One-Piece Zirconia Oral Implants for Single Tooth Replacement: Five-Year Results from a Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population Clinical Procedure
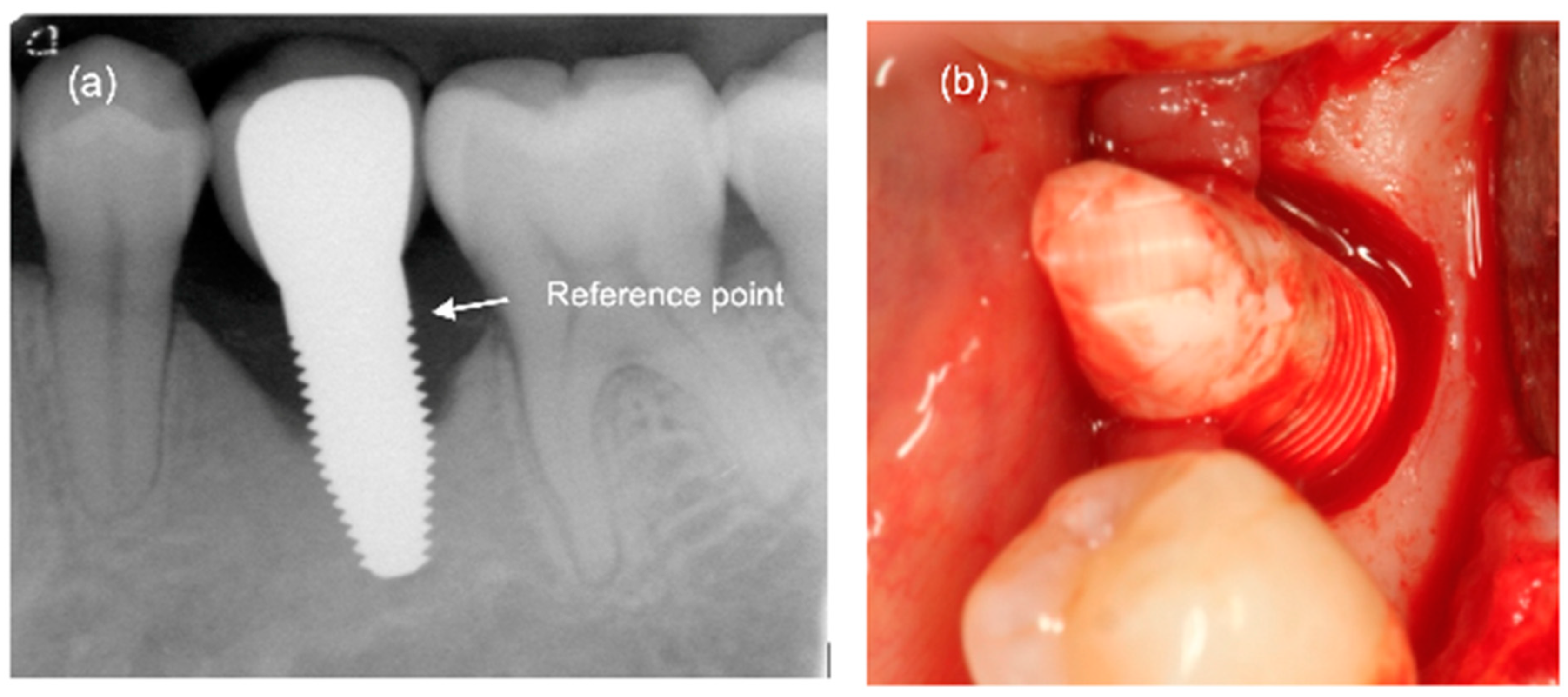
2.2. Clinical and Radiographic Assessment
2.3. Statistical Analysis
3. Results
3.1. Evaluation of Clinical Parameters (Figure 2)
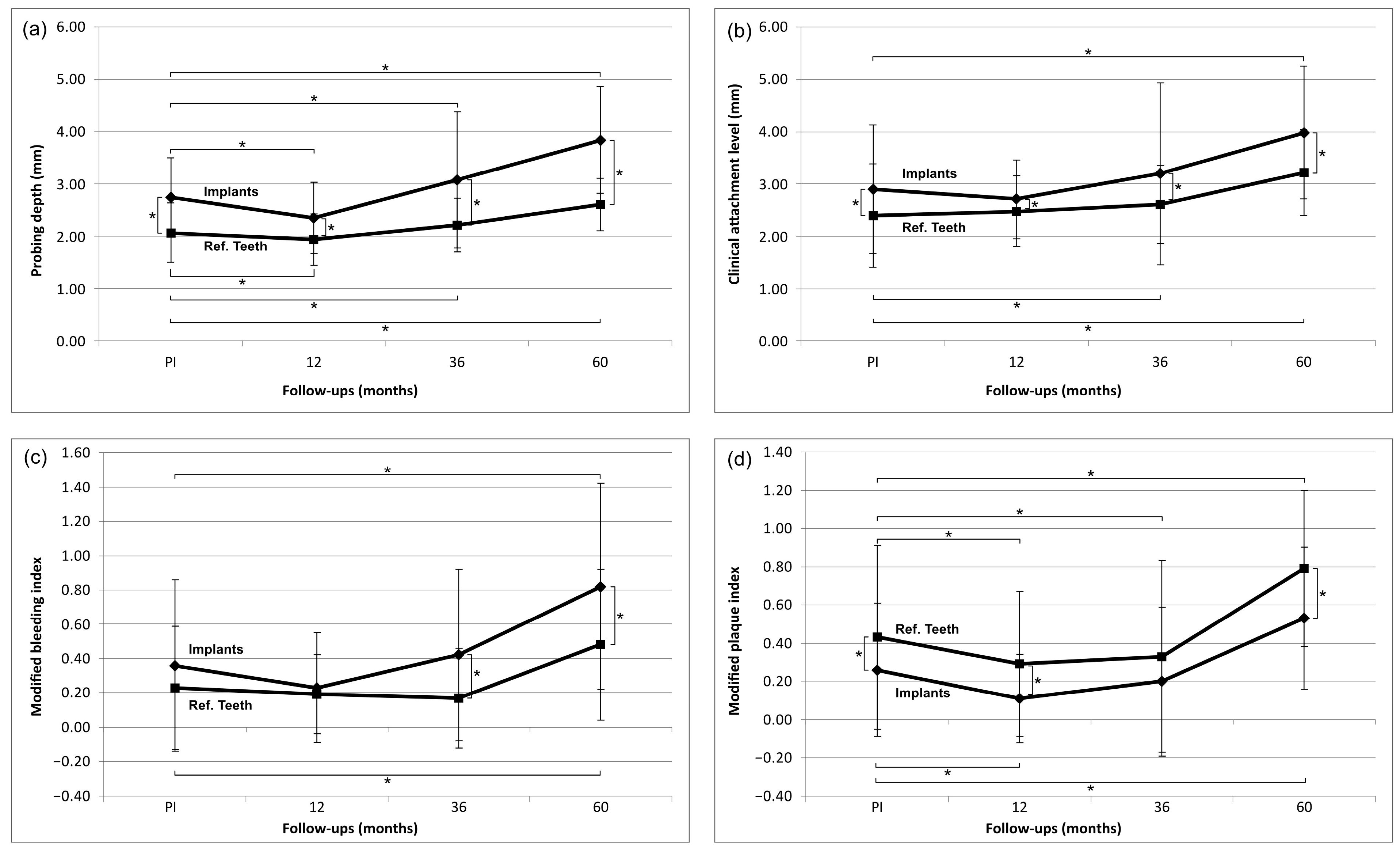
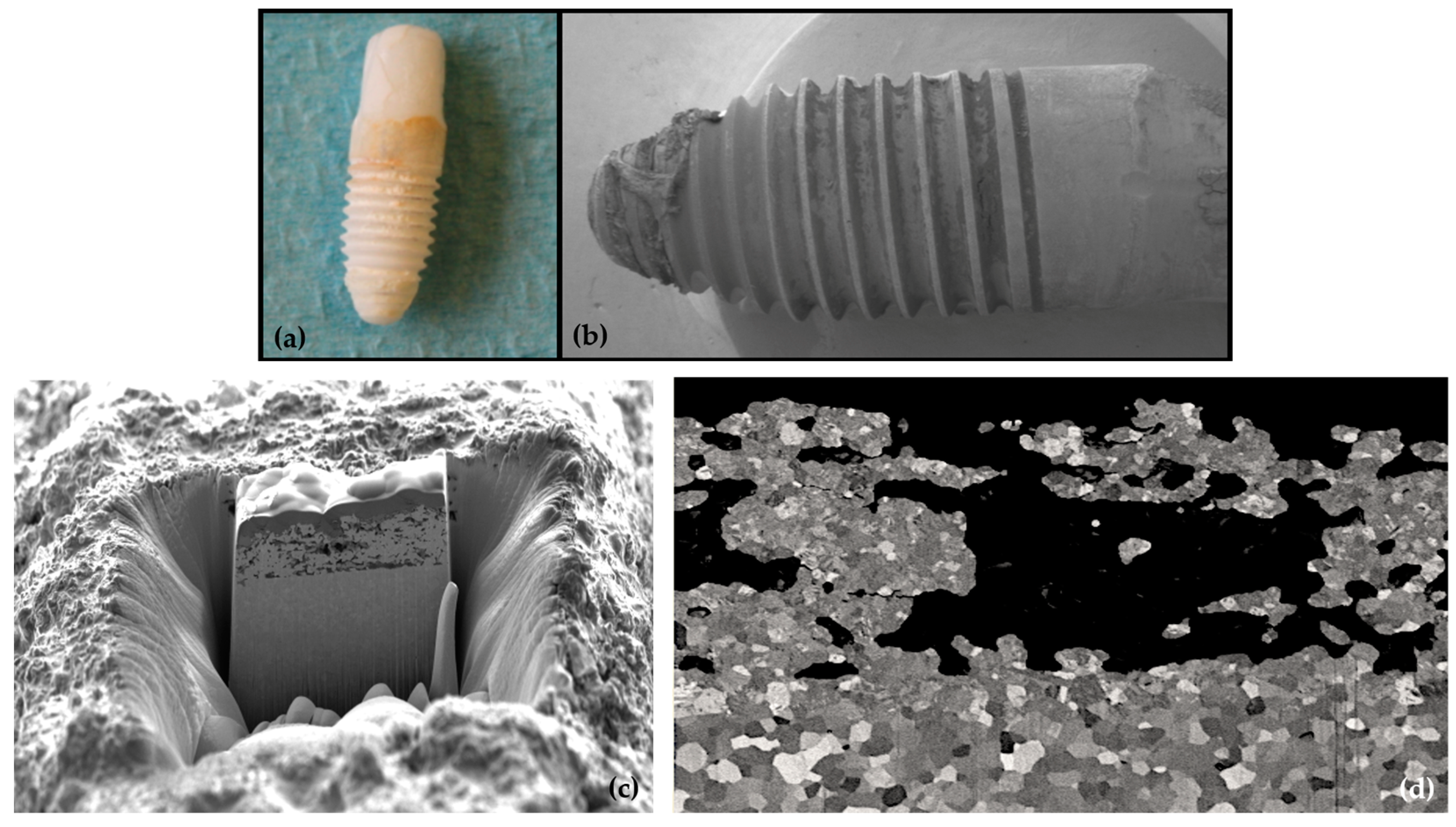
3.2. Biological Complications
3.3. Marginal Bone Remodelling
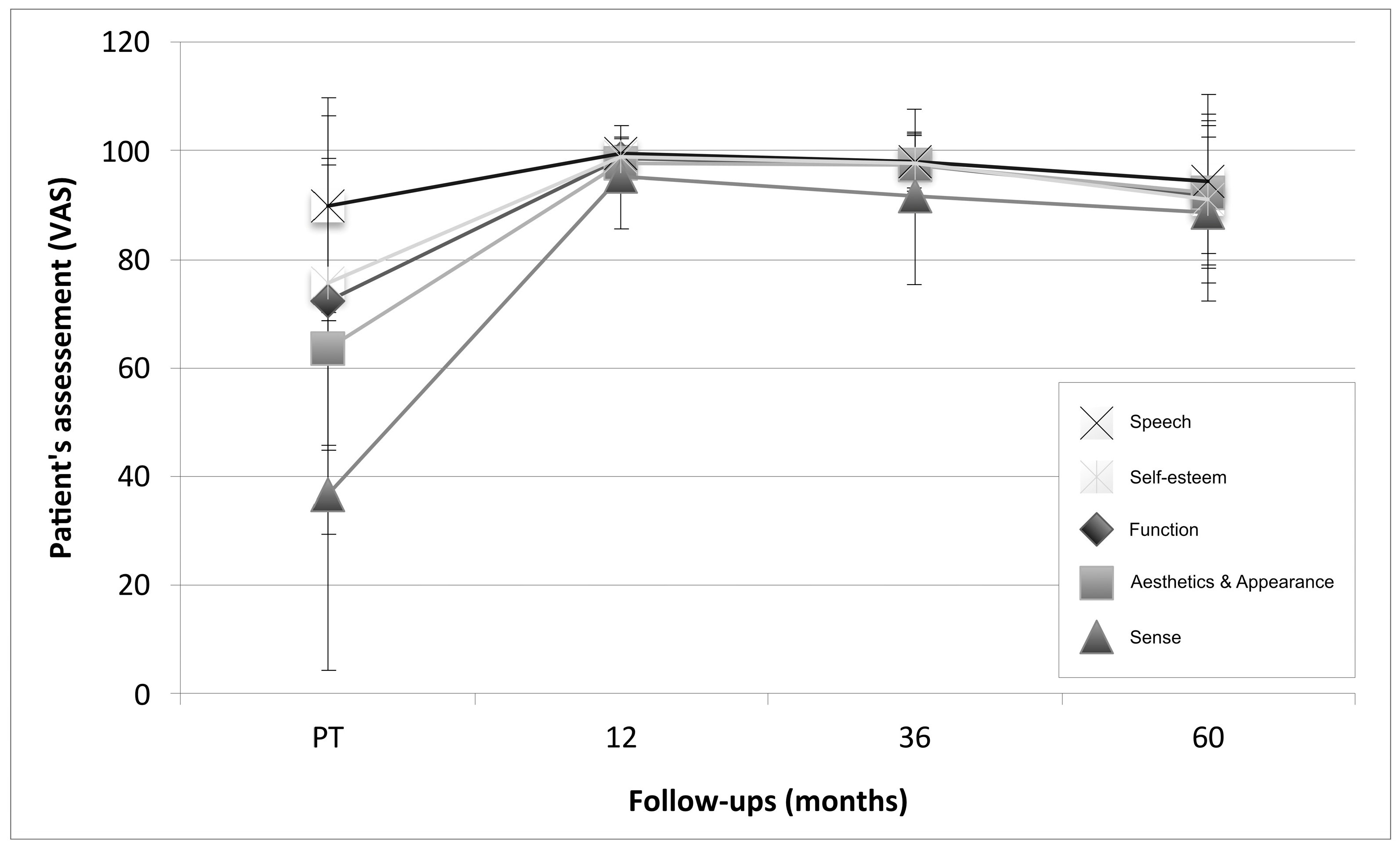
3.4. Patient Assessment: Patient-Reported Outcome Measures (PROMs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
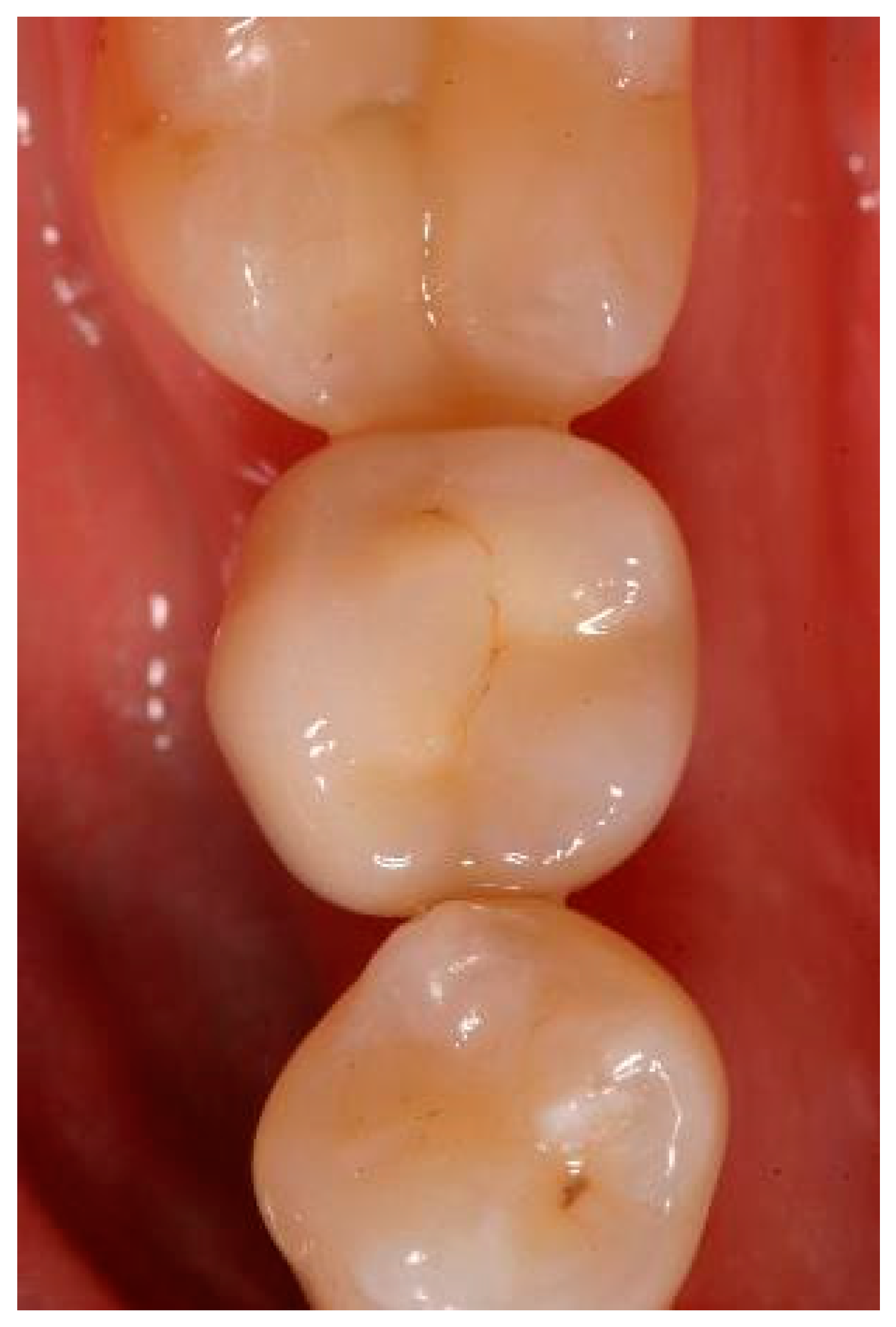
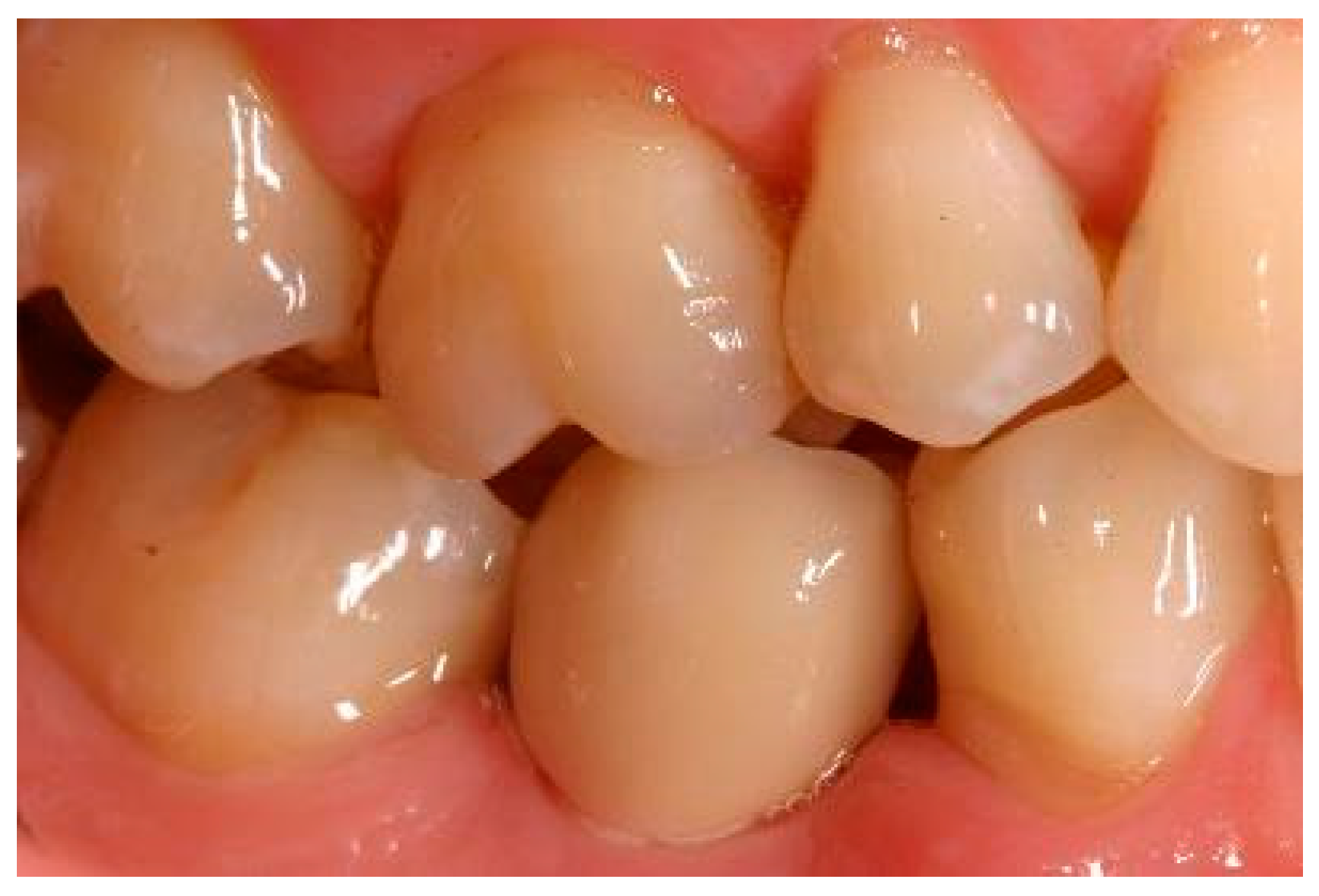
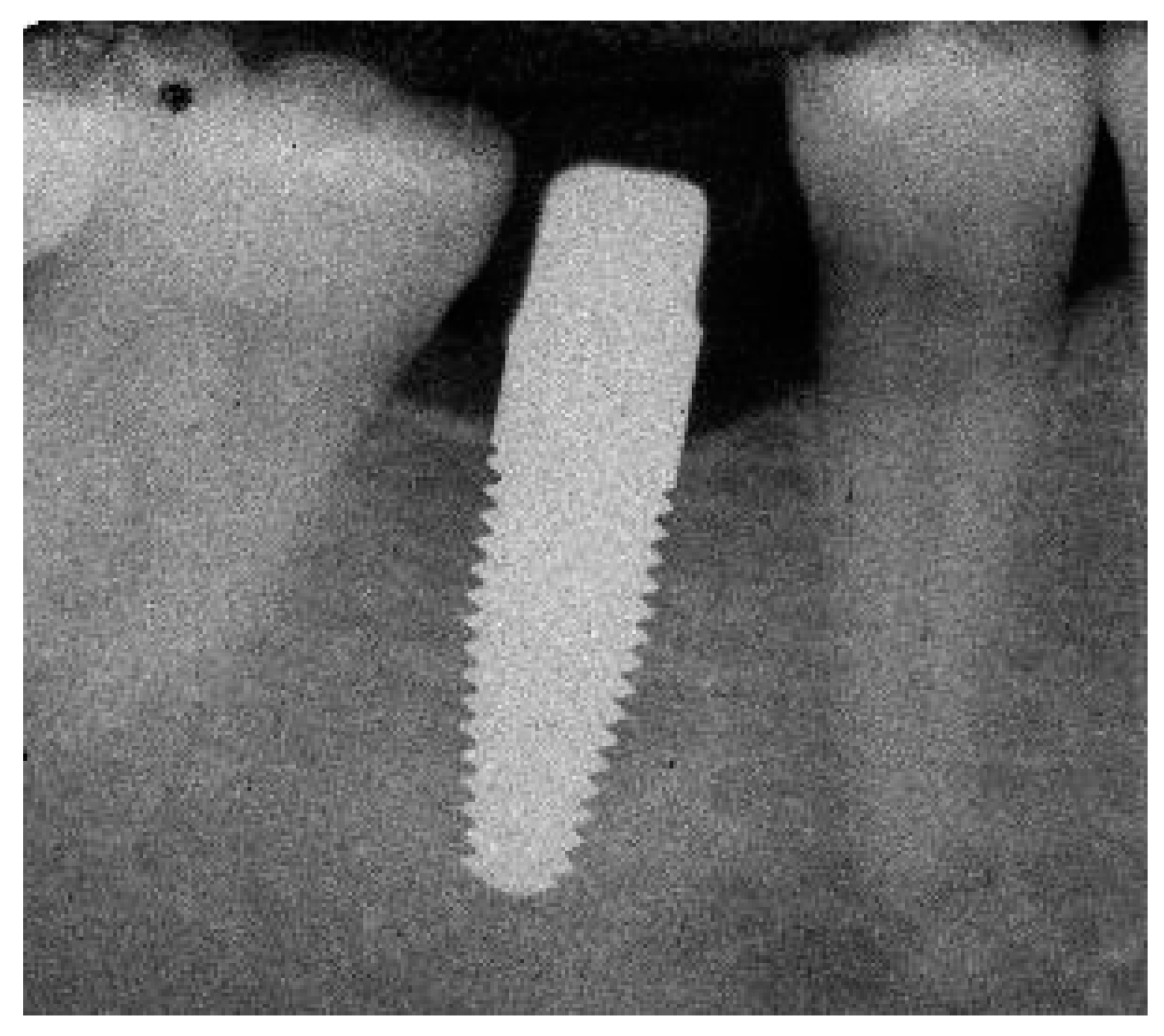
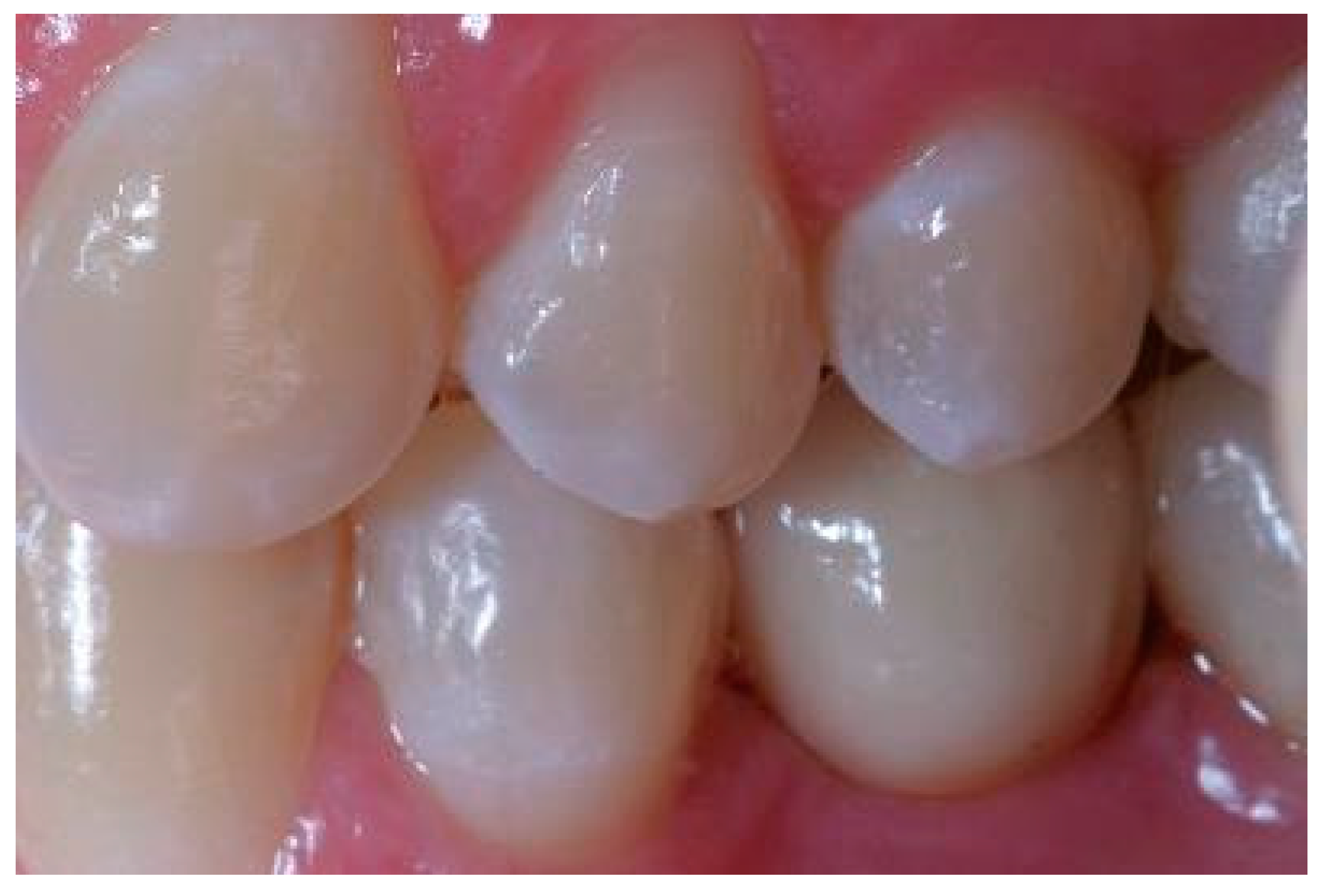
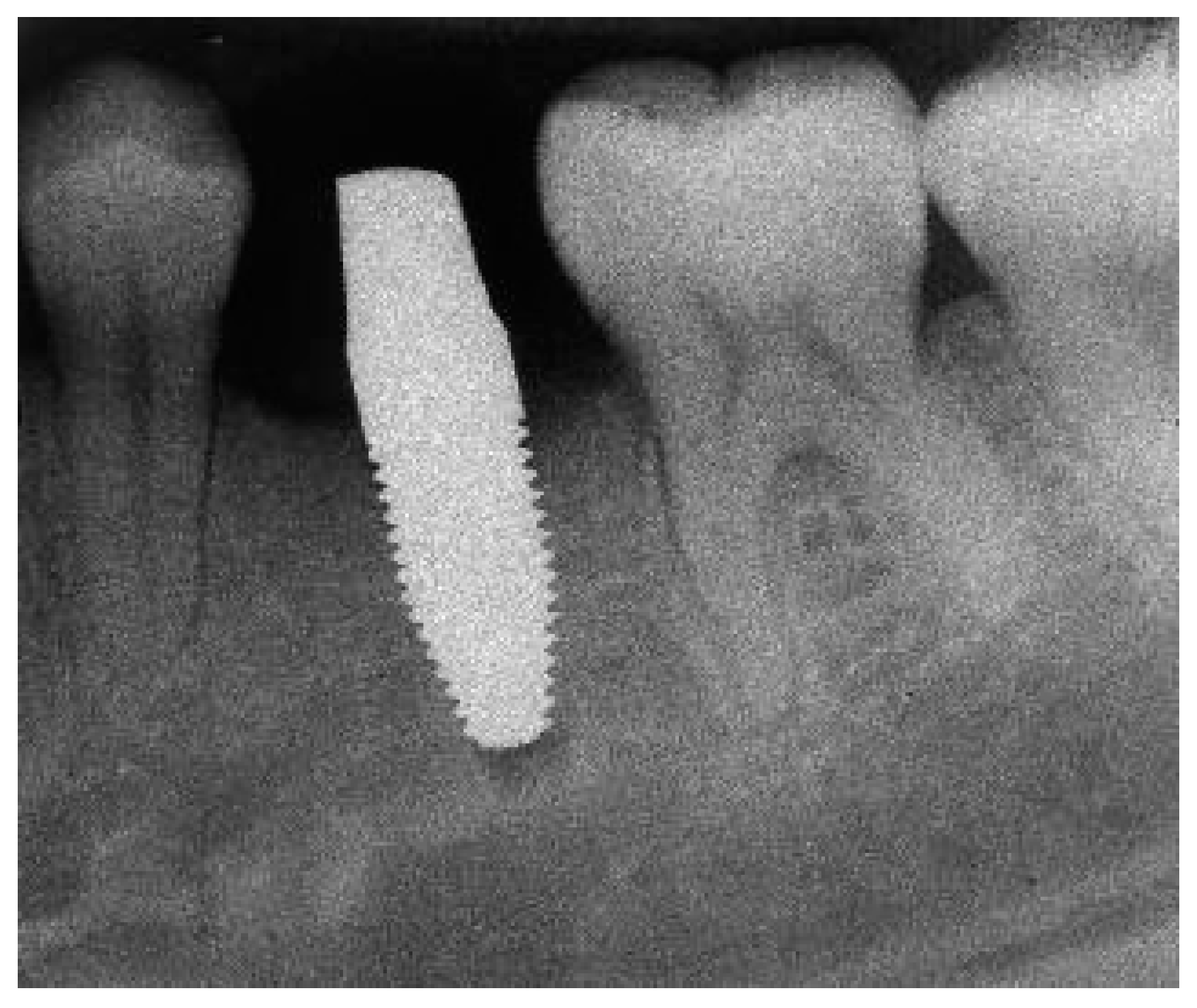
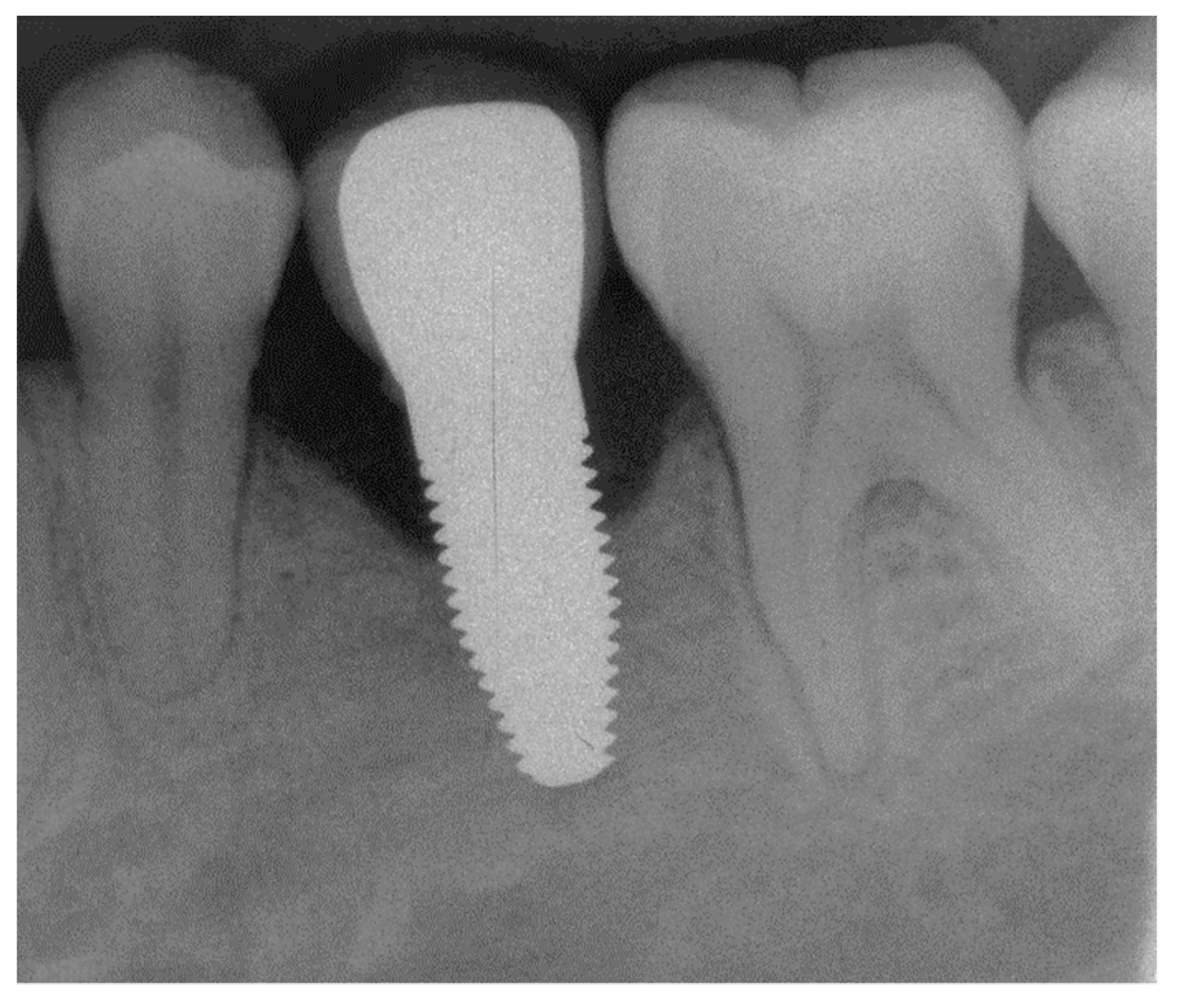
