Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HEK-293 Cell Culture
2.3. HEK-293 Cell Line Transfection
2.4. Preparation of Cell Lysates
2.5. Determination of Protein Concentration by Bradford Method
2.6. β-d-N-Acetyl-Hexosaminidase Enzyme Assay
2.7. Affinity Chromatography on Concanavalin A-Sepharose
2.8. DEAE-Chromatography
2.9. SDS-PAGE and Immunoblot Analysis
2.10. Immobilization of β-d-N-Acetyl-Hexosaminidase A
2.11. Biochemical Characterization of Immobilized β-d-N-Acetyl-Hexosaminidase A
3. Results and Discussion
3.1. HexA Purification by Affinity and DEAE Chromatography
3.2. SDS-PAGE and Immunoblot Analysis
3.3. HexA Immobilization on PLA Films
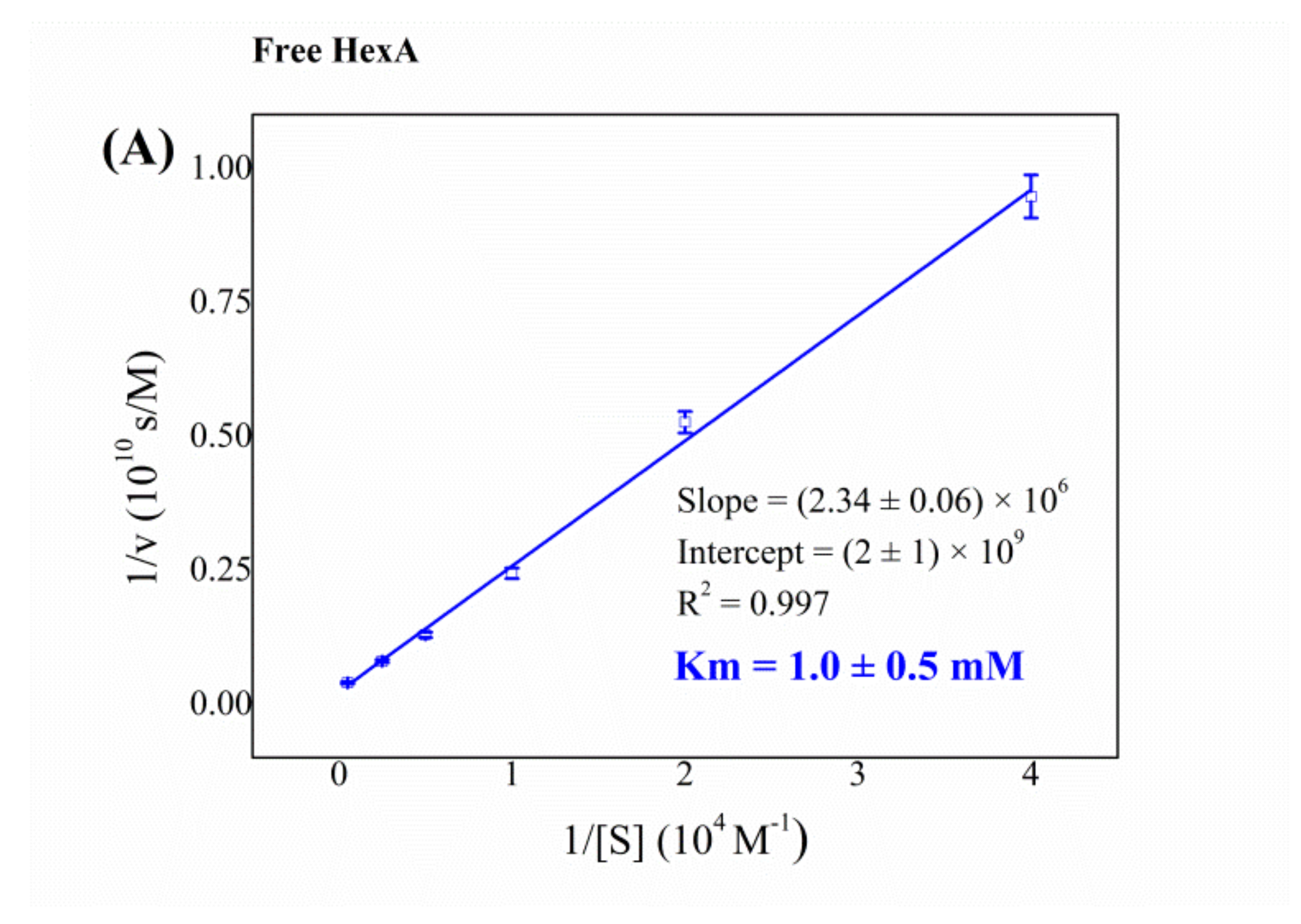
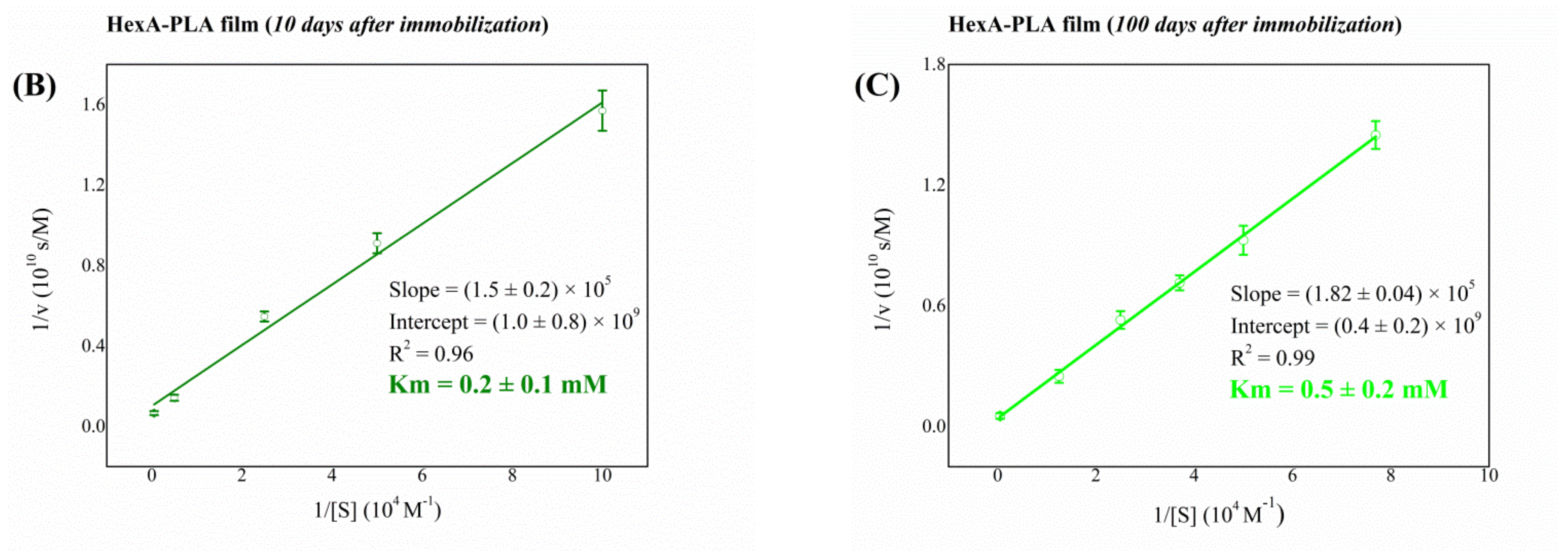
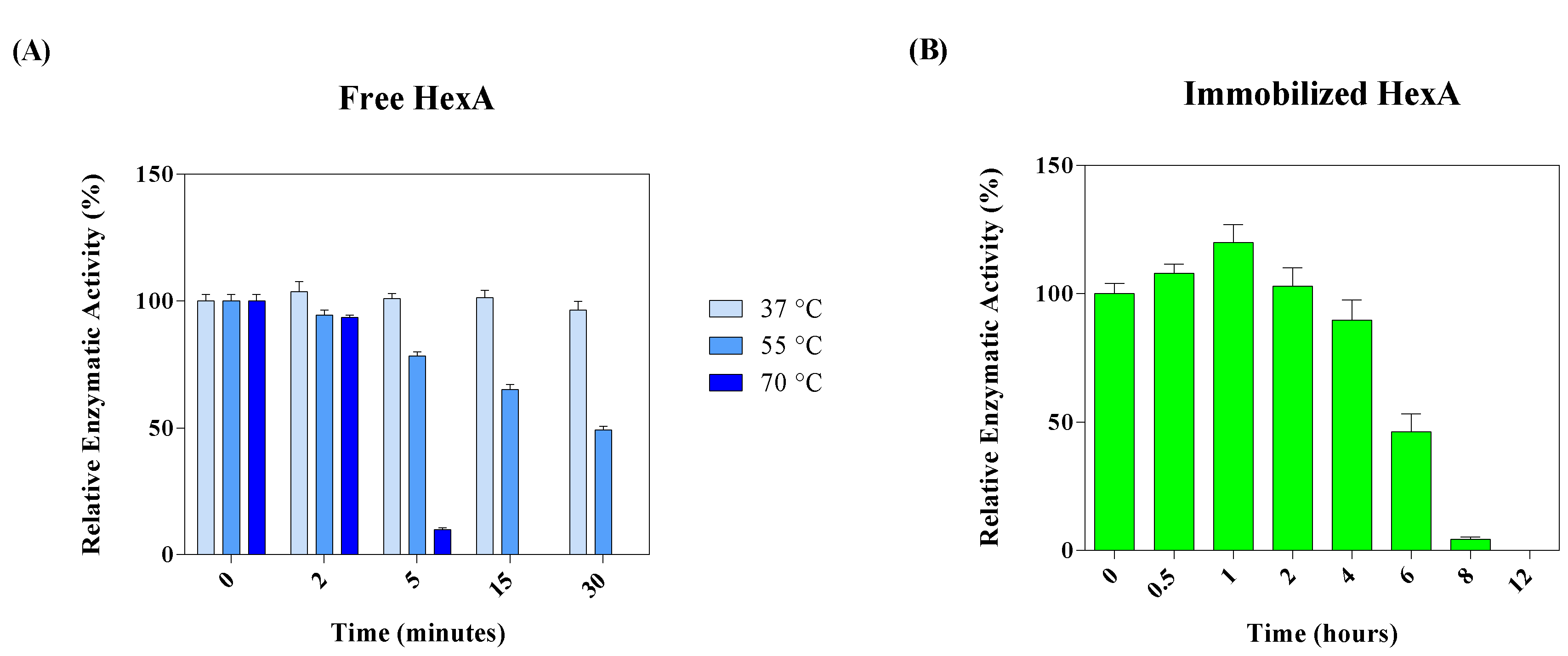
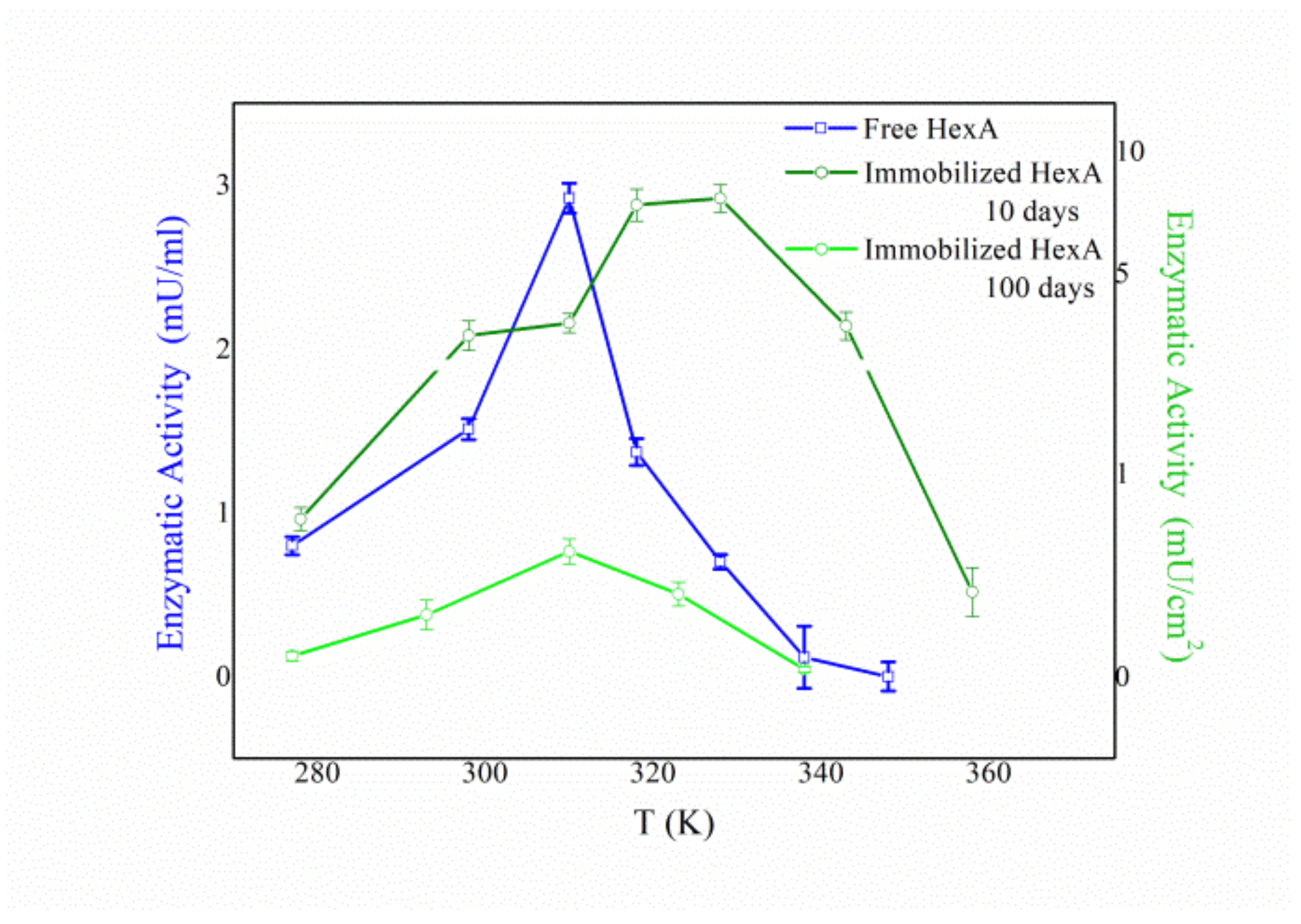
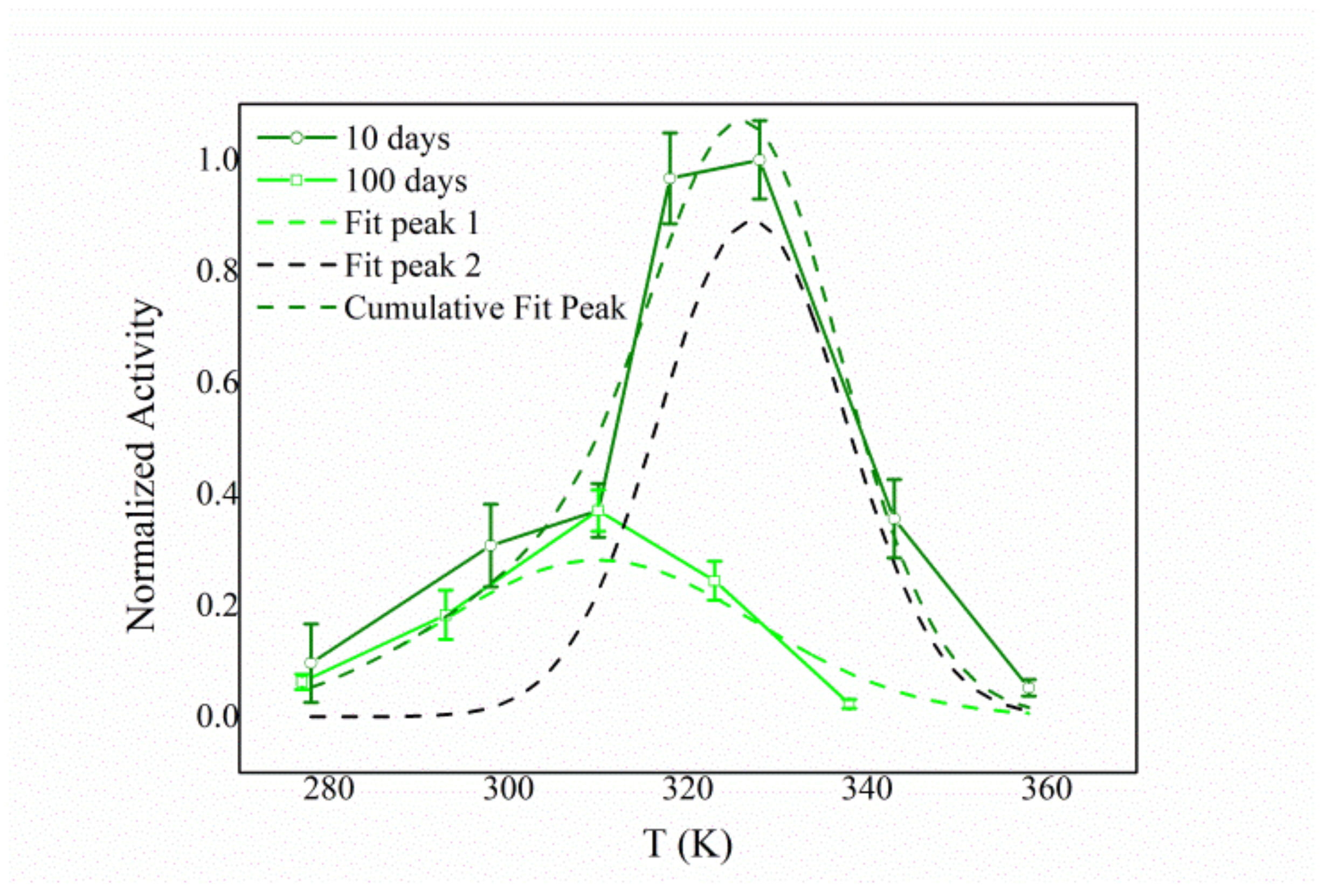
3.4. Biochemical Characterization of the Free and Immobilized HexA Enzyme on PLA Films
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neufeld, E.F. Natural history and inherited disorders of a lysosomal enzyme, beta-hexosaminidase. J. Biol. Chem. 1989, 264, 10927–10930. [Google Scholar] [CrossRef]
- Bojarová, P.; Kulik, N.; Slámová, K.; Hubálek, M.; Kotik, M.; Cvačka, J.; Pelantová, H.; Křen, V. Selective β-N-acetylhexosaminidase from Aspergillus versicolor—A tool for producing bioactive carbohydrates. Appl. Microbiol. Biotechnol. 2019, 103, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Bojarová, P.; Petrásková, L.; Křen, V. β-N-acetylhexosaminidase: What’s in a name…? Biotechnol. Adv. 2010, 28, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, N.O.; Roseman, S. The chitin catabolic cascade in the marine bacterium Vibrio furnissii: Molecular cloning, isolation, and characterization of a periplasmic chitodextrinase. J. Biol. Chem. 1996, 271, 33414–33424. [Google Scholar] [CrossRef] [PubMed]
