Green Synthesis of Platinum Nanoparticles for Biomedical Applications
Abstract
1. Introduction
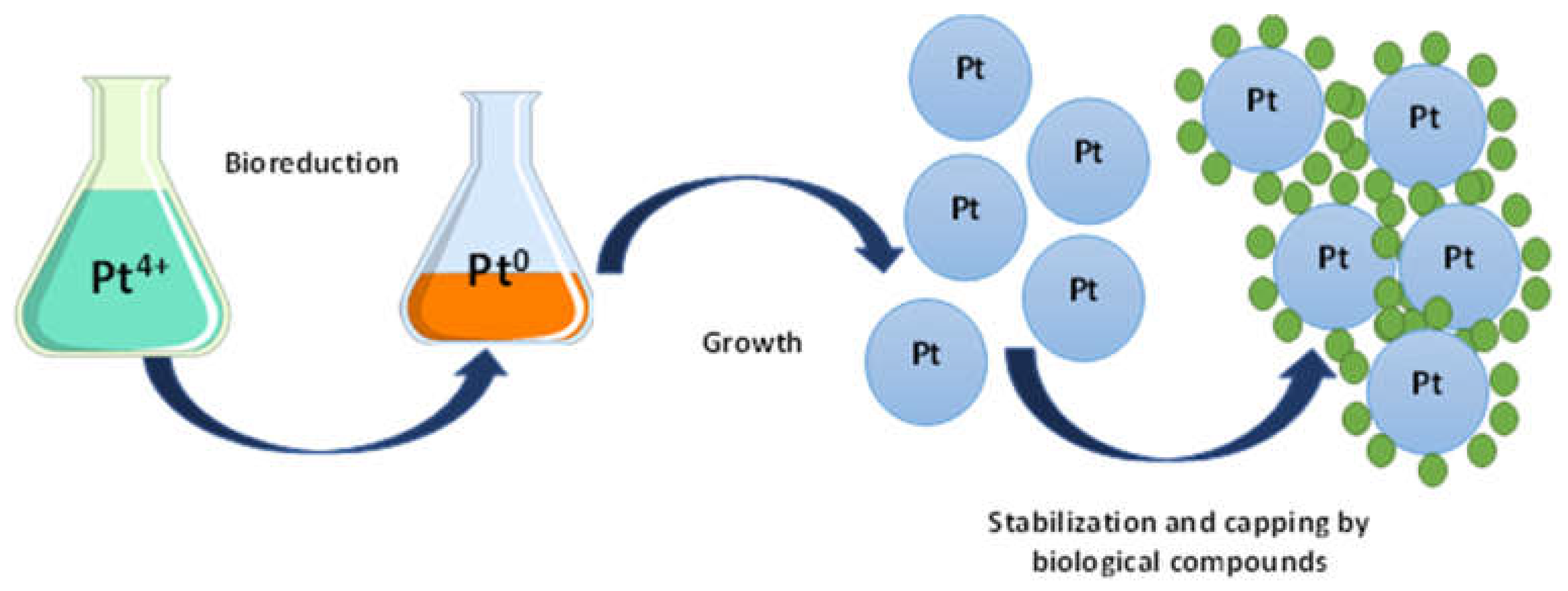
2. The Proposed Mechanism of PtNPs Synthesis
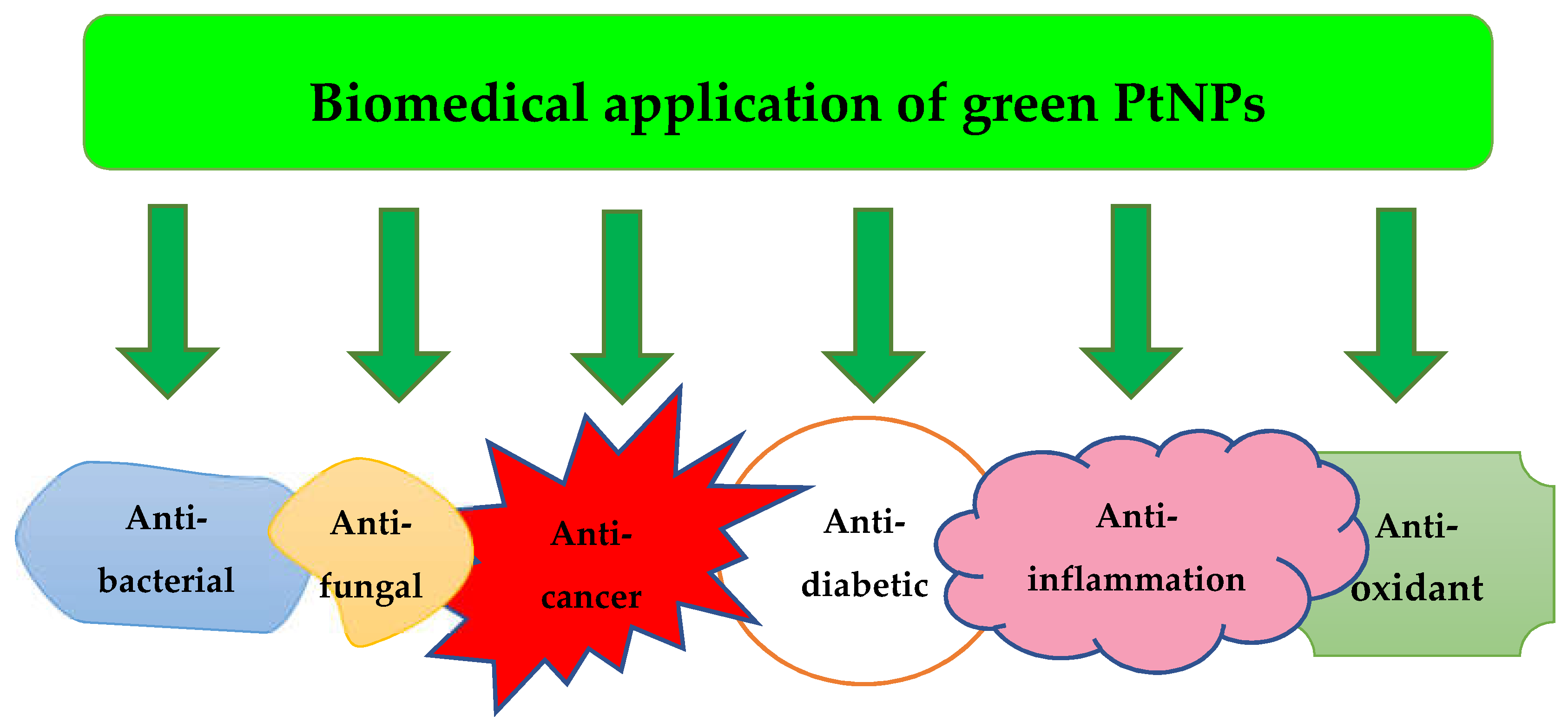
3. Application of Green PtNPs
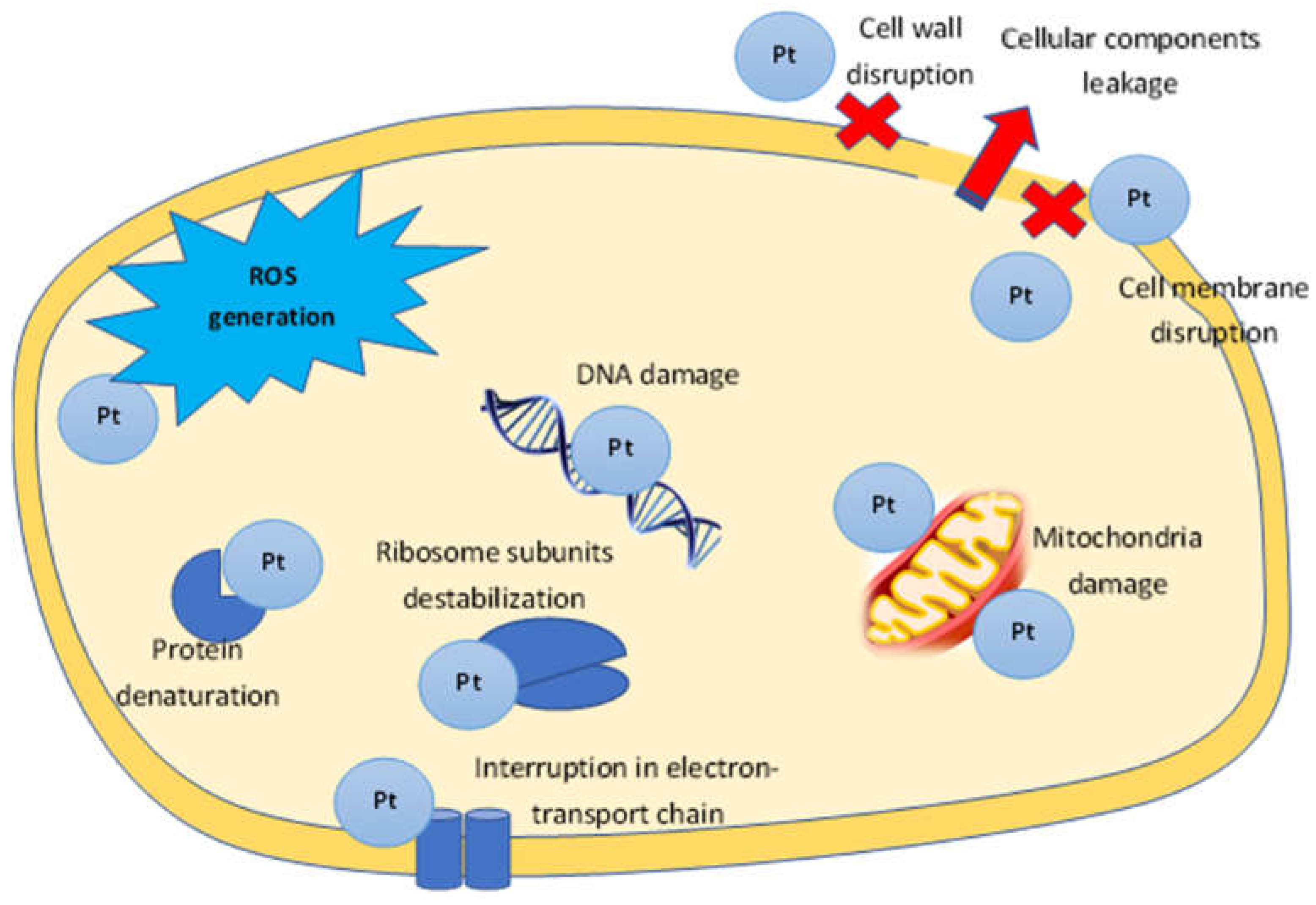
3.1. Antibacterial Activity
3.2. Anti-Fungal Activity
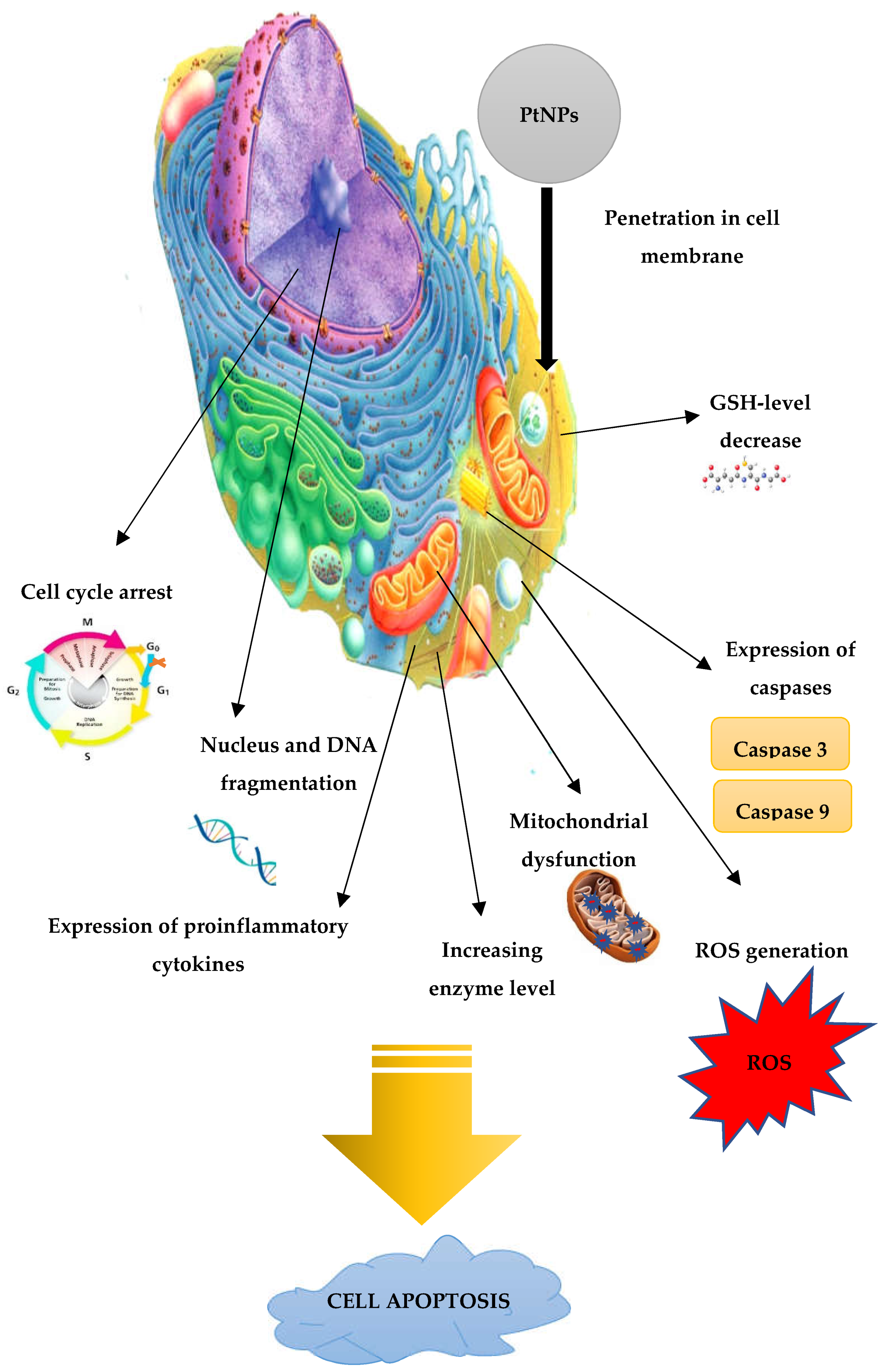
3.3. Anti-Cancer Activity
3.4. Antioxidant Activity
3.5. Anti-Diabetic Activity
3.6. Anti-Inflammation Activity
3.7. Other Application
4. Toxicology of «Green» Platinum Nanoparticles
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Maťatkova, O.; Michailidu, J.; Miskovska, A.; Kolouchova, I.; Masak, J.; Cejkov, A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Alhumaimess, M.S.; Alsohaimi, I.H.; Essawy, A.A.; Hussein, M.F.; Alshammari, H.M.; Aldosari, O.F. Biogenic-mediated synthesis of the Cs2O−MgO/MPC nanocomposite for biodiesel production from olive oil. ACS Omega 2020, 5, 27811–27822. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Mohd. Setapar, S.H.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef] [PubMed]
- Qi, С.; Musetti, S.; Fu, L.-H.; Zhu, Y.-J.; Huang, L. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications. Chem. Soc. Rev. 2019, 48, 2698. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Lee, K.G.; Lee, S.Y. Advances in microbial biosynthesis of metal nanoparticles. Appl. Microbiol. Biotechnol. 2016, 100, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kim, J.-O.; Seo, Y.B.; Kang, M.-j.; Kim, G.-D. Biogenic synthesis of metallic nanoparticles and their antibacterial applications. J. Life Sci. 2021, 31, 862–872. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- McDonald, D.; Hunt, L.B. A History of Platinum and its Allied Metals; Distributed by Europa Publicat; Europa Editions: New York, NY, USA, 1982; pp. 4–5. [Google Scholar]
- Odularu, A.T.; Ajibade, P.A.; Mbese, J.Z.; Oyedeji, O.O. Developments in Platinum-Group Metals as Dual Antibacterial and Anticancer Agents. J. Chem. 2019, 5459461. [Google Scholar] [CrossRef]
- Barnard, C.F.J. Platinum anti-cancer agents twenty years of continuing development. Platin. Met. Rev. 1989, 33, 162–167. [Google Scholar]
- Lewis, R. From basic research to cancer drug; the story of cisplatin. Science 2009, 13, 11. [Google Scholar]
- Wilson, J.J.; Lippard, S.J. Synthetic methods for the preparation of platinum anticancer complexes. Chem. Rev. 2014, 114, 4470–4495. [Google Scholar] [CrossRef] [PubMed]
- Bendale, Y.; Bendale, V.; Paul, S. Evaluation of cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis. Integr. Med. Res. 2017, 6, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Vukoja, D.; Vlainic, J.; Ljolic Bilic, V.; Martinaga, L.; Rezic, I.; Brlek Gorski, D.; Kosalec, I. Innovative Insights into In vitro activity of colloidal platinum nanoparticles against ESBL-Producing Strains of Escherichia coli and Klebsiella pneumoniae. Pharmaceutics 2022, 14, 1714. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of «naked» metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A. Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles. Green Process. Synth. 2020, 9, 386–398. [Google Scholar] [CrossRef]
- Subramaniyan, S.A.; Sheet, S.; Vinothkannan, M.; Yoo, D.J.; Lee, Y.S.; Belal, S.A.; Shim, K.S. One-pot facile synthesis of Pt nanoparticles using cultural filtrate of microgravity simulated grown P. chrysogenum and their activity on bacteria and cancer cells. J. Nanosci. Nanotechnol. 2018, 18, 3110–3125. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hong, Y.N.; Weyers, A.Y.; Kim, S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pantidos, N.; Horsfall, L.E. Biological Synthesis of Metallic Nanoparticles by Bacteria, Fungi and Plants. J. Nanomed. Nanotechnol. 2014, 5. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Gungor, A.A.; Ince, S.; Babagil, A. Green synthesis and characterisation of platinum nanoparticles using quail egg yolk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 172, 43–47. [Google Scholar] [CrossRef]
- Riddin, T.L.; Govender, Y.; Gericke, M.; Whiteley, C.G. Two different hydrogenase enzymes from sulphate-reducing bacteria are responsible for the bioreductive mechanism of platinum into nanoparticles. Enzyme Microb. Technol. 2009, 45, 267–273. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.H. Green Synthesis, Characterization and Uses of Palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Uttam, C.B. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Periasamy, J.A.; Subbarayan, V. Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J. Mater. Sci. Mater. Med. 2015, 26, 7. [Google Scholar] [CrossRef]
- Sahin, B.; Aygün, A.; Gündüz, H.; Sahin, K.; Demir, E.; Akocak, S.; Sen, F. Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids Surf. B Biointerfaces 2018, 163, 119–124. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Preis, E.; Bakowsky, U.; Azzazy, H.M.E.-S. Platinum Nanoparticles: Green Synthesis and Biomedical Applications. Molecules 2020, 25, 4981. [Google Scholar] [CrossRef]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent Advances in Green Synthesis, Characterization, and Applications of Bioactive Metallic Nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Aruna, K.; Kavitha, K. Synthesis and Characterization of Platinum Nanoparticles (Pt-NPs) from Centella Asiatica L Leaf Extract. Ann. Rom. Soc. Cell Biol. 2021, 25, 16–22. [Google Scholar]
- Rosas-Medellín, D.; Pérez-Salcedo, K.Y.; Morales-Acosta, D.; Rodríguez-Varela, F.J.; Escobar, B. Green synthesis of Pt nanoparticles and their application in the oxygen reduction reaction. J. Mater. Res. 2021, 36, 4131–4140. [Google Scholar] [CrossRef]
