The Influence of Saliva pH on the Fracture Resistance of Two Types of Implant-Supported Bis-Acrylic Resin Provisional Crowns—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
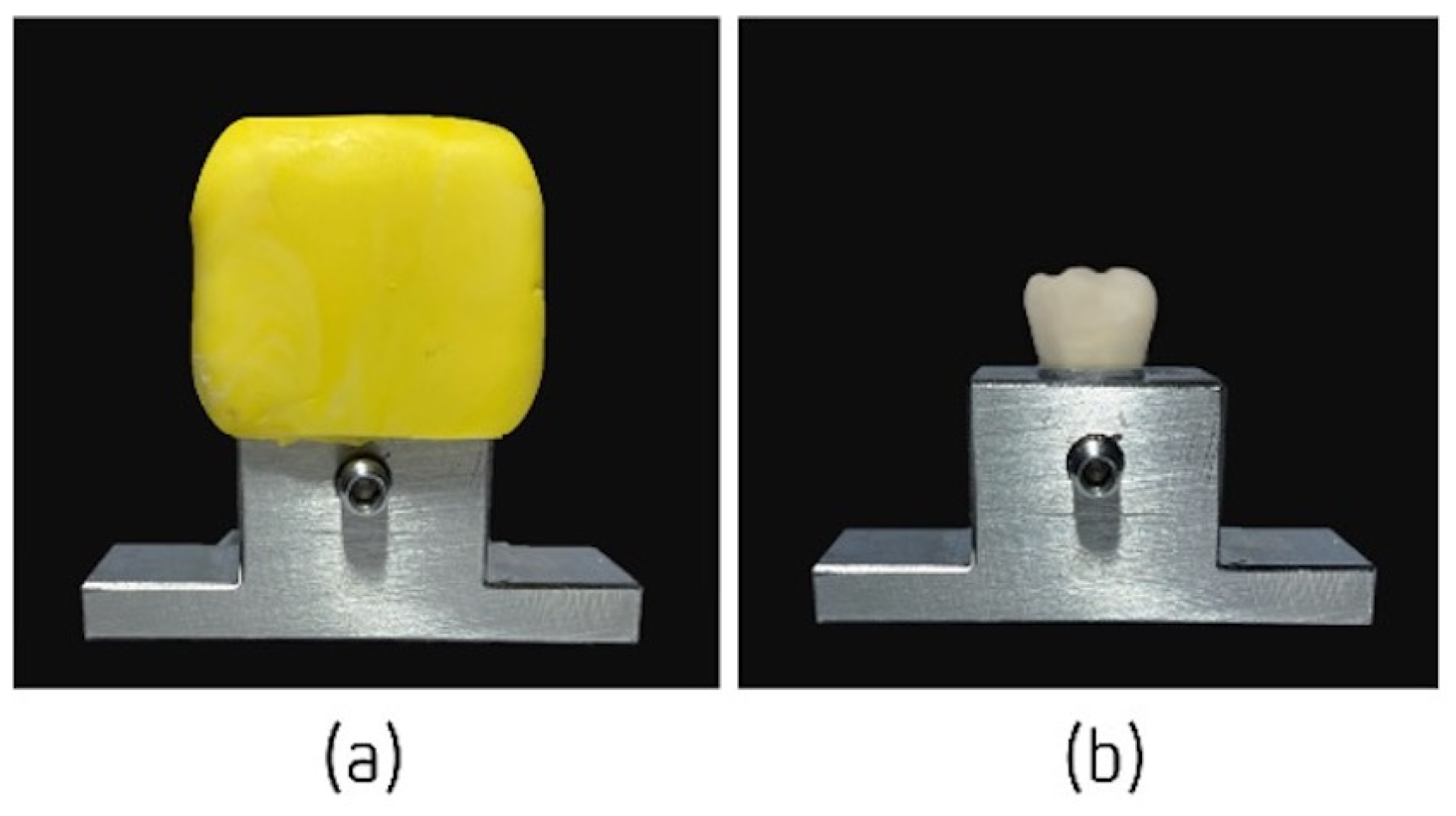
2.2.1. Preparation of the Sample
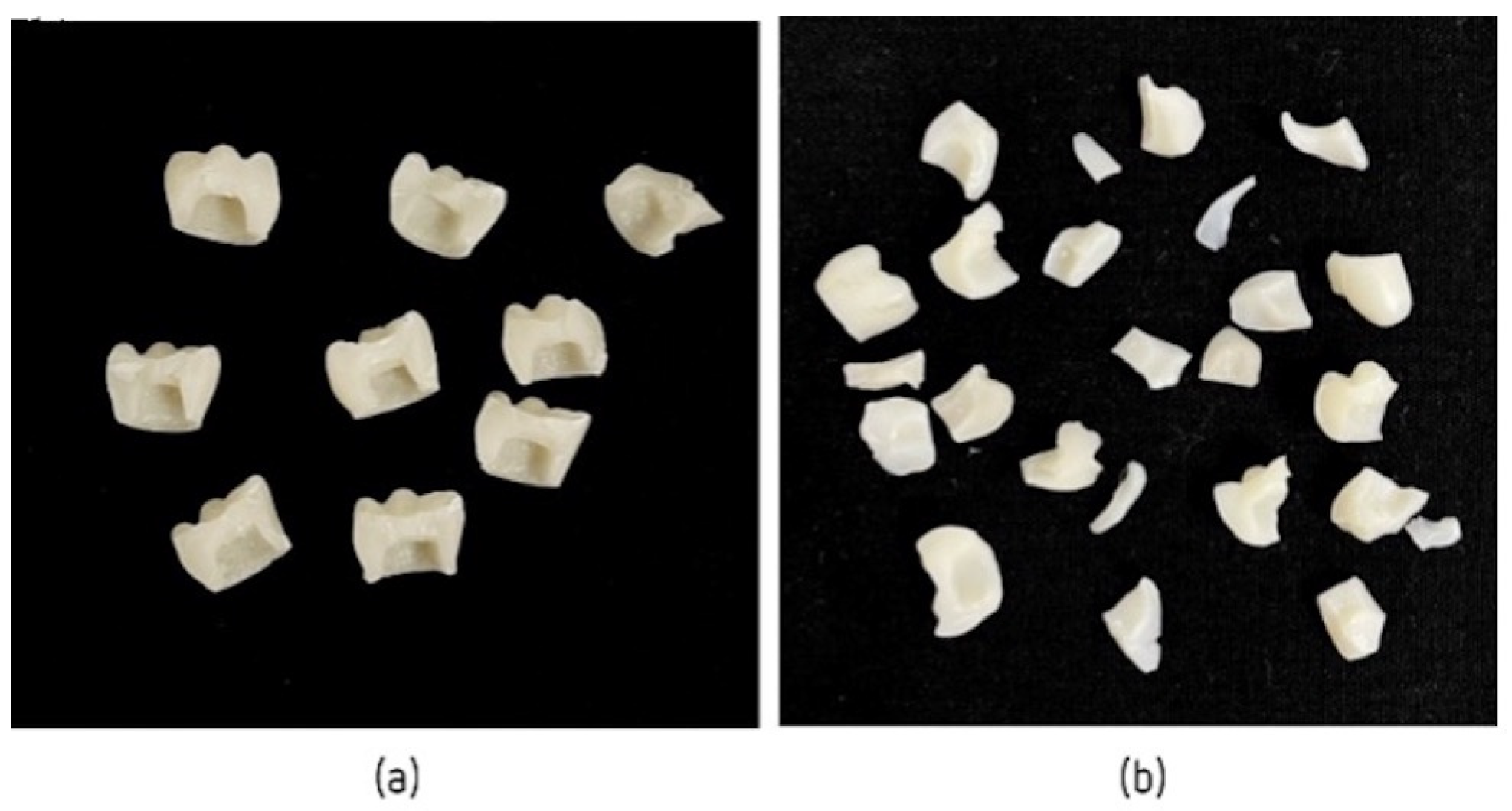
2.2.2. Elaboration of Temporary Crowns
2.3. Compression Tests to Measure the Fracture Resistance of Different Temporary Crowns
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jivraj, S.; Chee, W. Transitioning patients from teeth to implants. Br. Dent. J. 2006, 201, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Proussaefs, P. Immediate provisionalization with a CAD/CAM interim abutment and crown: A guided soft tissue healing technique. J. Prosthet. Dent. 2015, 113, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Santosa, R.E. Provisional restoration options in implant dentistry. Aust. Dent. J. 2007, 52, 234–242, quiz 254. [Google Scholar] [CrossRef] [PubMed]
- Priest, G. Esthetic potential of single-implant provisional restorations: Selection criteria of available alternatives. J. Esthet. Restor. Dent. 2006, 18, 326–338. [Google Scholar] [CrossRef]
- Cho, S.C.; Shetty, S.; Froum, S.; Elian, N.; Tarnow, D. Fixed and removable provisional options for patients undergoing implant treatment. Compend. Contin. Educ. Dent. 2007, 28, 604–608, quiz 609, 624. [Google Scholar]
- Abdullah, A.O.; Tsitrou, E.A.; Pollington, S. Comparative in vitro evaluation of CAD/CAM vs. conventional provisional crowns. J. Appl. Oral Sci. 2016, 24, 258–263. [Google Scholar] [CrossRef]
- Rosentritt, M.; Hahnel, S.; Engelhardt, F.; Behr, M.; Preis, V. In vitro performance and fracture resistance of CAD/CAM-fabricated implant supported molar crowns. Clin. Oral Investig. 2017, 21, 1213–1219. [Google Scholar] [CrossRef]
