprofloxacin or benzalkonium chloride [34] | 3-log reduction in S. aureus cell concentration | ||
| Enzymes with polyphenols | |||
| Laccase with poly(catechol) and poly- (p-phenylenediamine) [35] | Cotton, wool, and polyethylene terephthalate | 10-100 fold decrease in both E. coli and S. aureus cell concentration | Catechol and p-phenylenediamine polymerization |
| Enzymes with antimicrobial peptides | |||
| Laccase with KLWWMIRRWG- bromophenylalanine-3,4-dihydroxyphenylalanine-G and KLWWMIRRWG- bromophenylalanine-G [36] | Polystyrene | Inhibition of E. coli growth; MIC is 100 μg/mL | Increasing amounts of functional groups for immobilization of antimicrobial peptides |
| His6-OPH–polyelectrolyte complexes (polyglutamic acid (PLE50) with polymyxins [37] | Fibrous materials | Complex of polymyxin B with His6-OPH decreases the viability of both B. subtilis and E. coli cells | Hydrolysis of QS-signaling molecules and boosting of antibiotic action |
| Thermolysin in combination with polymyxin B [38] | Bacterial cellulose | Inhibition of Pseudomonas sp. growth | Thermolysin modified polymyxin confirmation and improved its antibacterial action |
| Enzymes with metal nanoparticles | |||
| α-Amylase [39] | Ag–enzyme nanoaggregates | 5.4 and 6.1 log reduction in S. aureus and E. coli cell concentration, respectively; 80% removal of cell biofilms | Degradation of the polysaccharides in biofilms and reducing cell attachment |
| α-Amylase [40] | Ag-nanoparticles | Significant inhibition of Klebsiella pneumoniae and S. aureus biofilm formation | Boosting of Ag-nanoparticles’ antibacterial action |
| His6-OPH–polyelectrolyte complexes (PEGylated derivatives of polyglutamic acid, PEG-PLE50) with Zn or Ta nanoparticles [37] | Fibrous materials (70% viscose and 30% polyester); activated carbon layer between polyester nonwoven fabrics (30% cotton and 70% meta polyaramide); fiber covered by poly(vinylidene difluoride)-co-poly(tetrafluoro- ethylene) membrane | Gradually decreasing of concentrations of viable B. subtilis and E. coli cells | Ta nanoparticles in combination with His6-OPH significantly increased the rate of cell death |
| His6-OPH/PLE50 [41] with Ta nanoparticles | Bacterial cellulose or fibrous materials (70% viscose and 30% polyester) modified by poly(4-hydroxybutyrate | Bacterial death (B. subtilis, E. coli), especially in the case of E. coli cells (up to 9-fold) | Synergetic effects of His6-OPH with Ta nanoparticles result in up to 4-fold harder elimination of bacterial cells |
2.1. Oxidoreductases in the Functionalized Materials with Antimicrobial Activity
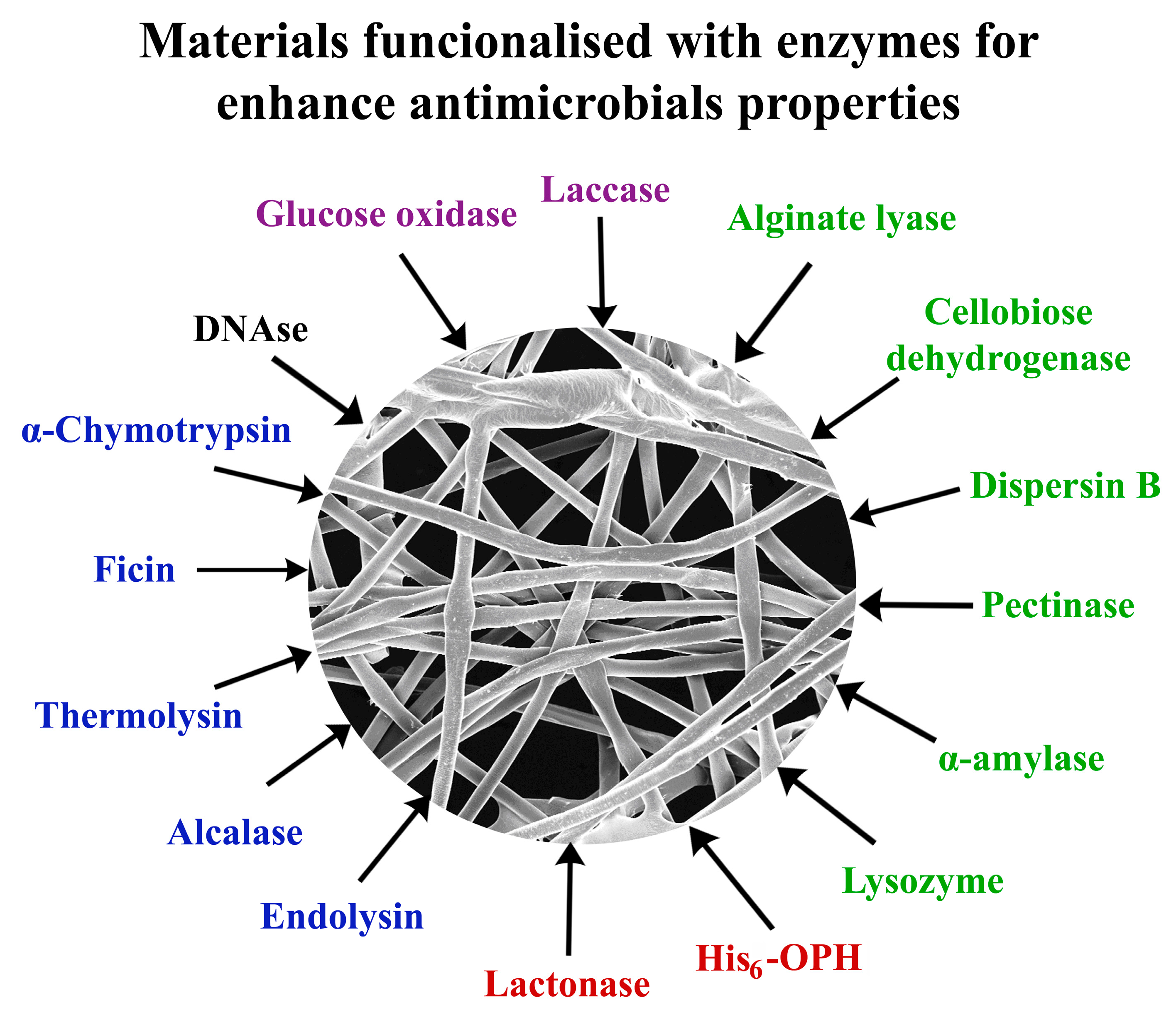
2.2. Carbohydrases in the Functionalized Materials with Antimicrobial Activity
2.2.1. Lysozyme
2.2.2. α-Amylase and Pectinase
2.2.3. Dispersin B and Alginate Lyase
2.3. Proteolytic Enzymes in the Functionalized Materials with Antimicrobial Activity
2.3.1. α-Chymotrypsin and Ficin
2.3.2. Endolysins
2.4. Quorum Quenching Enzymes in the Functionalized Materials with Antimicrobial Activity
| AMP | With or w/o Bacterial Cellulose | w/o His6-OPH | With His6-OPH | Decrease (Times) |
|---|---|---|---|---|
| Pseudomonas sp. | ||||
| Bacitacin | – | 5.27 ± 0.31 | 1.89 ± 0.06 | 2.79 |
| Indolicidin | – | 37.6 ± 1.9 | 0.24 ± 0.03 | 156.67 |
| Temporin A | – | 9.4 ± 0.7 | 0.41 ± 0.05 | 22.93 |
| Colistin | + | 0.92 ± 0.05 | 0.03 ± 0.004 | 30.67 |
| Polymyxin B | + | 0.87 ± 0.01 | 0.23 ± 0.03 | 3.78 |
| Indolicidin | + | 1.39 ± 0.03 | 0.52 ± 0.05 | 2.67 |
| Temporin | + | 0.45 ± 0.01 | 0.24 ± 0.004 | 1.87 |
| Bacillus subtilis | ||||
| Bacitacin | – | 0.02 ± 0.001 | 0.02 ± 0.002 | 1 |
| Indolicidin | – | 4.66 ± 0.61 | 2.62 ± 0.22 | 1.78 |
| Temporin A | – | 2.15 ± 0.31 | 0.60 ± 0.13 | 3.58 |
| Colistin | + | 1.23 ± 0.04 | 0.28 ± 0.006 | 4.39 |
| Polymyxin B | + | 1.03 ± 0.03 | 0.08 ± 0.002 | 12.87 |
| Indolicidin | + | 13.3 ± 0.81 | 2.45 ± 0.05 | 5.43 |
