Virus-like Particles for TEM Regulation and Antitumor Therapy
Abstract
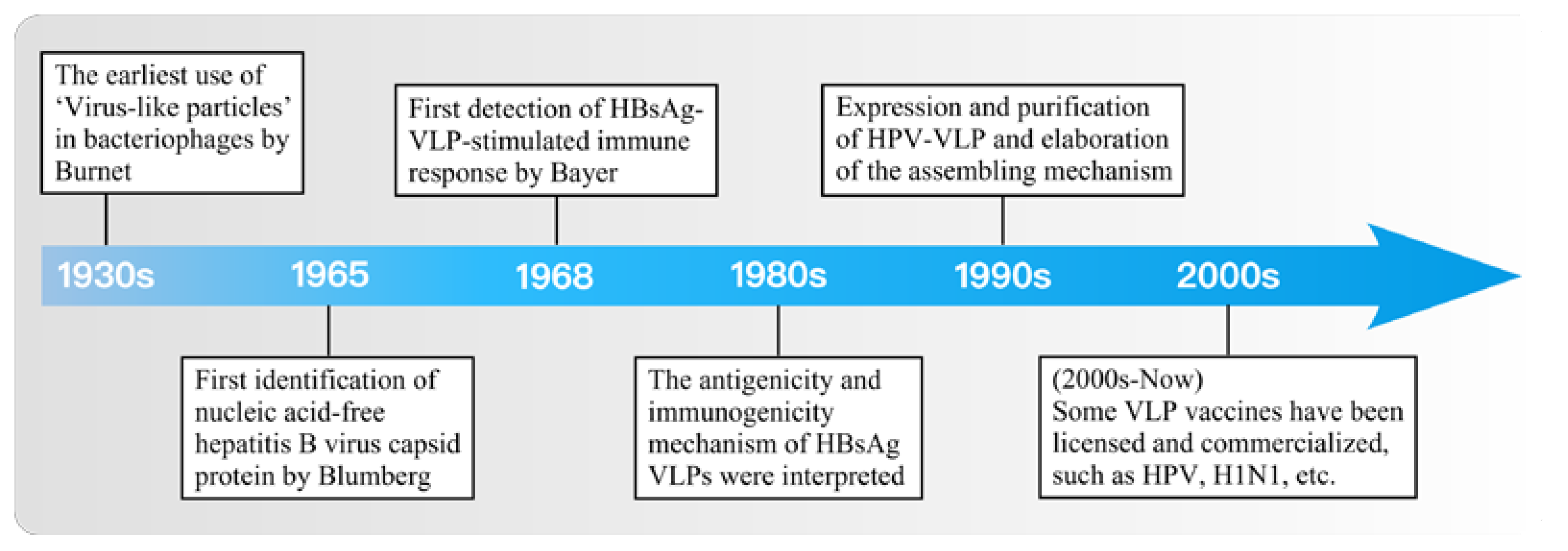
1. Introduction
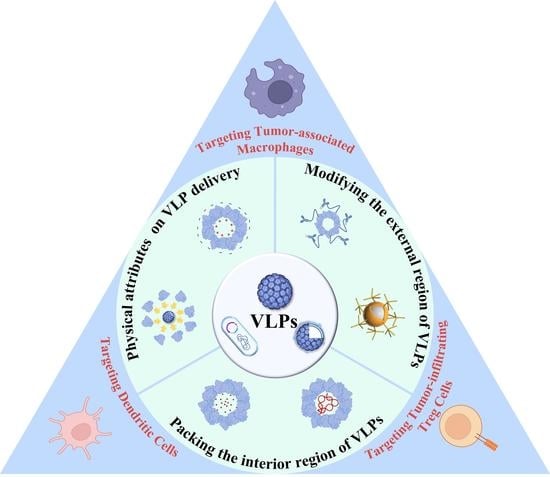
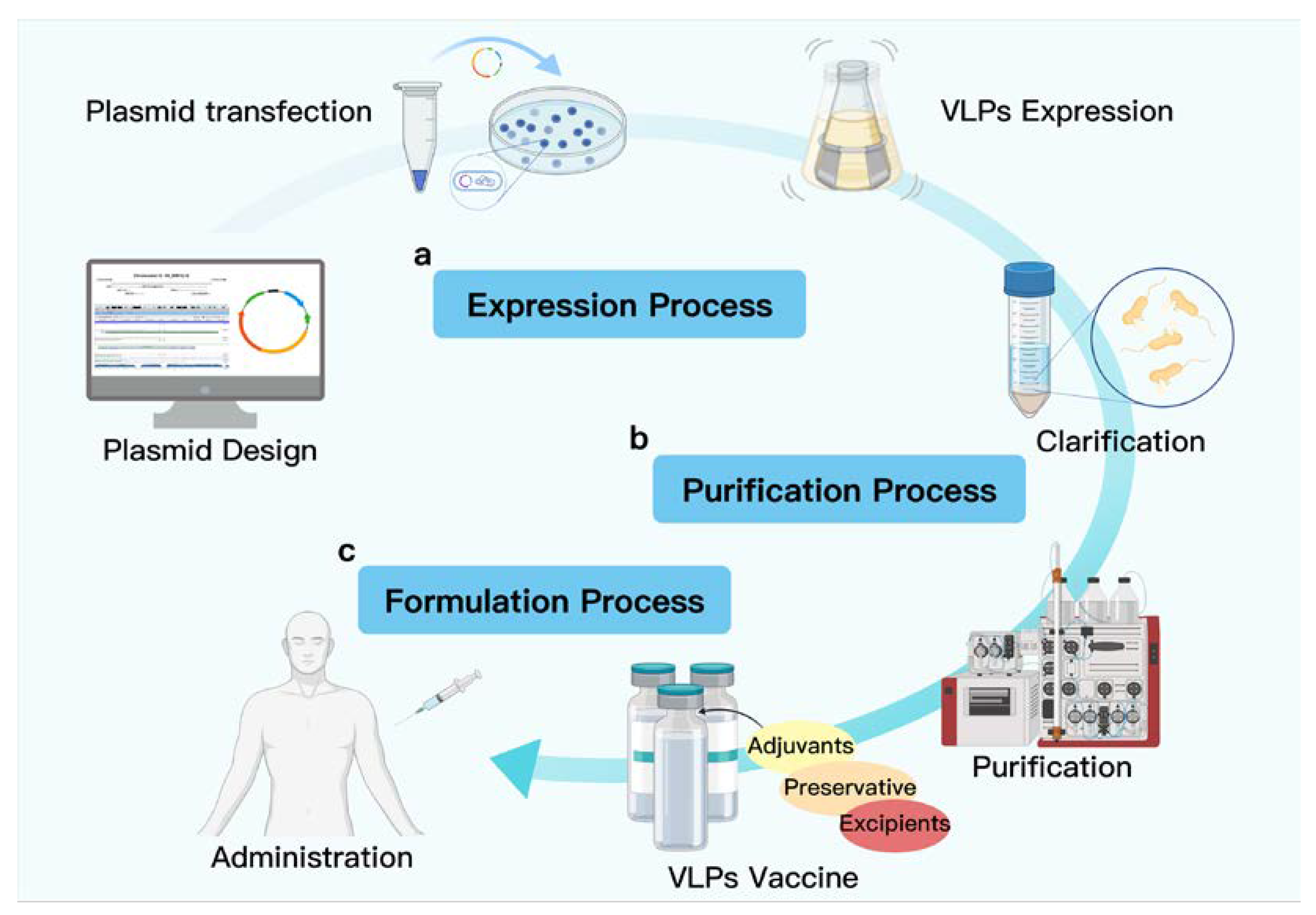
2. Research and Development of VLP-Based Vaccine Design
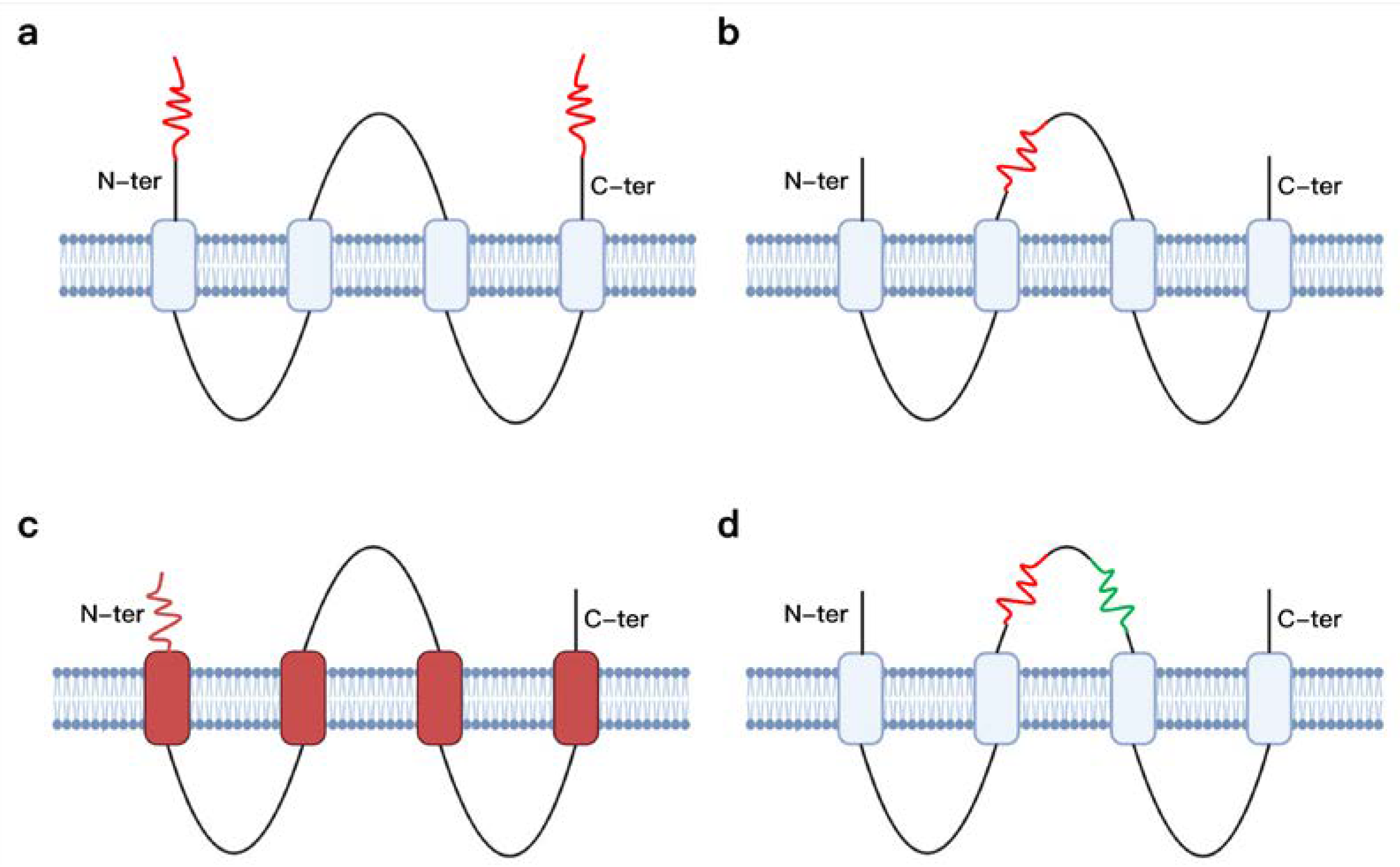
2.1. Impact of Physical Attributes on VLP Delivery
2.2. Modifying the External Region of VLPs for Robust Humoral and Cellular Immunity
2.3. Packing the Interior Region of VLPs for Effective Adjuvant Effect
3. VLPs for TEM Regulation against Cancer
3.1. Targeting Dendritic Cells
3.2. Targeting Tumor-Associated Macrophages
3.3. Targeting Tumor-Infiltrating Treg Cells
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- White, A.C.; Lowry, W.E. Refining the Role for Adult Stem Cells as Cancer Cells of Origin. Trends Cell Biol. 2015, 25, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer Incidence and Mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-Assembled Peptide and Protein Nanostructures for Anti-Cancer Therapy: Targeted Delivery, Stimuli-Responsive Devices and Immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef]
- Lazzati, A.; Epaud, S.; Ortala, M.; Katsahian, S.; Lanoy, E. Effect of Bariatric Surgery on Cancer Risk: Results from an Emulated Target Trial Using Population-Based Data. Br. J. Surg. 2022, 109, 433–438. [Google Scholar] [CrossRef]
- Wu, J.; Waxman, D.J. Immunogenic Chemotherapy: Dose and Schedule Dependence and Combination with Immunotherapy. Cancer Lett. 2018, 419, 210–221. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Zhang, Y.; Artzi, N. Local Triple-Combination Therapy Results in Tumour Regression and Prevents Recurrence in a Colon Cancer Model. Nat. Mater 2016, 15, 1128–1138. [Google Scholar] [CrossRef]
- Tao, Z.; Dang, X.; Huang, X.; Muzumdar, M.D.; Xu, E.S.; Bardhan, N.M.; Song, H.; Qi, R.; Yu, Y.; Li, T.; et al. Early Tumor Detection Afforded by in Vivo Imaging of Near-Infrared II Fluorescence. Biomaterials 2017, 134, 202–215. [Google Scholar] [CrossRef]
- Baker, A.E.G.; Bahlmann, L.C.; Tam, R.Y.; Liu, J.C.; Ganesh, A.N.; Mitrousis, N.; Marcellus, R.; Spears, M.; Bartlett, J.M.S.; Cescon, D.W.; et al. Benchmarking to the Gold Standard: Hyaluronan-Oxime Hydrogels Recapitulate Xenograft Models with In Vitro Breast Cancer Spheroid Culture. Adv. Mater. 2019, 31, 1901166. [Google Scholar] [CrossRef] [PubMed]
- Gilam, A.; Conde, J.; Weissglas-Volkov, D.; Oliva, N.; Friedman, E.; Artzi, N.; Shomron, N. Local MicroRNA Delivery Targets Palladin and Prevents Metastatic Breast Cancer. Nat. Commun. 2016, 7, 12868. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Abuzar, S.M.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Oxaliplatin-Loaded Chemically Cross-Linked Hydrogels for Prevention of Postoperative Abdominal Adhesion and Colorectal Cancer Therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bagley, A.F.; Hill, S.; Rogers, G.S.; Bhatia, S.N. Plasmonic Photothermal Heating of Intraperitoneal Tumors through the Use of an Implanted Near-Infrared Source. ACS Nano 2013, 7, 8089–8097. