Biocompatibility and Biological Corrosion Resistance of Ti–39Nb–6Zr+0.45Al Implant Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation and Biological Corrosion Resistance Test
2.2. In Vitro Experiment
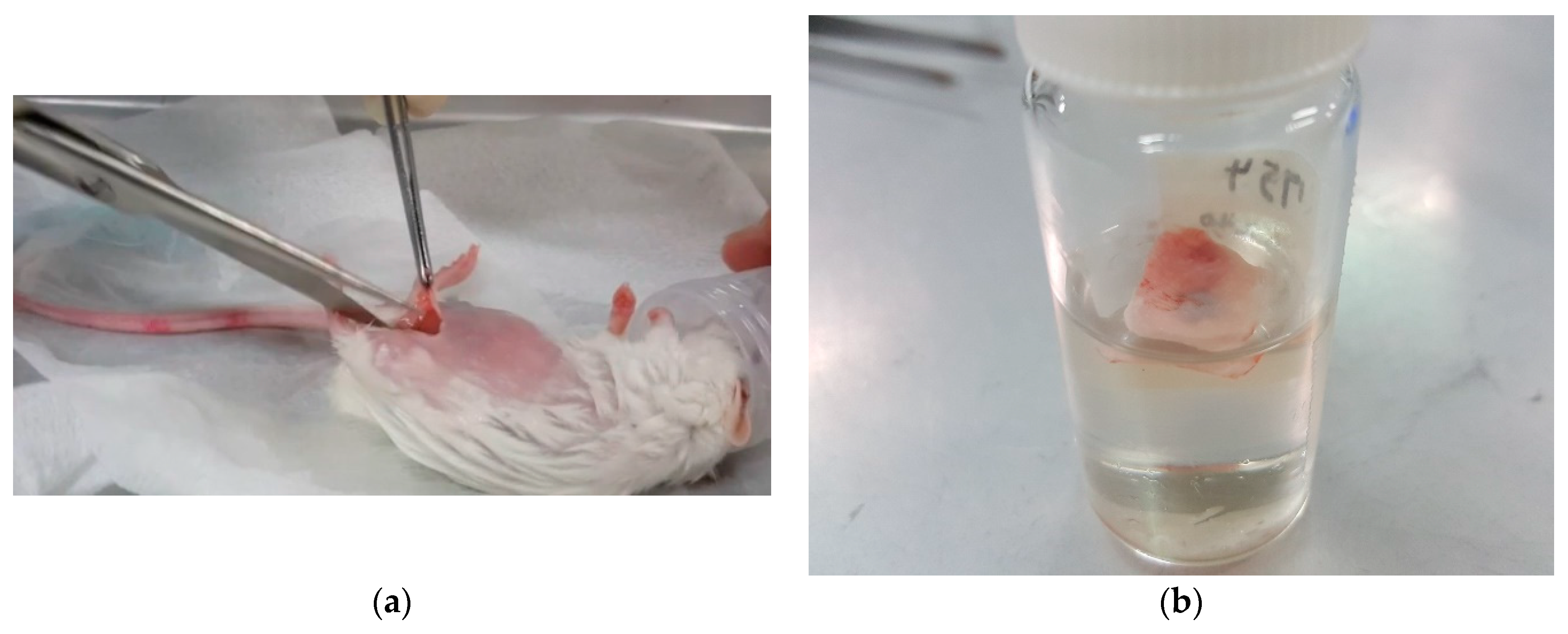
2.3. In Vivo Experiment
3. Results and Discussion
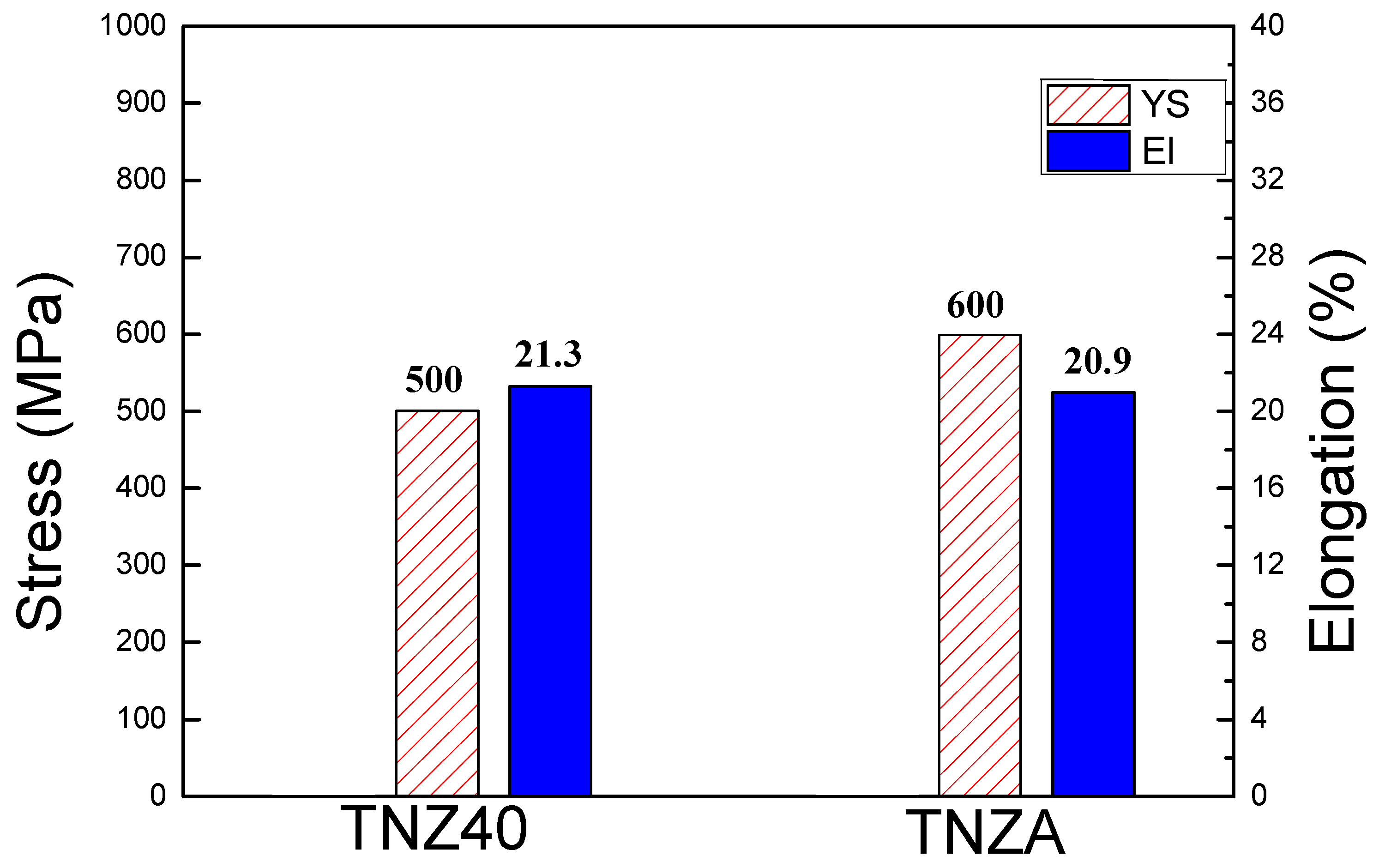
3.1. Change in Yield Strength (YS)
3.2. Biological Corrosion Resistance