| Temporin A | + | 1.58 ± 0.01 | 1.06 ± 0.002 | 1.49 |
3. Metal–Organic Frameworks Functionalized by Enzymes as Antimicrobials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered nanomaterials for antimicrobial applications: A review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-free antibacterial strategies enabled by nanomaterials: Progress and perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface design for antibacterial materials: From fundamentals to advanced strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar]
- Sheard, D.E.; O’Brien-Simpson, N.M.; Wade, J.D.; Separovic, F. Combating bacterial resistance by combination of antibiotics with antimicrobial peptides. Pure Appl. Chem. 2019, 91, 199–209. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Fanaei Pirlar, R.; Emaneini, M.; Beigverdi, R.; Banar, M.B.; van Leeuwen, W.; Jabalameli, F. Combinatorial effects of antibiotics and enzymes against dual-species Staphylococcus aureus and Pseudomonas aeruginosa biofilms in the wound-like medium. PLoS ONE 2020, 15, e0235093. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dey, A.; Sarkar, T.; Ray, R.R.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M.; Simal-Gandara, J. Immobilized enzymes as potent antibiofilm agent. Biotechnol. Prog. 2022, 38, e3281. [Google Scholar] [CrossRef]
- Leonarta, F.; Lee, C.K. Nanofibrous membrane with encapsulated glucose oxidase for self-sustained antimicrobial applications. Membranes 2021, 11, 997. [Google Scholar] [CrossRef]
- Liu, Q.; Xun, G.; Feng, Y. The state-of-the-art strategies of protein engineering for enzyme stabilization. Biotechnol. Adv. 2019, 37, 530–537. [Google Scholar] [CrossRef]
- Qing, R.; Hao, S.; Smorodina, E.; Jin, D.; Zalevsky, A.; Zhang, S. Protein Design: From the Aspect of Water Solubility and Stability. Chem. Rev. 2022, 122, 14085–14179. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, M.; Valle, F.; Cantelli, A.; Bottoni, A.; Zerbetto, F.; Fasoli, E.; Calvaresi, M. Identification and preparation of stable water dispersions of protein—Carbon nanotube hybrids and efficient design of new functional materials. Carbon 2019, 147, 70–82. [Google Scholar] [CrossRef]
- Lyagin, I.V.; Efremenko, E.N. Biomolecular engineering of biocatalysts hydrolyzing neurotoxic organophosphates. Biochimie 2018, 144, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.V.; Efremenko, E.N.; Varfolomeev, S.D. Enzymatic biosensors for determination of pesticides. Russ. Chem. Rev. 2017, 86, 339–355. [Google Scholar] [CrossRef]
- Califano, D.; Patenall, B.L.; Kadowaki, M.A.; Mattia, D.; Scott, J.L.; Edler, K.J. Enzyme-functionalized cellulose beads as a promising antimicrobial material. Biomacromolecules 2021, 22, 754–762. [Google Scholar] [CrossRef]
- Bösiger, P.; Tegl, G.; Richard, I.M.; Le Gat, L.; Huber, L.; Stagl, V.; Mensah, A.; Guebitz, G.M.; Rossi, R.M.; Fortunato, G. Enzyme functionalized electrospun chitosan mats for antimicrobial treatment. Carbohydr. Polym. 2018, 181, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface modification of polyester fabric using plasma-dendrimer for robust immobilization of glucose oxidase enzyme. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Alonso-González, M.; Corral-González, A.; Felix, M.; Romero, A.; Martin-Alfonso, J.E. Developing active poly (vinyl alcohol)-based membranes with encapsulated antimicrobial enzymes via electrospinning for food packaging. Int. J. Biol. Macromol. 2020, 162, 913–921. [Google Scholar] [CrossRef]
- Yeon, K.M.; You, J.; Adhikari, M.D.; Hong, S.G.; Lee, I.; Kim, H.S.; Kim, L.N.; Nam, J.; Kwon, S.J.; Kim, M.I.; et al. Enzyme-immobilized chitosan nanoparticles as environmentally friendly and highly effective antimicrobial agents. Biomacromolecules 2019, 20, 2477–2485. [Google Scholar] [CrossRef]