- Alt, V.; Hannig, M.; Wöstmann, B.; Balkenhol, M. Fracture strength of temporary fixed partial dentures: CAD/CAM versus directly fabricated restorations. Dent. Mater. 2011, 27, 339–347. [Google Scholar] [CrossRef]
- Mendes, J.M.; Botelho, P.C.; Mendes, J.; Barreiros, P.; Aroso, C.; Silva, A.S. Comparison of Fracture Strengths of Three Provisional Prosthodontic CAD/CAM Materials: Laboratory Fatigue Tests. Appl. Sci. 2021, 11, 9589. [Google Scholar] [CrossRef]
- Lee, H.; Son, K.; Lee, D.-H.; Kim, S.-Y.; Lee, K.-B. Comparison of Wear of Interim Crowns in Accordance with the Build Angle of Digital Light Processing 3D Printing: A Preliminary In Vivo Study. Bioengineering 2022, 9, 417. [Google Scholar] [CrossRef]
- Branco, A.C.; Colaço, R.; Figueiredo-Pina, C.G.; Serro, A.P. A State-of-the-Art Review on the Wear of the Occlusal Surfaces of Natural Teeth and Prosthetic Crowns. Materials 2020, 13, 3525. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, R.F.; Garrido, L.M.; Soares, A.F.; Rodriguez-Medina, A.D.; Mondelli, J.; de Lucena, F.S.; Furuse, A.Y. Effect of simulated brushing on surface roughness and wear of bis-acryl-based materials submitted to different polishing protocols. J. Clin. Exp. Dent. 2022, 14, e168–e176. [Google Scholar] [CrossRef]
- Akova, T.; Ozkomur, A.; Uysal, H. Effect of food-simulating liquids on the mechanical properties of provisional restorative materials. Dent. Mater. 