- Riekenberg, S.; Flockenhaus, B.; Vahrmann, A.; Müller, M.C.; Leippe, M.; Kieß, M.; Scholze, H. The β-N-acetylhexosaminidase of Entamoeba histolytica is composed of two homologous chains and has been localized to cytoplasmic granules. Mol. Biochem. Parasitol. 2004, 138, 217–225. [Google Scholar] [CrossRef]
- Gooday, G.W. Physiology of microbial degradation of chitin and chitosan. In Biochemistry of Microbial Degradation; Springer: Dordrecht, The Netherland, 1994; pp. 279–312. [Google Scholar]
- Koo, I.C.; Ohol, Y.M.; Wu, P.; Morisaki, J.H.; Cox, J.S.; Brown, E.J. Role for lysosomal enzyme β-hexosaminidase in the control of mycobacteria infection. Proc. Natl. Acad. Sci. USA 2008, 105, 710–715. [Google Scholar] [CrossRef]
- Scigelova, M.; Crout, D.H. Microbial β-N-acetylhexosaminidases and their biotechnological applications. Enzym. Microb. Technol. 1999, 25, 3–14. [Google Scholar] [CrossRef]
- Schlumbaum, A.; Mauch, F.; Vögeli, U.; Boller, T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986, 324, 365–367. [Google Scholar] [CrossRef]
- Nyffenegger, C.; Nordvang, R.T.; Zeuner, B.; Łężyk, M.; Difilippo, E.; Logtenberg, M.J.; Schols, H.A.; Meyer, A.S.; Mikkelsen, J.D. Backbone structures in human milk oligosaccharides: Trans-glycosylation by metagenomic β-N-acetylhexosaminidases. Appl. Microbiol. Biotechnol. 2015, 99, 7997–8009. [Google Scholar] [CrossRef]
- Visnapuu, T.; Teze, D.; Kjeldsen, C.; Lie, A.; Duus, J.Ø.; André-Miral, C.; Pedersen, L.H.; Stougaard, P.; Svensson, B. Identification and Characterization of a β-N-Acetylhexosaminidase with a Biosynthetic Activity from the Marine Bacterium Paraglaciecola hydrolytica S66T. Int. J. Mol. Sci. 2020, 21, 417. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Gahl, W.A. Lysosomal storage diseases. Transl. Sci. Rare Dis. 2017, 2, 1–71. [Google Scholar] [CrossRef]