- Cattò, C.; Secundo, F.; James, G.; Villa, F.; Cappitelli, F. α-Chymotrypsin immobilized on a low-density polyethylene surface successfully weakens Escherichia coli biofilm formation. Int. J. Mol. Sci. 2018, 19, 4003. [Google Scholar] [CrossRef] [PubMed]
- Asker, D.; Awad, T.S.; Baker, P.; Howell, P.L.; Hatton, B.D. Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials 2018, 167, 168–176. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Zeng, K.; Xia, Y.; Xu, W.; Wang, R.; Guo, J.; Xie, H. Functional immobilization of a biofilm-releasing glycoside hydrolase dispersin B on magnetic nanoparticles. Appl. Biochem. Biotechnol. 2021, 194, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, N.; Wang, Q.; Wang, P.; Yu, Y. Development of an eco-friendly antibacterial textile: Lysozyme immobilization on wool fabric. Bioprocess Biosyst. Eng. 2020, 43, 1639–1648. [Google Scholar] [CrossRef]
- Biswas, T.T.; Yu, J.; Nierstrasz, V.A. Sequential inkjet printing of lysozyme and tyrosinase on polyamide fabric: Sustainable enzyme binding on textile surface. Adv. Mater. Interfaces 2022, 9, 2200723. [Google Scholar] [CrossRef]
- Coradi, M.; Zanetti, M.; Valério, A.; de Oliveira, D.; da Silva, A.; Ulson, S.M.D.A.G.; de Souza, A.A.U. Production of antimicrobial textiles by cotton fabric functionalization and pectinolytic enzyme immobilization. Mater. Chem. Phys. 2018, 208, 28–34. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Taheri-Kafrani, A.; Asadnia, M.; Razmjou, A. Bienzymatic modification of polymeric membranes to mitigate biofouling. Sep. Purif. Technol. 2020, 237, 116464. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Ludwig, R.; Schneider-Stickler, B. Co-immobilization of cellobiose dehydrogenase and deoxyribonuclease I on chitosan nanoparticles against fungal/bacterial polymicrobial biofilms targeting both biofilm matrix and microorganisms. Mater. Sci. Eng. C 2020, 108, 110499. [Google Scholar] [CrossRef]
- Seijsing, F.; Nilebäck, L.; Öhman, O.; Pasupuleti, R.; Ståhl, C.; Seijsing, J.; Hedhammar, M. Recombinant spider silk coatings functionalized with enzymes targeting bacteria and biofilms. MicrobiologyOpen 2020, 9, e993. [Google Scholar] [CrossRef]
- Tavakolian, M.; Okshevsky, M.; van de Ven, T.G.; Tufenkji, N. Developing antibacterial nanocrystalline cellulose using natural antibacterial agents. ACS Appl. Mater. Interfaces 2018, 10, 33827–33838. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Enhanced clearing of wound-related pathogenic bacterial biofilms using protease-functionalized antibiotic nanocarriers. ACS Appl. Mater. Interfaces 2019, 11, 43902–43919. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Tripathi, M.; Pandey, N.; Agrawal, A.K.; Gade, S.; Anjum, M.M.; Tilak, R.; Sing, S. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Int. J. Pharm. 2019, 563, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Li, X.; Lee, B.S.; Jung, S.; Lee, M.-S. Enhancing the thermo-stability and anti-biofilm activity of alginate lyase by immobilization on low molecular weight chitosan nanoparticles. Int. J. Mol. Sci. 2019, 20, 4565. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasileva, O.S.; Akhmetov, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-biofilm and wound-healing activity of chitosan-immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef]
- Su, J.; Noro, J.; Silva, S.; Fu, J.; Wang, Q.; Ribeiro, A.; Silva, C.; Cavaco-Paulo, A. Antimicrobial coating of textiles by laccase in situ polymerization of catechol and p-phenylenediamine. React. Funct. Polym. 2019, 136, 25–33. [Google Scholar] [CrossRef]
- Corrales-Ureña, Y.R.; Souza-Schiaber, Z.; Lisboa-Filho, P.N.; Marquenet, F.; Noeske, P.L.M.; Gätjen, L.; Rischka, K. Functionalization of hydrophobic surfaces with antimicrobial peptides immobilized on a bio-interfactant layer. RSC Adv. 2020, 10, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Stepanov, N.; Frolov, G.; Efremenko, E. Combined modification of fiber materials by enzymes and metal nanoparticles for chemical and biological protection. Int. J. Mol. Sci. 2022, 23, 1359. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Stepanov, N.; Presnov, D.; Efremenko, E. Bacterial cellulose containing combinations of antimicrobial peptides with various QQ enzymes as a prototype of an “enhanced antibacterial” dressing: In silico and in vitro data. Pharmaceutics 2020, 12, 1155. [Google Scholar] [CrossRef]