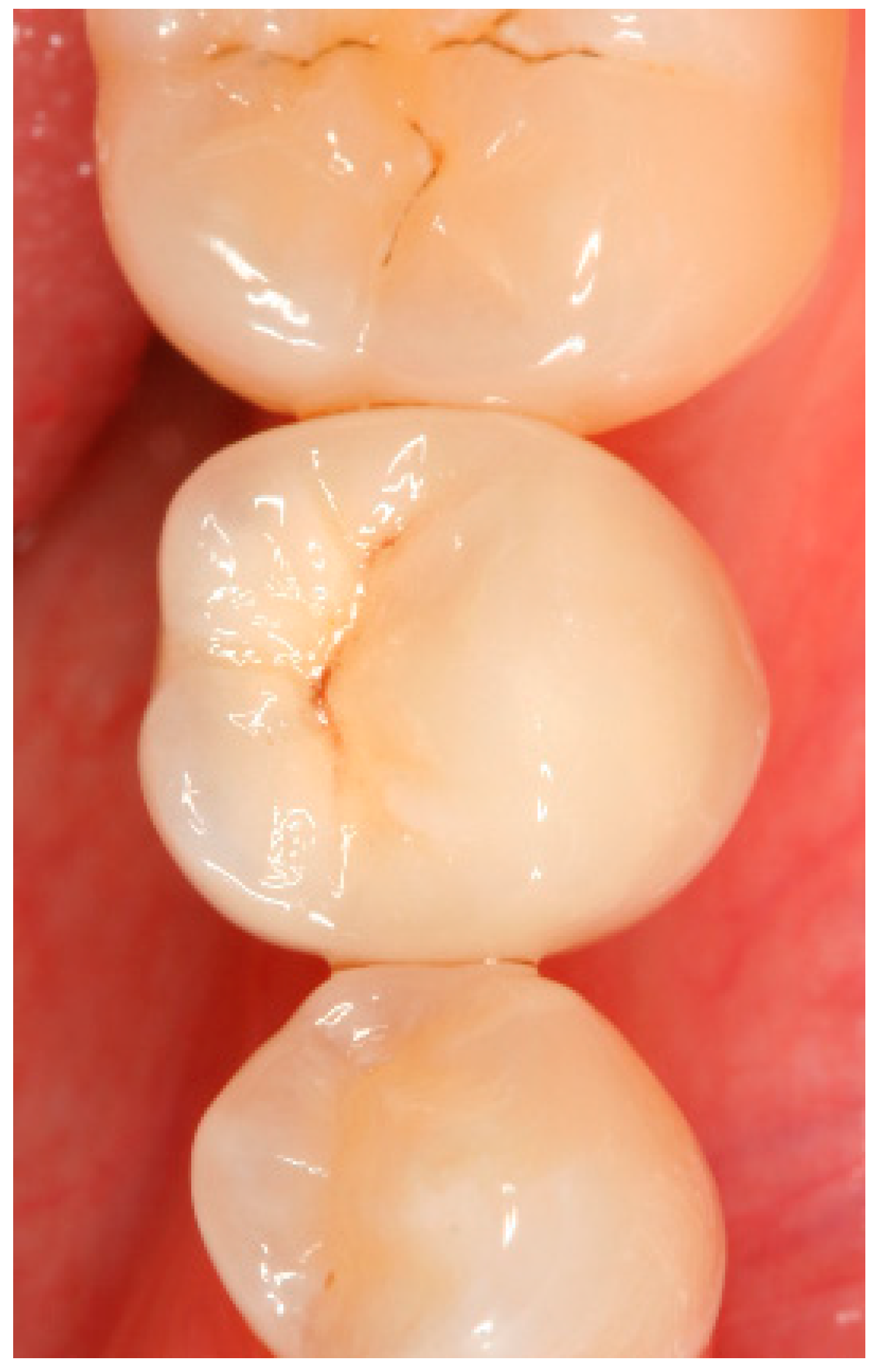
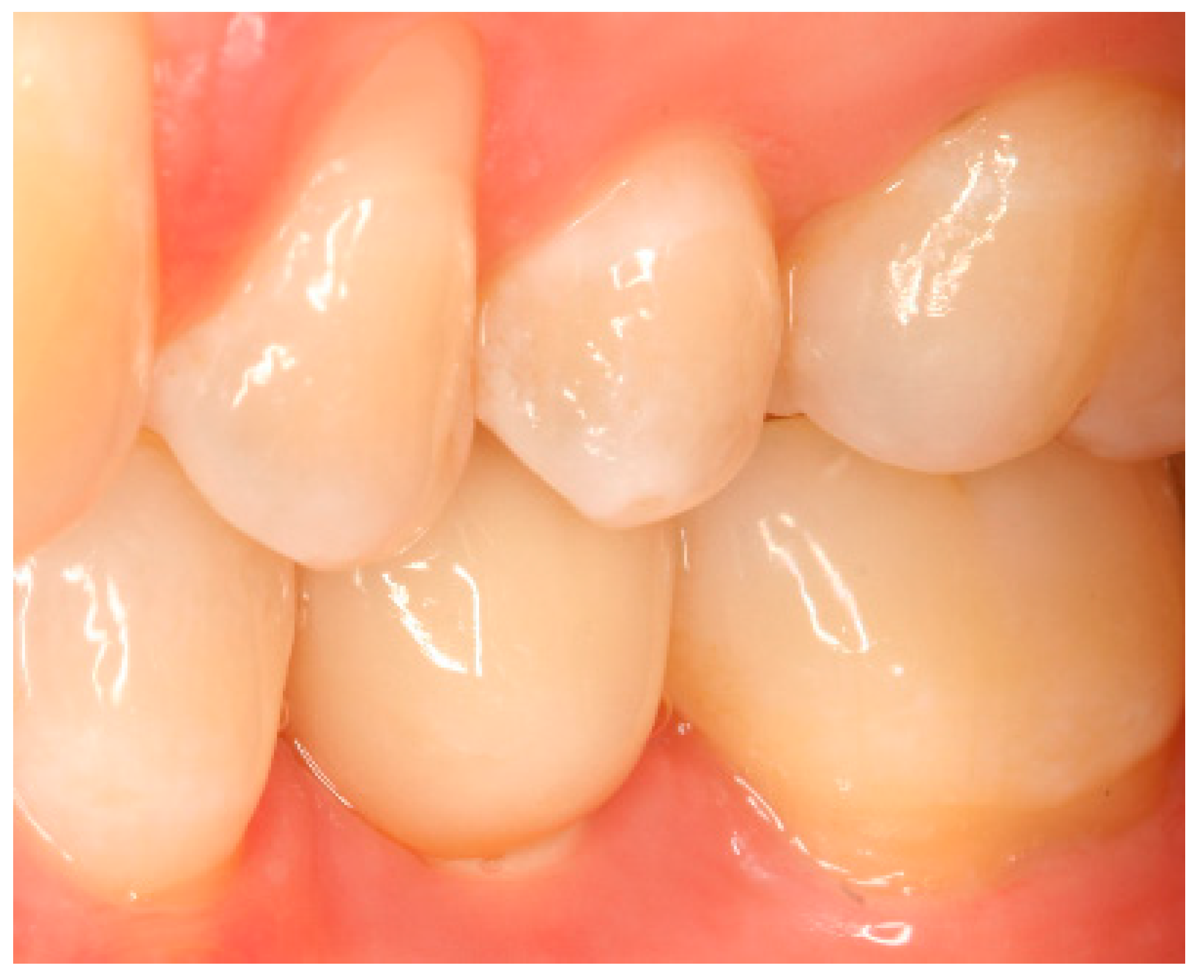
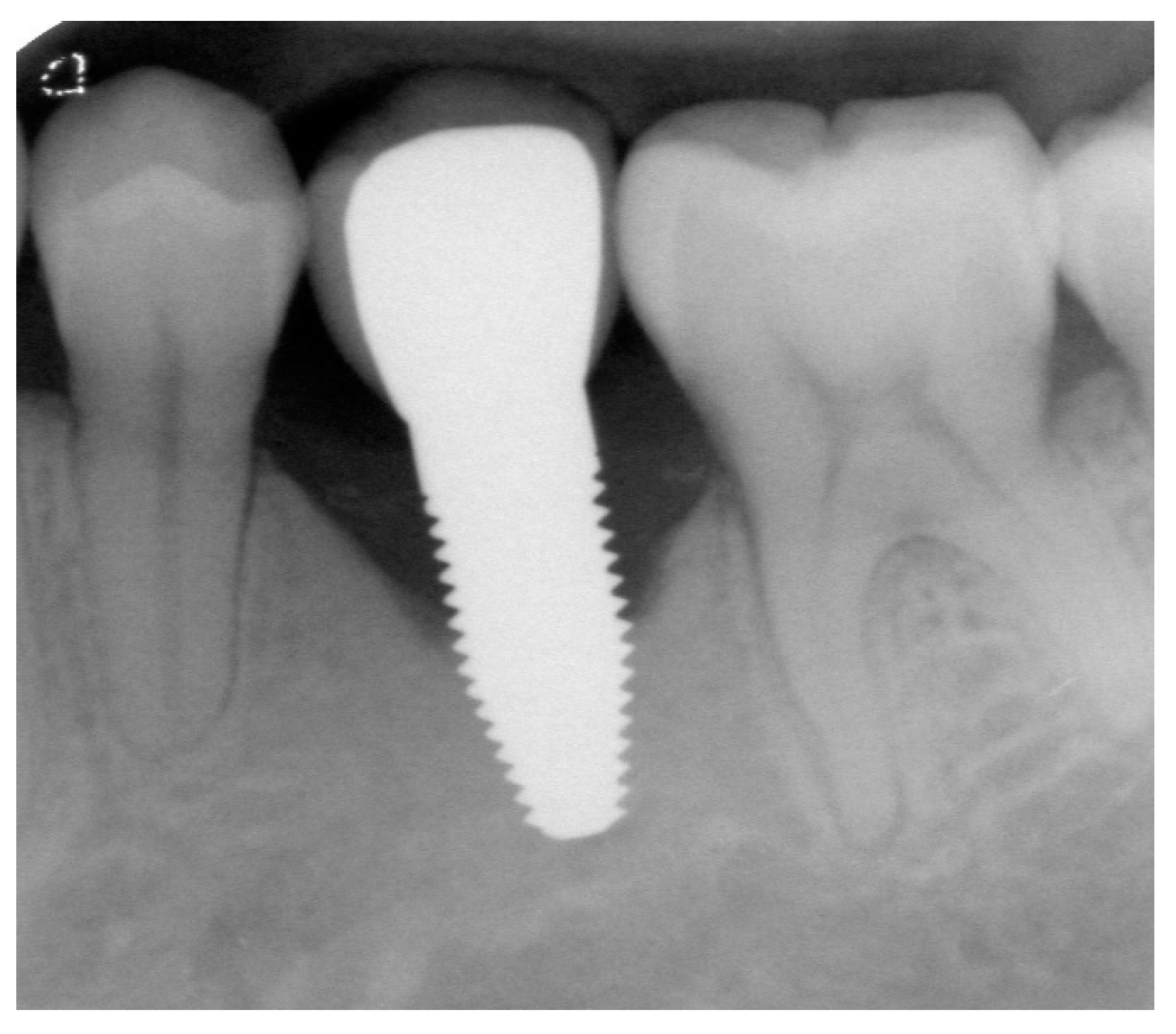
Appendix A. Exemplary Photographs and Radiographs from a Patient at Different Examination Time Points and a Positive Outcome
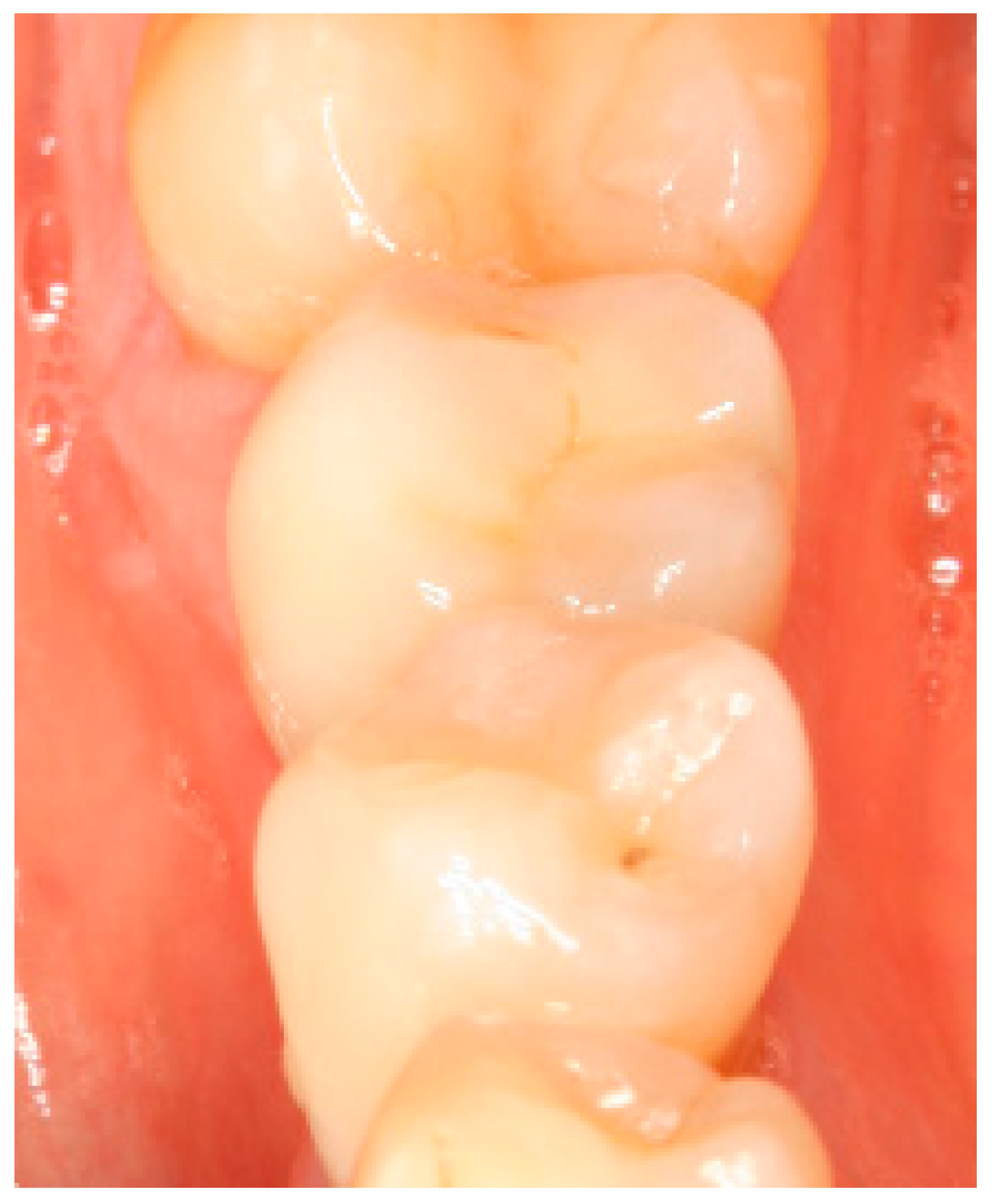
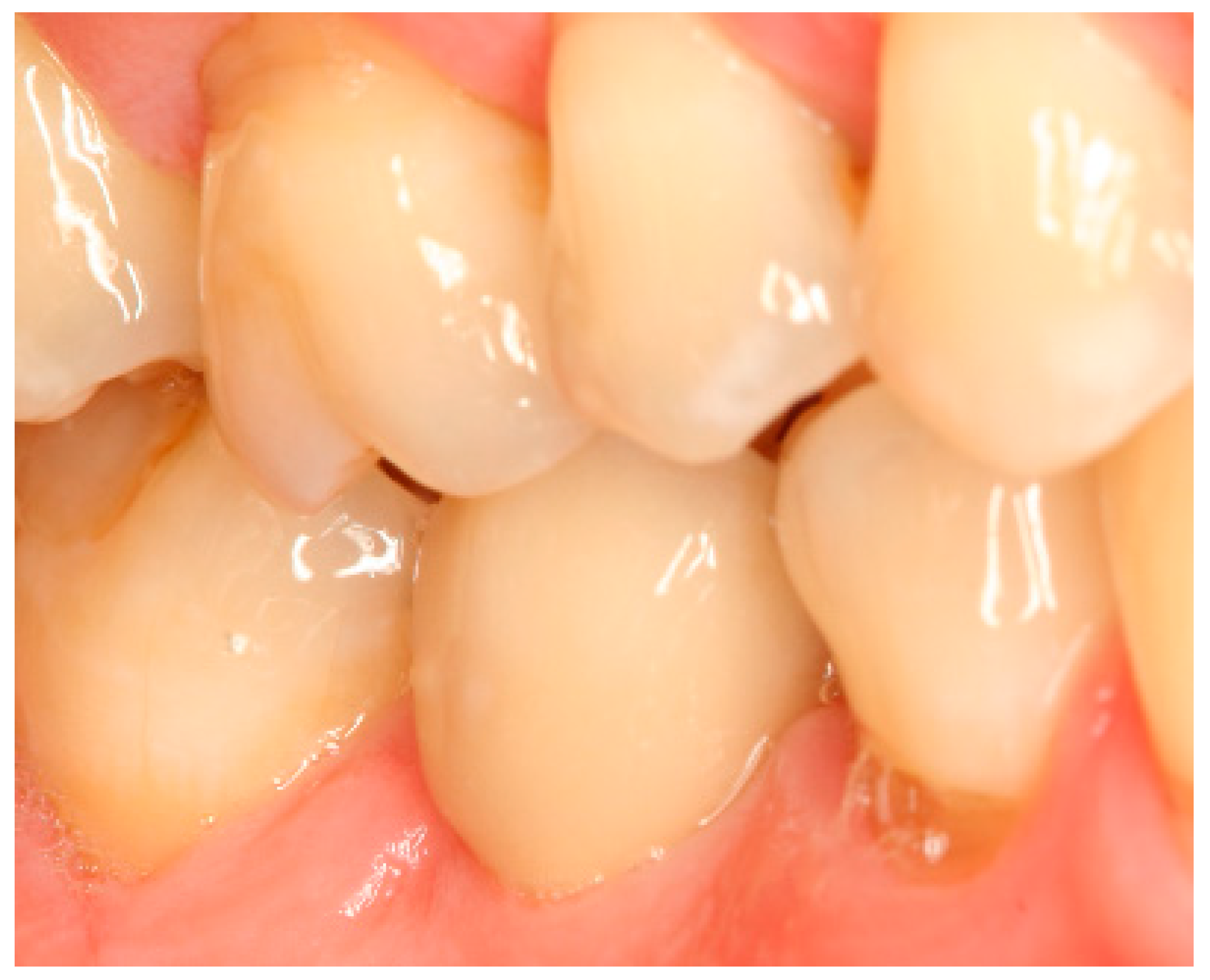
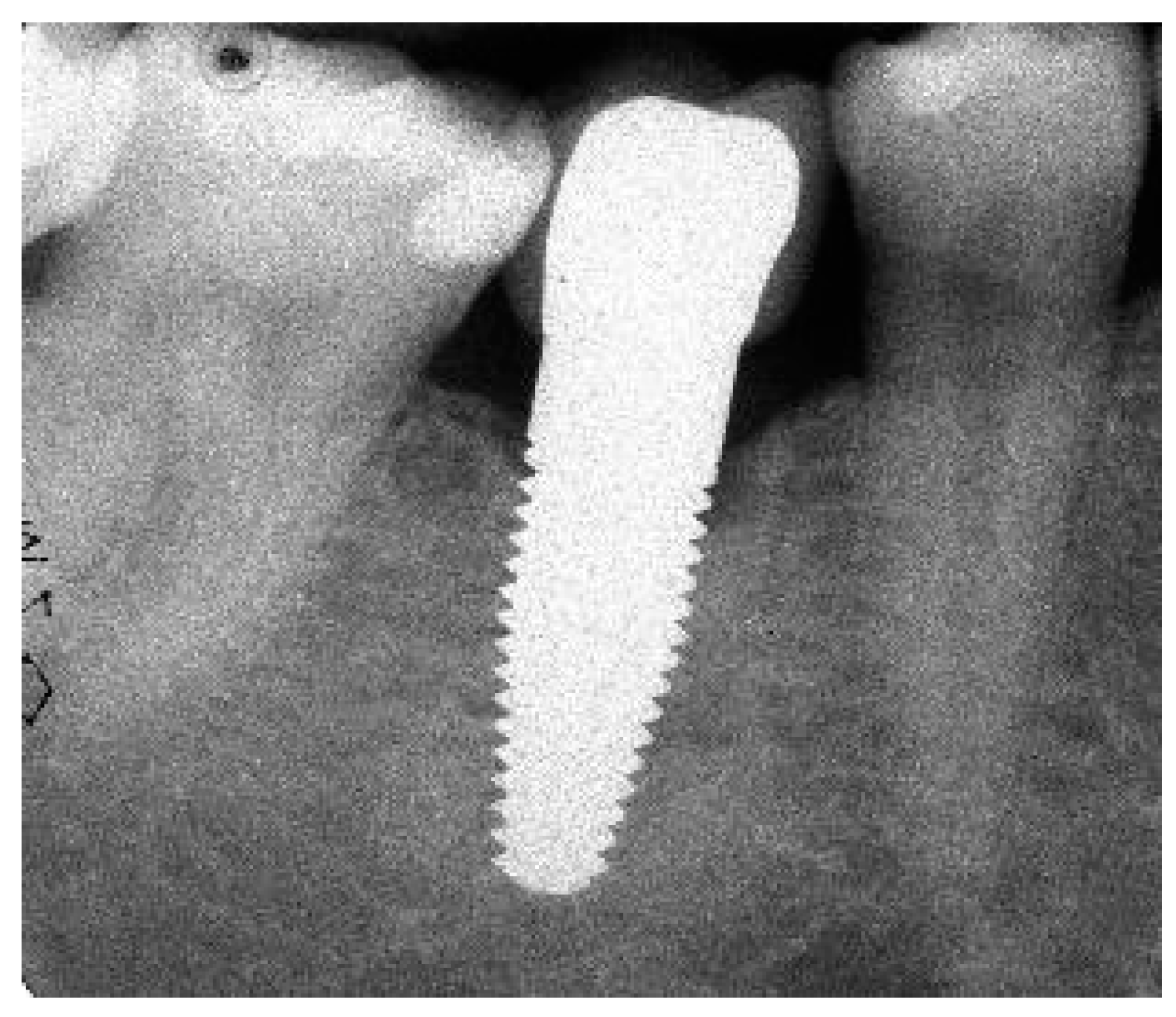
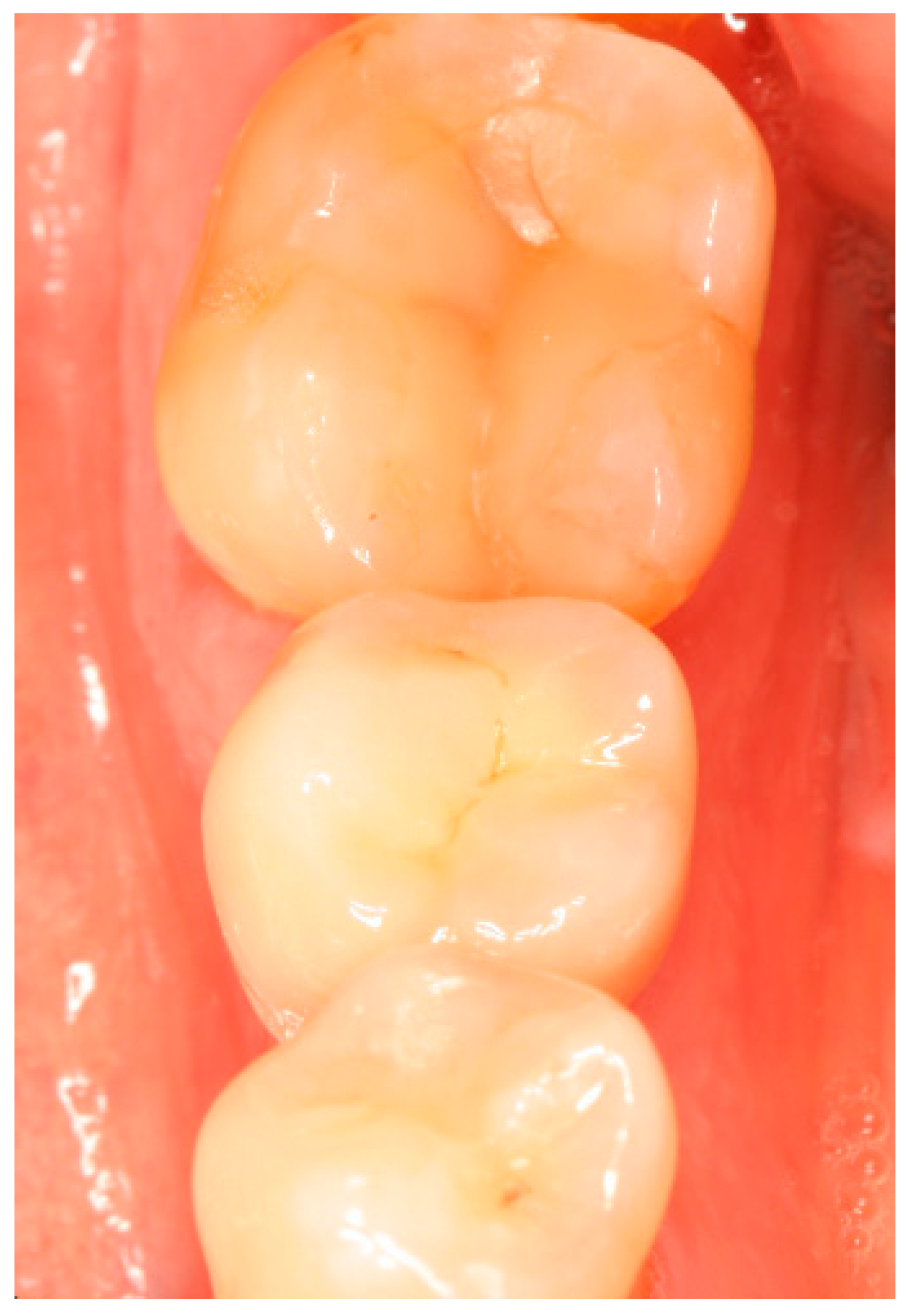
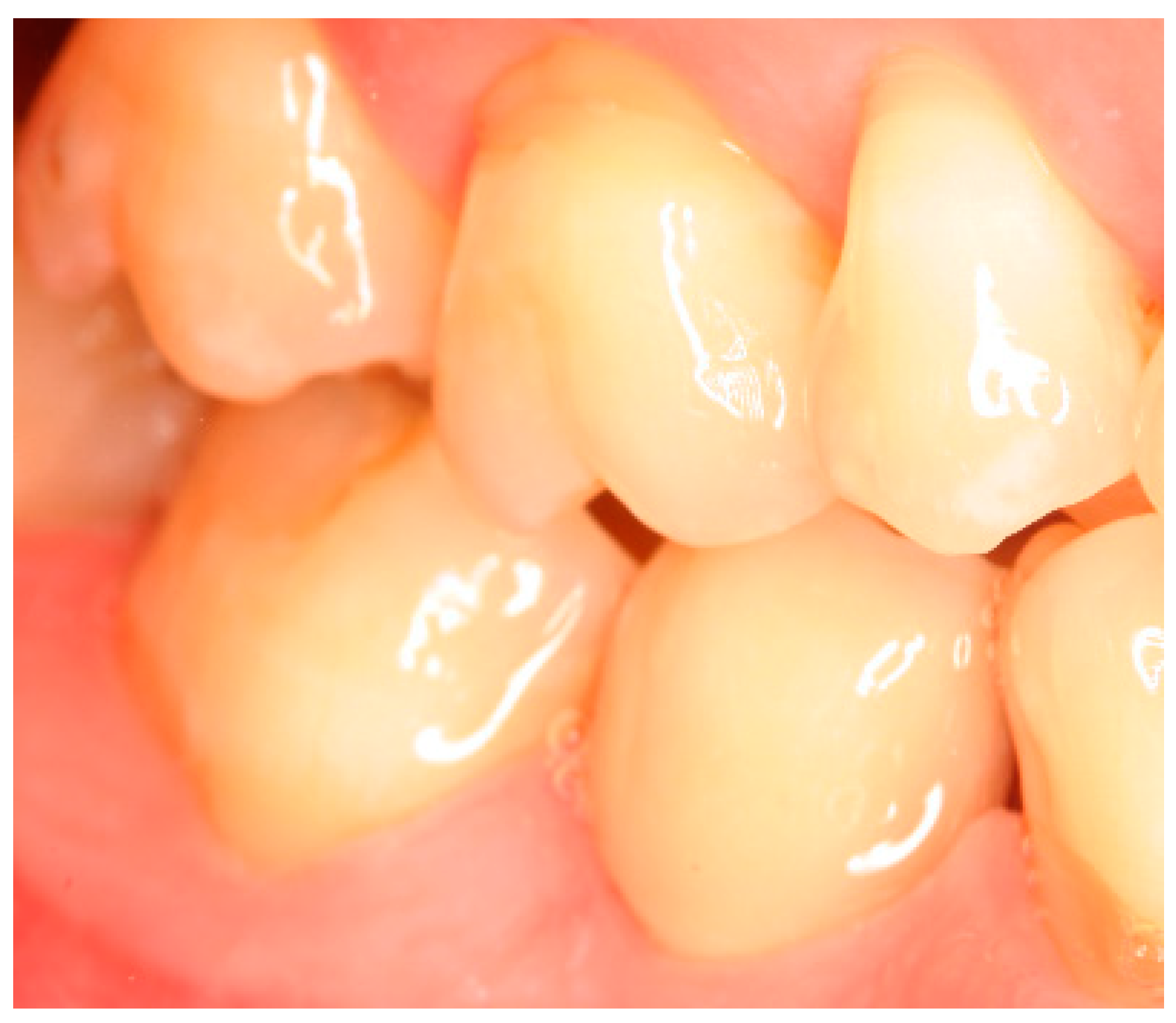
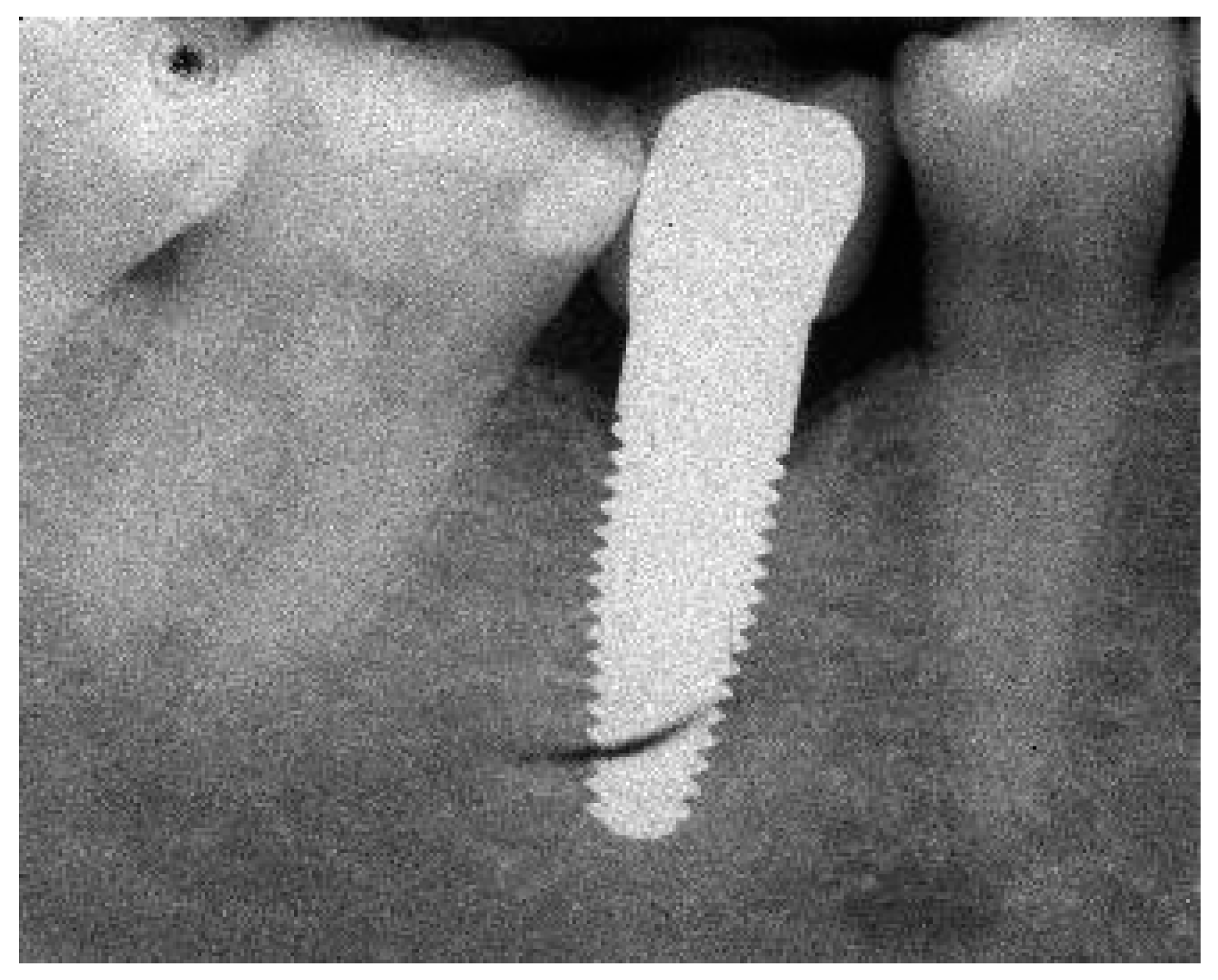
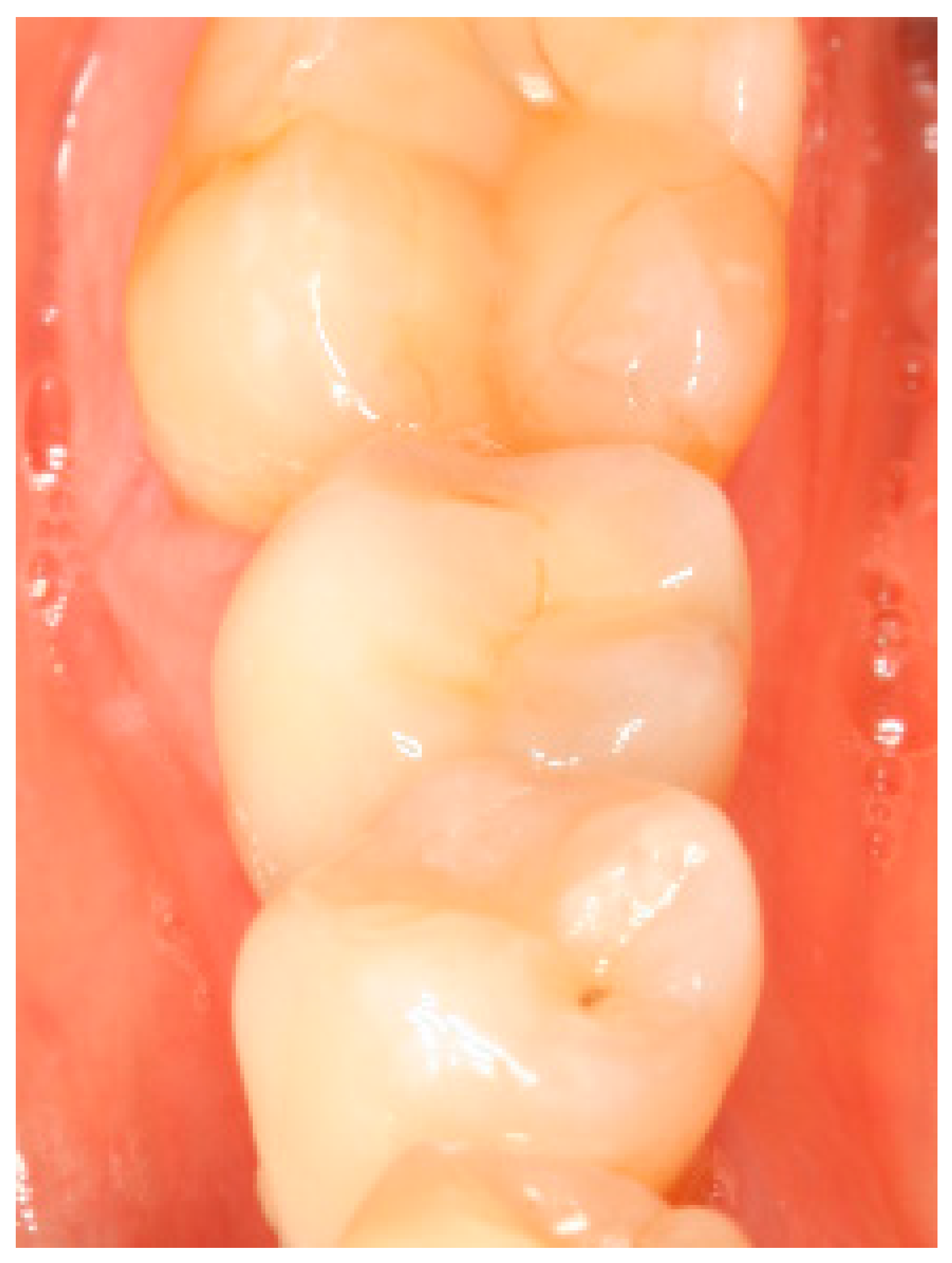
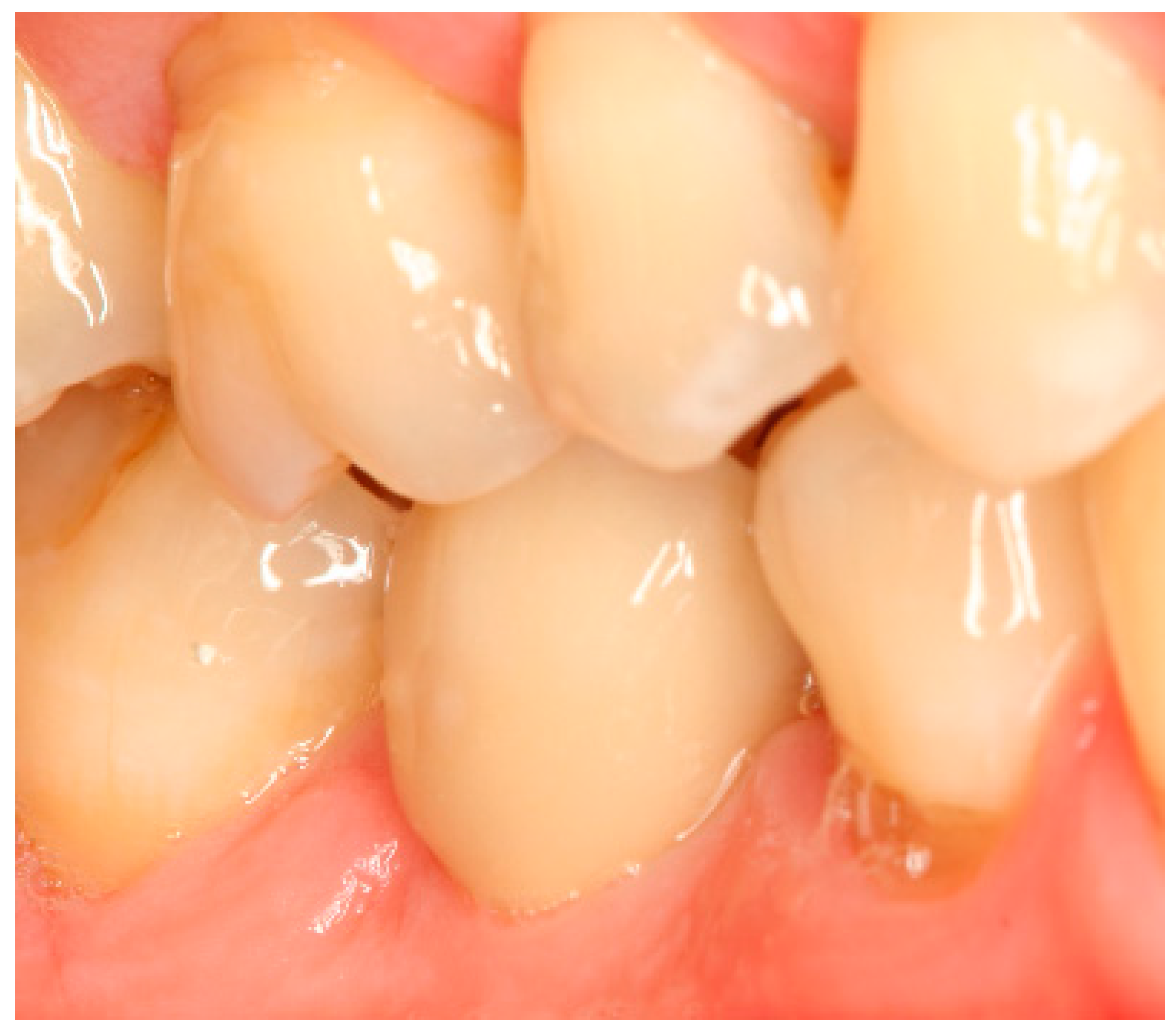

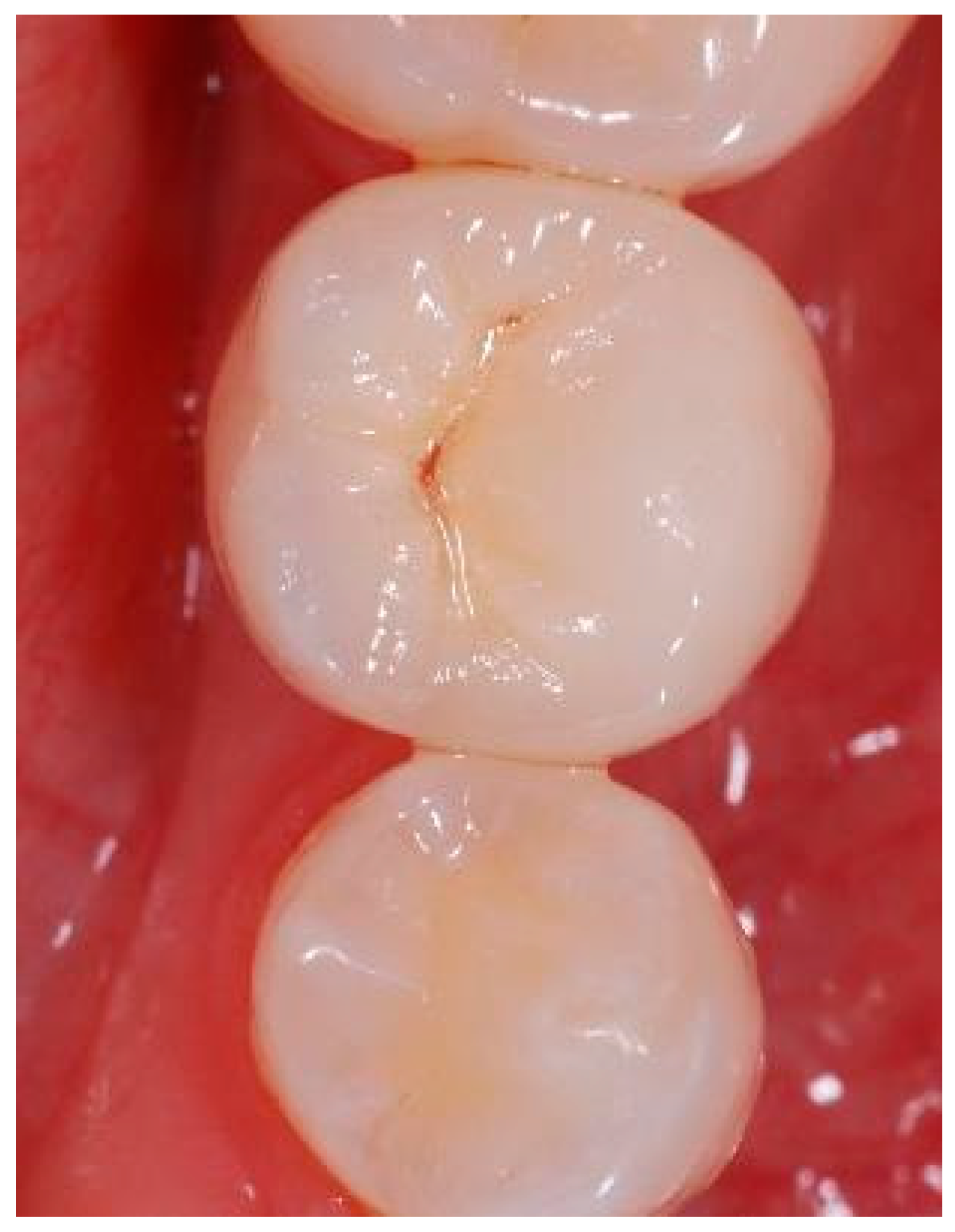
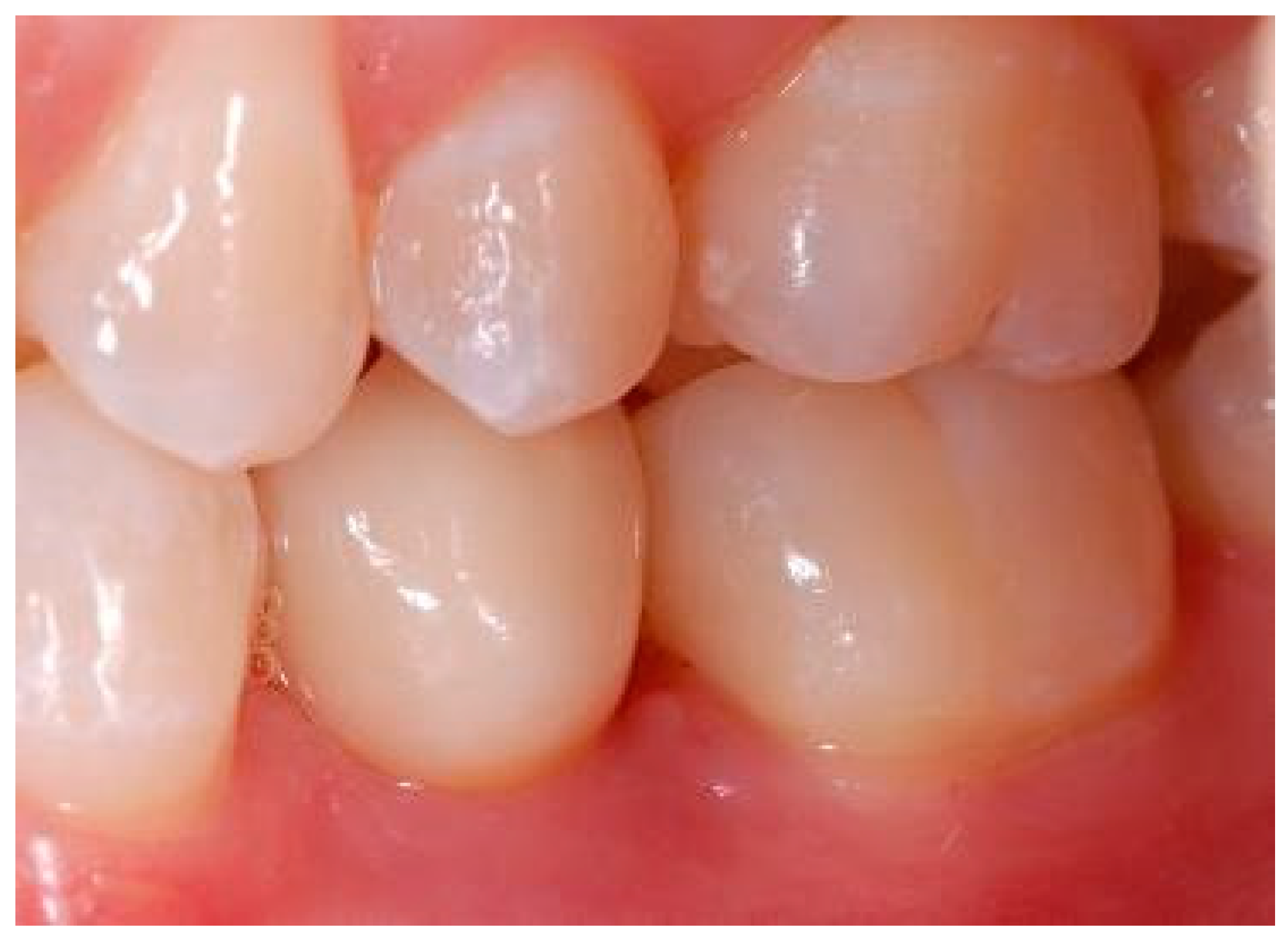
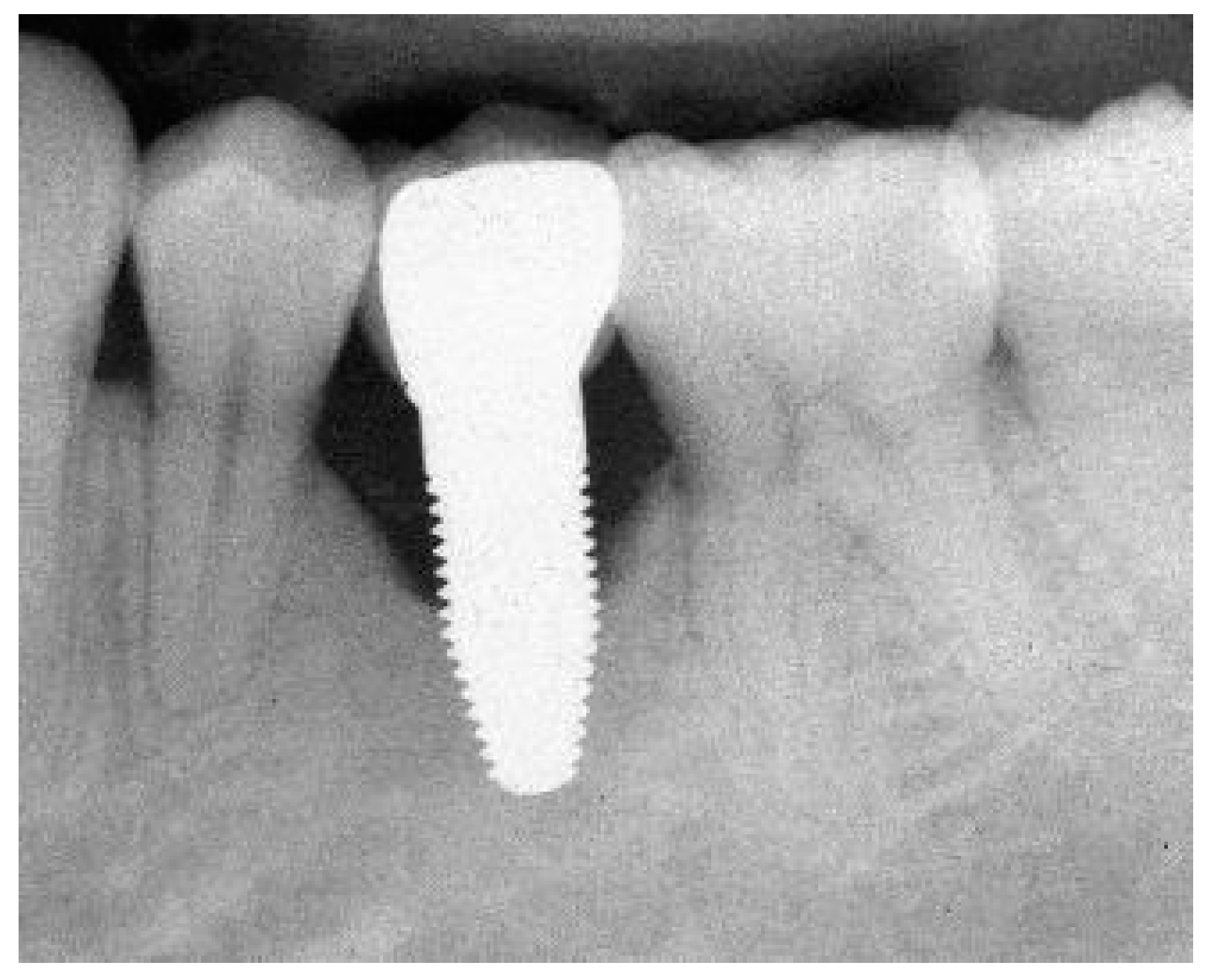
Appendix B. Exemplary Photographs and Radiographs from a Patient at Different Examination Time Points and a More Negative Outcome



References
