Quasi-Static Mechanical Properties and Continuum Constitutive Model of the Thyroid Gland
Abstract
1. Introduction
2. Materials and Methods
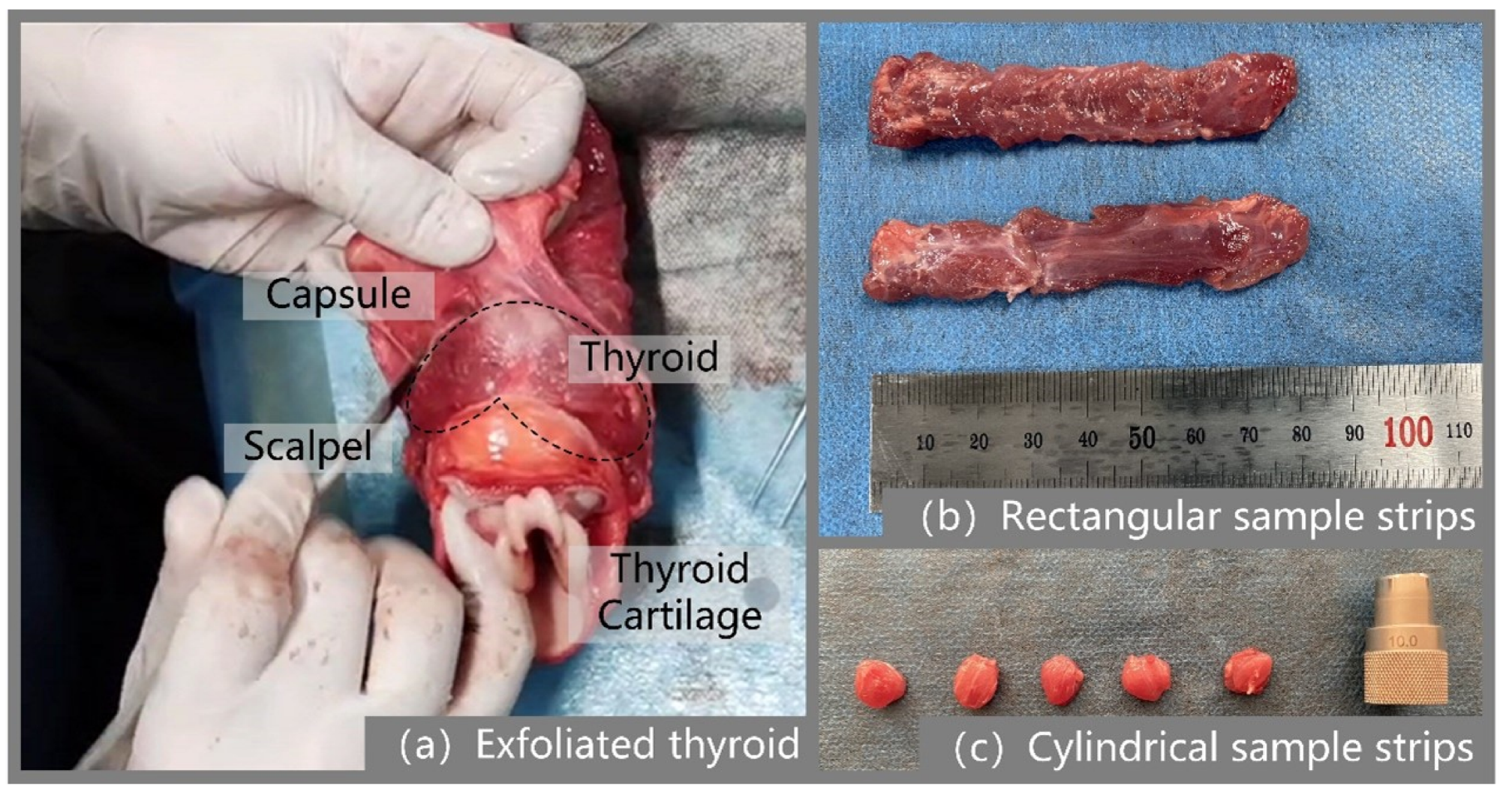
2.1. Specimen Preparation
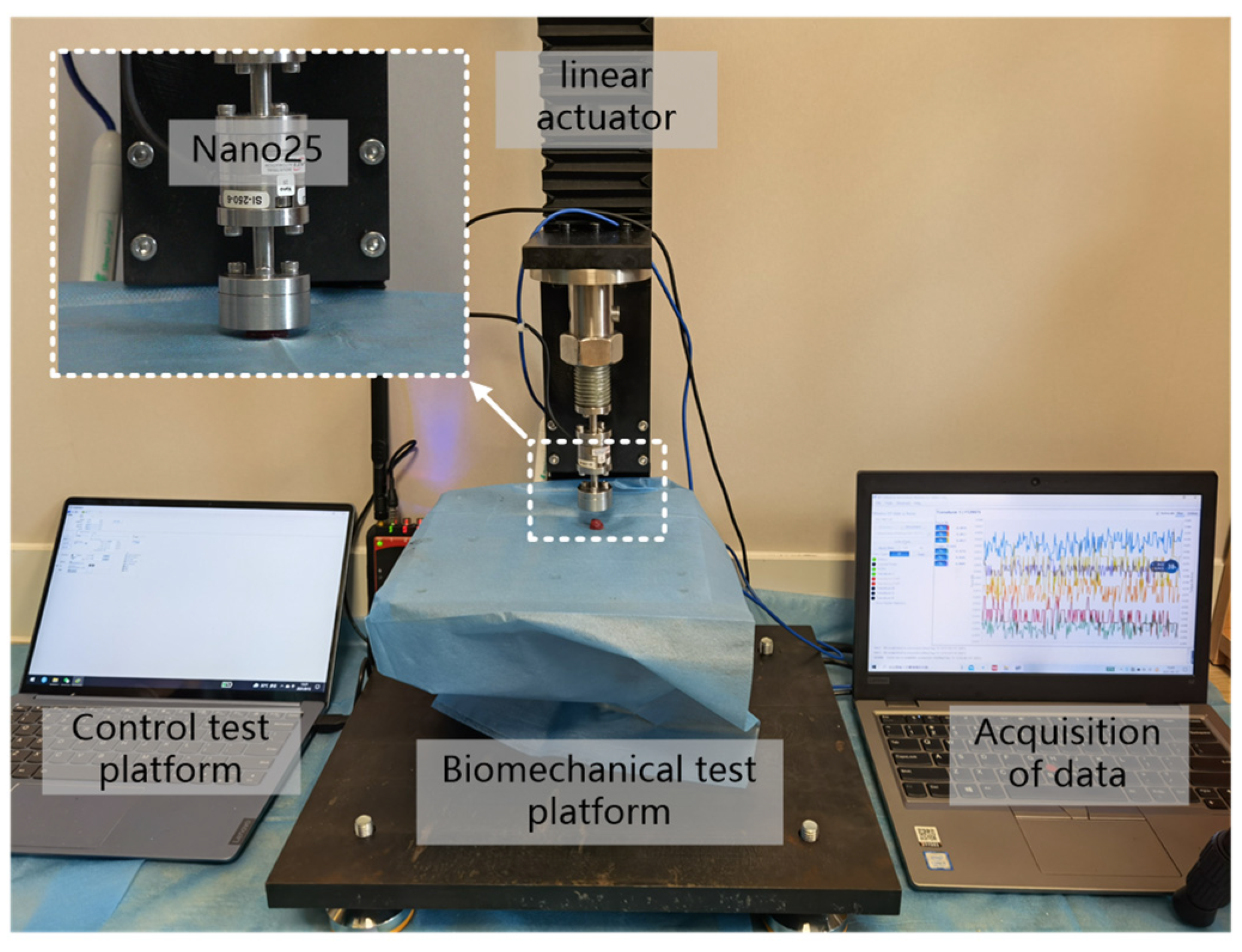
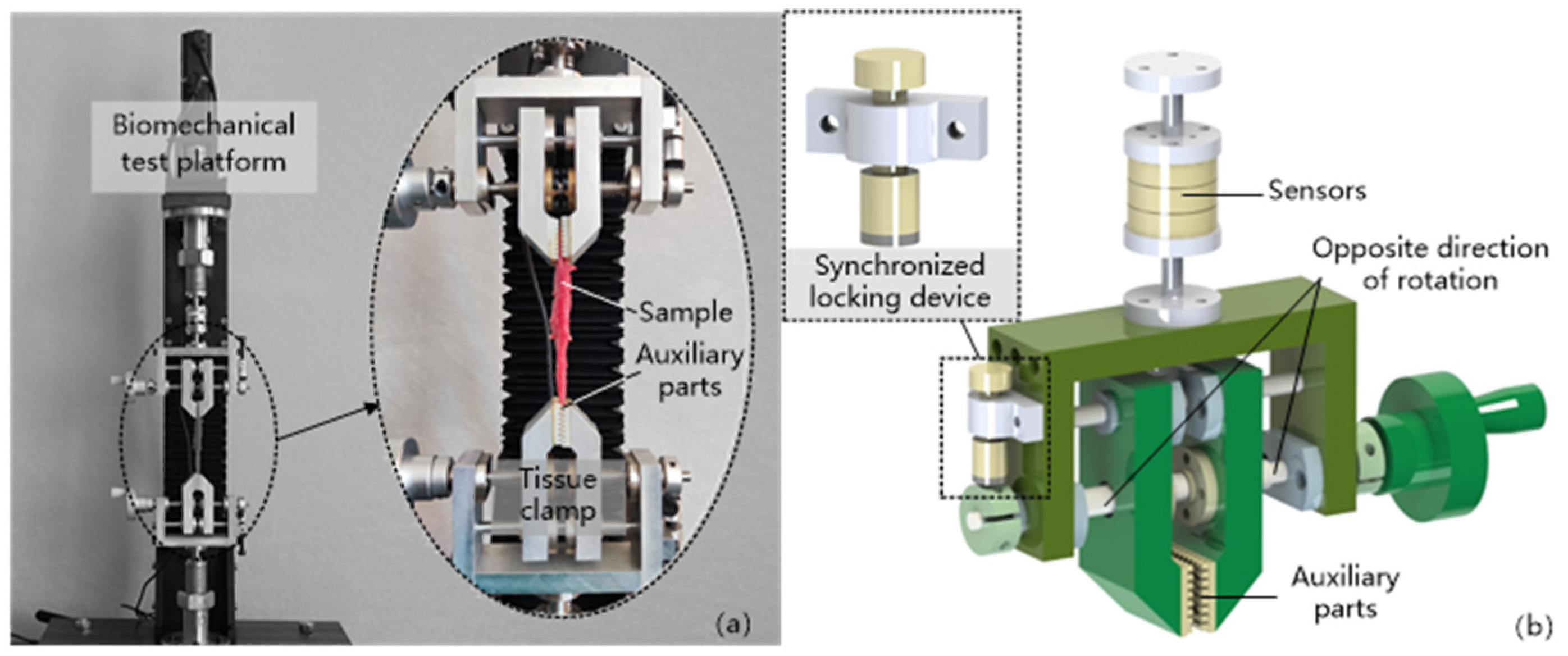
2.2. Uniaxial Compression Experiment
2.3. Hyperelastic Modeling