- Emiliani, C.; Beccari, T.; Tabilio, A.; Orlacchio, A.; Hosseini, R.; Stirling, J.L. An enzyme with properties similar to those of β-N-acetylhexosaminidase S is expressed in the promyelocytic cell line HL-60. Biochem. J. 1990, 267, 111–117. [Google Scholar] [CrossRef]
- Emiliani, C.; Orlacchio, A. Particular forms of β-N-acetylhexosaminidase in human leukaemic cells. Int. J. Biochem. 1992, 24, 539–544. [Google Scholar] [CrossRef]
- Ikonne, J.U.; Rattazzi, M.C.; Desnick, R.J. Characterization of Hex S, the major residual beta hexosaminidase activity in type O Gm2 gangliosidosis (Sandhoff-Jatzkewitz disease). Am. J. Hum. Genet. 1975, 27, 639. [Google Scholar]
- Sandhoff, K.; Andreae, U.; Jatzkewitz, H. Deficient hexosaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Life Sci. 1968, 7, 283–288. [Google Scholar] [CrossRef]
- Neote, K.; Brown, C.A.; Mahuran, D.J.; Gravel, R.A. Translation initiation in the HEXB gene encoding the beta-subunit of human beta-hexosaminidase. J. Biol. Chem. 1990, 265, 20799–20806. [Google Scholar] [CrossRef]
- Lemieux, M.J.; Mark, B.L.; Cherney, M.M.; Withers, S.G.; Mahuran, D.J.; James, M.N. Crystallographic structure of human β-hexosaminidase A: Interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis. J. Mol. Biol. 2006, 359, 913–929. [Google Scholar] [CrossRef]
- Martino, S.; Marconi, P.; Tancini, B.; Dolcetta, D.; De Angelis, M.G.; Montanucci, P.; Bregola, G.; Sandhoff, K.; Bordignon, C.; Emiliani, C.; et al. A direct gene transfer strategy via brain internal capsule reverses the biochemical defect in Tay-Sachs disease. Hum. Mol. Genet. 2005, 14, 2113–2123. [Google Scholar] [CrossRef]
