Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Resins
2.2. Cell Culture
2.3. Cytotoxicity and Cell Cycle Assays
2.4. Apoptosis/Necrosis
2.5. Oxidative Stress
2.6. HSP70 Expression
2.7. MiR-9 Assay
2.8. Statistical Analysis
3. Results
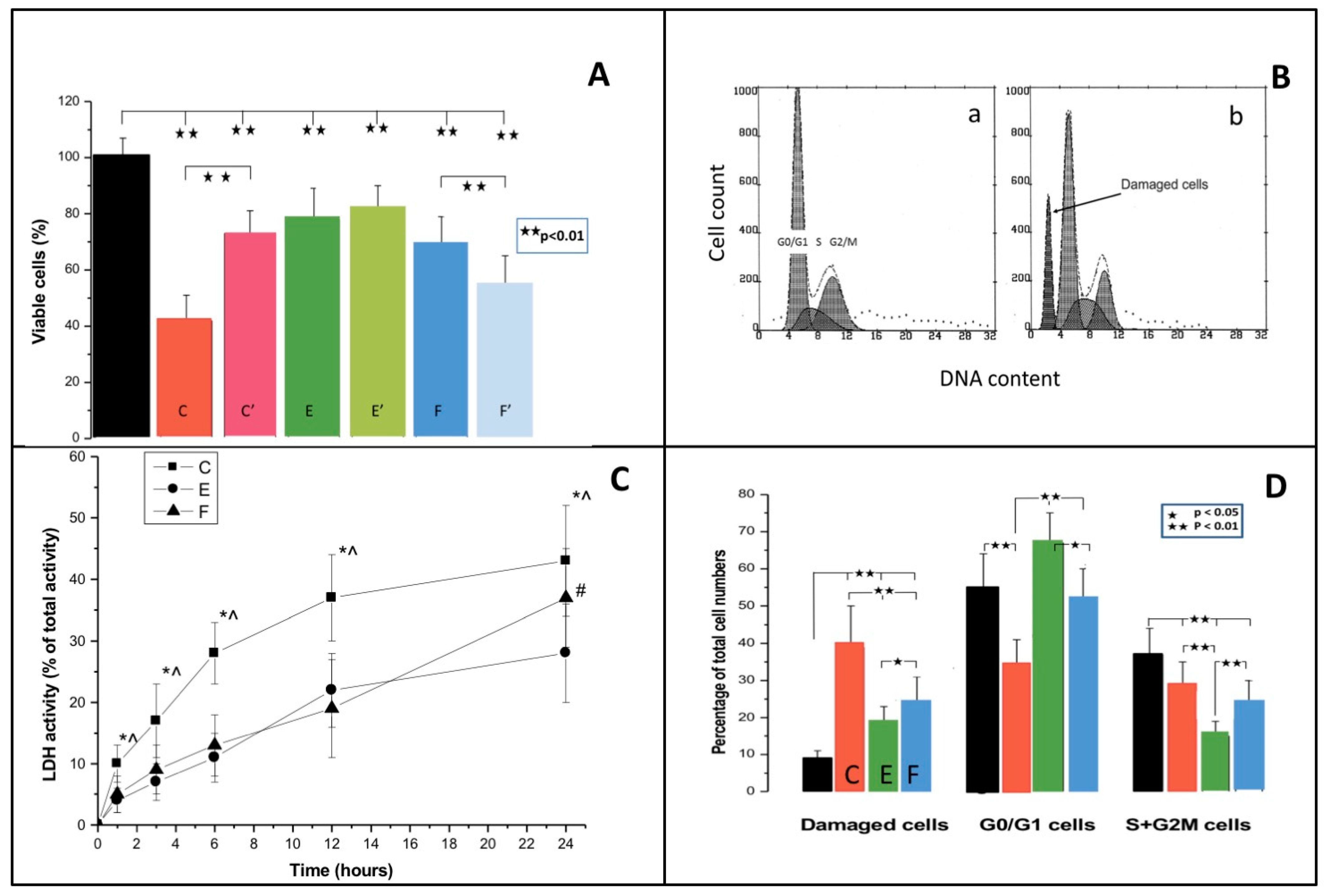
3.1. MTT Test
3.2. Cell Cycle
3.3. Cell Membrane Damage
3.4. Apoptosis/Necrosis
3.5. Oxidative Stress
3.6. MiR-9 and HSP70
3.7. Binary Scatter Plots of Oxidative Stress and HSP70
4. Discussion
4.1. Resin Cytotoxicity
Delayed Cytotoxicity and Cell Cycle
4.2. Oxidative Stress
4.3. Necrosis and Apoptosis
4.4. MiR-9 Expression
4.5. HSP70 Expression and HSP70/DCF Assay
4.6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahadevan, G.; Valiyaveettil, S. Understanding the interactions of poly (methyl methacrylate) and poly(vinyl chloride) nanoparticles with BHK-21 cell line. Sci. Rep. 2021, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Shih, W.-C.; Wang, Y.-L.; Tsai, Y.-L.; Chen, Y.-J.; Chang, M.-C.; Jeng, J.-H. Cytotoxicity and genotoxicity of DMABEE, a co-photoinitiator of resin polymerization, on CHO-K1 cells: Role of redox and carboxylesterase. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2020, 108, 2088–2098. [Google Scholar] [CrossRef]
- Kraus, D.; Wolfgarten, M.; Enkling, N.; Helfgen, E.-H.; Frentzen, M.; Probstmeier, R.; Winter, J.; Stark, H. In-vitro cytocompatibility of dental resin monomers on osteoblast-like cells. J. Dent. 2017, 65, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.O.; Magalhães, L.M.D.; Corrêa, J.D.; Dutra, W.O.; Gollob, K.J.; Silva, T.A.; Horta, M.C.R.; Souza, P.E.A. Composite-derived monomers affect cell viability and cytokine expression in human leukocytes stimulated with Porphyromonas gingivalis. J. Appl. Oral Sci. 2019, 27, e20180529. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 2013, 34, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Saxena, P.; Pant, V.A.; Pant, A.B. Release and toxicity of dental resin composite. Toxicol. Int. 2012, 19, 225–234. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins—A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef]
- Ponce-Bravo, S.; Ledesma-Montes, C.; Martínez-Rivera, J.-L.; Garcés-Ortíz, M. Toxicity test of a dental commercial composite. J. Clin. Exp. Dent. 2015, 7, e289–e292. [Google Scholar] [CrossRef]
- Schneider, T.R.; Hakami-Tafreshi, R.; Tomasino-Perez, A.; Tayebi, L.; Lobner, D. Effects of dental composite resin monomers on dental pulp cells. Dent. Mater. J. 2019, 38, 579–583. [Google Scholar] [CrossRef]