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Rahmani Moghadam, E.; Raei, M.; Kalantari, M.; Tavakol, S.; Mohammadinejad, R.; Najafi, M.; et al. Progress in Natural Compounds/SiRNA Co-Delivery Employing Nanovehicles for Cancer Therapy. ACS Comb. Sci. 2020, 22, 669–700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rao, J.; Pu, K. Recent Progress on Semiconducting Polymer Nanoparticles for Molecular Imaging and Cancer Phototherapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Zahir, N.; Sun, R.; Gallahan, D.; Gatenby, R.A.; Curtis, C. Characterizing the Ecological and Evolutionary Dynamics of Cancer. Nat. Genet. 2020, 52, 759–767. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Choi, S.H. Tumor-Associated Macrophages in Cancer: Recent Advancements in Cancer Nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Darcy, P.W.; Khan, I.Z.; Moon, C.S.; Kornberg, A.E.; Schneider, V.S.; Alvarez, Y.; Eleso, O.; Zhu, C.; Schernthanner, M.; et al. TCR-Vγδ Usage Distinguishes Protumor from Antitumor Intestinal Γδ T Cell Subsets. Science 2022, 377, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kuen, D.-S.; Kim, B.-S.; Chung, Y. IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Immune Netw. 2020, 20, e6. [Google Scholar] [CrossRef]
- Tang, T.; Huang, X.; Zhang, G.; Hong, Z.; Bai, X.; Liang, T. Advantages of Targeting the Tumor Immune Microenvironment over Blocking Immune Checkpoint in Cancer Immunotherapy. Sig. Transduct. Target. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Ma, J.; Huang, L.; Hu, D.; Zeng, S.; Han, Y.; Shen, H. The Role of the Tumor Microbe Microenvironment in the Tumor Immune Microenvironment: Bystander, Activator, or Inhibitor? J. Exp. Clin. Cancer Res. 2021, 40, 327. [Google Scholar] [CrossRef]
- Caldeira, J.C.; Perrine, M.; Pericle, F.; Cavallo, F. Virus-Like Particles as an Immunogenic Platform for Cancer Vaccines. Viruses 2020, 12, 488. [Google Scholar] [CrossRef]
- He, J.; Yu, L.; Lin, X.; Liu, X.; Zhang, Y.; Yang, F.; Deng, W. Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds. Viruses 2022, 14, 1905. [Google Scholar] [CrossRef]
- Yan, D.; Wei, Y.-Q.; Guo, H.-C.; Sun, S.-Q. The Application of Virus-like Particles as Vaccines and Biological Vehicles. Appl. Microbiol. Biotechnol. 2015, 99, 10415–10432. [Google Scholar] [CrossRef]
- Hyman, P.; Trubl, G.; Abedon, S.T. Virus-Like Particle: Evolving Meanings in Different Disciplines. PHAGE 2021, 2, 11–15. [Google Scholar] [CrossRef]