- Konishi, Y.; Tsukiyama, T.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S. Intracellular recovery of gold by microbial reduction of AuCl4 - ions using the anaerobic bacterium Shewanella algae. Hydrometallurgy 2006, 81, 24–29. [Google Scholar] [CrossRef]
- Gautam, A.; Guleria, P.; Kumar, V. Platinum nanoparticles: Synthesis strategies and applications. Nanoarchitectonics 2020, 1, 70–86. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Bunge, M.; Sobjerg, L.S.; Rotaru, A.; Gauthier, D.; Lindhardt, A.T.; Hause, G. Formation of palladium(0) nanoparticles at microbial surfaces. Biotechnol. Bioeng. 2010, 107, 206–215. [Google Scholar] [CrossRef]
- Capeness, M.J.; Edmundson, M.C.; Horsfall, L.E. Nickel and platinum group metal nanoparticle production by Desulfovibrio alaskensis G20. New Biotechnol. 2015, 32, 727–731. [Google Scholar] [CrossRef]
- Govender, Y.; Riddin, T.; Gericke, M.; Whiteley, C.G. On the enzymatic formation of platinum nanoparticles. J. Nanopart Res. 2010, 12, 261–271. [Google Scholar] [CrossRef]
- Gaidhani, S.V.; Yeshvekar, R.K.; Shedbalkar, U.U.; Bellare, J.H.; Chopade, B.A. Bio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticus. Process Biochem. 2014, 49, 2313–2319. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohnoa, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J. Biotech. 2007, 128, 648–653. [Google Scholar] [CrossRef]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic platinum vanoparticles for application in nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef]
- Kato, Y.; Suzuki, M. Synthesis of Metal Nanoparticles by Microorganisms. Crystals 2020, 10, 589. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Constanti, M. Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li) by Pseudomonas aeruginosa SM1. J. Nanopart. Res. 2012, 14, 831. [Google Scholar] [CrossRef]
- Baskaran, B.; Muthukumarasamy, A.; Chidambaram, S.; Sugumaran, A.; Ramachandran, K.; Manimuthu, T.R. Cytotoxic potentials of biologically fabricated platinum nanoparticles from Streptomyces sp. on MCF-7 breast cancer cells. IET Nanobiotechnol. 2017, 11, 241–246. [Google Scholar] [CrossRef]
- Eramabadi, P.; Masoudi, M.; Makhdoumi, A.; Mashreghi, M. Microbial cell lysate supernatant (CLS) alteration impact on platinum nanoparticles fabrication, characterization, antioxidant and antibacterial activity. Mater. Sci. Eng. C 2020, 117, 111292. [Google Scholar] [CrossRef]
- Riddin, T.L.; Gericke, M.; Whiteley, C.G. Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 2006, 17, 3482–3489. [Google Scholar] [CrossRef]
- Govender, Y.; Riddin, T.; Gericke, M. Bioreduction of platinum salts into nanoparticles: A mechanistic perspective. Biotechnol. Lett. 2009, 31, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Ahmad, A. Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2012, 97, 27–31. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Moreno-Velasquez, S.D.; Vilchis-Nestor, A.R.; Berumen, E.A.; Borja, M.A. Production of Platinum Nanoparticles and Nanoaggregates Using Neurospora crassa. J. Microbiol. Biotechnol. 2012, 22, 1000–1004. [Google Scholar] [CrossRef]
- Borse, V.; Kaler, A.; Banerjee, U.C. Microbial Synthesis of Platinum Nanoparticles and Evaluation of Their Anticancer Activity. J. Emerg. Trends Electr. Electron. 2015, 11, 26–31. [Google Scholar]
- Ito, R.; Kuroda, K.; Hashimoto, H.; Ueda, M. Recovery of platinum(0) through the reduction of platinum ions by hydrogenase-displaying yeast. AMB Expr. 2016, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jing, Y.; Guo, A.; Li, X.; Li, Q.; Liu, W.; Zhang, X. Biosynthesis of Platinum Nanoparticles with Cordyceps Flower Extract: Characterization, Antioxidant Activity and Antibacterial Activity. Nanomaterials 2022, 12, 1904. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Mashael, A.R.; Bin-Meferij, M. Cyanobacteria—A Promising Platform in Green Nanotechnology: A Review on Nanoparticles Fabrication and Their Prospective Applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef]
- Tyagi, R.; Kaushik, B.; Kumar, J. Antimicrobial activity of some cyanobacteria. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 463–470. [Google Scholar]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum (IV)-chloride complex. Langmuir 2006, 22, 7318–7323. [Google Scholar] [CrossRef]
- Chun, A.L. Green platinum nanoparticles. Nat. Nanotechnol. 2006. [Google Scholar] [CrossRef]
- Brayner, R.; Barberousse, H.; Hemadi, M.; Djedjat, C.; Yéprémian, C.; Coradin, T.; Livage, J.; Fiévet, F.; Couté, A. Cyanobacteria as Bioreactors for the Synthesis of Au, Ag, Pd, and Pt Nanoparticles via an Enzyme-Mediated Route. J. Nanosci. Nanotechnol. 2007, 7, 2696–2708. [Google Scholar] [CrossRef]
- Karthic, A.; Wagh, N.S.; Lakkakula, J.R. Algae-assisted synthesis of nanoparticles Nanobiotechnology Microbes and Plant Assisted Synthesis of Nanoparticles, Mechanisms and Applications. In Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–165. [Google Scholar]
- Arya, A.; Gupta, K.; Chundawat, T.S. In Vitro Antimicrobial and Antioxidant Activity of Biogenically Synthesized Palladium and Platinum Nanoparticles Using Botryococcus braunii. Turk. J. Pharm. Sci. 2020, 17, 299–306. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Prakash, S.; Ahila, N.K.; Vinoj, G.; Selvam, S.; Kumar, G.; Kannapiran, E.; Babu Rajendran, R. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. [Google Scholar] [CrossRef]