- Eteti, G.; Orsini, G.; Salvatore, V.; Efocaroli, S.; Emazzotti, M.C.; Eruggeri, A.; Mattioli-Belmonte, M.; Efalconi, M. HEMA but not TEGDMA induces autophagy in human gingival fibroblasts. Front. Physiol. 2015, 6, 275. [Google Scholar] [CrossRef]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.-A.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-L. The effects of cytotoxicity and genotoxicity induced by 2,2-bis[4-(acryloxypropoxy)phenyl]propane via caspases in human gingival fibroblasts. Toxicol. Ind. Health 2014, 30, 755–764. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Papachristou, E.; Koidis, P.; Geurtsen, W. Effects of resinous monomers on the odontogenic differentiation and mineralization potential of highly proliferative and clonogenic cultured apical papilla stem cells. Dent. Mater. 2012, 28, 327–339. [Google Scholar] [CrossRef]
- Styllou, M.; Reichl, F.-X.; Styllou, P.; Urcan, E.; Rothmund, L.; Hickel, R.; Högg, C.; Scherthan, H. Dental composite components induce DNA-damage and altered nuclear morphology in gingiva fibroblasts. Dent. Mater. 2015, 31, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Ginzkey, C.; Zinnitsch, S.; Steussloff, G.; Koehler, C.; Hackenberg, S.; Hagen, R.; Kleinsasser, N.H.; Froelich, K. Assessment of HEMA and TEGDMA induced DNA damage by multiple genotoxicological endpoints in human lymphocytes. Dent. Mater. 2015, 31, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Wellner, P.; Hickel, R.; Reichl, F. Synergistic interaction caused to human gingival fibroblasts from dental monomers. Dent. Mater. 2012, 28, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiyasat, A.S.; Darmani, H.; Milhem, M.M. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin. Oral Investig. 2005, 9, 21–25. [Google Scholar] [CrossRef]
- Ilie, N.; Kreppel, I.; Durner, J. Effect of radical amplified photopolymerization (RAP) in resin-based composites. Clin. Oral Investig. 2014, 18, 1081–1088. [Google Scholar] [CrossRef]
- Luczaj-Cepowicz, E.; Marczuk-Kolada, G.; Pawinska, M.; Obidzinska, M.; Holownia, A. Evaluation of cytotoxicity and pH changes generated by various dental pulp capping materials—An in vitro study. Folia Histochem. et Cytobiol. 2017, 55, 86–93. [Google Scholar] [CrossRef][Green Version]
- Ipek, S.; Üstündağ, A.; Eke, B.C. Three-dimensional (3D) cell culture studies: A review of the field of toxicology. Drug Chem. Toxicol. 2022, 1–11. [Google Scholar] [CrossRef]