- Lorusso, F.; Noumbissi, S.; Francesco, I.; Rapone, B.; Khater, A.G.A.; Scarano, A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials 2020, 13, 5534. [Google Scholar] [CrossRef]
- Kohal, R.-J.; Dennison, D.K. Clinical Longevity of Zirconia Implants with the Focus on Biomechanical and Biological Outcome. Curr. Oral Health Rep. 2020, 7, 344–351. [Google Scholar] [CrossRef]
- Carossa, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Pera, F. Full-Arch Rehabilitation Using Trans-Mucosal Tissue-Level Implants with and without Implant-Abutment Units: A Case Report. Dent. J. 2022, 10, 116. [Google Scholar] [CrossRef]
- Mombelli, A.; Hashim, D.; Cionca, N. What Is the Impact of Titanium Particles and Biocorrosion on Implant Survival and Complications? A Critical Review. Clin. Oral Implant. Res. 2018, 29, 37–53. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic Steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2017, 2017, 9246721. [Google Scholar] [CrossRef]
- Manzano, G.; Herrero, L.R.; Montero, J. Comparison of Clinical Performance of Zirconia Implants and Titanium Implants in Animal Models: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 311–320. [Google Scholar] [CrossRef]
- Bormann, K.-H.; Gellrich, N.-C.; Kniha, H.; Dard, M.; Wieland, M.; Gahlert, M. Biomechanical Evaluation of a Microstructured Zirconia Implant by a Removal Torque Comparison with a Standard Ti-SLA Implant. Clin. Oral Impl. Res. 2012, 23, 1210–1216. [Google Scholar] [CrossRef]
- Janner, S.F.M.; Gahlert, M.; Bosshardt, D.D.; Roehling, S.; Milz, S.; Higginbottom, F.; Buser, D.; Cochran, D.L. Bone Response to Functionally Loaded, Two-Piece Zirconia Implants: A Preclinical Histometric Study. Clin. Oral Impl. Res. 2018, 29, 277–289. [Google Scholar] [CrossRef]