- Suffian, I.F.B.M.; Al-Jamal, K.T. Bioengineering of Virus-like Particles as Dynamic Nanocarriers for in Vivo Delivery and Targeting to Solid Tumours. Adv. Drug Deliv. Rev. 2022, 180, 114030. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, M. A Smart Viral Vector for Targeted Delivery of Hydrophobic Drugs. Sci. Rep. 2021, 11, 7030. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kwon, Y.-M.; Lee, Y.-T.; Kim, M.-C.; Hwang, H.; Ko, E.-J.; Lee, Y.; Choi, H.-J.; Kang, S.-M. Virus-Like Particles Are a Superior Platform for Presenting M2e Epitopes to Prime Humoral and Cellular Immunity against Influenza Virus. Vaccines 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Z.-H.; Li, Y.-X.; Xu, H.-L.; Fang, W.-H.; He, F. Targeted Delivery of Nanovaccine to Dendritic Cells via DC-Binding Peptides Induces Potent Antiviral Immunity in Vivo. IJN 2022, 17, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, N.K.; Kim, I.-S. Bioengineered Protein-Based Nanocage for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 157–171. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major Findings and Recent Advances in Virus–like Particle (VLP)-Based Vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Lima, T.M.; Souza, M.O.; Castilho, L.R. Purification of Flavivirus VLPs by a Two-Step Chomatographic Process. Vaccine 2019, 37, 7061–7069. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Kumar, D.N.; Shaik, R.A.; Eid, B.G.; Abdel-Naim, A.B.; Md, S.; Ahmad, A.; Agrawal, A.K. Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy. IJMS 2022, 23, 10068. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent Developments in Functionalized Polymer Nanoparticles for Efficient Drug Delivery System. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, Y.; Im, G.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic Nanoparticles Applied as Functional Therapeutics. Adv. Funct. Mater. 2021, 31, 2008171. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines Against Emerging Infectious Diseases. Front. Microbiol. 2022, 12, 790121. [Google Scholar] [CrossRef] [PubMed]
- Syomin, B.V.; Ilyin, Y.V. Virus-Like Particles as an Instrument of Vaccine Production. Mol Biol 2019, 53, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, B.; Yin, S.; Li, X.; Zhao, D.; Wang, W.; Bi, J.; Su, Z. In Vitro Preparation of Uniform and Nucleic Acid Free Hepatitis B Core Particles through an Optimized Disassembly-Purification-Reassembly Process. Protein Expr. Purif. 2021, 178, 105747. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Gomes, A.C.; Cabral-Miranda, G.; Krueger, C.C.; Leoratti, F.M.; Stein, J.V.; Bachmann, M.F. Delivering Adjuvants and Antigens in Separate Nanoparticles Eliminates the Need of Physical Linkage for Effective Vaccination. J. Control. Release 2017, 251, 92–100. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Ye, D.; Zimmermann, T.; Demina, V.; Sotnikov, S.; Ried, C.L.; Rahn, H.; Stapf, M.; Untucht, C.; Rohe, M.; Terstappen, G.C.; et al. Trafficking of JC Virus-like Particles across the Blood–Brain Barrier. Nanoscale Adv. 2021, 3, 2488–2500. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Tamminen, K.; Heinimäki, S.; Gröhn, S.; Blazevic, V. Internalization and Antigen Presentation by Mouse Dendritic Cells of Rotavirus VP6 Preparations Differing in Nanostructure. Mol. Immunol. 