- Sathiyaraj, G.; Vinosha, M.; Sangeetha, D.; Manikandakrishnan, M.; Palanisamy, S.; Sonaimuthu, M.; Manikandan, R.; You, S.G.; Marimuthu, N. Prabhu Bio-directed synthesis of Pt-nanoparticles from aqueous extract of red algae Halymenia dilatata and their biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 18, 126434. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Al Mamun, M.S.M.; Ara, H. Review on platinum nanoparticles: Synthesis, characterization, and applications. Microchem. J. 2021, 171, 106840. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Chakraborty, S.; Abbasi, T.; Abbasi, S.A. A Clean-Green Synthesis of Platinum Nanoparticles Utilizing a Pernicious Weed Lantana (Lantana Camara). Am. J. Appl. Sci. 2016, 9, 84–90. [Google Scholar] [CrossRef]
- Zheng, B.; Kong, T.; Jing, X.; Odoom-Wubah, T.; Li, X.; Sun, D.; Lu, F.; Zheng, Y.; Huang, J.; Li, Q. Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism. J. Colloid Interface Sci. 2013, 396, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Sadalage, P.S.; Dar, M.A.; Bhor, R.D.; Bhalerao, B.M.; Kamble, P.N.; Paiva-Santos, A.C.; Nimbalkar, M.S.; Sonawane, K.D.; Pai, K.; Patil, P.S.; et al. Optimization of biogenic synthesis of biocompatible platinum nanoparticles with catalytic, enzyme mimetic and antioxidant activities. Food Biosci. 2022, 50, 102024. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Biofabrication, characterization, and possible bio-reduction mechanism of platinum nanoparticles mediated by agro-industrial waste and their catalytic activity. J. Ind. Eng. Chem. 2015, 22, 185–191. [Google Scholar] [CrossRef]
- Rokade, S.S.; Joshi, K.A.; Mahajan, K.; Tomar, G.; Dubal, D.S.; Parihar, V.S.; Kitture, R.; Bellare, J.; Ghosh, S. Novel Anticancer Platinum and Palladium Nanoparticles from Barleria prionitis. Glob J. Nanomed. 2017, 2, 555600. [Google Scholar]
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Nancy, A. Green synthesis of platinum nanoparticles using Azadirachta indica – An eco-friendly approach. Mater. Lett. 2016, 170, 175–178. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, E.-Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst. Eng. 2010, 33, 159. [Google Scholar] [CrossRef]
- Rehman, K.; Khan, S.U.; Tahir, K.; Zaman, U.; Khan, D.; Nazir, S.; Khan, W.U.; Khan, M.I.; Ullah, K.; Anjum, S.I.; et al. Sustainable and green synthesis of novel acid phosphatase mediated platinum nanoparticles (ACP-PtNPs) and investigation of its in vitro antibacterial, antioxidant, hemolysis and photocatalytic activities. J. Environ. Chem. Eng. 2022, 10, 107623. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Saravanan, P.; Sreedhar, B.; Keerthi Devi, D.; Sashidhar, R.B. A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum Gossypium). Colloids Surf B Biointerfaces 2011, 83, 291–298. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Laboratory scale medicinal plants mediated green synthesis of biocompatible nanomaterials and their versatile biomedical applications. Saudi J. Biol. Sci. 2022, 29, 3848–3870. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Mahato, K.; Srivastava, A.; Mauryac, P.K.; Chandra, P. Phytofabricated metallic nanoparticles and their clinical applications. RSC Adv. 2016, 6, 105996–106010. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Tammina, S.K. Green synthesis of nano platinum using naturally occurring polyphenols. RSC Adv. 2013, 3, 4033. [Google Scholar] [CrossRef]
- Karthik, R.; Sasikumar, R.; Chen, S.-M.; Govindasamy, M.; Kumar, J.V.; Muthuraj, V. Green Synthesis of Platinum Nanoparticles Using Quercus Glauca Extract and Its Electrochemical Oxidation of Hydrazine in Water Samples. Int. J. Electrochem. Sci. 2016, 11, 8245–8255. [Google Scholar] [CrossRef]
- Soundarrajan, C.; Sankari, A.; Dhandapani, P.; Maruthamuthu, S.; Ravichandran, S.; Sozhan, G.; Palaniswamy, N. Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst. Eng. 2012, 35, 827–833. [Google Scholar] [CrossRef]
- Ganaie, U.; Abbasi, T.; Abbasi, S.A. Biomimetic synthesis of platinum nanoparticles utilizing a terrestrial weed Antigonon leptopus. Part. Sci. Technol. 2018, 36, 681–688. [Google Scholar] [CrossRef]
- Nellore, J.; Pauline, C.; Amarnath, K. Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish. J. Neurodegener. Dis. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Chopade, B.A. Novel platinum–palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int. J. Nanomed. 2015, 10, 7477–7490. [Google Scholar]
- Fahmy, S.A.; Fawzy, I.M.; Saleh, B.M.; Issa, M.Y.; Bakowsky, U.; Azzazy, H.M.E.-S. Green Synthesis of Platinum and Palladium Nanoparticles Using Peganum harmala L. Seed Alkaloids: Biological and Computational Studies. Nanomaterials 2021, 11, 965. [Google Scholar] [CrossRef]
- Ramachandiran, D.; Elangovan, M.; Rajesh, K. Structural, optical, biological and photocatalytic activities of platinum nanoparticles using salix tetrasperma leaf extract via hydrothermal and ultrasonic methods. Optik 2021, 244, 167494. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Fawzy, M.; Hosny, M.; El-Monaem, E.M.A.; Tamer, T.M.; Omer, A.M. Green synthesis of platinum nanoparticles using Atriplex halimus leaves for potential antimicrobial, antioxidant, and catalytic applications. Arab. J. Chem. 2022, 15, 103517. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arab. J. Chem. 2019, 12, 330–349. [Google Scholar] [CrossRef]
- Kumar, P.V.; Kala, S.M.J.; Prakash, K.S. Green synthesis derived Pt-nanoparticles using Xanthium strumarium leaf extract and their biological studies. J. Environ. Chem. Eng. 2019, 7, 103146. [Google Scholar] [CrossRef]
- Dobrucka, R. Biofabrication of platinum nanoparticles using Fumariae herba extract and their catalytic properties. Saudi J. Biol. Sci. 2019, 26, 31–37. [Google Scholar] [CrossRef]