- Ferreres, G.; Bassegoda, A.; Hoyo, J.; Torrent-Burgues, J.; Tzanov, T. Metal–enzyme nanoaggregates eradicate both gram-positive and gram-negative bacteria and their biofilms. ACS Appl. Mater. Interfaces 2018, 10, 40434–40442. [Google Scholar] [CrossRef]
- Abeleda, H.E.P.; Javier, A.P.; Murillo, A.Q.M.; Baculi, R.Q. Alpha-amylase conjugated biogenic silver nanoparticles as innovative strategy against biofilm-forming multidrug resistant bacteria. Biocatal. Agric. Biotechnol. 2020, 29, 101784. [Google Scholar] [CrossRef]
- Lyagin, I.; Maslova, O.; Stepanov, N.; Presnov, D.; Efremenko, E. Assessment of composite with fibers as a support for antibacterial nanomaterials: A case study of bacterial cellulose, polylactide and usual textile. Fibers 2022, 10, 70. [Google Scholar] [CrossRef]
- Yuan, M.; Ning, C.; Yang, S.; Liang, Q.; Mou, H.; Liu, Z. A new cold-active glucose oxidase from Penicillium: High-level expression and application in fish preservation. Front. Microbiol. 2020, 11, 606007. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Soltani Fard, E.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Angaheh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2022, 69, 939–950. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.; Zhang, M.; Zhou, S.; Cui, P.; Hu, H.; Jiang, P.; Wang, C.; Qui, L.; Wang, J. Glucose oxidase loaded thermosensitive hydrogel as an antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2022, 76, 103791. [Google Scholar] [CrossRef]
- Ji, Y.; Han, Z.; Ding, H.; Xu, X.; Wang, D.; Zhu, Y.; An, F.; Tang, S.; Zhang, H.; Deng, J.; et al. Enhanced eradication of bacterial/fungi biofilms by glucose oxidase-modified magnetic nanoparticles as a potential treatment for persistent endodontic infections. ACS Appl. Mater. Interfaces 2021, 13, 17289–17299. [Google Scholar] [CrossRef] [PubMed]
- Bayazidi, P.; Almasi, H.; Asl, A.K. Immobilization of lysozyme on bacterial cellulose nanofibers: Characteristics, antimicrobial activity and morphological properties. Int. J. Biol. Macromol. 2018, 107, 2544–2551. [Google Scholar] [CrossRef]
- Moshtaghi, H.; Rashidimehr, A.; Shareghi, B. Antimicrobial activity of nisin and lysozyme on foodborne pathogens Listeria monocytogenes, Staphylococcus aureus, Salmonella typhimurium, and Escherichia coli at different pH. J. Food Nutr. Res. 2018, 3, 193–201. [Google Scholar] [CrossRef]
- Silva, N.H.; Garrido-Pascual, P.; Moreirinha, C.; Almeida, A.; Palomares, T.; Alonso-Varona, A.; Vileva, C.; Freire, C.S. Multifunctional nanofibrous patches composed of nanocellulose and lysozyme nanofibers for cutaneous wound healing. Int. J. Biol. Macromol. 2020, 165, 1198–1210. [Google Scholar] [CrossRef]
- Diken Gür, S.; Bakhshpour, M.; Bereli, N.; Denizli, A. Antibacterial effect against both Gram-positive and Gram-negative bacteria via lysozyme imprinted cryogel membranes. J. Biomater. Sci. Polym. Ed. 2021, 32, 1024–1039. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in synthesis of microbial polysaccharides: Properties and development trends. Catalysts 2022, 12, 1377. [Google Scholar] [CrossRef]
- Długosz, O.; Matysik, J.; Matyjasik, W.; Banach, M. Catalytic and antimicrobial properties of α-amylase immobilised on the surface of metal oxide nanoparticles. J. Clust. Sci. 2021, 32, 1609–1622. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sarkar, T.; Dutta, B.; Ray, R.R. Antibiofilm activity of α-amylase from Bacillus subtilis and prediction of the optimized conditions for biofilm removal by response surface methodology (RSM) and artificial neural network (ANN). Appl. Biochem. Biotechnol. 2021, 193, 1853–1872. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.C.; Kim, M.; Sung, J.S.; Kadam, A.A. Efficient biofilms eradication by enzymatic-cocktail of pancreatic protease type-I and bacterial α-amylase. Polymers 2020, 12, 3032. [Google Scholar] [CrossRef] [PubMed]