2020, 123, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zinkhan, S.; Ogrina, A.; Balke, I.; Reseviča, G.; Zeltins, A.; de Brot, S.; Lipp, C.; Chang, X.; Zha, L.; Vogel, M.; et al. The Impact of Size on Particle Drainage Dynamics and Antibody Response. J. Control. Release 2021, 331, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Cai, X.; Yang, Y. Genetic Engineering Strategies for Construction of Multivalent Chimeric VLPs Vaccines. Expert Rev. Vaccines 2020, 19, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Dishlers, A.; Skrastina, D.; Renhofa, R.; Petrovskis, I.; Ose, V.; Lieknina, I.; Jansons, J.; Pumpens, P.; Sominskaya, I. The Hepatitis B Virus Core Variants That Expose Foreign C-Terminal Insertions on the Outer Surface of Virus-Like Particles. Mol. Biotechnol. 2015, 57, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Hyakumura, M.; Walsh, R.; Thaysen-Andersen, M.; Kingston, N.J.; La, M.; Lu, L.; Lovrecz, G.; Packer, N.H.; Locarnini, S.; Netter, H.J. Modification of Asparagine-Linked Glycan Density for the Design of Hepatitis B Virus Virus-Like Particles with Enhanced Immunogenicity. J. Virol. 2015, 89, 11312–11322. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Z.; Yang, Y.; Ma, X.; An, W.; Ma, G.; Su, Z.; Zhang, S. A Biomimetic VLP Influenza Vaccine with Interior NP/Exterior M2e Antigens Constructed through a Temperature Shift-Based Encapsulation Strategy. Vaccine 2020, 38, 5987–5996. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zheng, W.; Pan, S.; Zhang, S.; Xie, Y.; Guo, H.; Wang, G.; Li, Z.; Luo, M. A Virus-like Particle of the Hepatitis B Virus PreS Antigen Elicits Robust Neutralizing Antibodies and T Cell Responses in Mice. Antivir. Res. 2018, 149, 48–57. [Google Scholar] [CrossRef]
- Peyret, H.; Gehin, A.; Thuenemann, E.C.; Blond, D.; El Turabi, A.; Beales, L.; Clarke, D.; Gilbert, R.J.C.; Fry, E.E.; Stuart, D.I.; et al. Tandem Fusion of Hepatitis B Core Antigen Allows Assembly of Virus-Like Particles in Bacteria and Plants with Enhanced Capacity to Accommodate Foreign Proteins. PLoS ONE 2015, 10, e0120751. [Google Scholar] [CrossRef]
- Miyazaki, N.; Taylor, D.W.; Hansman, G.S.; Murata, K. Antigenic and Cryo-Electron Microscopy Structure Analysis of a Chimeric Sapovirus Capsid. J. Virol. 2016, 90, 2664–2675. [Google Scholar] [CrossRef]
- Stephanopoulos, N.; Francis, M.B. Choosing an Effective Protein Bioconjugation Strategy. Nat. Chem. Biol. 2011, 7, 876–884. [Google Scholar] [CrossRef]