2006, 22, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Gujjari, A.K.; Bhatnagar, V.M.; Basavaraju, R.M. Color stability and flexural strength of poly (methyl methacrylate) and bis-acrylic composite based provisional crown and bridge auto-polymerizing resins exposed to beverages and food dye: An in vitro study. Indian J. Dent. Res. 2013, 24, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Yanikoğlu, N.D.; Bayindir, F.; Kürklü, D.; Beşir, B. Flexural Strength of Temporary Restorative Materials Stored in Different Solutions. Open J. Stomatol. 2014, 4, 291–298. [Google Scholar] [CrossRef]
- Pytko-Polonczyk, J.J.; Jakubik, A.; Przeklasa-Bierowiec, A.; Muszynska, B. Artificial saliva and its use in biological experiments. J. Physiol. Pharmacol. 2017, 68, 807–813. [Google Scholar]
- Lussi, A.; Jaeggi, T.; Zero, D. The role of diet in the aetiology of dental erosion. Caries Res. 2004, 38 (Suppl. S1), 34–44. [Google Scholar] [CrossRef]
- Eckley, A.; Costa, H.O. Estudo comparativo do pH e do volume salivar em indivıduos com laringofaringite cronica por doença do refluxo gastroesofágica antes e após o tratamento. Rev. Bras. Otorrinolaringol. 2006, 72, 55–60. [Google Scholar] [CrossRef]
- Muley, B.Y.; Shaikh, S.R.; Tagore, M.M.; Khalikar, A.N. Effect of Dietary Simulating Solvents on the Mechanical Properties of Provisional Restorative Materials-An In Vitro Study. J. Indian Prosthodont. Soc. 2014, 14 (Suppl. S1), 98–105. [Google Scholar] [CrossRef]
- Queiroz, G.M.; Silva, L.F.; Ferreira, J.T.; Gomes, J.A.; Sathler, L. Electrochemical behavior and pH stability of artificial salivas for corrosion tests. Braz. Oral Res. 2007, 21, 209–215. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Reeponmaha, T.; Angwaravong, O.; Angwarawong, T. Comparison of fracture strength after thermo-mechanical aging between provisional crowns made with CAD/CAM and conventional method. J. Adv. Prosthodont. 