3. Results and Discussion
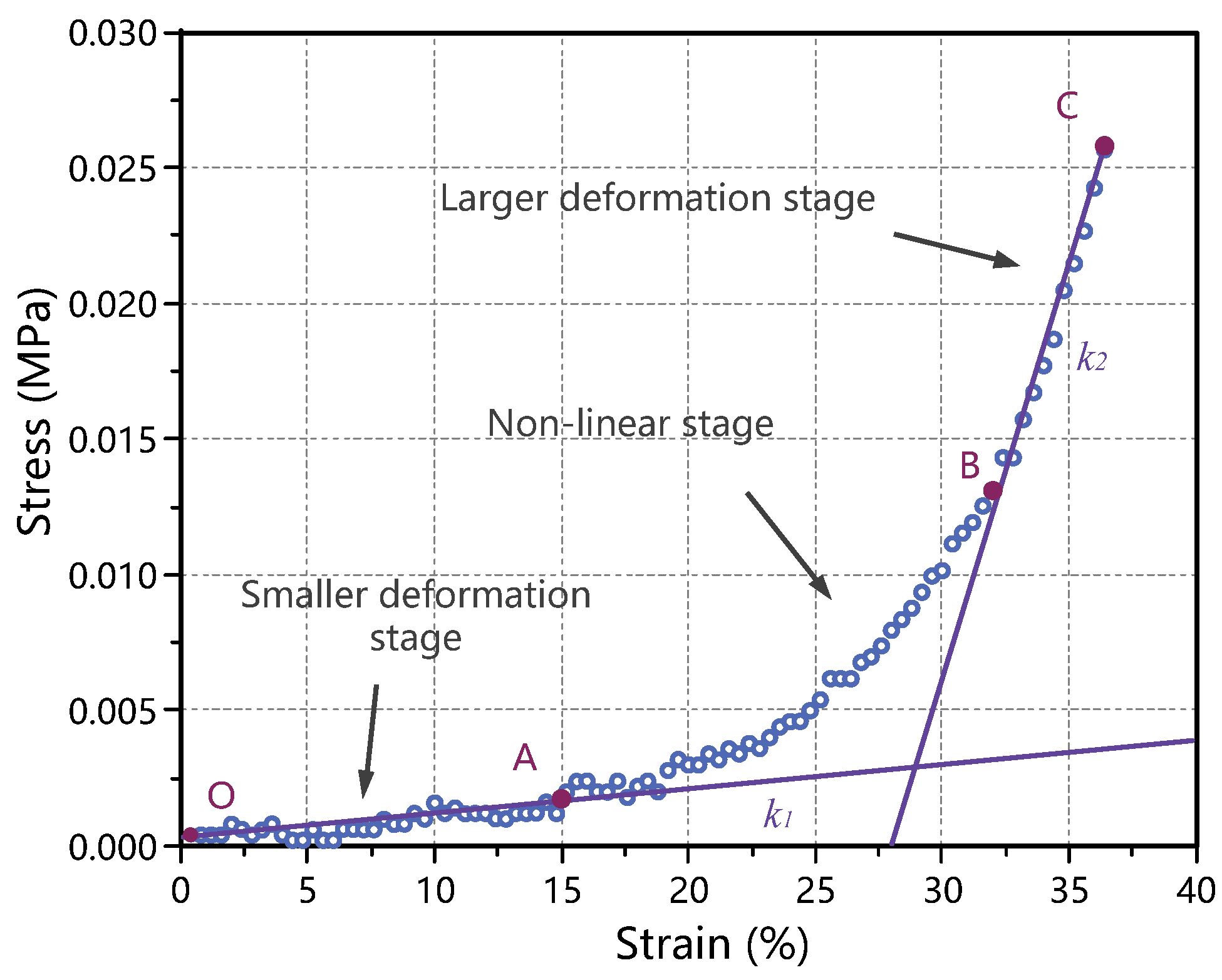
3.1. Quasi-Static Mechanical Properties
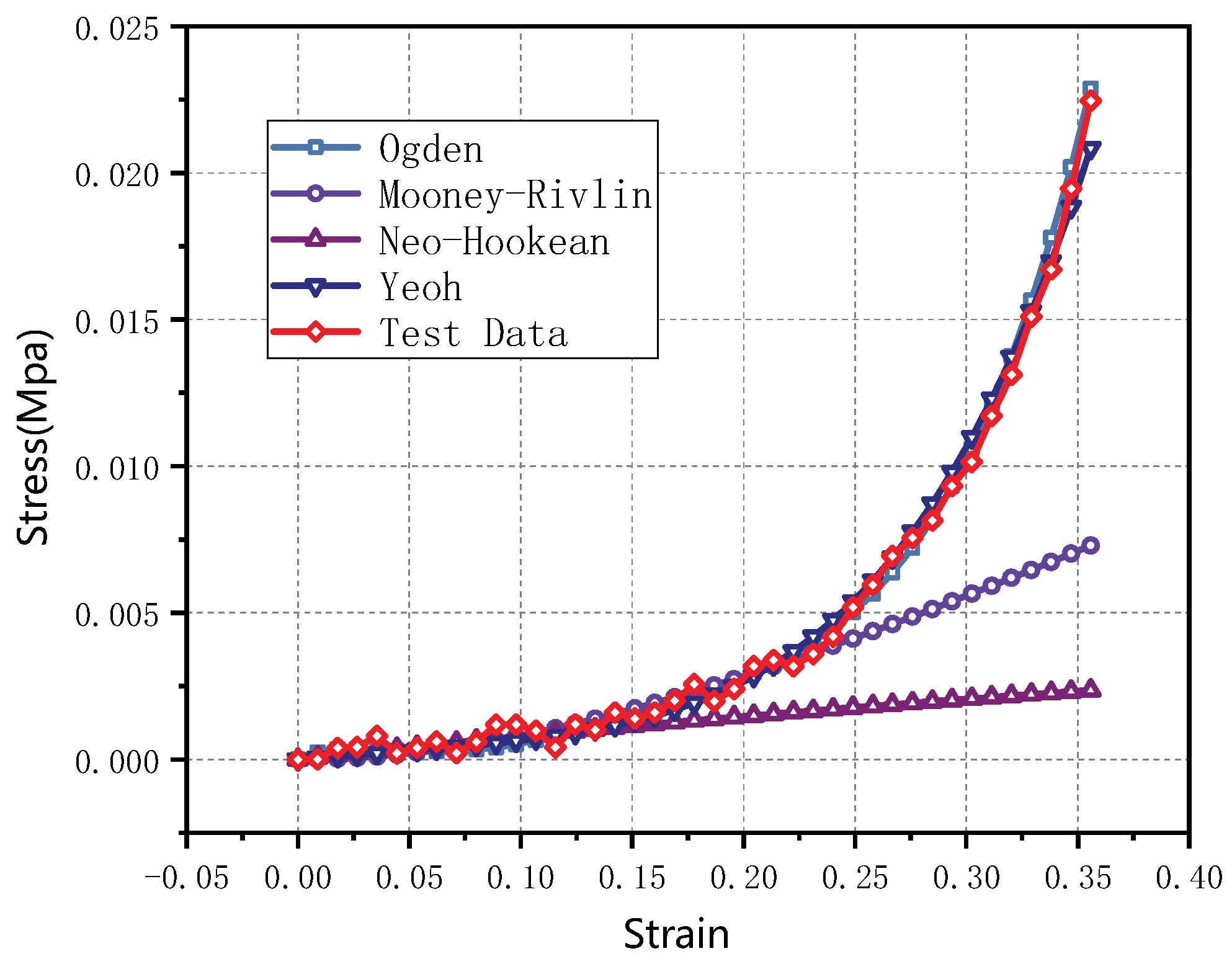
3.2. Hyperelastic Constitutive Model
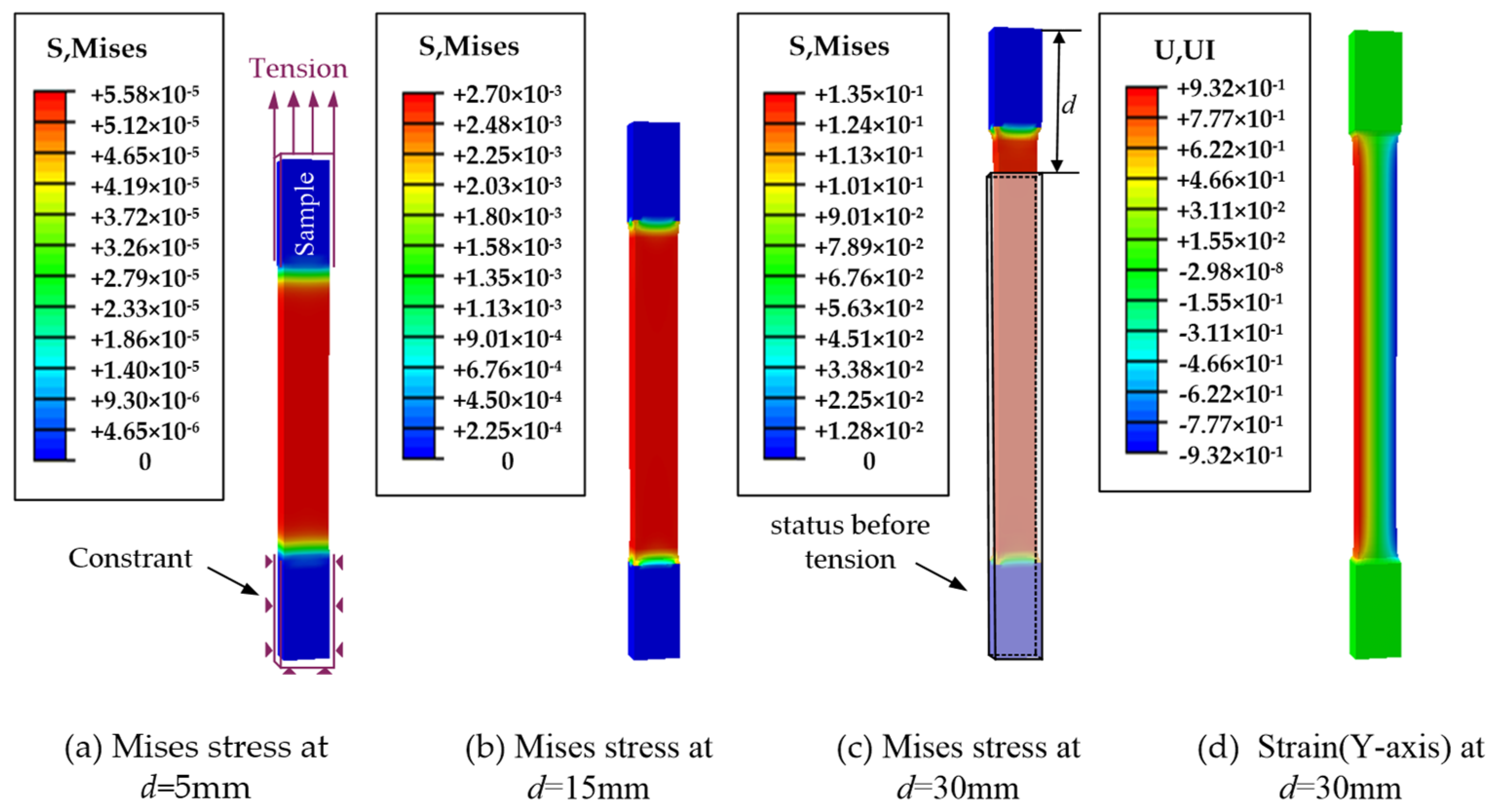
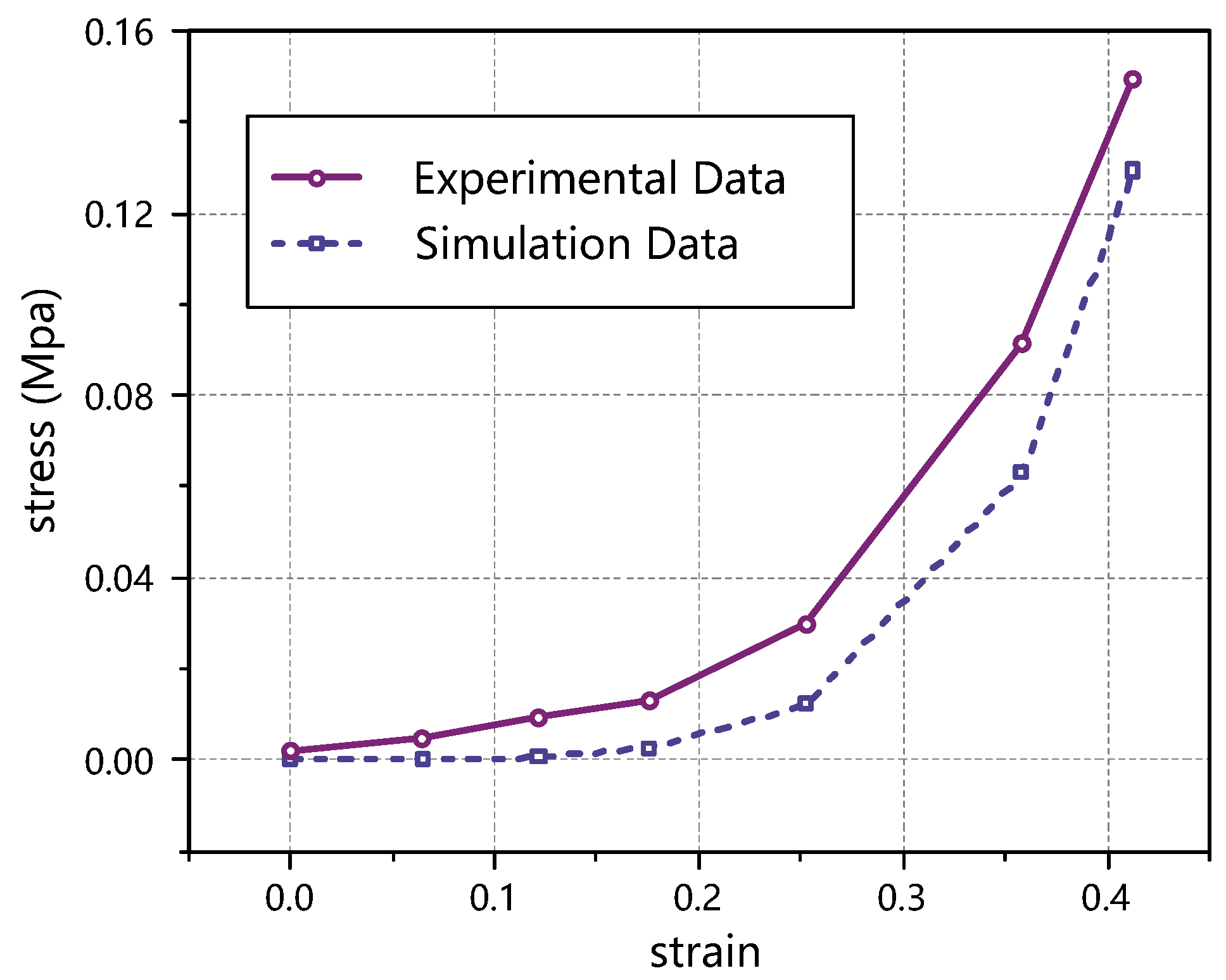
3.3. Verification by Stretching Thyroid Specimens
- Modeling. The stretched sample model was created in