- Sheny, D.S.; Philip, D.; Mathew, J. Synthesis of platinum nanoparticles using dried Anacardium occidentale leaf and its catalytic and thermal applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 267–271. [Google Scholar] [CrossRef]
- Chuang, Y.; Minghua, W.; Junde, Z.; Qiang, C. Bio-synthesis of peppermint leaf extract polyphenols capped nano-platinum and their in-vitro cytotoxicity towards colon cancer cell lines (HCT 116). Mater. Sci. Eng. C 2017, 77, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Selvi, A.M.; Palanisamy, S.; Jeyanthi, S.; Vinosh, M.; Mohandoss, S.; Tabars, M.; You, S.G.; Kannapiran, E.; Prabhu, N.M. Synthesis of Tragia involucrata mediated platinum nanoparticles for comprehensive therapeutic applications: Antioxidant, antibacterial and mitochondria-associated apoptosis in HeLa cells. Process Biochem. 2020, 98, 21–33. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; El-Fakharany, E.M.; Omer, A.M.; El-Monaem, E.M.A.; Randa, E. Khalifa, Abdelazeem S. Eltaweil Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves. J. Environ. Chem. Eng. 2022, 10, 106806. [Google Scholar] [CrossRef]
- Laura Castro, M.L.B.; Gonzalez, F.; Muñoz, J.A.; Ballester, A. Biosynthesis of silver and platinum nanoparticles using orange peel extract: Characterisation and applications. IET Nanobiotechnol. 2015, 9, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Shim, J.; Kim, K.; Oh, B.-T. Prunus yedoensis tree gum mediated synthesis of platinum nanoparticles with antifungal activity against phytopathogens. Mater. Lett. 2016, 174, 61–65. [Google Scholar] [CrossRef]
- Anyik, J.L.; Oluwafemi, O.S. Plant-mediated synthesis of platinum nanoparticles using water hyacinth as an efficient biomatrix source – An eco-friendly development. Mater. Lett. 2017, 196, 141–144. [Google Scholar]
- Kumar, M.N.; Govindh, B.; Annapurna, N. Green synthesis and characterization of platinum nanoparticles using Sapindus mukorossi gaertn. fruit pericarp. Asian J. Chem. 2017, 29, 2541–2544. [Google Scholar] [CrossRef]
- Fanoro, O.T.; Parani, S.; Maluleke, R.; Lebepe, T.C.; Varghese, R.J.; Mgedle, N.; Mavumengwana, V.; Oluwafemi, O.S. Biosynthesis of smaller-sized platinum nanoparticles using the leaf extract of combretum erythrophyllum and its antibacterial activities. Antibiotics 2021, 10, 1275. [Google Scholar] [CrossRef]
- Dobrucka, R.; Romaniuk-Drapała, A.; Kaczmarek, V. Evaluation of biological synthesized platinum nanoparticles using Ononidis radix extract on the cell lung carcinoma A549. Biomed. Microdevices 2019, 21, 75. [Google Scholar] [CrossRef]
- Rokade, S.S.; Joshi, K.A.; Mahajan, K.; Patil, S.; Tomar, G.; Dubal, D.S.; Parihar, V.S.; Kitture, R.; Bellare, J.R.; Ghosh, S. Gloriosa superba mediated synthesis of platinum and palladium nanoparticles for induction of apoptosis in breast cancer. Bioinorg. Chem. Appl. 2018, 2018, 4924186. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Gholami-Shabani, Z.; Akbarzadeh, A.; Riazi, G.; Razzaghi-Abyaneh, M. Biogenic approach using sheep milk for the synthesis of platinum nanoparticles: The role of milk protein in platinum reduction and stabilization. Int. J. Nanosci. Nanotechnol. 2016, 12, 199–206. [Google Scholar]
- Venu, R.; Ramulu, T.S.; Anandakumar, S.; Rani, V.S.; Kim, C.G. Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 384, 733–738. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Brandelli, A.; Ritter, A.C.; Veras, F.F. Antimicrobial activities of metal nanoparticles. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 337–363. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Luzala, M.M.; Muanga, C.K.; Kyana, J.; Safari, J.B.; Zola, E.N.; Mbusa, G.V.; Nuapia, Y.B.; Liesse, J.-M.I.; Nkanga, C.I.; Krause, R.W.M. A critical review of the antimicrobial and antibiofilm activities of green-synthesized plant-based metallic nanoparticles. Nanomaterials 2022, 12, 1841. [Google Scholar] [CrossRef]
- Chwalibog, A.; Sawosz, E.; Hotowy, A.; Szeliga, J.; Mitura, S.; Mitura, K.; Grodzik, M.; Orlowski, P.; Sokolowska, A. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomed. 2010, 5, 1085–1094. [Google Scholar] [CrossRef]
- Abalkhil, T.A.; Alharbi, S.A.; Salmen, S.H.; Wainwright, M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017, 31, 411–417. [Google Scholar] [CrossRef]
- Feng, Z.V.; Gunsolus, I.L.; Qiu, T.A.; Hurley, K.R.; Nyberg, L.H.; Frew, H.; Johnson, K.P.; Vartanian, A.M.; Jacob, L.M.; Lohse, S.E. Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria. Chem. Sci. 2015, 6, 5186–5196. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.B.; Ramani, A.; Ganapathy, V.; Anbazhagan, V. Preparation of self-assembled platinum nanoclusters to combat Salmonella typhi infection and inhibit biofilm formation. Colloids Surf. B Biointerfaces 2018, 171, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria — “A battle of the titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.; Gul, R.; Andleeb, A.; Ullah, S.; Shah, M.; Khanum, M.; Ullah, I.; Hano, C.; Abbasi, B.H. A detailed review on biosynthesis of platinum nanoparticles (PtNPs), their potential antimicrobial and biomedical applications. J. Saudi Chem. Soc. 2021, 25, 101297. [Google Scholar] [CrossRef]
- Nejdl, L.; Kudr, J.; Moulick, A.; Hegerova, D.; Ruttkay-Nedecky, B.; Gumulec, J. Platinum nanoparticles induce damage to DNA and inhibit DNA replication. PLoS ONE 2017, 12, e0180798. [Google Scholar] [CrossRef]
- Sawosz, E.; Chwalibog, A.; Szeliga, J.; Sawosz, F.; Grodzik, M.; Rupiewicz, M. Visualization of gold and platinum nanoparticles interacting with Salmonella Enteritidis and Listeria monocytogenes. Int. J. Nanomed. 2010, 5, 631–637. [Google Scholar]