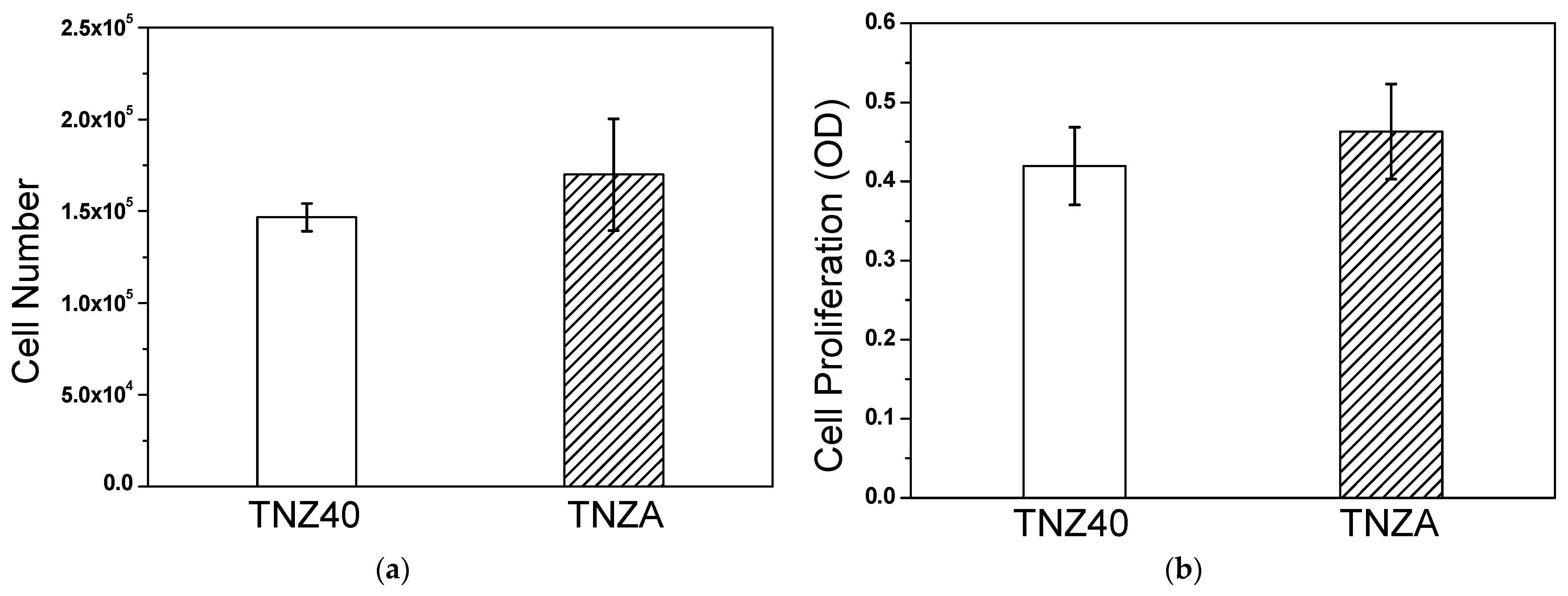
3.3. In Vitro Properties
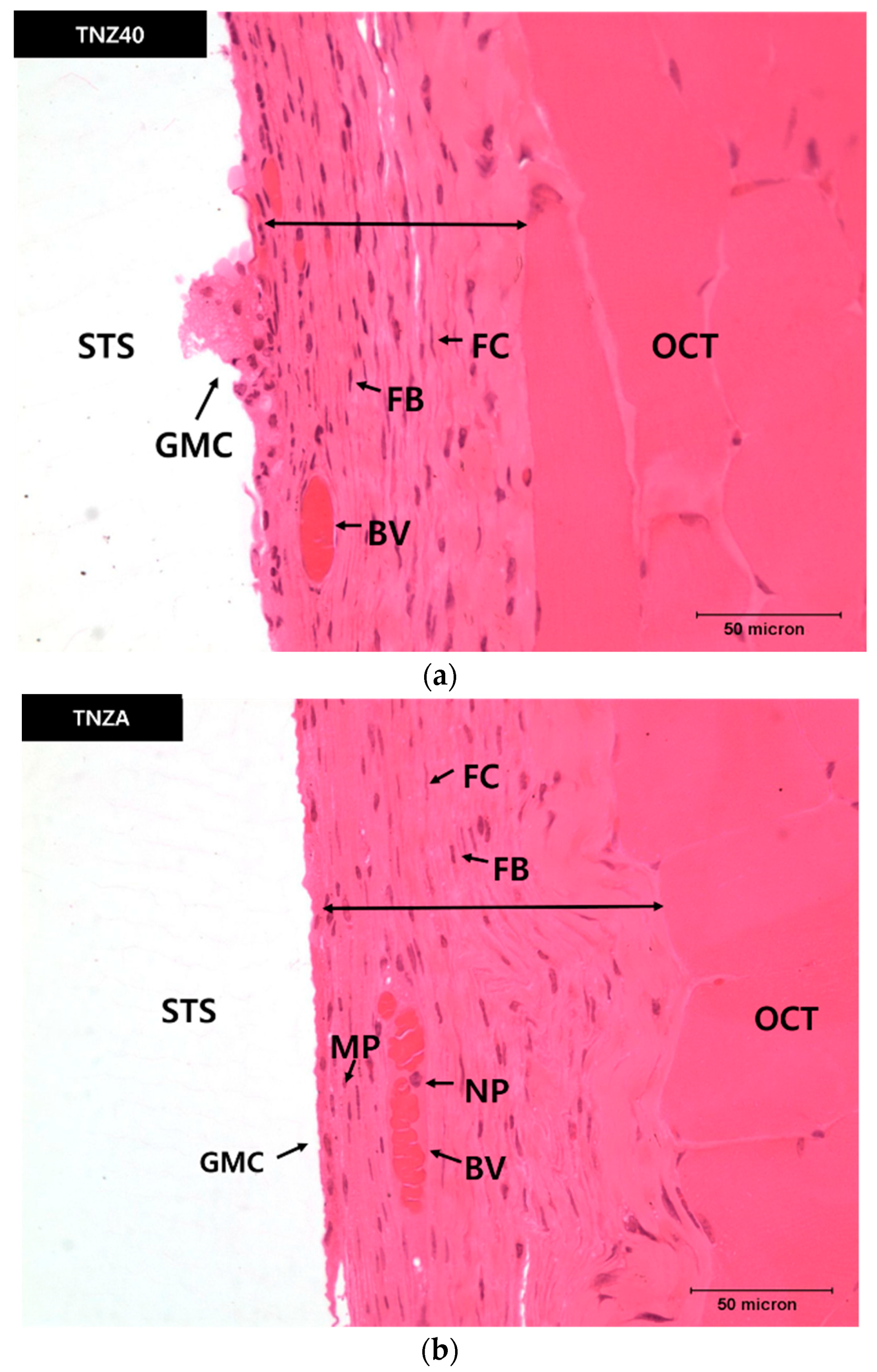
3.4. In Vivo Properties
4. Conclusions
- The YS was improved by approximately 20% owing to the deformation of the lattices by the formation of a substitutional solid solution and resulting obstruction of the dislocation movement by the Al alloying element added to the TNZ40 alloy. Thus, it can be utilized as a biocompatible implant material.
- The Icorr values of the TNZA and TNZ40 alloys were 2.104 and 3.157 μA/cm2, respectively. Pitting was not observed, because these two alloys consisted of stable passive films up to 1.5 V. This confirmed that the corrosion resistance of the TNZA alloy was better than that of the TNZ40 alloy and that the elution of Al ions does not lead to problems.
- The degrees of cell proliferation and absorbance were similar to those of the TNZ40 alloy, according to the evaluation of the biocompatibility of the TNZA alloy through the in vitro experiments (cell proliferation and measurement of the absorbance). According to the in vitro experiments, no harmful effects of the addition of a small amount of Al were observed.