- Cachon-Gonzalez, M.B.; Wang, S.Z.; Lynch, A.; Ziegler, R.; Cheng, S.H.; Cox, T.M. Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 10373–10378. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Chakrabarti, L.; Rizvanov, A.A. New Approaches to Tay-Sachs Disease Therapy. Front. Physiol. 2018, 9, 1663. [Google Scholar] [CrossRef]
- Beck, M. New therapeutic options for lysosomal storage disorders: Enzyme replacement, small molecules and gene therapy. Hum. Genet. 2007, 121, 1–22. [Google Scholar] [CrossRef]
- Futerman, A.H.; Van Meer, G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 2004, 5, 554. [Google Scholar] [CrossRef]
- Matern, D.; Gavrilov, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Tortorelli, S. Newborn screening for lysosomal storage disorders. In Seminars in Perinatology; WB Saunders: Philadelphia, PA, USA, 2005; Volume 39, pp. 206–216. [Google Scholar]
- Ono, H.; Sugiura, C.; Narita, A.; Ohno, K.; Saito, Y.; Maegaki, Y.; Murakami, N.; Nanba, E. Clinical characteristics of early juvenile GM2 gangliosidosis: A case report. No Hattatsu Brain Dev. 2017, 49, 203–206. [Google Scholar]
- Platt, F.M.; Boland, B.; van der Spoel, A.C. Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012, 199, 723–734. [Google Scholar] [CrossRef]
- Regier, D.S.; Proia, R.L.; D’Azzo, A.; Tifft, C.J. The GM1 and GM2 Gangliosidoses: Natural History and Progress toward Therapy. Pediatr. Endocrinol. Rev. 2016, 13, 663–673. [Google Scholar]
- Urbanelli, L.; Magini, A.; Polchi, A.; Polidoro, M.; Emiliani, C. Recent developments in therapeutic approaches for lysosomal storage diseases. Recent Pat. CNS Drug Discov. 2011, 6, 1–19. [Google Scholar] [CrossRef]