2020, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Karaokutan, I.; Sayin, G.; Kara, O. In vitro study of fracture strength of provisional crown materials. J. Adv. Prosthodont. 2015, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Aroso, C.; Ustrell, R.; Braga, A.C.; Mendes, J.M.; Escuin, T. The influence of saliva pH value on the retention and durability of bar-clip attachments. J. Adv. Prosthodont. 2015, 7, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Takaki, P.; Vieira, M.; Bommarito, S. Maximum bite force analysis in different age groups. Int. Arch. Otorhinolaryngol. 2014, 18, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Levartovsky, S.; Peleg, G.; Matalon, S.; Tsesis, I.; Rosen, E. Maximal Bite Force Measured via Digital Bite Force Transducer in Subjects with or without Dental Implants—A Pilot Study. Appl. Sci. 2022, 12, 1544. [Google Scholar] [CrossRef]
- Cosme, D.C.; Baldisserotto, S.M.; Canabarro Sde, A.; Shinkai, R.S. Bruxism and voluntary maximal bite force in young dentate adults. Int. J. Prosthodont. 2005, 18, 328–332. [Google Scholar] [PubMed]
- Haselton, D.R.; Diaz-Arnold, A.M.; Vargas, M.A. Flexural strength of provisional crown and fixed partial denture resins. J. Prosthet. Dent. 2002, 87, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Koumjian, J.H.; Nimmo, A. Evaluation of fracture resistance of resins used for provisional restorations. J. Prosthet. Dent. 1990, 64, 654–657. [Google Scholar] [CrossRef]
- Singh, A.; Garg, S. Comparative Evaluation of Flexural Strength of Provisional Crown and Bridge Materials-An Invitro Study. J. Clin. Diagn. Res. 2016, 10, ZC72–ZC77. [Google Scholar] [CrossRef]



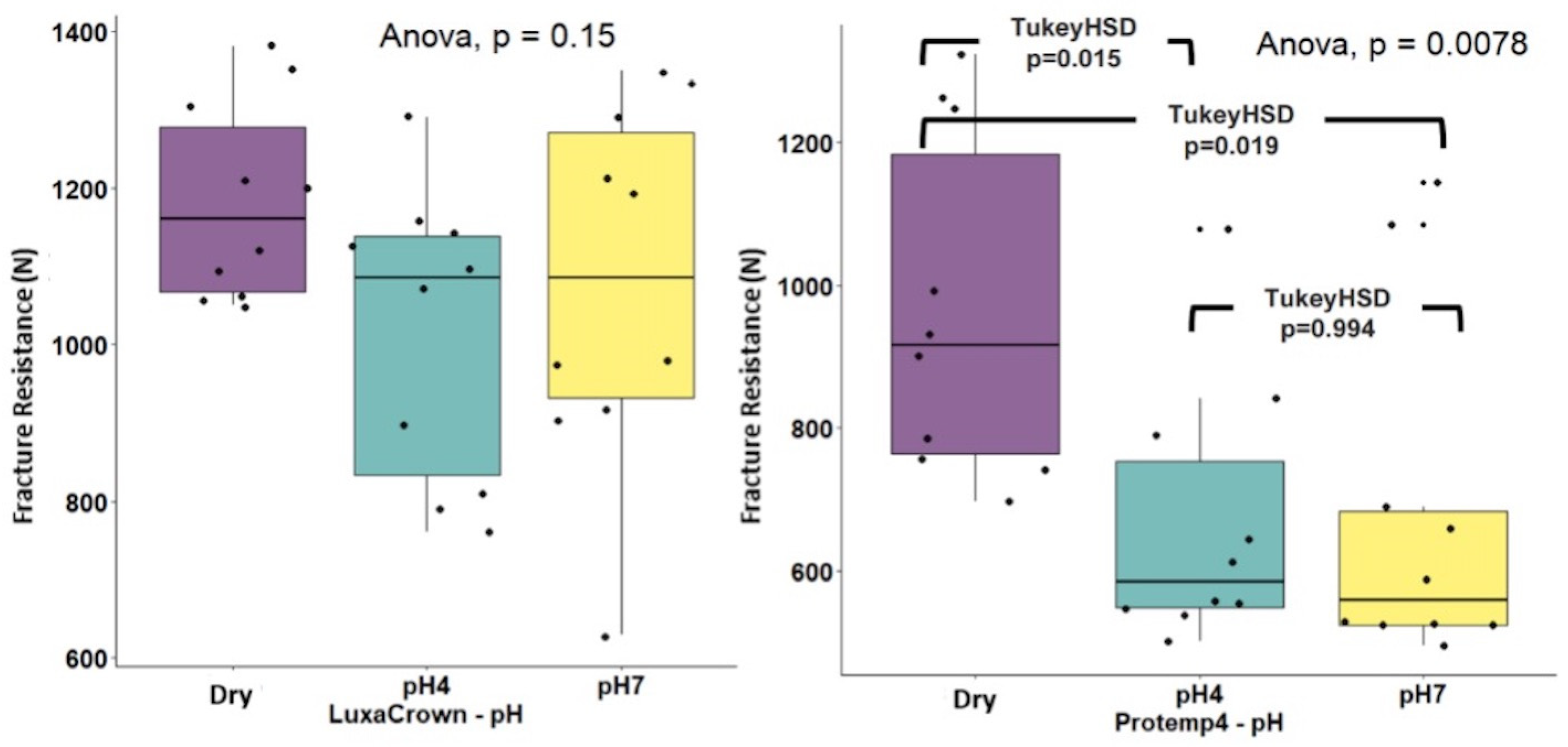


| Crowns | Control | pH 4 | pH 7 | Subtotal Crowns | ANOVA * pH | ANOVA ** Crowns × pH |