- Khafaei, M.; Rezaie, E.; Mohammadi, A.; Gerdehsang, P.S.; Ghavidel, S.; Kadkhoda, S.; Zahra, A.Z.; Forouzanfar, N.; Arabameri, H.; Tavallaie, M. miR-9: From function to therapeutic potential in cancer. J. Cell. Physiol. 2019, 234, 14651–14665. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, M.R.; Valpuesta, J.M. Hsp70 chaperone: A master player in protein homeostasis. F1000Research 2018, 7, 1497. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Rousselle, C.; Robert-Nicoud, M.; Ronot, X. Flow cytometric analysis of DNA content of living and fixed cells: A comparative study using various fixatives. Histochem. J. 1998, 30, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, M.-L.; Lecoeur, H.; Prévost, M. Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: A reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry 2001, 44, 65–72. [Google Scholar] [CrossRef]
- Ubezio, P.; Civoli, F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic. Biol. Med. 1994, 16, 509–516. [Google Scholar] [CrossRef]
- Aranha, A.M.F.; Giro, E.M.A.; Hebling, J.; Lessa, F.; Costa, C.A.D.S. Effects of light-curing time on the cytotoxicity of a restorative composite resin on odontoblast-like cells. J. Appl. Oral Sci. 2010, 18, 461–466. [Google Scholar] [CrossRef]
- Nalçaci, A.; Ulusoy, N.; Atakol, O.; Nalçacı, A. Time-based Elution of TEGDMA and BisGMA from Resin Composite Cured with LED, QTH and High-intensity QTH Lights. Oper. Dent. 2006, 31, 197–203. [Google Scholar] [CrossRef]
- Reichl, F.-X.; Esters, M.; Simon, S.; Seiss, M.; Kehe, K.; Kleinsasser, N.; Folwaczny, M.; Glas, J.; Hickel, R. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch. Toxicol. 2005, 80, 370–377. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular Toxicology of Substances Released from Resin–Based Dental Restorative Materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef]
- Atsumi, T.; Ishihara, M.; Kadoma, Y.; Tonosaki, K.; Fujisawa, S. Comparative radical production and cytotoxicity induced by camphorquinone and 9-fluorenone against human pulp fibroblasts. J. Oral Rehabil. 2004, 31, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, C.; D’Aurora, M.; Di Giacomo, V.; Stuppia, L.; Cataldi, A.; Gatta, V. Transcriptome modifications in human gingival fibroblasts exposed to 2-hydroxyethyl methacrylate. Gene 2016, 582, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, B.W.; Simon-Santamaria, J.; Örtengren, U.; Jensen, E.; Bruun, J.-A.; Michelsen, V.B.; Sørensen, K.K. Dose- and time-dependent effects of triethylene glycol dimethacrylate on the proteome of human THP-1 monocytes. Eur. J. Oral Sci. 2018, 126, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Lussi, A.; Gruber, R.; Ilie, N.; Price, R.B.; Schmalz, G. Effect of the degree of conversion of resin-based composites on cytotoxicity, cell attachment, and gene expression. Dent. Mater. 2019, 35, 1173–1193. [Google Scholar] [CrossRef] [PubMed]