- Muslim, D.S.N.; Hasan, A.M.; Mahdi, D.N.Z. Antibiofilm and antiadhesive properties of pectinase purified from Pseudomonas stutzeri isolated from spoilt orange. Adv. Environ. Biol. 2016, 10, 91. [Google Scholar]
- Hu, J.; Zhang, C.; Zhou, L.; Hu, Q.; Kong, Y.; Song, D.; Cheng, Y.; Zhang, Y. A smart hydrogel for on-demand delivery of antibiotics and efficient eradication of biofilms. Sci. China Mater. 2021, 64, 1035–1046. [Google Scholar] [CrossRef]
- Moreno-Couranjou, M.; Mauchauffé, R.; Bonot, S.; Detrembleur, C.; Choquet, P. Anti-biofouling and antibacterial surfaces via a multicomponent coating deposited from an up-scalable atmospheric-pressure plasma-assisted CVD process. J Mater. Chem. B 2018, 6, 614–623. [Google Scholar] [CrossRef]
- Nileback, L.; Widhe, M.; Seijsing, J.; Bysell, H.; Sharma, P.K.; Hedhammar, M. Bioactive silk coatings reduce the adhesion of Staphylococcus aureus while supporting growth of osteoblast-like cells. ACS Appl. Mater. Interfaces 2019, 11, 24999–25007. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Paetzold, B.; Ferrar, T.; Mazzolini, R.; Torrents, E.; Serrano, L.; Lluch-Senar, M. Characterization of different alginate lyases for dissolving Pseudomonas aeruginosa biofilms. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, W.; Zhang, M.; Zhang, L.; Shen, Y.; Huang, S.; Li, M.; Pan, Y.; Zhou, L.; Zhang, K. The inhibitory effects of ficin on Streptococcus mutans biofilm formation. Biomed Res. Int. 2021, 2021, 6692328. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D. Effect of dalbavancin on Staphylococcal biofilms when administered alone or in combination with biofilm-detaching compounds. Front. Microbiol. 2020, 11, 553. [Google Scholar] [CrossRef]
- Love, M.J.; Abeysekera, G.S.; Muscroft-Taylor, A.C.; Billington, C.; Dobson, R.C. On the catalytic mechanism of bacteriophage endolysins: Opportunities for engineering. Biochim. Biophys. Acta—Proteins Proteom. 2020, 1868, 140302. [Google Scholar] [CrossRef] [PubMed]
- Baliga, P.; Goolappa, P.T.; Shekar, M.; Kallappa, G.S. Cloning, characterization, and antibacterial properties of endolysin LysE against planktonic cells and biofilms of Aeromonas hydrophila. Probiotics Antimicrob. Proteins 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kour, A.; Panda, J.J.; Harjai, K.; Chhibber, S. Exploring endolysin-loaded alginate-chitosan nanoparticles as future remedy for Staphylococcal Infections. AAPS PharmSciTech 2020, 21, 233. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Wang, J.; Zhao, Y.; Zhong, Q.; Li, G.; Fu, Z.; Lu, S. Phage endolysin LysP108 showed promising antibacterial potential against methicillin-resistant Staphylococcus aureus. Front. Cell Infect. Microbiol. 2021, 11, 668430. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Gurevich, D.; Dor, S.; Erov, M.; Dan, Y.; Moy, J.C.; Mairesse, O.; Dafny-Yelin, M.; Adler-Abramovich, L.; Afriat-Jurnou, L. Directed enzyme evolution and encapsulation in peptide nanospheres of quorum quenching lactonase as an antibacterial treatment against plant pathogen. ACS Appl. Mater. Interfaces 2021, 13, 2179–2188. [Google Scholar] [CrossRef]
- Sakr, M.M.; Elkhatib, W.F.; Aboshanab, K.M.; Mantawy, E.M.; Yassien, M.A.; Hassouna, N.A. In vivo evaluation of a recombinant N-acylhomoserine lactonase formulated in a hydrogel using a murine model infected with MDR Pseudomonas aeruginosa clinical isolate, CCASUP2. AMB Express 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Gupta, K.; Chhibber, S. Biofunctionalization of silver nanoparticles with lactonase leads to altered antimicrobial and cytotoxic properties. Front. Mol. Biosci. 2019, 6, 63. [Google Scholar] [CrossRef]