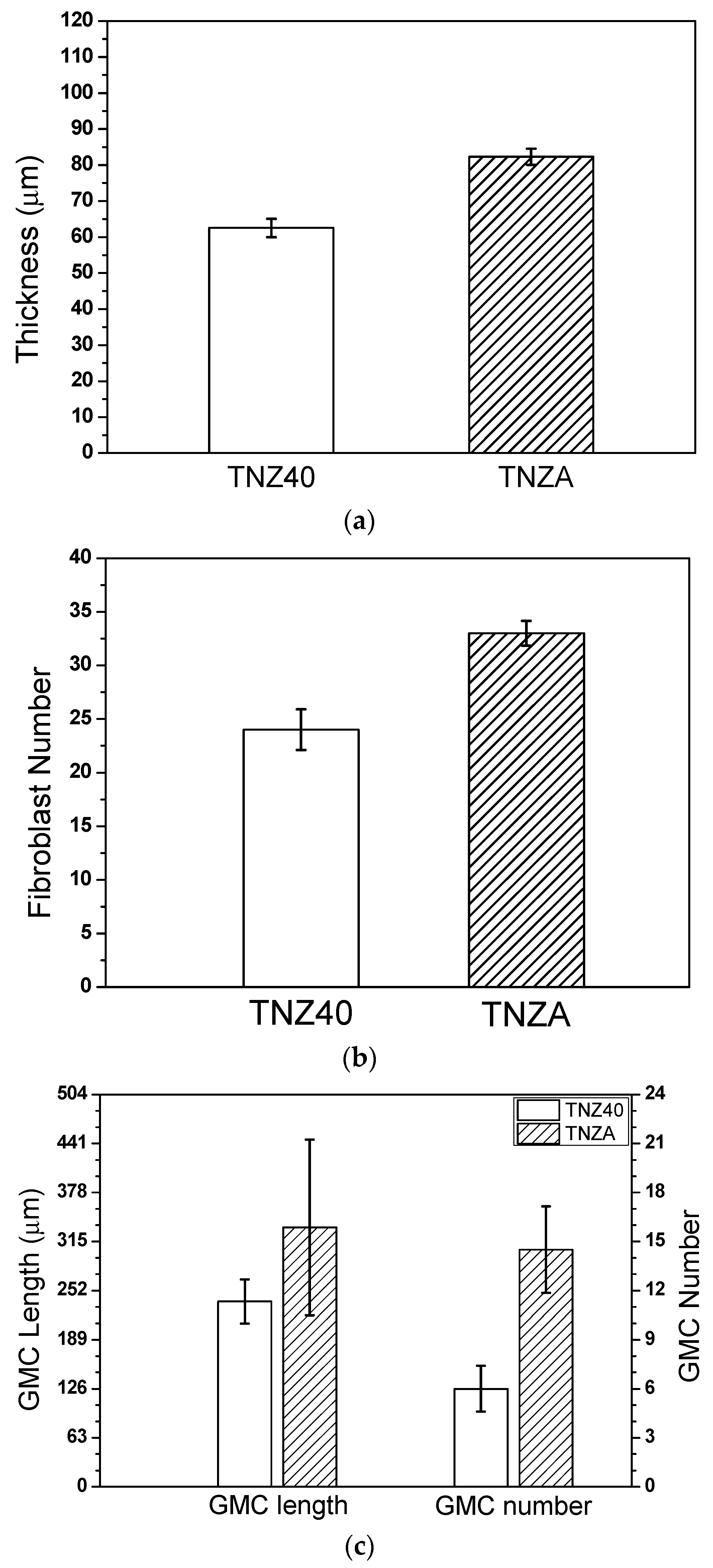
- According to the measurement of the thicknesses of the newly created fibrous connective tissue membranes four weeks after inserting the specimens of TNZA and TNZ40 into the mouse abdominal subcutaneous tissues, although the membrane thickness, number of fiber cells, and number and length of the GMCs of the TNZ40 alloy were smaller than those of the TNZA alloy, the differences were not significant. In addition, no tissue reactions such as tissue necrosis and bleeding around the inserted specimen were observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.W.; Ulrickson, M.A.; Causey, R.A. Use of titanium in fusion components. J. Nucl. Mater. 1994, 813–817, 212–215. [Google Scholar] [CrossRef]
- Williams, D.F. Tissue-biomaterial interactions. J. Mater. Sci. 1987, 22, 3421–3445. [Google Scholar] [CrossRef]
- Thair, L.; Mudali, U.K.; Bhuvaneswaran, N.; Nair, K.G.M.; Asokamano, R.; Raj, B. Nitrogen ion implantation and in vitro corrosion behavior of as-cast Ti-6Al-7Nb alloy. Corros. Sci. 2002, 44, 2439–2457. [Google Scholar] [CrossRef]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef]
- Niinomi, M. Metals for Biomedical Devices, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 122–156. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M.; Kuroda, D.; Fukunaga, K.; Morinaga, M.; Kato, Y.; Yashiro, T.; Suzuki, A. Corrosion wear fracture of new β type biomedical titanium alloys. Mater. Sci. Eng. 1999, 263, 193–199. [Google Scholar] [CrossRef]
- Shim, J.D.; Seok, H.K. A State of the Art on Research and Development of Biomedical Titanium Alloys. Biomater. Res. 2011, 15, 176–183. [Google Scholar]
- Laing, P.G.; Fergosun, A.B.; Hodge, E.S. Tissue reaction in rabbit muscle exposed to metallic implants. J. Biomed. Mater. Res. 1967, 1, 135–149. [Google Scholar] [CrossRef]
- Morais, L.S.; Serra, G.G.; Muller, C.A.; Andrade, L.R.; Palermo, E.F.A.; Elias, C.N.; Meyers, M. Titanium alloy mini-implants for orthodontic anchorage: Immediate loading and metal ion release. Acta Biomaterilia 2007, 3, 331–339. [Google Scholar] [CrossRef]
- Li, D.-J.; Ohsaki, K.; Li, K.; Cui, P.-C.; Ye, Q.; Baba, K.; Wang, G.-C.; Tenshin, S.; Takano-Yamamoto, T. Thickness of fibrous capsule after implantation of hydroxyapatite in subcutaneous tissue in rats. J. Biomed. Mater. Res. 1999, 45, 322–326. [Google Scholar] [CrossRef]