- Kohal, R.J.; Bächle, M.; Att, W.; Chaar, S.; Altmann, B.; Renz, A.; Butz, F. Osteoblast and Bone Tissue Response to Surface Modified Zirconia and Titanium Implant Materials. Dent. Mater. 2013, 29, 763–776. [Google Scholar] [CrossRef]
- Bethke, A.; Pieralli, S.; Kohal, R.J.; Burkhardt, F.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Fracture Resistance of Zirconia Oral Implants in Vitro: A Systematic Review and Meta-Analysis. Materials 2020, 13, 562. [Google Scholar] [CrossRef]
- Burkhardt, F.; Spies, B.C.; Riemer, L.; Adolfsson, E.; Doerken, S.; Kohal, R. Fracture Resistance and Crystal Phase Transformation of a One- and a Two-piece Zirconia Implant with and without Simultaneous Loading and Aging—An in Vitro Study. Clin. Oral Impl. Res. 2021, 32, 1288–1298. [Google Scholar] [CrossRef]
- Spies, B.C.; Nold, J.; Vach, K.; Kohal, R.-J. Two-Piece Zirconia Oral Implants Withstand Masticatory Loads: An Investigation in the Artificial Mouth. J. Mech. Behav. Biomed. Mater. 2016, 53, 1–10. [Google Scholar] [CrossRef]