- Dor, S.; Prusky, D.; Afriat-Jurnou, L. Bacterial quorum-quenching lactonase hydrolyzes fungal mycotoxin and reduces pathogenicity of penicillium expansum—Suggesting a mechanism of bacterial antagonism. J. Fungus 2021, 7, 826. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, P.; Wu, J.; Yan, W.; Xie, S.; Sun, X.; Ye, B.-C.; Chu, X. N-acylhomoserine lactonase-based hybrid nanoflowers: A novel and practical strategy to control plant bacterial diseases. J. Nanobiotechnol. 2022, 20, 347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Xuan, C.G.; Lu, C.H.; Guo, S.; Yu, J.F.; Asif, M.; Jiang, W.J.; Zhou, Z.G.; Luo, Z.Q.; Zhang, L.Q. AidB, a novel thermostable N-acylhomoserine lactonase from the bacterium Bosea sp. Appl. Environ. Microbiol. 2019, 85, e02065-19. [Google Scholar] [CrossRef] [PubMed]
- Sirotkina, M.; Efremenko, E.N. Rhodococcus lactonase with organophosphate hydrolase (OPH) activity and His6-tagged OPH with lactonase activity: Evolutionary proximity of the enzymes and new possibilities in their application. Appl. Microbiol. Biotechnol. 2014, 98, 2647–2656. [Google Scholar] [CrossRef]
- Maslova, O.; Aslanli, A.; Stepanov, N.; Lyagin, I.; Efremenko, E. Catalytic characteristics of new antibacterials based on hexahistidine-containing organophosphorus hydrolase. Catalysts 2017, 7, 271. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Novel approach to Quorum Quenching: Rational design of antibacterials in combination with hexahistidine-tagged organophosphorus hydrolase. Biol. Chem. 2018, 399, 869–879. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Charges’ interaction in polyelectrolyte (nano)complexing of His6-oph with peptides: Unpredictable results due to imperfect or useless concept? Int. J. Biol. Macromol. 2019, 140, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. “Universal” antimicrobial combination of bacitracin and His6-OPH with lactonase activity, acting against various bacterial and yeast cells. Int. J. Mol. Sci. 2022, 23, 9400. [Google Scholar] [CrossRef]
- Aslanli, A.; Stepanov, N.; Razheva, T.; Podorozhko, E.A.; Lyagin, I.; Lozinsky, V.I.; Efremenko, E.N. Enzymatically functionalized composite materials based on nanocellulose and poly(vinylalcohol) cryogel and possessing antimicrobial activity. Materials 2019, 12, 3619. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC—Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–organic frameworks for biomedical applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal–organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef]
- Li, T.; Qiu, H.; Liu, N.; Li, J.; Bao, Y.; Tong, W. Construction of self-activated cascade metal− organic framework/enzyme hybrid nanoreactors as antibacterial agents. Colloids Surf. B Biointerfaces 2020, 191, 111001. [Google Scholar] [CrossRef]
- Qin, J.; Feng, Y.; Cheng, D.; Liu, B.; Wang, Z.; Zhao, Y.; Wei, J. Construction of a mesoporous ceria hollow sphere/enzyme nanoreactor for enhanced cascade catalytic antibacterial therapy. ACS Appl. Mater. Interfaces 2021, 13, 40302–40314. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Chen, Y.L.; Feng, P.F.; Wang, C.C.; Li, X.K.; Liu, L.L.; Tang, Y. Hierarchically porous MOF-based microneedles for glucose-responsive infected diabetic wound treatment. Mater. Chem. Front. 2022, 6, 680–688. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, S.; Liu, Y.; Meng, J.; Wang, M.; Sun, Y.; Xia, L.; He, Z.; Hu, W.; Ren, L.; et al. Antibacterial cascade catalytic glutathione-depleting MOF nanoreactors. ACS Appl. Mater. Interfaces 2022, 14, 11104–11115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, Z.; Zhang, Y.; Liu, Z.; Sun, Y.; Ren, J.; Qu, X. Two-dimensional metal–organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano 2019, 13, 5222–5230. [Google Scholar] [CrossRef]