- Patel, K.G.; Swartz, J.R. Surface Functionalization of Virus-Like Particles by Direct Conjugation Using Azide−Alkyne Click Chemistry. Bioconjugate Chem. 2011, 22, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, G.; Saul, A.; MacLennan, C.A.; Micoli, F. Click Chemistry Applied to the Synthesis of Salmonella Typhimurium O-Antigen Glycoconjugate Vaccine on Solid Phase with Sugar Recycling. Bioconjugate Chem. 2015, 26, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xuan, B.; Ye, X.; Huang, Z.; Qian, Z. A Modular Vaccine Development Platform Based on Sortase-Mediated Site-Specific Tagging of Antigens onto Virus-Like Particles. Sci. Rep. 2016, 6, 25741. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, W.; Mao, H.; Zhang, W.; Yin, G.; Zhang, X.; Li, F. Switch from Polymorphic to Homogenous Self-Assembly of Virus-Like Particles of Simian Virus 40 through Double-Cysteine Substitution. Small 2020, 16, 2004484. [Google Scholar] [CrossRef]
- Singh, S.K.; Thrane, S.; Janitzek, C.M.; Nielsen, M.A.; Theander, T.G.; Theisen, M.; Salanti, A.; Sander, A.F. Improving the Malaria Transmission-Blocking Activity of a Plasmodium Falciparum 48/45 Based Vaccine Antigen by SpyTag/SpyCatcher Mediated Virus-like Display. Vaccine 2017, 35, 3726–3732. [Google Scholar] [CrossRef]
- Brune, K.D.; Howarth, M. New Routes and Opportunities for Modular Construction of Particulate Vaccines: Stick, Click, and Glue. Front. Immunol. 2018, 9, 1432. [Google Scholar] [CrossRef]
- Pulendran, B.S.; Arunachalam, P.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Hills, R.A.; Howarth, M. Virus-like Particles against Infectious Disease and Cancer: Guidance for the Nano-Architect. Curr. Opin. Biotechnol. 2022, 73, 346–354. [Google Scholar] [CrossRef]
- Mohsen, M.; Gomes, A.; Vogel, M.; Bachmann, M. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef]
- Jobsri, J.; Allen, A.; Rajagopal, D.; Shipton, M.; Kanyuka, K.; Lomonossoff, G.P.; Ottensmeier, C.; Diebold, S.S.; Stevenson, F.K.; Savelyeva, N. Plant Virus Particles Carrying Tumour Antigen Activate TLR7 and Induce High Levels of Protective Antibody. PLoS ONE 2015, 10, e0118096. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of Double-Stranded RNA and Activation of NF-ΚB by Toll-like Receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Terhuja, M.; Saravanan, P.; Tamilselvan, R.P. Comparative Efficacy of Virus like Particle (VLP) Vaccine of Foot-and-Mouth-Disease Virus (FMDV) Type O Adjuvanted with Poly I:C or CpG in Guinea Pigs. Biologicals 2015, 43, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Choi, S.-Y.; Jung, N.-C.; Song, J.-Y.; Seo, H.G.; Lee, H.S.; Lim, D.-S. The Effect of the Tumor Microenvironment and Tumor-Derived Metabolites on Dendritic Cell Function. J. Cancer 2020, 11, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, N.; Wu, J.; Wang, X.; Wang, W.; Liu, Y.-J.; Chen, J. Tumor Microenvironment-Related Dendritic Cell Deficiency: A Target to Enhance Tumor Immunotherapy. Pharmacol. Res. 2020, 159, 104980. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Jarvis, C.M.; Hincapie, R.; McKay, C.S.; Schimer, J.; Sanhueza, C.A.; Xu, K.; Diehl, R.C.; Finn, M.G.; Kiessling, L.L. Glycan-Modified Virus-like Particles Evoke T Helper Type 1-like Immune Responses. ACS Nano 2021, 15, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Peers-Adams, A.; Win, S.J.; Scullion, S.; Wilson, M.; Young, V.L.; Jennings, P.; Ward, V.K.; Baird, M.A.; Young, S.L. Antigen Incorporated In Virus-like Particles Is Delivered to Specific Dendritic Cell Subsets That Induce An Effective Antitumor Immune Response In Vivo. J. Immunother. 2013, 36, 11–19. [Google Scholar] [CrossRef]