- Jeyapaul, U.; Kala, M.J.; Bosco, A.J.; Piruthiviraj, P.; Easuraja, M. An eco-friendly approach for synthesis of platinum nanoparticles using leaf extracts of Jatropa gossypifolia and Jatropa glandulifera and their antibacterial activity. OJCHEG 2018, 34, 783–790. [Google Scholar] [CrossRef]
- Sharma, K.D. Antibacterial activity of biogenic platinum nanoparticles: An invitro study. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 801–808. [Google Scholar] [CrossRef]
- Gupta, K.; Chundawat, T.S. Bio-inspired synthesis of platinum nanoparticles from fungus Fusarium oxysporum: Its characteristics, potential antimicrobial, antioxidant and photocatalytic activities. Mater. Res. Express 2019, 6, 1050d6. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Raman, T.; Anbazhagan, V. Platinum nanoparticles inhibit bacteria proliferation and rescue zebrafish from bacterial infection. RSC Adv. 2016, 6, 44415–44424. [Google Scholar] [CrossRef]
- Muraro, P.C.L.; Anjos, J.F.; Pinheiro, L.D.S.M.; Gomes, P.; Sagrillo, M.R.; da Silva, W.L. Green synthesis, antimicrobial, and antitumor activity of platinum nanoparticles: A review. Disciplinarum Scientia. Série: Naturais e Tecnológicas 2021, 22, 169–178. [Google Scholar] [CrossRef]
- Rajathi, F.A.A.; Nambaru, V.R.M.S. Phytofabrication of nano-crystalline platinum nanoparticles by leaves of Cerbera manghas and its antibacterial efficacy. Int. J. Pharm. Bio. Sci. 2014, 5, 619–628. [Google Scholar]
- Godugu, D.; Beedu, S.R. Biopolymer-mediated synthesis and characterisation of platinum nanocomposite and its anti-fungal activity against A. parasiticus and A. flavus. Micro Nano Lett. 2018, 13, 1491–1496. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Govind, P. Some important anticancer herbs: A review. IRJP 2011, 2, 45–52. [Google Scholar]
- Khan, I.; Paul, S.; Jakhar, R.; Bhardwaj, M.; Han, J.; Kang, S.C. Novel quercetin derivative TEF induces ER stress and mitochondria-mediated apoptosis in human colon cancer HCT-116 cells. Biomed. Pharmacother. 2016, 84, 789–799. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Wang, A.; Raza, M.; Jan, A.U.; Tahir, K.; Qipeng, Y. Bio-fabrication of catalytic platinum nanoparticles and their in vitro efficacy against lungs cancer cells line (A549). J. Photochem. Photobiol. B Biol. 2017, 173, 368–375. [Google Scholar]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. Anticancer properties of platinum nanoparticles and retinoic acid: Combination therapy for the treatment of human neuroblastoma cancer. Int. J. Mol. Sci. 2020, 21, 6792. [Google Scholar] [CrossRef]
- Loan, T.T.; Do, L.T.; Yoo, H. Platinum Nanoparticles Induce Apoptosis on Raw 264.7 Macrophage Cells. J. Nanosci. Nanotechnol. 2018, 18, 861–864. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and improving platinum anticancer drugs–phenanthriplatin. Anticancer Res. 2014, 34, 471–476. [Google Scholar]
- Tait, S.W.G.; Ichim, G.; Green, D.R. Die another way–non-apoptotic mechanisms of cell death. J. Cell. Sci. 2014, 127, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Kutwin, M.; Sawosz, E.; Jaworski, S.; Hinzmann, M.; Wierzbicki, M.; Hotowy, A.; Grodzik, M.; Winnicka, A.; Chwalibog, A. Investigation of platinum nanoparticle properties against U87 glioblastoma multiforme. Arch. Med. Sci. 2017, 6, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Ali, D.; Alarifi, S.; Alkahtani, S.; Almansour, M. Green platinum nanoparticles interaction with HEK293 cells: Cellular toxicity, apoptosis, and genetic damage. Dose Response 2018, 16, 1559325818807382. [Google Scholar] [CrossRef] [PubMed]
- Toufektchan, E.; Toledo, F. The guardian of the genome revisited: P53 downregulates genes required for telomere maintenance, DNA repair, and centromere structure. Cancers 2018, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Murrell, G.A.; Trickett, A.; Wang, M.X. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim. Biophys. Acta Mol. Cell Res. 2003, 1641, 35–41. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP 1317and cytochome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, B.; Lai, H.; Liu, G.; Ghia, E.M.; Widhopf, G.F.; Zhang, Z.; Wu, C.C.N.; Chen, L.; Wu, R.; et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti -cancer-stem-cell therapy. Proc. Natl. Acad. Sci. USA 2014, 111, 17266–17271. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Pavagadhi, S.; Mahadevan, A.; Balasubramanian, R. Biosynthesis of gold nanoparticles and related cytotoxicity evaluation using A549cells. Ecotoxicol. Environ. Saf. 2015, 114, 232–240. [Google Scholar] [CrossRef]
- Mohammadi, H.; Abedi, A.; Akbarzade, A. Evaluation of synthesized platinum nanoparticles on the MCF-7 and HepG-2 cancer cell lines. Int. Nano Lett. 2013, 3, 28. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K. LCytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. The effects of apigenin-biosynthesized ultra-small platinum nanoparticles on the human monocytic THP-1 cell line. Cells 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M.R.; Mirzaei, S.A.; Soleimanpour, H.; Dehghani, S.; et al. Platinum nanoparticles in biomedicine: Preparation, anti-cancer activity, and drug delivery vehicles. Front. Pharmacol. 2022, 13, 797804. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; Meyskens, F.L. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. O. J. Am. Assoc. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: Inflammatory and cytotoxic effects. Int. J. Nanomed. 2011, 6, 2821. [Google Scholar]
- Uehara, T.; Kikuchi, Y.; Nomura, Y. Caspase activation accompanying cytochrome c release from mitochondria is possibly involved in nitric oxide-induced neuronal apoptosis in SH-SY5Y cells. J. Neurochem. 1999, 72, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, J.P.; Almeida, A.; Stewart, V.; Peuchen, S.; Land, J.M.; Clark, J.B.; Heales, S.J. Nitric oxide-mediated mitochondrial damage in the brain: Mechanisms and implications for neurodegenerative diseases. J. Neurochem. 1997, 68, 2227–2240. [Google Scholar] [CrossRef] [PubMed]