- Issa, Y.; Watts, D.C.; Brunton, P.A.; Waters, C.M.; Duxbury, A.J. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent. Mater. 2004, 20, 12–20. [Google Scholar] [CrossRef]
- Nomura, Y.; Teshima, W.; Kawahara, T.; Tanaka, N.; Ishibashi, H.; Okazaki, M.; Arizono, K. Genotoxicity of dental resin polymerization initiators in vitro. J. Mater. Sci. Mater. Med. 2006, 17, 29–32. [Google Scholar] [CrossRef]
- Randolph, L.D.; Steinhaus, J.; Möginger, B.; Gallez, B.; Stansbury, J.; Palin, W.M.; Leloup, G.; Leprince, J.G. Photopolymerization of highly filled dimethacrylate-based composites using Type I or Type II photoinitiators and varying co-monomer ratios. Dent. Mater. 2016, 32, 136–148. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzì, R.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Zhang, Y.; Zhang, H.-N.; Wang, Y.-B.; Wang, W. TGF-β1-induced epithelial–mesenchymal transition in lung cancer cells involves upregulation of miR-9 and downregulation of its target, E-cadherin. Cell. Mol. Biol. Lett. 2017, 22, 1–10. [Google Scholar] [CrossRef]
- Detassis, S.; Grasso, M.; Del Vescovo, V.; Denti, M.A. microRNAs Make the Call in Cancer Personalized Medicine. Front. Cell Dev. Biol. 2017, 5, 86. [Google Scholar] [CrossRef]
- Qu, B.; Jia, Y.; Liu, Y.; Wang, H.; Ren, G.; Wang, H. The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: A literature review. Cell Stress Chaperon. 2015, 20, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Wataha, J.C.; Kaga, M.; Lockwood, P.E.; Volkmann, K.R.; Sano, H. Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethal concentrations. J. Dent. Res. 2002, 81, 265–269. [Google Scholar] [CrossRef] [PubMed]


| Resin | Chemical Constituents |
|---|---|
| C | Bisphenol-A-glycidyl methacrylate (BisGMA); Triethylene glycol dimethacrylate (TEGDMA); Camphorquinone (CQ) |
| E | Bisphenol-A-glycidyl methacrylate (BisGMA); Triethylene glycol dimethacrylate (TEGDMA) |
| F | Urethane dimethacrylate (UDMA); Bisphenol-A- polyethethylene glycol diether dimethacrylate (BisEMA) |
| Biochemical Indices of Resin Toxicity | Control | C | E | F | Significance |
|---|---|---|---|---|---|
| MTT test (% of control) | |||||
| Freshly-cured resin | 100 ± 7 | 42 ± 9 ** | 78 ± 11 **## | 69 ± 10 **## | Toxicity C >> E = F |
| Preincubated resin | 73 ± 8 **++ | 81 ± 9 **## | 54 ± 11 **##^^ | F > E > E | |
| LDH (% of total activity) | |||||