ABAQUS according to the structure and dimensions (80 mm × 15 mm × 5 mm) of the thyroid sample prepared in Figure 1b.
- Properties. The thyroid material properties were defined by the hyperelastic constitutive model established above.
- Loading. The lower end of the fixed thyroid-like strip is shown in Figure 8a. The upper end of the sample was stretched at a rate of 2 mm/s.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosko, A.J.; Gay, B.L.; Reyes-Gastelum, D.; Hamilton, A.S.; Ward, K.C.; Haymart, M.R. Surgeons’ Attitudes on Total Thyroidectomy vs Lobectomy for Management of Papillary Thyroid Microcarcinoma. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Oquendo, Y.A.; Chua, Z.; Coad, M.M.; Nisky, I.; Jarc, A.M.; Wren, S.M.; Lendvay, T.S.; Okamura, A.M. Robot-Assisted Surgical Training Over Several Days in a Virtual Surgical Environment with Divergent and Convergent Force Fields. Proc. Hamlyn Symp. Med. Robot. 2021, 81–82. [Google Scholar]
- Vinck, E.E.; Smood, B.; Barros, L.; Palmen, M. Robotic cardiac surgery training during residency: Preparing residents for the inevitable future. Laparosc. Endosc. Robot. Surg. 2022, 5, 75–77. [Google Scholar] [CrossRef]
- Ujala, Z.; Zarafshan, Z. Surgical resident training in Pakistan and benefits of simulation based training. JPMA. J. Pak. Med. Assoc. 2020, 70, 904–908. [Google Scholar]
- Strength of Biological Materials; Williams & Wilkins: Philadelphia, PA, USA, 1970; p. 582.