- Roussel, B.D.; Kruppa, A.J.; Miranda, E.; Crowther, D.C.; Lomas, D.A.; Marciniak, S.J. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013, 12, 105–118. [Google Scholar] [CrossRef]
- Aygun, A.; Gülbagca, F.; Ozer, L.Y.; Ustaoglu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Atalar, M.N.; Alma, M.H.; Sen, F. Biogenic platinum nanoparticles using black cumin seed and their potential usages antimicrobial and anticancer agent. J. Pharm. Biomed. Anal. 2020, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.S.; Aziz, A.A.; Baharak, M.A.D.; Mehrdel, B.; Khaniabadi, P.M. Rapid sonochemically-assisted green synthesis of highly stable and biocompatible platinum nanoparticles. Surf. Interfaces. 2020, 20, 100635. [Google Scholar] [CrossRef]
- Jha, B.; Rao, M.; Chattopadhyay, A.; Bandyopadhyay, A.; Prasad, K.; Jha, A.K. Punica granatum fabricated platinum nanoparticles: A therapeutic pill for breast cancer. AIP Conf. Proc. 1953, 2018, 030087. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Halder, M.; Sarkar, A.; Pal, P.; Das, A.; Kundu, S.; Mandal, D.; Bhattacharjee, S. Investigating in vitro and in vivo anti-tumor activity of Curvularia-based platinum nanoparticles. J. Environ. Pathol. Toxicol. Oncol. 2022, 41, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-P.; Shen, J.; Qin, C.-Y.; Li, Y.-M.; Gao, L.-J.; Zheng, J.; Feng, Y.-L.; Yan, Z.; Zhou, X.; Cao, J.-M. Platinum nanoparticles promote breast cancer cell metastasis by disrupting endothelial barrier and inducing intravasation and extravasation. Nano Res. 2022, 15, 7366–7377. [Google Scholar] [CrossRef]
- Biosynthesized Platinum Nanoparticles Inhibit the Proliferation of Human Lung-Cancer Cells in vitro and Delay the Growth of a Human Lung-Tumor Xenograft in vivo -In vitro and in vivo Anticancer Activity of bio-Pt NPs- Yogesh Bendale*, Vineeta Bendale, Rammesh Natu, Saili Paul. J. Pharmacopunct. 2016, 19, 114–121. [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Sadras, S.R. The antioxidant and catalytic activities of green synthesized gold nanoparticles from Piper longum fruit extract. Process Saf. Environ. Prot. 2016, 100, 288–294. [Google Scholar] [CrossRef]
- Chakraborthy, A.; Ramani, P.; Sherlin, H.; Premkumar, P.; Natesan, A. Antioxidant and pro-oxidant activity of Vitamin C in oral environment. Indian J. Dent. Res. 2014, 25, 499. [Google Scholar] [CrossRef]
- Rajaram, K.; Aiswarya, D.; Sureshkumar, P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater. Lett. 2015, 138, 251–254. [Google Scholar] [CrossRef]
- Chen, G.; Guo, M. Rapid screening for α-glucosidase inhibitors from Gymnema sylvestre by affinity ultrafiltration–HPLC-MS. Front. Pharmacol. 2017, 8, 228. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Gu, J.; Chen, S. Biosynthesis of polyphenol-stabilised nanoparticles and assessment of anti-diabetic activity. J. Photochem. Photobiol. B. 2017, 169, 96–100. [Google Scholar] [CrossRef]
- Bag, J.; Mukherjee, S.; Tripathy, M. Platinum as a novel nanoparticle for wound healing model in Drosophila melanogaster. J. Clust. Sci. 2022. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Shirahata, S.; Hamasaki, T.; Haramaki, K.; Nakamura, T.; Abe, M.; Yan, H.; Kinjo, T.; Nakamichi, N.; Kabayama, S.; Shirahata, K.T. Anti-diabetes effect of water containing hydrogen molecule and Pt nanoparticles. BMC Proc. 2011, 5, P18. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehma, M.; Yoshihisa, Y.; Miyamoto, Y.; Shimizu, T. The anti-inflammatory effects of platinum nanoparticles on the lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages. Inflamm. Res. 2012, 61, 1177–1185. [Google Scholar] [CrossRef]
- Zhu, S.; Zeng, M.; Feng, G.; Wu, H. Platinum nanoparticles as a therapeutic agent against dextran sodium sulfate-induced colitis in mice. Int. J. Nanomed. 2019, 14, 8361–8378. [Google Scholar] [CrossRef]
- Moglianetti, M.; Luca, E.D.; Pedone, D.; Marotta, R.; Pompa, P.P. Platinum nanozymes recover cellular ros homeostasis in oxidative stressmediated disease model. Nanoscale 2016, 8, 3739–3752. [Google Scholar] [CrossRef]
- Takamiya, M.; Miyamoto, Y.; Yamashita, T.; Deguchi, K.; Ohta, Y.; Abe, K. Strong neuroprotection with a novel platinum nanoparticle against ischemic Stroke- and tissue plasminogen activator-related brain damages in mice. Neuroscience 2012, 221, 47–55. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Honda, A.; Zhao, Q.-L.; Makino, T.; Abe, R.; Matsui, K. Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp. Dermatol. 2010, 19, 1000–1006. [Google Scholar] [CrossRef]
- Onizawa, S.; Aoshiba, K.; Kajita, M.; Miyamoto, Y.; Nagai, A. Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. Ther. 2009, 22, 340–349. [Google Scholar] [CrossRef]
- Shibuya, S.; Ozawa, Y.; Watanabe, K.; Izuo, N.; Toda, T. Palladium and platinum nanoparticles attenuate aging-like skin atrophy via antioxidant activity in mice. PLoS ONE 2014, 9, e109288. [Google Scholar] [CrossRef] [PubMed]
- Gharibshahi, E.; Saion, E. Influence of dose on particle size and optical properties of colloidal platinum nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Bui, N.Q.; Moorthy, M.S.; Lee, K.D.; Oh, J. Synthesis and in vitro performance of polypyrrole-coated iron-platinum nanoparticles for photothermal therapy and photoacoustic imaging. Nanoscale Res. Lett. 2017, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Miao, H.; Ma, S.; Zhang, L.; You, C.; Tang, F.; Yang, C.; Tian, X.; Wang, F.; Luo, Y.; et al. FePt-Cys nanoparticles induce ROS-dependent cell toxicity, and enhance chemo-radiation sensitivity of NSCLC cells in vivo and in vitro. Cancer Lett. 2018, 418, 27–40. [Google Scholar] [CrossRef]