- de Avila, E.D.; Avila-Campos, M.J.; Vergani, C.E.; Spolidório, D.M.P.; Mollo Jr, F. de A. Structural and Quantitative Analysis of a Mature Anaerobic Biofilm on Different Implant Abutment Surfaces. J. Prosthet. Dent. 2016, 115, 428–436. [Google Scholar] [CrossRef]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial Colonization of Zirconia Ceramic Surfaces: An in Vitro and in Vivo Study. Int. J. Oral Maxillofac. Implant. 2002, 17, 793–798. [Google Scholar]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial Adhesion on Commercially Pure Titanium and Zirconium Oxide Disks: An In Vivo Human Study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic Contact Dermatitis Caused by Titanium Screws and Dental Implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.-L.; Giannobile, W.V. Is Metal Particle Release Associated with Peri-Implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-Temperature Degradation of Zirconia and Implications for Biomedical Implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Comisso, I.; Arias-Herrera, S.; Gupta, S. Zirconium Dioxide Implants as an Alternative to Titanium: A Systematic Review. J. Clin. Exp. Dent. 2021, 13, e511–e519. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical Performance of Zirconia Implants: A Meta-Review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone Tissue Responses to Surface-Modified Zirconia Implants: A Histomorphometric and Removal Torque Study in the Rabbit. Clin. Implant. Dent. Relat. Res. 2005, 7, s13–s20. [Google Scholar] [CrossRef]
- Adilstam, F.; Iverhed, M. Patent EP1670378A1. June 2006. Available online: https://patents.google.com/patent/EP1670378A1/en?inventor=Fredrik+Adilstam (accessed on 1 December 2022).