- Sandhoff, K.; Harzer, K. Gangliosides and gangliosidoses: Principles of molecular and metabolic pathogenesis. J. Neurosci. 2013, 33, 10195–10208. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Brooks, D.A.; Evangelista, M.; Hein, L.K.; Hopwood, J.J.; Meikle, P.J. Prediction of neuropathology in mucopolysaccharidosis I patients. Mol. Genet. Metab. 2005, 84, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Muro, S. Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives. Adv. Drug Deliv. Rev. 2007, 118, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Beck, M. Treatment strategies for lysosomal storage disorders. Dev. Med. Child Neurol. 2018, 60, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, M.; Dwek, R.A.; Butters, T.D.; Platt, F.M. Storage solutions: Treating lysosomal disorders of the brain. Nat. Rev. Neurosci. 2005, 6, 713–725. [Google Scholar] [CrossRef]
- Tropak, M.B.; Mahuran, D. Lending a helping hand, screening chemical libraries for compounds that enhance β-hexosaminidase A activity in GM2 gangliosidosis cells. FEBS J. 2007, 274, 4951–4961. [Google Scholar] [CrossRef]
- Akeboshi, H.; Kasahara, Y.; Tsuji, D.; Itoh, K.; Sakuraba, H.; Chiba, Y.; Jigami, Y. Production of human β-hexosaminidase A with highly phosphorylated N-glycans by the overexpression of the Ogataea minuta MNN4 gene. Glycobiology 2009, 19, 1002–1009. [Google Scholar] [CrossRef]
- Li, M. Enzyme replacement therapy: A review and its role in treating lysosomal storage diseases. Pediatr. Ann. 2018, 47, e191–e197. [Google Scholar] [CrossRef]
- Mühlstein, A.; Gelperina, S.; Kreuter, J. Development of nanoparticle-bound arylsulfatase B for enzyme replacement therapy of mucopolysaccharidosis VI. Die Pharm. Int. J. Pharm. Sci. 2013, 68, 549–554. [Google Scholar]