|---|---|---|---|---|---|---|
| LuxaCrown® | 1182.00 (125.59) | 1015.00 (184.58) | 1077.00 (233.57) | 1091.33 (193.17) | F(2.27) = 5.833 (p = 0.150) η2 = 0.132 | F(2.54) = 1.051 (p = 0.357) η2 = 0.037 |
| ProtempTM 4 | 963.54 (235.37) | 666.66 (182.98) | 679.59 (239.44) | 768.93 (254.92) | F(2.27) = 2.048 (p = 0.0078) η2 = 0.302 | |
| Subtotal pH | 1072.77 (215.11) | 840.83 (252.85) | 876.80 (308.53) | 930.13 (276.96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa-Santos, S.; Silva, A.S.; Sousa-Santos, P.; Vale, T.; Mendes, J.M. The Influence of Saliva pH on the Fracture Resistance of Two Types of Implant-Supported Bis-Acrylic Resin Provisional Crowns—An In Vitro Study. J. Funct. Biomater. 2023, 14, 62. https://doi.org/10.3390/jfb14020062
Sousa-Santos S, Silva AS, Sousa-Santos P, Vale T, Mendes JM. The Influence of Saliva pH on the Fracture Resistance of Two Types of Implant-Supported Bis-Acrylic Resin Provisional Crowns—An In Vitro Study. Journal of Functional Biomaterials. 2023; 14(2):62. https://doi.org/10.3390/jfb14020062
Chicago/Turabian StyleSousa-Santos, Sofia, António Sérgio Silva, Primavera Sousa-Santos, Teresa Vale, and José Manuel Mendes. 2023. "The Influence of Saliva pH on the Fracture Resistance of Two Types of Implant-Supported Bis-Acrylic Resin Provisional Crowns—An In Vitro Study" Journal of Functional Biomaterials 14, no. 2: 62. https://doi.org/10.3390/jfb14020062
APA StyleSousa-Santos, S., Silva, A. S., Sousa-Santos, P., Vale, T., & Mendes, J. M. (2023). The Influence of Saliva pH on the Fracture Resistance of Two Types of Implant-Supported Bis-Acrylic Resin Provisional Crowns—An In Vitro Study. Journal of Functional Biomaterials, 14(2), 62. https://doi.org/10.3390/jfb14020062