- Gomes, A.C.; Flace, A.; Saudan, P.; Zabel, F.; Cabral-Miranda, G.; Turabi, A.E.; Manolova, V.; Bachmann, M.F. Adjusted Particle Size Eliminates the Need of Linkage of Antigen and Adjuvants for Appropriated T Cell Responses in Virus-Like Particle-Based Vaccines. Front. Immunol. 2017, 8, 226. [Google Scholar] [CrossRef]
- Lin, K.; Roosinovich, E.; Ma, B.; Hung, C.-F.; Wu, T.-C. Therapeutic HPV DNA Vaccines. Immunol. Res. 2010, 47, 86–112. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Umezu, D.; Okada, N.; Sakoda, Y.; Adachi, K.; Ojima, T.; Yamaue, H.; Eto, M.; Tamada, K. Inhibitory Functions of PD-L1 and PD-L2 in the Regulation of Anti-Tumor Immunity in Murine Tumor Microenvironment. Cancer Immunol. Immunother. 2019, 68, 201–211. [Google Scholar] [CrossRef]
- Cai, H.; Shukla, S.; Steinmetz, N.F. The Antitumor Efficacy of CpG Oligonucleotides Is Improved by Encapsulation in Plant Virus-Like Particles. Adv. Funct. Mater. 2020, 30, 1908743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Zheng, H.; He, Y.; Jia, H.; Zhang, L.; Lin, C.; Chen, S.; Zheng, J.; Yang, Q.; et al. In Situ Biomimetic Nanoformulation for Metastatic Cancer Immunotherapy. Acta Biomater. 2021, 134, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.I.; Barsoumian, H.B.; Sezen, D.; Verma, V.; Patel, R.; Wasley, M.; Hu, Y.; Dunn, J.D.; He, K.; Chen, D.; et al. Addition of TLR9 Agonist Immunotherapy to Radiation Improves Systemic Antitumor Activity. Transl. Oncol. 2021, 14, 100983. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.N.R.; Bittner, Z.; Liu, X.; Dang, T.-M.; Radsak, M.P.; Brunner, C. Bruton’s Tyrosine Kinase: An Emerging Key Player in Innate Immunity. Front. Immunol. 2017, 8, 1454. [Google Scholar] [CrossRef]
- Gagliani, N.; Magnani, C.F.; Huber, S.; Gianolini, M.E.; Pala, M.; Licona-Limon, P.; Guo, B.; Herbert, D.R.; Bulfone, A.; Trentini, F.; et al. Coexpression of CD49b and LAG-3 Identifies Human and Mouse T Regulatory Type 1 Cells. Nat. Med. 2013, 19, 739–746. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T Cells in Cancer Immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef]
- Palameta, S.; Manrique-Rincón, A.J.; Toscaro, J.M.; Semionatto, I.F.; Fonseca, M.C.; Rosa, R.S.M.; Ruas, L.P.; Oliveira, P.S.L.; Bajgelman, M.C. Boosting Antitumor Response with PSMA-Targeted Immunomodulatory VLPs, Harboring Costimulatory TNFSF Ligands and GM-CSF Cytokine. Mol. Ther.-Oncolytics 2022, 24, 650–662. [Google Scholar] [CrossRef]