- Tsai, T.L.; Lai, Y.H.; Hw Chen, H.; Su, W.C. Overcoming radiation resistance by iron-platinum metal alloy nanoparticles in human copper transport 1-overexpressing cancer cells via mitochondrial disturbance. Int. J. Nanomed. 2021, 16, 2071–2085. [Google Scholar] [CrossRef]
- Peng, S.; Sun, Y.; Luo, Y.; Ma, S.; Sun, W.; Tang, G.; Li, S.; Zhang, N.; Ren, J.; Xiao, Y.; et al. MFP-FePt-GO nanocomposites promote radiosensitivity of non-small cell lung cancer via activating mitochondrial-mediated apoptosis and impairing DNA damage repair. Int. J. Biol. Sci. 2020, 16, 2145–2158. [Google Scholar] [CrossRef]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Koayashi, K.; Furusawa, Y.; Lesech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 085103. [Google Scholar] [CrossRef]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.T.; Pereira, I.A. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2016, 108, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Deng, H.H.; Lin, F.L.; Xu, X.W.; Weng, S.H.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. In situ growth of porous platinum nanoparticles on graphene oxide for colorimetric detection of cancer cells. Anal. Chem. 2014, 86, 2711–2718. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, Z.; Yi, C.; Xu, Z. Synthesis of platinum nanoparticles templated by dendrimers terminated with alkyl chain. Chem. Comm. J. 2018, 54, 9143–9146. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, W.; Kim, W.; Yoo, H.; Shin, H.C.; Jeon, S. Colorimetric detection of penicillin antibiotic residues in pork using hybrid magnetic nanoparticles and penicillin class-selective, antibody-functionalized platinum nanoparticles. Anal. Methods 2015, 7, 7639–7645. [Google Scholar] [CrossRef]
- Ji, X.; Lau, H.Y.; Ren, X.; Peng, B.; Zhai, P.; Feng, S.P.; Chan, P.K. Highly sensitive metabolite biosensor based on organic electrochemical transistor integrated with microfluidic channel and poly (N-vinyl-2-pyrrolidone) capped platinum nanoparticles. Adv. Mater. Technol. 2016, 1, 1600042. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhuo, Y.; Yuan, R.; Chai, Y.Q. A nanohybrid of platinum nanoparticles-porous ZnO-hemin with electrocatalytic activity to construct an amplified immunosensor for detection of influenza. Biosens. Bioelectron. 2016, 78, 321–327. [Google Scholar] [CrossRef]
- Dutta, G.; Nagarajan, S.; Lapidus, L.J.; Lillehoj, P.B. Enzyme-free electrochemical immunosensor based on methylene blue and the electro-oxidation of hydrazine on Pt nanoparticles. Biosens. Bioelectron. 2017, 92, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, M.; Hou, L.; Chen, G.; Tang, D. Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal. Chim. Acta. 2013, 776, 79–86. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Peroxidase activity of biogenic platinumnanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuat B 2018, 254, 690–700. [Google Scholar] [CrossRef]
- Prasek, M.; Sawosz, E.; Jaworski, S. Influence of nanoparticles of platinum on chicken embryo development and brain morphology. Nanoscale Res. Lett. 2013, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Gu, J.L.; Cao, J.M. The acute toxic effects of platinum nanoparticles on ion channels, transmembrane potentials of cardiomyocytes in vitro and heart rhythm in vivo in mice. Int. J. Nanomed. 2019, 14, 5595–5609. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Moglianetti, M.; Pompa, P.P.; Bardi, G. Platinum nanoparticles decrease reactive oxygen species and modulate gene expression without alteration of immune responses in THP-1 Monocytes. Nanomaterials 2018, 8, 392. [Google Scholar] [CrossRef]
- Gunes, S.; He, Z.; Malone, R.; Cullen, P.J.; Curtin, J.F. Platinum nanoparticles inhibit intracellular ROS generation and protect against cold atmospheric plasma-induced cytotoxicity. Nanomed. Nanotechnol. Biol. Med. 2021, 36. [Google Scholar] [CrossRef] [PubMed]
- Almarzoug, M.H.A.; Ali, D.; Alarifi, S.; Alkahtani, S.; Alhadheq, A.M. Platinum nanoparticles induced genotoxicity and apoptotic activity in human normal and cancer hepatic cells via oxidative stress-mediated Bax/Bcl-2 and caspase-3 expression. Environ. Toxicol. 2020, 35, 930–941. [Google Scholar] [CrossRef]
- Chlumsky, O.; Purkrtova, S.; Michova, H.; Sykorova, H.; Slepicka, P.; Fajstavr, D.; Ulbrich, P.; Viktorova, J.; Demnerova, K. Antimicrobial properties of palladium and platinum nanoparticles: A new tool for combating food-borne pathogens. Int. J. Mol. Sci. 2021, 22, 7892. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sun, J.; Li, S.; Shi, J.; Gao, H.; Leong, W.S.; Wu, Y.; Li, M.; Liu, C.; Li, P.; et al. Blood-triggered generation of platinum nanoparticle functions as an anti-cancer agent. Nat. Commun. 2020, 11, 567. [Google Scholar] [CrossRef]
- Hashimoto, M.; Yanagiuchi, H.; Kitagawa, H.; Honda, Y. Inhibitory effect of platinum nanoparticles on biofilm formation of oral bacteria. Nano Biomed. 2017, 9, 77–82. [Google Scholar]
- Kotov, N.A. Inorganic nanoparticles as protein mimics. Science 2010, 330, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Robert-Genthon, M.; Casabona, M.G.; Neves, D.; Couté, Y.; Cicéron, F.; Elsen, S. Unique features of a Pseudomonas aeruginosa α2-macroglobulin homolog. mBio 2013, 4, e00309-13. [Google Scholar] [CrossRef]