- Peng, L.; Yang, X.; Wang, S.; Chan, Y.K.; Chen, Y.; Yang, Z.; Mao, Y.; Li, L.; Yang, W.; Deng, Y. Bimetal metal–organic framework domino micro-reactor for synergistic antibacterial starvation/chemodynamic therapy and robust wound healing. Nanoscale 2022, 14, 2052–2064. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Y.; Liu, T.; Wang, W.; Zhao, Y.; Sun, Y.; Zhang, Y.; Qin, W.; Chen, Z.; Lu, C.; et al. A triple-enhanced chemodynamic approach based on glucose-powered hybrid nanoreactors for effective bacteria killing. Nano Res. 2022, 1–13. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Li, Y.; Dong, Y.; Meng, J.; Zhang, S.; Xia, L.; He, Z.; Ren, L.; Chen, Z.; et al. Triple-synergistic MOF-nanozyme for efficient antibacterial treatment. Bioact. Mater. 2022, 17, 289–299. [Google Scholar] [CrossRef]
- Wang, P.; Peng, L.; Lin, J.; Li, Y.; Luo, Q.; Jiang, S.; Tian, H.; Zhang, Y.; Liu, X.; Liu, J. Enzyme hybrid virus-like hollow mesoporous CuO adhesive hydrogel spray through glucose-activated cascade reaction to efficiently promote diabetic wound healing. Chem. Eng. J. 2021, 415, 128901. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, S.; Liu, H.; Chen, H.; Zhou, J.; Chen, Z.; Zhou, X.; Xie, Z.; Kuang, Q.; Zheng, L. Biomimetic metal–organic framework composite-mediated cascade catalysis for synergistic bacteria killing. ACS Appl. Mater. Interfaces 2020, 12, 36996–37005. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, D.Y.; Liu, Y.; Huang, F.; Tian, S.; Ren, Y.; Liu, J.; An, Y.; van der Mei, H.C.; Busscher, H.J.; et al. In-biofilm generation of nitric oxide using a magnetically-targetable cascade-reaction container for eradication of infectious biofilms. Bioact. Mater. 2022, 14, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Nong, W.; Chen, Y.; Lv, D.; Yan, Y.; Zheng, X.; Shi, X.; Xu, Z.; Guan, W.; Wu, J.; Guan, Y. Metal-organic framework based nanozyme hybrid for synergistic bacterial eradication by lysozyme and light-triggered carvacrol release. Chem. Eng. J. 2022, 431, 134003. [Google Scholar] [CrossRef]
- Lee, I.; Cheon, H.J.; Adhikari, M.D.; Tran, T.D.; Yeon, K.M.; Kim, M.I.; Kim, J. Glucose oxidase-copper hybrid nanoflowers embedded with magnetic nanoparticles as an effective antibacterial agent. Int. J. Biol. Macromol. 2020, 155, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Gascón Pérez, V.P.; Sánchez-Sánchez, M. Environmentally friendly enzyme immobilization on MOF materials. Methods Mol Biol. 2020, 2100, 271–296. [Google Scholar] [CrossRef]
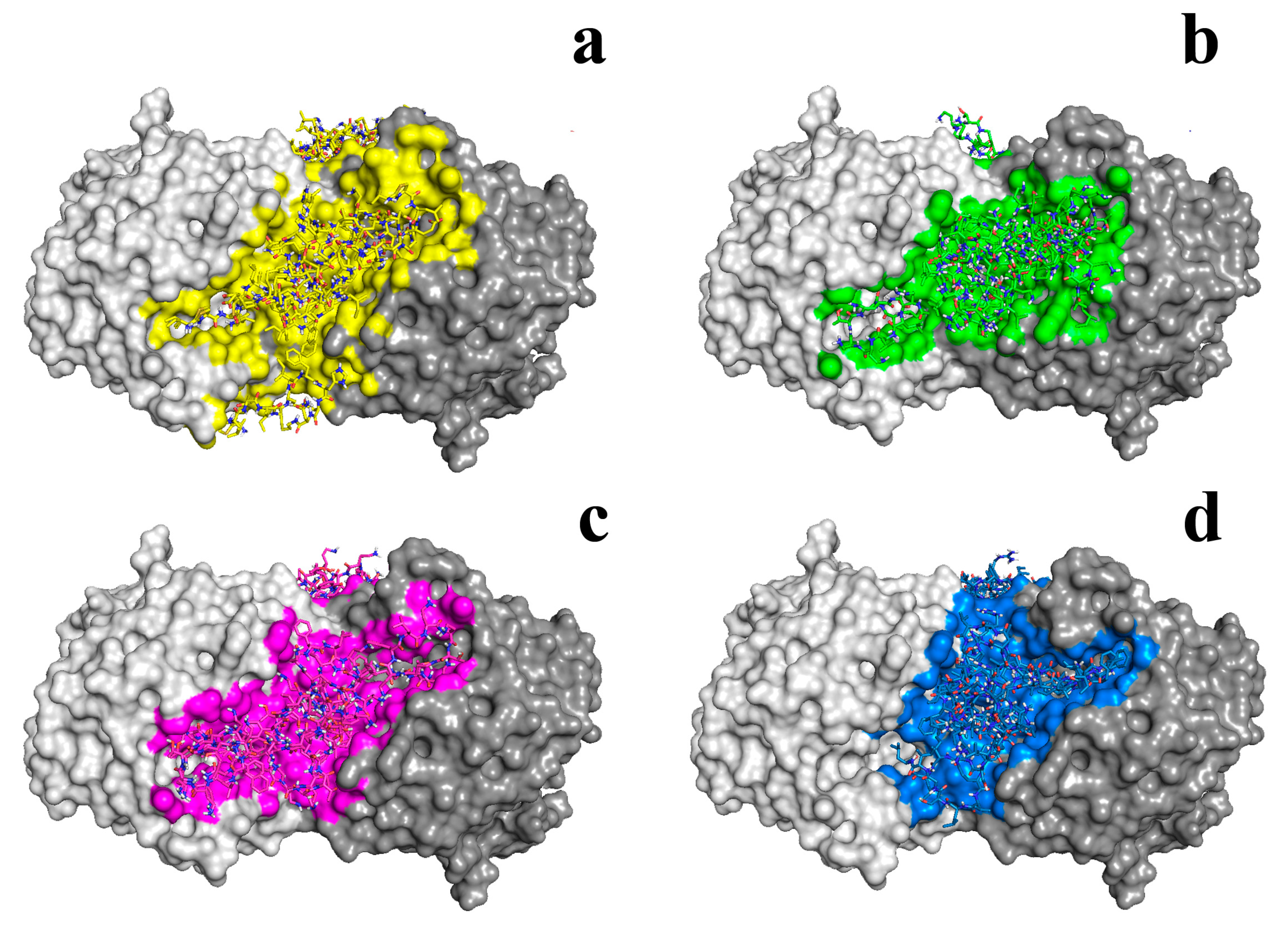
| MOF [Reference] | Antibacterial Properties | Effect of Action |
|---|---|---|
| MIL@GOx-MIL NRs (NH2-BDC and FeCl3·6H2O) [83] | 5μg/mL MIL@GOx-MIL inhibits S. aureus growth. No biofilms formation was revealed at 80 µg/mL | The enzyme catalyzed conversion of glucose to gluconic acid reducing pH from 7.4 to 4 and MIL NRs continually produced H2O2 and toxic hydroxyl radicals (HO-) |
| GOx in mesoporous CeO2 hollow sphere [84] | 100 μg/mL MOF efficiently eliminate 99.9% bacteria in the wound tissues (E. coli and S. aureus) | Production of highly toxic HO- radicals via a cascade catalytic reaction; Gluconic acid decreased the pH value that boosting the peroxidase-like catalytic performance of mesoporous CeO2 |
| MOF (GOx@Fe-ZIF-TA) Fe-doped zeolitic imidazolate framework (ZIF) etching by tannic acid [85] | Complete inhibition of E. coli growth was observed | Transformation of surplus glucose to gluconic acid and H2O2, which was transferred by Fe(II) to antibacterial OH− in infected diabetic wounds |
| MnFe2O4@MIL/Au&GOx [86] | Inhibition of bacterial growth; MIC values for E. coli and S. aureus were 125 and 31.2 μg/mL, respectively | Continuous conversion of glucose into gluconic acid and H2O2. MnFe2O4@MIL/Au demonstrated increased peroxidase (POD)-like activity and catalyzed transformation of H2O2 to large amounts of toxic-reactive oxygen species |