- Sumitomo, N.; Noritake, K.; Morikawa, T.; Hattori, K.; Niwa, S.; Sato, K.; Niinomi, M. Experiment study on fracture fixation with low rigidity titanium alloy. J. Mater. Sci. 2008, 19, 1581–1586. [Google Scholar] [CrossRef]
- Cheal, E.J.; Spector, M.; Hayes, W.C. Role of loads and prosthesis material properties on the mechanics of the proximal femur after total hip arthroplasty. Clin. Orthop. Relat. Res. 1992, 10, 405–422. [Google Scholar] [CrossRef]
- Lee, D.-G.; Mi, X.; Eom, T.K.; Lee, Y.T. Bio-Compatible Properties of Ti–Nb–Zr Titanium Alloy with Extra Low Modulus. J. Biomat. Tissue Eng. 2016, 6, 798–801. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lim, K.R.; Lee, Y.T.; Lee, D.-G.; Lee, J.H.; Kim, S.E. Effect of Aging Time and Temperature on Microstructure and Mechanical Properties of Ti-39Nb-6Zr Alloy. Korean J. Met. Mater. 2016, 54, 925–930. [Google Scholar]
- Lee, Y.T. Titanium; S&M Media Corp.: Seoul, Korea, 2009; pp. 59–127. [Google Scholar]
- Yang, Y.L.; Wang, W.Q.; Li, F.L.; Li, W.Q.; Zhang, Y.Q. The Effect of Aluminum Equivalent and Molybdenum Equivalent on the Mechanical Properties of High Strength and High Toughness Titanium Alloys. Mater. Sci. Forum 2009, 618–619, 169–172. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy, 3rd ed.; McGraw-Hill: New York, NY, USA, 1990; pp. 72–104. [Google Scholar]
- Jiang, X.J.; Jing, R.; Liu, C.Y.; Ma, M.Z.; Liu, R.P. Structure and mechanical properties of TiZr binary alloy after Al addition. Mater. Sci. Eng. 2013, 586, 301–305. [Google Scholar] [CrossRef]
- Wapner, K.L. Implications of Metallic Corrosion in Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 1991, 271, 12–20. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, K.M.; Lee, K.K.; Ryu, C.N.; Oh, T.W.; Kim, S.H.; Yoon, T.L. Effects of Nb Addition on Corrosion Resistance and Cytotoxicity Behavior of Ti Alloys. Korean J. Mater. Res. 2003, 13, 761–768. [Google Scholar]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, P.; Vasilescu, E.; Osiceanu, P.; Drob, S.I.; Popa, M.V. Electrochemical and Corrosion Behaviour of a New Titanium Base Alloy in Simulated Human Electrolytes. Int. J. Electrochem. Sci. 2013, 8, 10733–10745. [Google Scholar]