- Thibault, L.E.; Gennarelli, T.A. Biomechanics of Diffuse Brain Injuries. In Proceedings of the 10th International Technical Conference on Experimental Safety Vehicles (ESV), Oxford, UK, 1–4 July 1985; p. 185. [Google Scholar]
- Duma, S.M.; Stitzel, J.D.; Hardy, W.N.; Sparks, J.L. Characterizing the Biomechanical Response of the Liver; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2010. [Google Scholar]
- Pervin, F.; Chen, W.W.; Weerasooriya, T. Dynamic compressive response of bovine liver tissues. J. Mech. Behav. Biomed. Mater. 2011, 4, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Umale, S.; Deck, C.; Bourdet, N.; Dhumane, P.; Soler, L. Jacques Marescaux, Remy Willinger. Experimental mechanical characterization of abdominal organs: Liver, kidney & spleen. J. Mech. Behav. Biomed. Mater. 2013, 17, 22–33. [Google Scholar] [PubMed]
- Brunon, A.; Bruyere-Garnier, K.; Coret, M. Mechanical characterization of liver capsule through uniaxial quasi-static tensile tests until failure. J. Biomech. 2010, 43, 2221–2227. [Google Scholar] [CrossRef]
- Kemper, A.R.; Santago, A.C.; Stitzel, J.D.; Sparks, J.L.; Duma, S.M. Biomechanical response of human spleen in tensile loading. J. Biomech. 2012, 45, 348–355. [Google Scholar] [CrossRef]
- Yarpuzlu, B.; Ayyildiz, M.; Tok, O.E.; Aktas, R.G.; Basdogan, C. Correlation between the mechanical and histological properties of liver tissue. J. Mech. Behav. Biomed. Mater. 2014, 29, 403–416. [Google Scholar] [CrossRef]
- Kugler, M.; Hostettler, A.; Soler, L.; Borzacchiello, D.; Chinesta, F.; George, D.; Rémond, Y. Numerical simulation and identification of macroscopic vascularised liver behaviour: Case of indentation tests. Biomed. Mater. Eng. 2017, 28, S107. [Google Scholar] [CrossRef]
- Brinker, S.; Klatt, D. Demonstration of concurrent tensile testing and magnetic resonance elastography. J. Mech. Behav. Biomed. Mater. 2016, 63, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shen, Y.; Chen, X.; Zhu, Y.; Zheng, Y.; Zhang, X.; Guo, Y.; Wang, T.; Chen, S. Viscoelastic properties of normal rat liver measured by ultrasound elastography: Comparison with oscillatory rheometry. Biorheology 2016, 53, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Matin, Z.; Ghahfarokhi; Zand, M.M.; Tehrani, M.S.; Wendland, B.R.; Dargazany, R. A visco-hyperelastic constitutive model of short- and long-term viscous effects on isotropic soft tissues. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, 234, 3–17. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, Y.; Cao, Y. A porohyperviscoelastic model for the shear wave elastography of the liver. J. Mech. Phys. Solids 2021, 150, 104339. [Google Scholar] [CrossRef]
- Mattei, G.; Ahluwalia, A. Sample, testing and analysis variables affecting liver mechanical properties: A review. Acta Biomater. 2016, 45, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Pervin, F.; Chen, W.W. Effect of inter-species, gender, and breeding on the mechanical behavior of brain tissue. Neuroimage 2011, 54, S98–S102. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, T.; Yan, W.; Chen, Y.; Wang, G. Mechanical response of porcine hind leg muscles under dynamic tensile loading. J. Mech. Behav. Biomed. Mater. 2020, 115, 104279. [Google Scholar] [CrossRef]
- Fan, L.; Yao, J.; Wang, L.; Xu, D.; Tang, D. Optimization of Left Ventricle Pace Maker Location Using Echo-Based Fluid-Structure Interaction Models. Front. Physiol. 2022, 13, 215. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.H.; Ma, X.Q. Parametric characterization of the Mooney-Rivlin hyperelastic intrinsic model of rubber. Noise Vib. Control 2018, A02, 4. [Google Scholar]