- Wraith, J.E. Limitations of enzyme replacement therapy: Current and future. J. Inherit. Metab. Dis. 2006, 29, 442–447. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Muro, S. Strategies for delivery of therapeutics into the central nervous system for treatment of lysosomal storage disorders. Drug Deliv. Transl. Res. 2012, 2, 169–186. [Google Scholar] [CrossRef]
- Schuster, T.; Mühlstein, A.; Yaghootfam, C.; Maksimenko, O.; Shipulo, E.; Gelperina, S.; Kreuter, J.; Gieselmann, V.; Matzner, U. Potential of surfactant-coated nanoparticles to improve brain delivery of arylsulfatase A. J. Control. Release 2017, 253, 1–10. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Romero-Fernández, M.; Paradisi, F. General Overview on Immobilization Techniques of Enzymes for Biocatalysis. Catal. Immobil. Methods Appl. 2020, 409–435. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.; Dinu, C. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Enzymes and bioconversions of industrial, pharmaceutical, and biotechnological significance. Org. Process Res. Dev. 2010, 15, 224–230. [Google Scholar] [CrossRef]
- Polchi, A.; Magini, A.; Mazuryk, J.; Tancini, B.; Gapiński, J.; Patkowski, A.; Giovagnoli, S.; Emiliani, C. Rapamycin loaded solid lipid nanoparticles as a new tool to deliver mTOR inhibitors: Formulation and in vitro characterization. Nanomaterials 2016, 6, 87. [Google Scholar] [CrossRef]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Luo, G.; Liu, Q.; Wang, Y. Pharmacokinetics and biodistribution of surface modification polymeric nanoparticles. Arch. Pharm. Res. 2008, 31, 547–554. [Google Scholar] [CrossRef]