- Simons, B.W.; Cannella, F.; Rowley, D.T.; Viscidi, R.P. Bovine Papillomavirus Prostate Cancer Antigen Virus-like Particle Vaccines Are Efficacious in Advanced Cancers in the TRAMP Mouse Spontaneous Prostate Cancer Model. Cancer Immunol. Immunother. 2020, 69, 641–651. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Liu, Y.; Zhang, X.; Shan, W.; Ye, S.; Zhou, X.; Ge, Y.; Wang, X.; Ren, L. Nanoparticle-Based Co-Delivery of SiRNA and Paclitaxel for Dual-Targeting of Glioblastoma. Nanomedicine 2020, 15, 1391–1409. [Google Scholar] [CrossRef]
- Spice, A.J.; Aw, R.; Bracewell, D.G.; Polizzi, K.M. Synthesis and Assembly of Hepatitis B Virus-Like Particles in a Pichia Pastoris Cell-Free System. Front. Bioeng. Biotechnol. 2020, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Steinmetz, N.F. Doxorubicin-Loaded Physalis Mottle Virus Particles Function as a PH-Responsive Prodrug Enabling Cancer Therapy. Biotechnol. J. 2020, 15, 2000077. [Google Scholar] [CrossRef] [PubMed]
| Platforms | Pros | Cons | Ref. |
|---|---|---|---|
| Virus-Like Particles | (i) Highly ordered structures with stability at the nanoscale. (ii) Uniform size and shape distribution through self-assembly. (iii) Three distinct interfaces available for functionalization (external, internal, and inter-subunit). (iv) Both genetic and chemical modifications are available. (v) Repeatable structure means that a single modification allows the whole particle to be arranged in a constant manner. | (i) Viruses with mutant epitopes do not elicit an effective immune response. (ii) High immunity is easily eliminated by triggering an immune response. (iii) The purification step is complex. | [35,36,37] |
| Lipid-based nanoparticles | (i) Strong membrane fusion capability. (ii) Broad adaptability to the drugs contained. | (i) Poor or no immunogenicity. (ii) Toxicity (cytotoxicity and genotoxicity). (iii) The use of organic solvents in production may be detrimental to large-scale production. | [38,39,40,12] |
| Polymer nanoparticles | (i) Simple and scalable synthesis. (ii) High transfection rate. (iii) Prepared with widespread and accessible natural polymers. | ||
| Inorganic nanoparticles | (i) Simple to synthesize. (ii) Excellent magnetic, optical, and electrical properties (ideal materials for building integrated diagnosis and therapy). |
| Study | Conditions | Interventions | Phases | Completion Date | NCT Number |
|---|---|---|---|---|---|
| A Study to Compare Immune Response of V503 to Gardasil in 16- to 26-year-old Men (V503-020) | Papilloma Viral Infection | V503 vs. GARDASIL | Phase 3 | 22 April 2015 | NCT02114385 |
| Trial of a Chikungunya Vaccine, PXVX0317 CHIKV-VLP, in Healthy Adults | Chikungunya Virus Infection | CHIKV VLP/unadjuvanted vs. CHIKV VLP/adjuvanted vs. Placebo | Phase 2 | 21 September 2020 | NCT03483961 |
| Serologic Assay Validation, Proficiency Testing, Safety, and Immunogenicity of Norovirus GI.1/GII.4 Bivalent Virus-Like Particle Vaccine | Norovirus, Healthy Participants | NoV GI.1/GII.4 Bivalent VLP Vaccine | Phase 2 | 9 September 2015 | NCT02475278 |