| 1 h | 10 ± 3 * | 4 ± 2 | 5 ± 3 * | C—Rapid increase, saturable | |
| 3 h | 17 ± 6 ** | 7 ± 3 ** | 9 ± 4 ** | E—Slow increase, saturable | |
| 6 h | 28 ± 5 ** | 11 ± 4 ** | 13 ± 5 ** | F—Slow increase, linear | |
| 12 h | 37 ± 7 ** | 22 ± 6 ** | 19 ± 8 ** | ||
| 24 h | 43 ± 9 ** | 28 ± 8 ** | 37 ± 8 ** | ||
| Cell cycle (%) | |||||
| Cells with damaged DNA | 8 ± 3 | 39 ± 11 ** | 18 ± 5 **## | 24 ± 7 **##^ | Increased C > F > E |
| Pre-DNA synthesis/ resting | 55 ± 9 | 34 ± 7 ** | 67 ± 8 ## | 52 ± 9 ##^ | Variable E > C = F |
| Proliferation | 37 ± 7 | 27 ± 8 ** | 15 ± 4 **## | 24 ± 6 **^^ | Decreased E > F > C |
| Oxidative stress (relative units) | 100 ± 34 | 951 ± 111 ** | 877 ± 132 ** | 1215 ± 201 **##^^ | F > E = C |
| Apoptosis/Necrosis (%) | |||||
| Naive cells | 93 ± 7 | 18 ± 6 ** | 21 ± 11 ** | 20 ± 5 ** | C-predominantly necrosis |
| Necrotic cells | 2 ± 3 | 75 ± 10 ** | 48 ± 14 **# | 73 ± 11 ** | |
| Apoptotic cells | 2 ± 2 | 2 ± 2 | 3 ± 2 | 1 ± 2 | D - some apoptosis |
| Necrotic/apoptotic cells | 3 ± 2 | 5 ± 4 | 28 ± 13 **# | 16 ± 7 **##^^ | F-mostly necrosis |
| miR-9 (fold change) | 1 | 1.76 * | 1.92 * | 3.39 * | Increased F > C = E |
| HSP70 (relative units) | 100 ± 17 | 733 ± 76 ** | 112 ± 23 ## | 177 ± 28 ## | Increased C >> (E = F normal) |
| DCF/HSP70 (relative units) | |||||
| Scatterplot area | 100 ± 14 | 167 ± 26 ** | 127 ± 17 | 131 ± 19 * | Increased C > E = F |
| Central tendency line (slope act) | 1.04 ± 0.12 | 1.15 ± 0.11 | 0.9 ± 0.14 | 1.00 ± 0.15 | |
| Trend (vector) line (slope at) | - | 0.05 ± 0.14 κκ | 1.19 ± 0.18 κ− | 1.11 ± 0.25 | C ≠ E = F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulek, J.; Luczaj-Cepowicz, E.; Marczuk-Kolada, G.; Rosłan, M.; Holownia, A. Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts. J. Funct. Biomater. 2022, 13, 56. https://doi.org/10.3390/jfb13020056
Sulek J, Luczaj-Cepowicz E, Marczuk-Kolada G, Rosłan M, Holownia A. Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts. Journal of Functional Biomaterials. 2022; 13(2):56. https://doi.org/10.3390/jfb13020056
Chicago/Turabian StyleSulek, Jolanta, Elzbieta Luczaj-Cepowicz, Grazyna Marczuk-Kolada, Maciej Rosłan, and Adam Holownia. 2022. "Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts" Journal of Functional Biomaterials 13, no. 2: 56. https://doi.org/10.3390/jfb13020056
APA StyleSulek, J., Luczaj-Cepowicz, E., Marczuk-Kolada, G., Rosłan, M., & Holownia, A. (2022). Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts. Journal of Functional Biomaterials, 13(2), 56. https://doi.org/10.3390/jfb13020056