- Shilo, M.; Sharon, A.; Baranes, K.; Motiei, M.; Lellouche, J.P.M.; Popovtzer, R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: An in-vitro endothelial cell model. J. Nanobiotechnol. 2015, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood–brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Emiliani, C.; Beccari, T.; Stirling, J.L.; Sciarra, R.; Orlacchio, A. New Biospecific Chromatographies of β-Hexosaminidases. In Macromolecular Biorecognition; Humana Press: Totowa, NJ, USA, 1987; pp. 309–319. [Google Scholar]
- Robinson, D.; Stirling, J.L. N-acetyl-β-glucosaminidases in human spleen. Biochem. J. 1968, 107, 321. [Google Scholar] [CrossRef]
- LaemmLi, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Tacchi, S.; Caponi, S.; Pellegrino, R.M.; Luzi, F.; Cottone, F.; Fioretto, D.; Emiliani, C.; Di Michele, A. Covalent Immobilization of Proteases on Polylactic Acid for Proteins Hydrolysis and Waste Biomass Protein Content Valorization. Catalysts 2021, 11, 167. [Google Scholar] [CrossRef]
- Tancini, B.; Magini, A.; Bortot, B.; Polchi, A.; Urbanelli, L.; Sonnino, S.; Severini, G.M.; Emiliani, C. β-Hexosaminidase over-expression affects lysosomal glycohydrolases expression and glycosphingolipid metabolism in mammalian cells. Mol. Cell. Biochem. 2012, 363, 109–118. [Google Scholar] [CrossRef]
- Espejo-Mojica, A.J.; Mosquera, A.; Rodrfguez-Lopez, A.; Diaz, D.; Beltran, L.; Hernandez, F.L.; Alméciga-Diaz, C.J.; Barrera, L.A. Characterization of recombinant human lysosomal beta-hexosaminidases produced in the methylotrophic yeast Pichia pastoris. Univ. Sci. 2016, 21, 195–217. [Google Scholar] [CrossRef]
- Kresse, H.; Fuchs, W.; Glössl, J.; Holtfrerich, D.; Gilberg, W. Liberation of N-acetylglucosamine-6-sulfate by human beta-N-acetylhexosaminidase A. J. Biol. Chem. 1981, 256, 12926–12932. [Google Scholar] [CrossRef]