- Yeoh, O.H. Some Forms of the Strain Energy Function for Rubber. Rubber Chem. Technol. 2012, 66, 754–771. [Google Scholar] [CrossRef]
- Cheng, K.E.; Wang, F.; Cao, Z.; Kong, H.; Zhang, J.; Fan, Y. A continuous medium intrinsic model for skeletal muscle at variable strain rates. Med. Biomech. 2021, 36, 7. [Google Scholar]
- Ogden, R.W. Large Deformation Isotropic Elasticity-On the Correlation of Theory and Experiment for Incompressible Rubberlike Solids. Proc. R. Soc. A Math. 1972, 326, 26. [Google Scholar] [CrossRef]
- Stasio, L.D.; Liu, Y.; Moran, B. Large deformation near a crack tip in a fiber-reinforced neo-Hookean sheet with discrete and continuous distributions of fiber orientations—ScienceDirect. Theor. Appl. Fract. Mech. 2021, 114, 103020. [Google Scholar] [CrossRef]
- Chu, H.Y.; Sun, D.M.; Chen, Q.; Cai, L.G. Establishment of hyperelastic-viscoplastic model and structural dynamic response of rubber. J. Beijing Univ. Technol. 2019, 45, 10. [Google Scholar]
- Guan, G.Y.; Meng, Z.G.; Xie, L.; Peng, N. Evaluation of the fitting effect of the hyperelasticity constitutive model of polyurethane rubber. Q. J. Mech. 2021, 42, 10. [Google Scholar]
- Estermann, S.J.; Pahr, D.H.; Reisinger, A. Hyperelastic and viscoelastic characterisation of hepatic tissue under uniaxial tension in time and frequency domain. J. Mech. Behav. Biomed. Mater. 2020, 112, 104038. [Google Scholar] [CrossRef]
- Giannokostas, K.; Dimakopoulos, Y.; Tsamopoulos, J. Shear stress and intravascular pressure effects on vascular dynamics: Two-phase blood flow in elastic microvessels accounting for the passive stresses. Biomech. Model. Mechanobiol. 2022, 21, 1659–1684. [Google Scholar] [CrossRef]
- Su, P.; Yang, Y.; Xiao, J.; Song, Y. Corneal hyper-viscoelastic model: Derivations, experiments, and simulations. Acta Bioeng. Biomech. 2015, 17, 73–84. [Google Scholar]


| Test Group | ||
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| Mean | ||
| Variance |
| N-H | 2.357 | - | - | - | - | 0.564 |
| M-R | - | - | - | 0.661 | ||
| Ogden | −0.305 | 0.297 | 1.619 | 10.267 | −25 | 0.996 |
| Yeoh | 0.04 | - | - | 0.999 |
| Test Group | Initial Stress (MPa) | Last Stress (MPa) |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| Mean | ||
| Variance |
| Strain | Stress (MPa) | Absolute Error (MPa) | Relative Error (%) | |
|---|---|---|---|---|
| Simulation Data | Experimental Data | |||
| 0 | 0 | 0 | 0 | 0 |
| 0.064 | 16.16 | |||
| 0.121 | 21.97 | |||
| 0.175 | 37.46 | |||
| 0.253 | −0.0085 | 40.92 | ||
| 0.388 | −0.0112 | 15.05 | ||
| 0.413 | 0.1294 | 0.1489 | −0.0196 | 13.13 |
| Mean error | 20.67 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.; Yue, C.; Cui, L.; Zhang, Q.; Liu, B.; Liu, T. Quasi-Static Mechanical Properties and Continuum Constitutive Model of the Thyroid Gland. J. Funct. Biomater. 2022, 13, 283. https://doi.org/10.3390/jfb13040283
Su P, Yue C, Cui L, Zhang Q, Liu B, Liu T. Quasi-Static Mechanical Properties and Continuum Constitutive Model of the Thyroid Gland. Journal of Functional Biomaterials. 2022; 13(4):283. https://doi.org/10.3390/jfb13040283
Chicago/Turabian StyleSu, Peng, Chao Yue, Likun Cui, Qinjian Zhang, Baoguo Liu, and Tian Liu. 2022. "Quasi-Static Mechanical Properties and Continuum Constitutive Model of the Thyroid Gland" Journal of Functional Biomaterials 13, no. 4: 283. https://doi.org/10.3390/jfb13040283
APA StyleSu, P., Yue, C., Cui, L., Zhang, Q., Liu, B., & Liu, T. (2022). Quasi-Static Mechanical Properties and Continuum Constitutive Model of the Thyroid Gland. Journal of Functional Biomaterials, 13(4), 283. https://doi.org/10.3390/jfb13040283