- Madlum, K.N.; Khamees, E.J.; Abdulridha, S.A.; Naji, R.A. Antimicrobial and cytotoxic activity of platinum nanoparticles synthesized by laser ablation technique. J. Nanostruct. 2021, 11, 13–19. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Yu, P.L. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Katsumi, H.; Fukui, K.; Sato, K.; Maruyama, S.; Yamashita, S.; Mizumoto, E.; Kusamori, K.; Oyama, M.; Sano, M.; Sakane, T. Pharmacokinetics and preventive effects of platinum nanoparticles as reactive oxygen species scavengers on hepatic ischemia/reperfusion injury in mice. Metallomics 2014, 6, 1050–1056. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; La, H.; Yoo, H.; Choi, Y.; Do, J.T.; Park, C.; Kim, J.-H.; Hong, K. Anisotropic platinum nanoparticle-induced cytotoxicity, apoptosis, inflammatory response, and transcriptomic and molecular pathways in human acute monocytic leukemia cells. Int. J. Mol. Sci. 2020, 21, 440. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Platinum nanoparticles enhance exosome release in human lung epithelial adenocarcinoma cancer cells (A549): Oxidative stress and the ceramide pathway are key players. Int. J. Nanomedicine 2021, 16, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef]
- Konieczny, P.; Goralczyk, A.G.; Szmyd, R.; Skalniak, L.; Koziel, J.; Filon, F.L.; Crosera, M.; Cierniak, A.; Zuba-Surma, E.K.; Borowczyk, J.; et al. Effects triggered by platinum nanoparticles on primary keratinocytes. Int. J. Nanomed. 2013, 8, 3963–3975. [Google Scholar]
- Shoshan, M.S.; Vonderach, T.; Hattendorf, B.; Angew, H.W. Peptide-coated platinum nanoparticles with selective toxicity against liver cancer cells. Chem. Int. Ed. Engl. 2019, 58, 4901–4905. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Porcel, E.; Remita, H.; Marco, S.; Réfrégiers, M.; Dutertre, M.; Confalonieri, F.; Lacombe Li, S. Platinum nanoparticles: An exquisite tool to overcome radioresistance. Cancer Nano 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Poona, W.; Tavares, A.J.; McGilvray, I.D.; Warren, C.W. Chan Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022. [Google Scholar] [CrossRef]
- Gutiérrez de la Rosa, S.Y.; Muñiz Diaz, R.; Villalobos Gutiérrez, P.T.; Patakfalvi, R.; Gutiérrez Coronado, Ó. Functionalized platinum nanoparticles with biomedical applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef]
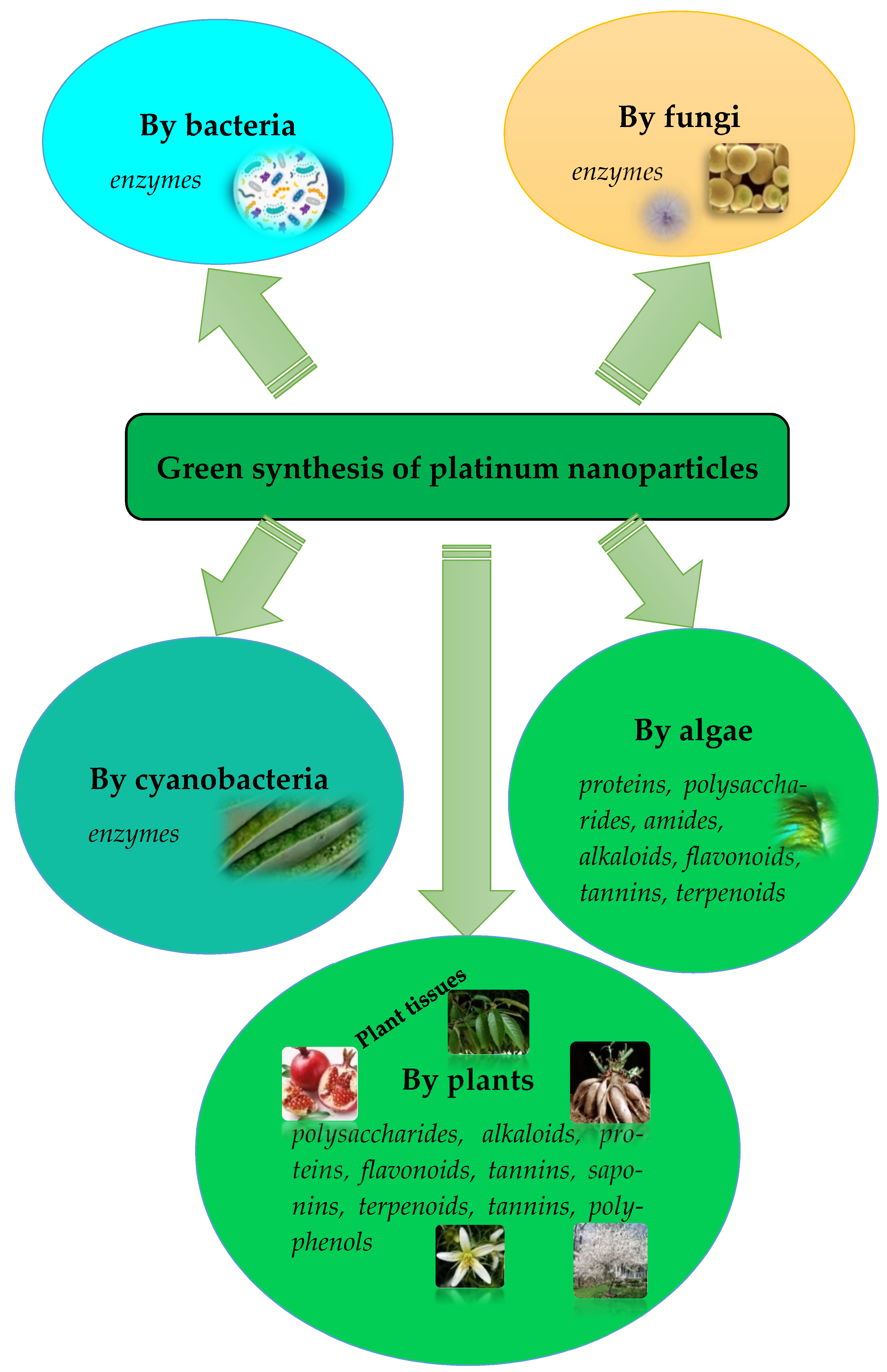
| Plant | Part of the Plant | Capping Agents | Reference |
|---|---|---|---|
| Cacumen platycladi | leaf extract | reducing sugars and flavonoids | [71] |
| Punica granatum | peel extract | polyphenols (ellagic acid, gallic acid, and quercetin) | [73] |
| Barleria prionitis | leaf extract | alkaloid, flavonoids, saponins, tannin, steroid, terpenoids, sterol, phenolic compound, glycosides | [74] |
| Cochlospermum gossypium (gum kondagogu) | aqueous medium containing gum | amino acids | [78] |
| Quercus glauca | leaf extract | flavonoids, tannins, carboxyl, amino and glycosides | [79] |
| Ocimum sanctum | leaf extract | ascorbic acid, gallic acid, terpenoids, proteins and amino acids | [80] |
| Antigonon leptopus | leaf stem and root extract | polysaccharides and proteins | [81] |
| Azadirachta indica | leaf extract | terpenoid | [75] |
| Terminalia chebula | fruit extract | polyphenolic content | [79] |
| Bacopa monnieri | leaf extract | amines, alcohols, ketones, aldehydes, and carboxylic acid | [85] |
| Dioscorea bulbifera | tuber extract | saponins, reducing sugars, ascorbic acid, citric acid, phenolics, and flavonoids | [86] |
| Peganum harmala | seed alkaloid fraction | alkaloid | [87] |
| Salix tetraspeama | leaf extract | polyphenols, polysaccharides, tannins, proteins, terpenoids | [88] |
| Atriplex halimus | leaf extract | glycosides, flavonoids, phenolic acids, and alkaloids | [89] |
| Date plants (Barni and Ajwa) | fruit extract | flavanols (polyphenols) | [90] |
| Xanthium strumarium | leaf extract | hydroxyl group | [91] |
| Fumariae herba | leaf extract | alkaloids (protopine), flavonoid compounds (quercetin-3,7-diglucoside, 3-arabinoglucoside, quercetin or rutin), and phenolic acids (p-coumaric acid, sinapic acid) | [92] |
| Anacardium occidentale | leaf extract | proteins, tannins, terpenoids, alkaloids, flavanols, phenols and glycosides | [93] |
| Mentha piperita | leaf extract | polyphenols | [94] |
| Tragia involucrata | leaf extract | polyphenols, alkaloids, flavonoids, and proteins | [95] |
| Polygonum salicifolium | leaf extract | glycosides, terpenoids, flavonoids, and alkaloids | [96] |
| Prunus × yedoensis | Gum extract | alkenes, alcohols, flavonoids, and amines | [98] |
| Eichhornia crassipes | leaf extract | polysaccharides | [99] |
| Sapindus mukorossi | aqueous extract of Soap nuts | saponins and flavonoids | [100] |
| Centella asiatica | leaf extract | flavonoids | [35] |
| Combretum erythrophyllum | leaf extract | proteins, flavonoids, amino acids, polyphenols, and carbohydrates | [101] |
| Ononidis radix | extract | isoflavonoids, phenolic acids | [102] |
| Gloriosa superba | tuber extract | alcoholic and phenolic compounds | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, E.O. Green Synthesis of Platinum Nanoparticles for Biomedical Applications. J. Funct. Biomater. 2022, 13, 260. https://doi.org/10.3390/jfb13040260
Mikhailova EO. Green Synthesis of Platinum Nanoparticles for Biomedical Applications. Journal of Functional Biomaterials. 2022; 13(4):260. https://doi.org/10.3390/jfb13040260
Chicago/Turabian StyleMikhailova, Ekaterina O. 2022. "Green Synthesis of Platinum Nanoparticles for Biomedical Applications" Journal of Functional Biomaterials 13, no. 4: 260. https://doi.org/10.3390/jfb13040260
APA StyleMikhailova, E. O. (2022). Green Synthesis of Platinum Nanoparticles for Biomedical Applications. Journal of Functional Biomaterials, 13(4), 260. https://doi.org/10.3390/jfb13040260