- Jemt, T. Regeneration of Gingival Papillae after Single-Implant Treatment. Int. J. Periodontics Restor. Dent. 1997, 17, 326–333. [Google Scholar]
- Mombelli, A.; Oosten, M.A.C.; Schürch, E.; Lang, N.P. The Microbiota Associated with Successful or Failing Osseointegrated Titanium Implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Östman, P.-O.; Hellman, M.; Albrektsson, T.; Sennerby, L. Direct Loading of Nobel Direct and Nobel Perfect One-Piece Implants: A 1-Year Prospective Clinical and Radiographic Study. Clin. Oral Implant. Res. 2007, 18, 409–418. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall/CRC Texts in Statistical Science; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-1-58488-039-4. [Google Scholar]
- Lang, N.P.; Berglundh, T.; Heitz-Mayfield, L.J.; Pjetursson, B.E.; Salvi, G.E.; Sanz, M. Consensus Statements and Recommended Clinical Procedures Regarding Implant Survival and Complications. Int. J. Oral Maxillofac. Implant. 2004, 19, 150–154. [Google Scholar]
- Kohal, R.-J.; Knauf, M.; Larsson, B.; Sahlin, H.; Butz, F. One-Piece Zirconia Oral Implants: One-Year Results from a Prospective Cohort Study. 1. Single Tooth Replacement. J. Clin. Periodontol. 2012, 39, 590–597. [Google Scholar] [CrossRef]
- Kohal, R.-J.; Spies, B.C.; Bauer, A.; Butz, F. One-Piece Zirconia Oral Implants for Single-Tooth Replacement: Three-Year Results from a Long-Term Prospective Cohort Study. J. Clin. Periodontol. 2018, 45, 114–124. [Google Scholar] [CrossRef]
- Grassi, F.R.; Capogreco, M.; Consonni, D.; Bilardi, G.; Buti, J.; Kalemaj, Z. Immediate Occlusal Loading of One-Piece Zirconia Implants: Five-Year Radiographic and Clinical Evaluation. Int. J. Oral Maxillofac. Implant. 2015, 30, 671–680. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Kohal, R.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia Implants Restored with Single Crowns or Fixed Dental Prostheses: 5-year Results of a Prospective Cohort Investigation. Clin. Oral Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef]
- Kohal, R.J.; Spies, B.C.; Vach, K.; Balmer, M.; Pieralli, S. A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results. J. Clin. Med. 2020, 9, 2585. [Google Scholar] [CrossRef]
- Lorenz, J.; Giulini, N.; Hölscher, W.; Schwiertz, A.; Schwarz, F.; Sader, R. Prospective Controlled Clinical Study Investigating Long-term Clinical Parameters, Patient Satisfaction, and Microbial Contamination of Zirconia Implants. Clin. Implant. Dent. Relat. Res. 2019, 21, 263–271. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and Outcome of Zirconia Dental Implants in Clinical Studies: A Meta-analysis. Clin. Oral Implant. Res. 2018, 29, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, I.; Lindhe, J. Probing Depth at Implants and Teeth. An Experimental Study in the Dog. J. Clin. Periodontol. 1993, 20, 623–627. [Google Scholar] [CrossRef]
- Gerber, J.A.; Tan, W.C.; Balmer, T.E.; Salvi, G.E.; Lang, N.P. Bleeding on Probing and Pocket Probing Depth in Relation to Probing Pressure and Mucosal Health around Oral Implants. Clin. Oral Implant. Res. 2009, 20, 75–78. [Google Scholar] [CrossRef]
- Lang, N.P.; Wetzel, A.C.; Stich, H.; Caffesse, R.G. Histologic Probe Penetration in Healthy and Inflamed Peri-Implant Tissues: Probing around Implants. Clin. Oral Implant. Res. 1994, 5, 191–201. [Google Scholar] [CrossRef]
- Listgarten, M.A.; Lang, N.P.; Schroeder, H.E.; Schroeder, A. Periodontal Tissues and Their Counterparts around Endosseous Implants: Periodontal versus “Peri-Implant” Tissues. Clin. Oral Implant. Res. 1991, 2, 1–19. [Google Scholar] [CrossRef]
- Kohal, R.-J.; Schwindling, F.S.; Bächle, M.; Spies, B.C. Peri-Implant Bone Response to Retrieved Human Zirconia Oral Implants after a 4-Year Loading Period: A Histologic and Histomorphometric Evaluation of 22 Cases: Peri-Implant Bone Response To Retrieved Human Zirconia Oral Implants. J. Biomed. Mater. Res. 2016, 104, 1622–1631. [Google Scholar] [CrossRef]
- Finne, K.; Rompen, E.; Toljanic, J. Clinical Evaluation of a Prospective Multicenter Study on 1-Piece Implants. Part 1: Marginal Bone Level Evaluation after 1 Year of Follow-Up. Int. J. Oral Maxillofac. Implant. 2007, 22, 226–234. [Google Scholar]
- Siepenkothen, T. Clinical Performance and Radiographic Evaluation of a Novel Single-Piece Implant in a Private Practice over a Mean of Seventeen Months. J. Prosthet. Dent. 2007, 97, S69–S78. [Google Scholar] [CrossRef]
- Albrektsson, T.; Gottlow, J.; Meirelles, L.; Östman, P.-O.; Rocci, A.; Sennerby, L. Survival of NobelDirect Implants: An Analysis of 550 Consecutively Placed Implants at 18 Different Clinical Centers. Clin. Implant. Dent. Rel. Res. 2007, 9, 65–70. [Google Scholar] [CrossRef]
- Nowzari, H.; Chee, W.; Yi, K.; Pak, M.; Chung, W.H.; Rich, S. Scalloped Dental Implants: A Retrospective Analysis of Radiographic and Clinical Outcomes of 17 NobelPerfectTM Implants in 6 Patients: Scalloped Dental Implants. Clin. Implant. Dent. Relat. Res. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Sennerby, L.; Rocci, A.; Becker, W.; Jonsson, L.; Johansson, L.-Å.; Albrektsson, T. Short-Term Clinical Results of Nobel Direct Implants: A Retrospective Multicentre Analysis. Clin. Oral Implant. Res. 2008, 19, 219–226. [Google Scholar] [CrossRef]
- Van de Velde, T.; Thevissen, E.; Persson, G.R.; Johansson, C.; De Bruyn, H. Two-Year Outcome with Nobel Direct® Implants: A Retrospective Radiographic and Microbiologic Study in 10 Patients. Clin. Implant. Dent. Relat. Res. 2009, 11, 183–193. [Google Scholar] [CrossRef]
- Zembić, A.; Johannesen, L.H.; Schou, S.; Malo, P.; Reichert, T.; Farella, M.; Hämmerle, C.H.F. Immediately Restored One-Piece Single-Tooth Implants with Reduced Diameter: One-Year Results of a Multi-Center Study: Immediately Restored One-Piece Implants. Clin. Oral Implant. Res. 2012, 23, 49–54. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.-S.; Wolkewitz, M.; Kohal, R. In Vivo Study of the Initial Bacterial Adhesion on Different Implant Materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef]
- Sanon, C. Lumière Sur La Zircone 3Y-TZP Utilisée En Implantologie Orale: Etude de La Relation Entre La Microstructure et La Durabilité; INS-Lyon: Villeurbanne, France, 2014. [Google Scholar]
- Song, F.; Parekh, S.; Hooper, L.; Loke, Y.; Ryder, J.; Sutton, A.; Hing, C.; Kwok, C.; Pang, C.; Harvey, I. Dissemination and Publication of Research Findings: An Updated Review of Related Biases. Health Technol. Assess. 2010, 14, 1–220. [Google Scholar] [CrossRef]

| Number of Patients | % | |
|---|---|---|
| 18–30 years | 16 | 24 |
| 31–40 years | 21 | 32 |
| 41–50 years | 17 | 26 |
| 51–70 years | 11 | 17 |
| Total | 65 | 100 |
| Upper Jaw | Lower Jaw | ||||
|---|---|---|---|---|---|
| Diameter | Length | Placed | Failed | Placed | Failed |
| Regular platform, ∅4.3 mm | 10 mm | 1 | 0 | 10 | 2 |
| 13 mm | 1 | 0 | 6 | 2 | |
| 16 mm | 3 | 0 | 0 | 0 | |
| Total | 5 | 0 | 16 | 4 | |
| Wide platform, ∅5.0 mm | 10 mm | 1 | 0 | 9 | 3 |
| 13 mm | 9 | 2 | 18 | 5 | |
| 16 mm | 3 | 0 | 5 | 0 | |
| Total | 13 | 2 | 32 | 12 | |
| Implant Insertion | 1 Year | 3 Years | 5 Years | |
|---|---|---|---|---|
| Followed patients | 65 | 61 | 56 | 48 |
| Patients with failed Implants | 0 | 3 | 6 | 13 |
| Missing forms | 0 | 1 | 3 | 4 |
| Total | 65 | 65 | 65 | 65 |
| Time Period | Total Implants | Failed Implants | Missing Forms | Cumulative Survival Rate (%) |
|---|---|---|---|---|
| Insertion to 1 year | 66 | 3 | 1 | 95.5 |
| 1 year to 3 years | 62 | 3 | 3 | 90.8 |
| 3 years to 5 years | 57 | 8 | 4 | 78.2 |
| 5 years | 48 |
| Cumulative Survival Rate | ||
|---|---|---|
| % | 95% CI | |
| Jaw type | ||
| Maxilla | 88.89 | 62.42–97.10 |
| Mandible | 74.23 | 59.08–84.47 |
| Ant-Post | ||
| Anterior | 100.00 | - |
| Posterior | 75.99 | 62.82–85.03 |
| Position | ||
| Posterior Mandible | 73.66 | 58.28–84.11 |
| Other positions | 89.47 | 64.08–97.26 |
| Smoking | ||
| No | 78.22 | 65.43–86.74 |
| Yes | 75.00 | 12.79–96.05 |
| Bruxism before treatment | ||
| No | 76.79 | 63.95–88.55 |
| Yes | 100.00 | |
| Bone quality | ||
| 1 | 100.00 | - |
| 2–3 | 77.61 | 65.12–86.10 |
| Bone quantity | ||
| A | 81.57 | 65.13–90.77 |
| B | 71.58 | 49.41–85.33 |
| C | 100.00 | - |
| D | - | - |
| Platform | ||
| RP | 80.67 | 56.31–92.28 |
| WP | 76.65 | 60.91–86.71 |
| Implant length | ||
| 10 mm | 75.63 | 50.95–89.08 |
| 13 mm | 73.20 | 54.79–85.07 |
| 16 mm | 100.00 | - |
| Flap design | ||
| No flap | 100.00 | - |
| Punch | 82.96 | 55.92–94.18 |
| Flap | 72.92 | 56.43–84.00 |
| Site | ||
| Immediate | 100.00 | - |
| Healed | 76.39 | 63.39–85.29 |
| Bone grafting | ||
| No | 75.92 | 59.85–86.26 |
| Yes | 82.13 | 59.03–92.91 |
| Insertion torque | ||
| ≤45 | 79.93 | 63.81–89.43 |
| >45 | 75.89 | 51.39–89.20 |
| Insertion to 1 Year | 1 Year to 3 Years | 3 Years to 5 Years | Total | |
|---|---|---|---|---|
| Pus | - | 12 | 23 (9) | 35 (9) |
| Plaque | - | - | 1 | 1 (0) |
| Peri-implantitis | - | 3 | 11 (3) | 14 (3) |
| Implant Insertion to Prosthesis Insertion | Implant Insertion to 1 Year Follow-Up | Implant Insertion to 3 Year Follow-Up | Implant Insertion to 5 Year Follow-Up | |||||
|---|---|---|---|---|---|---|---|---|
| Number | 59 | 56 | 55 | 41 | ||||
| Mean Value | −1.13 mm | −1.31 mm | −1.45 mm | −1.12 mm | ||||
| SD | 1.47 mm | 1.49 mm | 1.96 mm | 1.83 mm | ||||
| n | % | n | % | n | % | n | % | |
| >0 mm | 8 | 14 | 7 | 13 | 12 | 22 | 8 | 20 |
| 0 mm | 1 | 2 | 0 | 0 | 1 | 2 | 1 | 2 |
| −0.1–−1.0 mm | 23 | 39 | 20 | 36 | 13 | 24 | 12 | 29 |
| −1.1–−2.0 mm | 11 | 19 | 10 | 18 | 10 | 18 | 9 | 22 |
| −2.1–−3.0 mm | 9 | 15 | 11 | 20 | 7 | 13 | 6 | 15 |
| −3.1–−4.0 mm | 3 | 5 | 5 | 9 | 7 | 13 | 2 | 5 |
| < −4.0 mm | 4 | 7 | 3 | 5 | 5 | 9 | 3 | 7 |
| Difference | Correlation | |||||
|---|---|---|---|---|---|---|
| Implants a | Mean (SD) | 95% CI | p Value | r | p Value | |
| Jaw type | ||||||
| Maxilla | 9 | −0.63 (2.2) | −1.1 to 2.4 | 0.40 | ||
| Mandible | 32 | −1.26 (1.7) | ||||
| Ant-Post | ||||||
| Anterior | 3 | −1.45 (3.9) | −9.8 to 9.1 | 0.58 | ||
| Posterior | 38 | −1.10 (1.7) | ||||
| Position | ||||||
| Posterior Mandible | 31 | −1.21 (1.7) | −2.0 to 1.3 | 0.65 | ||
| Other positions | 10 | −0.84 (2.2) | ||||
| Smoking | ||||||
| No | 40 | −1.02 (1.7) | 0.59 | 7.67 | ||
| Yes | 1 | −5.15 (-) | ||||
| Bruxism before treatment | ||||||
| No | 38 | −1.24 (1.9) | −3.6 to 0.3 | 0.10 | ||
| Yes | 3 | −0.42 (1.0) | ||||
| Bone quality | ||||||
| 1 | 1 | −1.85 (-) | −4.5 to 3.0 | 0.53 | ||
| 2–3 | 40 | −1.10 (1.8) | ||||
| Bone quantity | ||||||
| A | 25 | −1.20 | 0.15 | 0.61 | ||
| B | 15 | −1.27 | ||||
| C | 1 | 2.90 | ||||
| D | 0 | - | ||||
| Bone level at placement | 41 | −0.36 | 0.36 | |||
| Platform | ||||||
| RP | 13 | −0.60 (1.7) | −0.5 to 2.0 | 0.09 | ||
| WP | 28 | −1.36 (1.9) | ||||
| Implant length | ||||||
| 10 mm | 13 | −1.03 (1.5) | −0.15 | 0.28 | ||
| 13 mm | 20 | −0.86 (1.6) | ||||
| 16 mm | 8 | −1.93 (2.7) | ||||
| Flap design | ||||||
| No flap | 3 | −3.55 (2.2) | 0.29 | 0.16 | ||
| Punch | 10 | −0.97 (2.0) | ||||
| Flap | 28 | −0.92 (1.6) | ||||
| Site | ||||||
| Immediate | 2 | −0.80 (5.2) | −45.7 to 46.3 | 0.95 | ||
| Healed | 39 | −1.14 (1.7) | ||||
| Bone grafting | ||||||
| No | 26 | −1.10 (1.7) | −1.2 to 1.4 | 0.88 | ||
| Yes | 15 | −1.16 (2.1) | ||||
| Insertion torque | ||||||
| ≤45 | 27 b | −1.11 (1.7) | −0.9 to 1.6 | 0.60 | ||
| >45 | 13 b | −1.46 (1.8) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohal, R.-J.; Burkhardt, F.; Chevalier, J.; Patzelt, S.B.M.; Butz, F. One-Piece Zirconia Oral Implants for Single Tooth Replacement: Five-Year Results from a Prospective Cohort Study. J. Funct. Biomater. 2023, 14, 116. https://doi.org/10.3390/jfb14020116
Kohal R-J, Burkhardt F, Chevalier J, Patzelt SBM, Butz F. One-Piece Zirconia Oral Implants for Single Tooth Replacement: Five-Year Results from a Prospective Cohort Study. Journal of Functional Biomaterials. 2023; 14(2):116. https://doi.org/10.3390/jfb14020116
Chicago/Turabian StyleKohal, Ralf-Joachim, Felix Burkhardt, Jerome Chevalier, Sebastian Berthold Maximilian Patzelt, and Frank Butz. 2023. "One-Piece Zirconia Oral Implants for Single Tooth Replacement: Five-Year Results from a Prospective Cohort Study" Journal of Functional Biomaterials 14, no. 2: 116. https://doi.org/10.3390/jfb14020116
APA StyleKohal, R.-J., Burkhardt, F., Chevalier, J., Patzelt, S. B. M., & Butz, F. (2023). One-Piece Zirconia Oral Implants for Single Tooth Replacement: Five-Year Results from a Prospective Cohort Study. Journal of Functional Biomaterials, 14(2), 116. https://doi.org/10.3390/jfb14020116