- Dewji, N.N.; De-Keyzer, D.R.; Stirling, J.L. Purification and characterization of β-N-acetylhexosaminidase I2 from human liver. Biochem. J. 1986, 234, 157–162. [Google Scholar] [CrossRef] [PubMed]
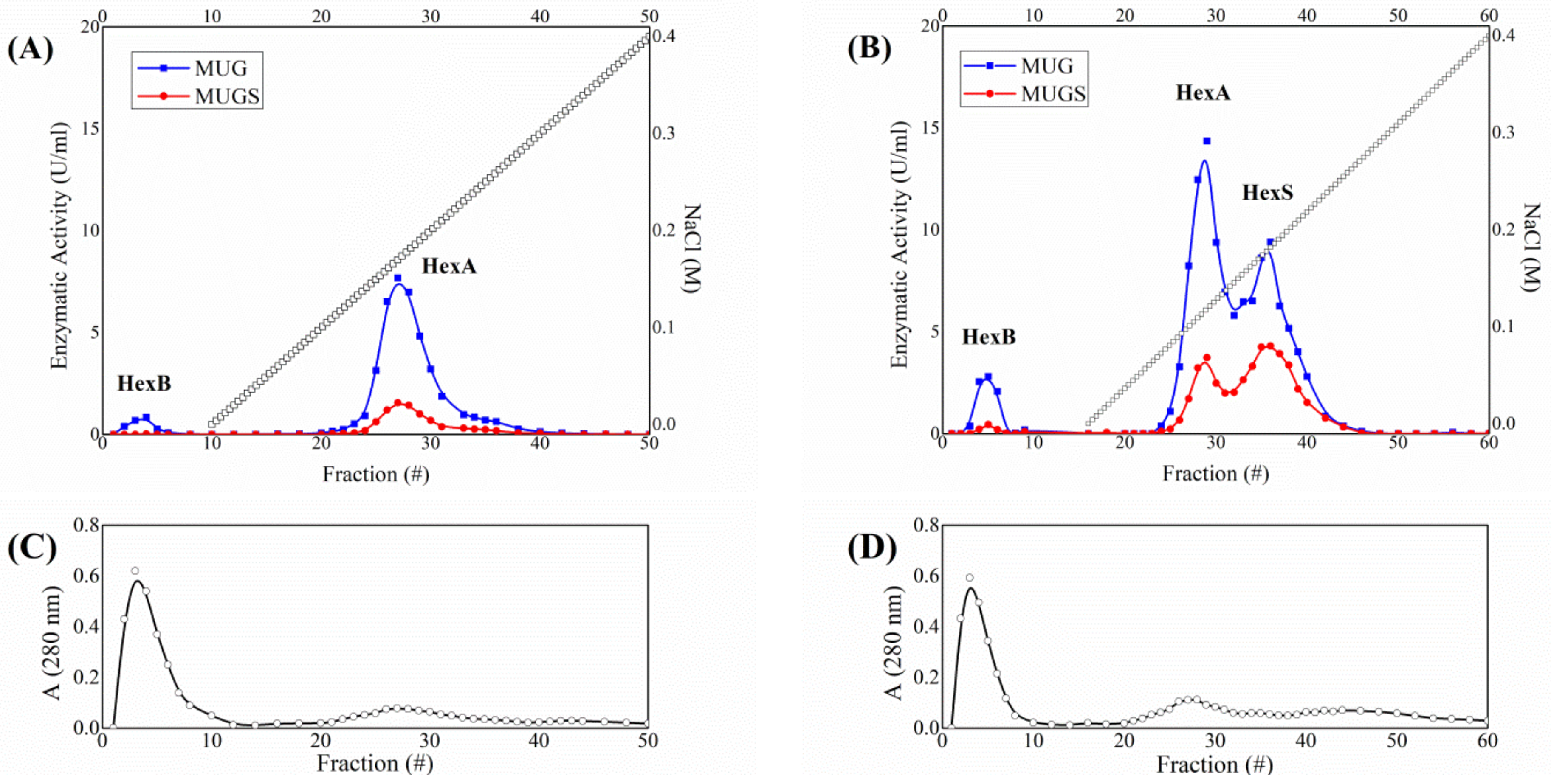
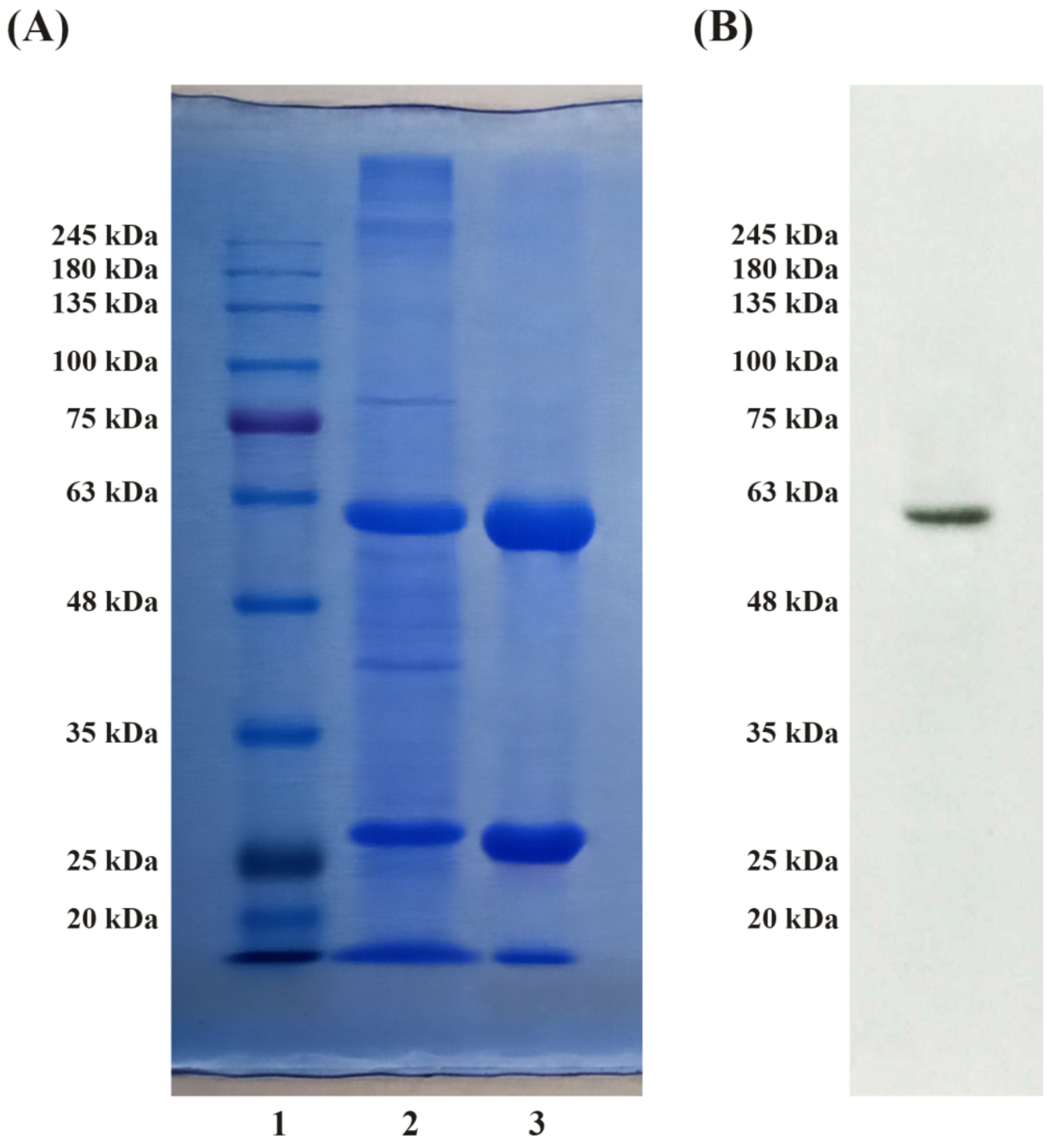


| Purification Step | HexA Specific Activity towards MUG (mU/mg) | Purification Ratio |
|---|---|---|
| Lysate HEK-HEXA | 42.5 | 1 |
| Affinity chromatography | 185 | 4.4 |
| Ion-exchange chromatography | 1250 | 29.4 |
| System | Km (mM) |
|---|---|
| Free HexA from HEK-293 cells | 1.0 |
| Free HexA from HL-60 cells [13] | 1.0 |
| Free HexA from human placenta [64] | 0.95 |
| Free HexA from human liver [65] | 0.8 |
| Immobilized HexA (10 days after immobilization) | 0.2 |
| Immobilized HexA (100 days after immobilization) | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Emiliani, C. Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications. J. Funct. Biomater. 2021, 12, 32. https://doi.org/10.3390/jfb12020032
Calzoni E, Cesaretti A, Montegiove N, Di Michele A, Emiliani C. Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications. Journal of Functional Biomaterials. 2021; 12(2):32. https://doi.org/10.3390/jfb12020032
Chicago/Turabian StyleCalzoni, Eleonora, Alessio Cesaretti, Nicolò Montegiove, Alessandro Di Michele, and Carla Emiliani. 2021. "Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications" Journal of Functional Biomaterials 12, no. 2: 32. https://doi.org/10.3390/jfb12020032
APA StyleCalzoni, E., Cesaretti, A., Montegiove, N., Di Michele, A., & Emiliani, C. (2021). Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications. Journal of Functional Biomaterials, 12(2), 32. https://doi.org/10.3390/jfb12020032