- Okazaki, Y.; Gotoh, E.; Manave, T.; Kobayashi, K. Comparison of metal concentrations in rat tibia tissues with various metallic implants. Biomaterials 2004, 25, 5913–5920. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Jaob, J.T.; Burgoyne, C.F.; McKinnon, S.J.; Tanji, T.M.; LaFleur, P.K.; Duzman, E. Biocompatibility response to modified Baerveldt glaucoma drains. J. Biomed. Mater. Res. 1998, 43, 99–107. [Google Scholar]
- Udipi, K.; Ornberg, R.L.; Thurmond, K.B., II; Settle, S.L.; Forster, D.; Riley, D. Modification of inflammatory response to implanted biomedical materials in vivo by surface bound superoxide dismutase mimics. J. Biomed. Mater. Res. 2000, 51, 549–560. [Google Scholar] [CrossRef]
- Ryhänen, J.; Kallioinen, M.; Tuukkanen, J.; Junila, J.; Niemelä, E.; Sandvik, P.; Serlo, W. In vivo biocompatibility evaluation of nickel-titanium shape memory metal alloy: Muscle and perineural tissue responses and encapsule membrane thickness. J. Biomed. Mater. Res. 1998, 41, 481–488. [Google Scholar] [CrossRef]

| Specimens | Cell Number (×103) | Cell Proliferation (×10−1) |
|---|---|---|
| TNZ40 | 146.7 ± 7.6 | 4.19 ± 0.49 |
| TNZA | 170.0 ± 30.4 | 4.63 ± 0.6 |
| Specimens | Thickness (μm) | Fibroblast Number | GMC Length (μm) | GMC Number |
|---|---|---|---|---|
| TNZ40 | 62.5 ± 2.6 | 24 ± 1.9 | 238 ± 28.2 | 6 ± 1.4 |
| TNZA | 82.3 ± 2.2 | 33 ± 1.2 | 333 ± 113 | 14 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-J.; Choi, Y.-S.; Hwang, Y.-H.; Cho, H.-W.; Lee, D.-G. Biocompatibility and Biological Corrosion Resistance of Ti–39Nb–6Zr+0.45Al Implant Alloy. J. Funct. Biomater. 2021, 12, 2. https://doi.org/10.3390/jfb12010002
Hwang Y-J, Choi Y-S, Hwang Y-H, Cho H-W, Lee D-G. Biocompatibility and Biological Corrosion Resistance of Ti–39Nb–6Zr+0.45Al Implant Alloy. Journal of Functional Biomaterials. 2021; 12(1):2. https://doi.org/10.3390/jfb12010002
Chicago/Turabian StyleHwang, Yu-Jin, Young-Sin Choi, Yun-Ho Hwang, Hyun-Wook Cho, and Dong-Geun Lee. 2021. "Biocompatibility and Biological Corrosion Resistance of Ti–39Nb–6Zr+0.45Al Implant Alloy" Journal of Functional Biomaterials 12, no. 1: 2. https://doi.org/10.3390/jfb12010002
APA StyleHwang, Y.-J., Choi, Y.-S., Hwang, Y.-H., Cho, H.-W., & Lee, D.-G. (2021). Biocompatibility and Biological Corrosion Resistance of Ti–39Nb–6Zr+0.45Al Implant Alloy. Journal of Functional Biomaterials, 12(1), 2. https://doi.org/10.3390/jfb12010002