| GOx in 2D Cu-TCPP(Fe) [87] | Inhibition of bacterial growth rates were 88% and 90% for E. coli and S. aureus | Conversion of glucose to gluconic acid and production of H2O2; pH decreasing and activation of the peroxidase-like activity of 2D Cu-TCPP(Fe) nanosheets with production of toxic OH− radicals |
| GOx in Cu/Zn bimetal MOF (Zn(NO3)2 and Cu(NO3)2 and 2-methylimidazole) [88] | Inhibition of bacterial growth of both E. coli and S. aureus was up to 90% | Generation of H2O2 and its further conversion to OH− radicals by the Cu2+ ions; that blocks the nutrient/energy supply for bacteria and triggers a Fenton(-like) reaction; glutathione depletion. All these reactions lead to highly efficient bactericidal effect through synergistic starvation/chemodynamic therapy. |
| ZIF-ICG@ZIF-GOx@MPN (Indocyanine green (ICG) and GOx were incorporated into homologous zeolitic imidazolate framework-8 (ZIF-8) nanoparticles coating with metal polyphenol network (MPN) composed by Fe3+ and tannic acid [89] | Killing of bacteria S. aureus and P.aeruginosa was with efficiency up to 99.7% | Robust OH− radical generation in combination with O2 under irradiation induce oxidative damage of pathogenic bacteria |
| ZIF8/Au-GOx (2-methylimidazole and Zn(NO3)2·-ZIF8) [90] | Inhibition of bacterial growth; MIC was 4 μg/mL for S. aureus and 8 μg/mL for E. coli | Generation of ROSand gluconic acid |
| GOx HvCuO@GOx (hybrid hollow virus-like mesoporous CuO nanospheres) [91] | Inhibition of bacterial growth of S. aureus. and E. coli; HSHvCuO@GOx dressing decreased amounts of bacterial cells down to 11.5% and 3.3% for 9 and 15 days during wound healing | HvCuO@GOx nanospheres can be efficiently adhered on bacterial surfaces and then activated by the high glucose concentration in biofilm matrix with further generation of toxic OH− radicals and release of Cu2+ |
| GOx with L-arginine in CuBDC (L-Arg/GOx@CuBDC) [92] | Fenton-like catalytic activity with production of toxic radicals ONOO− and NO | Inactivation of bacterial growth of E. coli and S. aureus was 97% at 38 μg/mL and 3.8 μg/mL, respectively |
| GOx with L-arginine in mesoporous Fe3O4@SiO2 [93] | 80 μg/mL MOF with GOx and L-arginine can reduce amounts of bacterial cells (S. aureus. and E. coli) in 1000-100 000 times | Generation of H2O2 and gluconic acid from glucose |
| Fe3O4@PVP@MIL-88B(Fe)–NH- lysozyme/carvacrol Polyvinylpyrrolidone (PVP) [94] | 100 μg/mL MOF can cause 100% inhibition of bacterial growth of E. coli and S. aureus when cell concentration was 106 CFU/mL | The lysozyme degrades the peptidoglycan on bacterial cell wall and carvacrol damages the cell membrane under near-infrared irradiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremenko, E.; Stepanov, N.; Aslanli, A.; Lyagin, I.; Senko, O.; Maslova, O. Combination of Enzymes with Materials to Give Them Antimicrobial Features: Modern Trends and Perspectives. J. Funct. Biomater. 2023, 14, 64. https://doi.org/10.3390/jfb14020064
Efremenko E, Stepanov N, Aslanli A, Lyagin I, Senko O, Maslova O. Combination of Enzymes with Materials to Give Them Antimicrobial Features: Modern Trends and Perspectives. Journal of Functional Biomaterials. 2023; 14(2):64. https://doi.org/10.3390/jfb14020064
Chicago/Turabian StyleEfremenko, Elena, Nikolay Stepanov, Aysel Aslanli, Ilya Lyagin, Olga Senko, and Olga Maslova. 2023. "Combination of Enzymes with Materials to Give Them Antimicrobial Features: Modern Trends and Perspectives" Journal of Functional Biomaterials 14, no. 2: 64. https://doi.org/10.3390/jfb14020064
APA StyleEfremenko, E., Stepanov, N., Aslanli, A., Lyagin, I., Senko, O., & Maslova, O. (2023). Combination of Enzymes with Materials to Give Them Antimicrobial Features: Modern Trends and Perspectives. Journal of Functional Biomaterials, 14(2), 64. https://doi.org/10.3390/jfb14020064