| A Study of V503 (A Multivalent Human Papillomavirus [HPV] L1 Virus-Like Particle [VLP] Vaccine) in Preadolescents and Adolescents (V503-002) | Cervical Cancers, Vulvar Cancer, Vaginal Cancer, Genital Lesions, PAP Test Abnormalities, HPV Infections | V503 | Phase 3 | 22 April 2021 | NCT00943722 |
| VRC 313: A Trivalent Virus-like Particle (VLP) Encephalitis Vaccine (WEVEE) in Healthy Adults | Venezuelan Equine Encephalitis, Western Equine Encephalitis, Eastern Equine Encephalitis, Alphavirus Infections | VRC-WEVVLP073-00-VP vs. VRC-GENMIX083-AL-VP | Phase 1 | 26 February 2020 | NCT03879603 |
| Safety and Immunogenicity of GSK Biologicals’ HPV-16/18 L1 VLP AS04 Vaccine (GSK-580299) in Healthy Female Children 4–6 Years Old | Infections, Papillomavirus | Cervarix vs. Priorix vs. Infanrix | Phase 3 | 6 October 2016 | NCT01627561 |
| A Study of Gardasil (V501) in Preadolescents and Adolescents (V501-018) | Human, Papillomavirus Infections | V501 vs. Placebo | Phase 3 | 1 June 2015 | NCT00092547 |
| Trial for Safety and Immunogenicity of a Chikungunya Vaccine, VRC-CHKVLP059-00-VP, in Healthy Adults | Chikungunya Virus Infection | VRC-CHKVLP059-00-VP vs. VRC-PBSPLA043-00-VP | Phase 2 | 6 March 2018 | NCT02562482 |
| Gardasil Vaccination in Post Stem Cell Transplant Patients | Gardasil Vaccine, Stem Cell Transplant, Immunogenicity | Gardasil | Phase 1 | 19 July 2016 | NCT01092195 |
| Safety and Immunogenicity of Norovirus Bivalent Virus-Like Particle Vaccine in Healthy Adults | Norovirus Prevention | Norovirus Bivalent VLP Vaccine vs. Placebo (Saline) | Phase 2 | 6 January 2016 | NCT02142504 |
| Safety and Immunogenicity of Norovirus GI.1/GII.4 Bivalent VLP Vaccine | Healthy Volunteers, Norovirus, Prevention | Hepatitis A Vaccine vs. Norovirus Bivalent VLP Vaccine | Phase 2 | 19 June 2015 | NCT02038907 |
| Safety and Immunogenicity of Norovirus GI.1/GII.4 Bivalent Virus-Like Particle Vaccine in an Elderly Population | Norovirus | Norovirus GI.1/GII.4 Bivalent VLP Vaccine vs. 0.9% sodium chloride (saline) | Phase 2 | 29 September 2017 | NCT02661490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Chi, Y.; Bao, J.; Zhao, X.; Zhang, J.; Wang, L. Virus-like Particles for TEM Regulation and Antitumor Therapy. J. Funct. Biomater. 2022, 13, 304. https://doi.org/10.3390/jfb13040304
Yang Z, Chi Y, Bao J, Zhao X, Zhang J, Wang L. Virus-like Particles for TEM Regulation and Antitumor Therapy. Journal of Functional Biomaterials. 2022; 13(4):304. https://doi.org/10.3390/jfb13040304
Chicago/Turabian StyleYang, Zhu, Yongjie Chi, Jiaxin Bao, Xin Zhao, Jing Zhang, and Lianyan Wang. 2022. "Virus-like Particles for TEM Regulation and Antitumor Therapy" Journal of Functional Biomaterials 13, no. 4: 304. https://doi.org/10.3390/jfb13040304
APA StyleYang, Z., Chi, Y., Bao, J., Zhao, X., Zhang, J., & Wang, L. (2022). Virus-like Particles for TEM Regulation and Antitumor Therapy. Journal of Functional Biomaterials, 13(4), 304. https://doi.org/10.3390/jfb13040304